Abstract
Mannose-capped lipoarabinomannan (ManLAM) is a complex lipoglycan abundantly present in the Mycobacterium tuberculosis cell envelope. Many biological properties have been ascribed to ManLAM, from directly interacting with the host and participating in the intracellular survival of M. tuberculosis, to triggering innate and adaptive immune responses, including the activation of CD1b-restricted T cells. Due to its structural complexity, ManLAM is considered a heterogeneous population of molecules which may explain its different biological properties. The presence of various modifications such as fatty acids, succinates, lactates, phosphoinositides and methylthioxylose in ManLAM have proven to correlate directly with its biological activity and may potentially be involved in the interactions between CD1b and the T cell population. To further delineate the specific ManLAM epitopes involved in CD1b-restricted T cell recognition, and their potential roles in mediating immune responses in M. tuberculosis infection, we established a method to resolve ManLAM into eight different isoforms based on their different isoelectric values. Our results show that a ManLAM isoform with an isoelectric value of 5.8 was the most potent in stimulating the production of interferon-γ in different CD1b-restricted T-cell lines. Compositional analyses of these isoforms of ManLAM revealed a direct relationship between the overall charge of the ManLAM molecule and its capacity to be presented to T cells via the CD1 compartment.
Keywords: CD1b, lipoarabinomannan, lipoglycans, Mycobacterium tuberculosis, T cells
Introduction
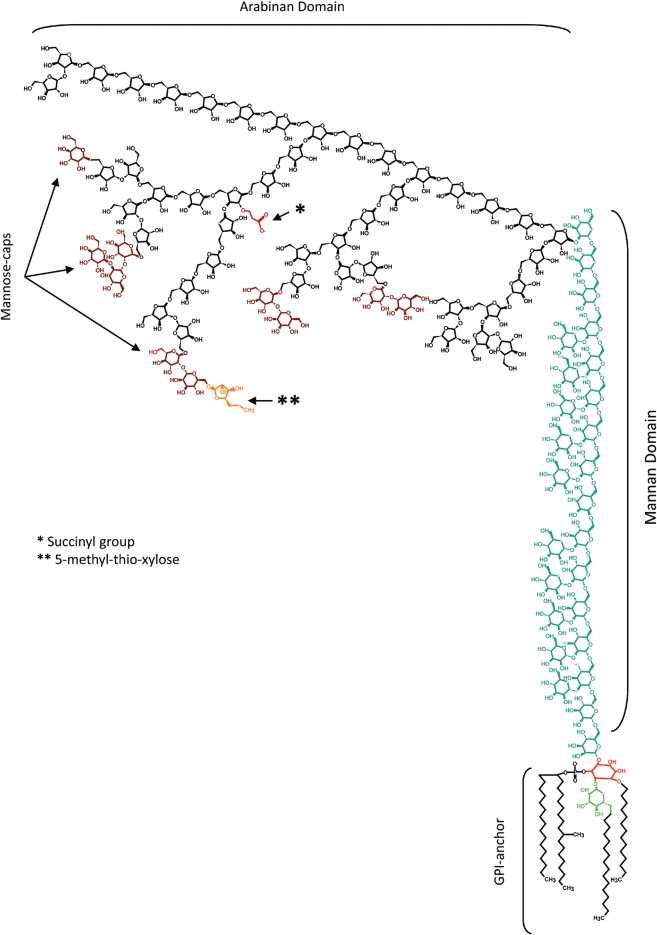
The complex surface of the Mycobacterium tuberculosis (M.tb) cell envelope is formed by cell wall and capsule-like components (Crick et al. 2003; Torrelles 2012). This lipid-rich low permeability matrix contributes to the difficulty in combating mycobacterial diseases by endowing M.tb with innate resistance to therapeutic agents and host defenses. The outermost components are mainly solvent-extractable non-covalently bound free lipids and carbohydrates associated with the mycolylarabinogalactan–peptidoglycan complex (cell wall core) of M.tb (Kolattukudy et al. 1997). We have shown that several of these outer cell envelope components are important for bacterial uptake and survival (Kang et al. 2005;Torrelles et al. 2006). Although the surface of M.tb contains α-glucan and some traces of xylan (Lemassu and Daffe 1994), it is particularly rich in mannose-containing molecules, such as mannose-capped lipoarabinomannan (ManLAM), lipomannan (LM), phosphatidyl-myo-inositol mannosides (PIMs), arabinomannan, mannan and mannoglycoproteins. ManLAM is a pivotal mycobacterial antigenic lipoglycan found in various compartments of the M.tb cell envelope, notably the cell wall proper and the cytoplasmic membrane (Torrelles and Schlesinger 2010). ManLAMs isolated from M.tb, M. leprae, M. bovis BCG and M. avium among others share the same basic structure characterized by a diacyl glyceryl phosphatidyl-myo-inositol anchor, mannan and arabinan domains, and manno-oligosaccharide caps (Figure 1; reviewed in Kaur et al. 2009).
Fig. 1.
ManLAM structure. ManLAM is a lipoglycan described as a tripartite structure containing a d-mannan and d-arabinan domains, a GPI anchor and terminal mannose-motifs. The d-mannan is composed of a linear [α-d-Manp(1 → 6)-α-d-Manp]n with single α-d-Manp branches at position C2 (blue resides). The d-arabinan domain (black residues) is composed of a linear α-d-Araf(1 → 5)-α-d-Araf with some branches at position C3. The non-reducing terminal of this d-arabinan is characterized by being mannose capped with mono-, di- or tri- α(1 → 2)-linked α-d-Manp (dark-red residues). A 5-methyl-thio-xylose (orange residue, double-asterisk) attached to position C6 of the terminal α-d-Manp of the capping has been also described. Succinyl residues (red chain, asterisk) have been described on position C2 of the 3,5-α-d-Araf. A mannose-containing phosphatidyl-myo-inositol anchor has been described where the myo-inositol (red residue) is linked at position C6 to the mannan core, at position C2 to a α-d-Manp (green residue) and at position C1 to a phosphodiacylglycerol containing palmitic acid (16:0) and TBST. A third acyl substituent can be found at position C6 of the α-d-Manp linked at the C2 position of the myo-inositol. A fourth acyl group may also be found at position C3 of the myo-inositol.
The majority of ManLAM biological functions have been attributed to its degree of mannose capping and acylation, i.e. fatty acids, succinates and lactates (Hunter et al. 1986; Chatterjee et al. 1993; Delmas et al. 1997; Khoo et al. 2001). All of these characteristics together define the high complexity present in the population of ManLAM molecules. As a consequence of this peculiar heterogeneity, it is difficult to address which features of ManLAM are responsible for its wide variety of biological functions. In this context, ManLAMs derived from different M.tb strains have been shown to minimize macrophage microbial activity by negatively modulating the production of nitric oxide, oxygenated radicals and inflammatory cytokines (Briken et al. 2004). In addition, ManLAM facilitates the survival of the bacillus inside the macrophage (Vergne et al. 2003) through the initial binding of M.tb to the surface macrophage mannose receptor (MR) via ManLAM (Kang et al. 2005). The capacity of ManLAM to stimulate CD1b-restricted T-cells, which can kill infected macrophages, shows that ManLAM can also play an important role in protective immunity against M.tb infection (Sieling et al. 1995; Stenger et al. 1997; Porcelli and Modlin 1999). In this context, binding of ManLAM to the MR (Schlesinger et al. 1994; Venisse et al. 1995) is also known to stimulate T cell clones with different fine specificities (Sieling et al. 1995). Some mycobacterial LMs can induce a strong pro-inflammatory response via Toll-like receptor 2 (TLR2) alone or in association with TLR1 or TLR6 (reviewed in Jo et al. 2007; Sasindran and Torrelles 2011). Moreover, M.tb LM having similar glycosylphosphatidyl-myo-inositol (GPI) anchor structural features to ManLAM is not capable of stimulating CD1-restricted T cells (Torrelles et al. 2011). However, other LMs can induce an anti-inflammatory response through a TLR-independent pathway (Quesniaux et al. 2004). In this context, Rajaram et al. (2011) recently identified a cellular mechanism whereby M.tb LM is capable of blocking tumor necrosis factor biosynthesis in human macrophages.
Based on the previous observations that mycobacterial lipoglycans in the form of ManLAM and its structural variants can stimulate T cells in the context of CD1b (Porcelli and Modlin 1999), we determined to resolve homogeneous subpopulations of ManLAM to allow for a comparison of different entities in relation to their capacity to stimulate CD1-restricted T cells. The fact that these CD1-restricted T cell lines have been shown to strongly recognize ManLAM from M. leprae but not from M.tb H37Rv supports the case for subtle structural differences contributing to their biological functions. Indeed, our studies comparing ManLAMs from different mycobacterial strains have revealed a truncated form from an ethambutol resistant strain of M.tb which stimulated strong proliferation of two different T cell lines in the context of human CD1b molecules (Torrelles et al. 2004).
In this present study, we established a methodology to study ManLAM heterogeneity based on the presence of charged groups such as succinates and phosphates, which allowed us to separate ManLAM into eight different isoforms, some of which were biochemically characterized, and out of these, one with an isoelectric point (pI) value of 5.8 was the only form of ManLAM capable of activating CD1-restricted T cells.
Results
Heterogeneity of ManLAM, LM and PIMs
Depending on the pH of their local environment, for every charged molecule, there is a specific pH at which the net charge that it carries is zero. This pI value is the characteristic property of every molecule that permits fractionation of complex mixtures in a pH gradient. Although a very effective technique applicable to proteins, this is not the case for carbohydrates, which are normally neutral. However, considering that ManLAM may be charged due to the carboxyl groups on its acyl substituents and phosphates, we exposed ManLAM to a separation by charge and size using two-dimensional (2D) electrophoresis (Torrelles et al. 2004). To avoid the precipitation of ManLAM and to maximize resolution, we tried different combinations of chaotropic agents, detergents and ampholytes. Our results (Figure 2) show that the combination of urea, 4-nonylphenolpolyethylenglycol and a mixture of ampholyte 4.5–5.4 and ampholyte 3–10 were optimal to solubilize and resolve ManLAM into eight discrete isoforms. The pI value of each isoform was determined to be 4.7, 4.8, 5.1, 5.3, 5.5, 5.7, 5.8 and 6.8 (Figure 2A). These results showed that the bands differed only in charge but not in size. All of the bands showed positive reactivity to the anti-LAM monoclonal antibody CS-35 (Figure 2A), which recognizes preferentially with the terminal Ara4, Ara5 and Ara6 motifs of LAM (Kaur et al. 2002; Rademacher et al. 2007). Structurally related LM and PIMs were also submitted to isoelectric focusing (IEF); interestingly, these two samples gave band patterns different from ManLAM (Figure 2B).
Fig. 2.
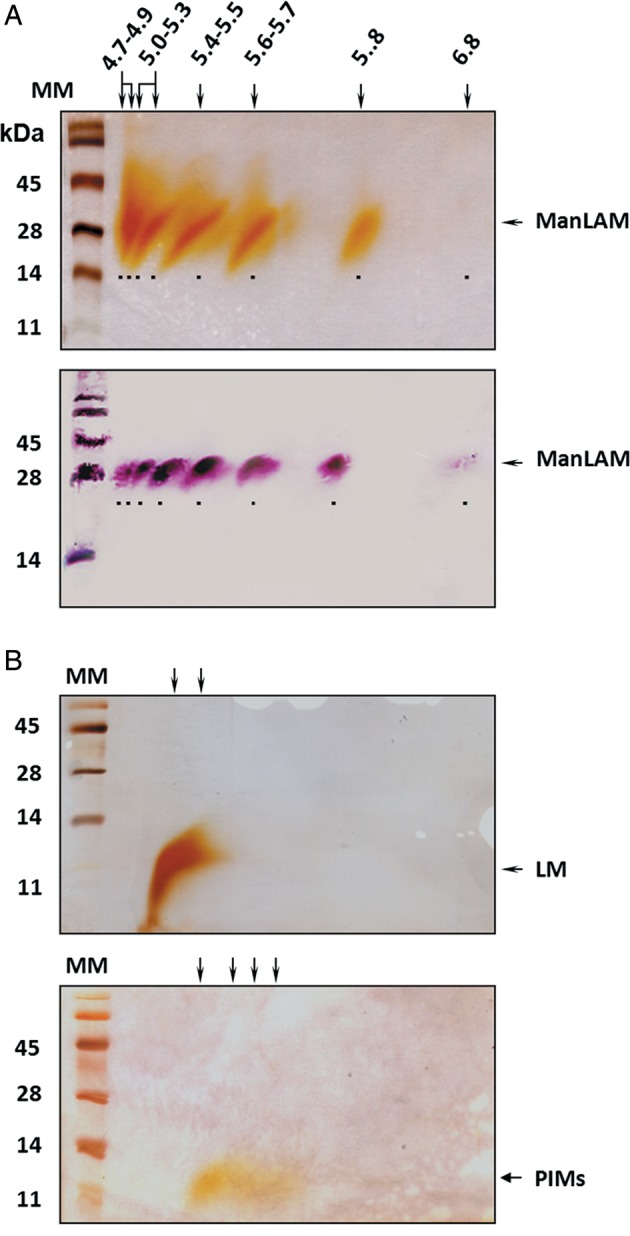
Resolution of ManLAM, LM and PIMs from M.tb by charge and size. (A) ManLAM was resolved into eight discrete isoforms based on pI. Western blot using anti-LAM monoclonal antibody CS-35 shows that all isoforms were immunoreactive. For better visualization, bands are underlined. Notice that the ManLAM pI 6.8 isoform is only visualized on the western blot due to its low abundance within the pre-IEF LAM population. This isoform is better visualized in Figure 4 after IEF. (B) Separation of LM and PIMs by charge showed that LM could be separated into two overlapping isoforms and PIMs into four diffused isoforms.
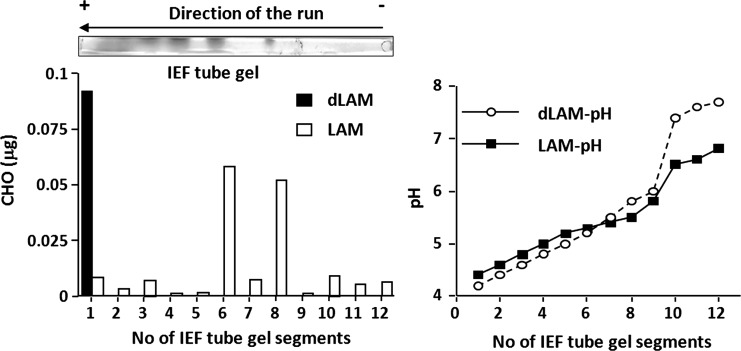
To determine whether the acylation of ManLAM controlled the behavior of these molecular populations in 1D and 2D sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE), ManLAM was deacylated and both ManLAM and deacylated ManLAM were subjected to IEF. Deacylation removed all acyl substitutions present in ManLAM (i.e. fatty acids, succinates and lactates). Deacylated ManLAM did not run in the first or second dimension, suggesting that the acyl groups are important in the observed separation by charge. To further establish if the separation by IEF was based on net charge or degree of acylation, the tube gels containing ManLAM and deacylated ManLAM were submitted to IEF and used to establish the pH gradient of the tube gel and the sugar composition after excising the tube gels into 12 different segments (Figure 3). Our results indicate that the pH range of the IEF gel containing ManLAM isoforms was from 4.38 to 6.77; however, for the IEF gel containing deacylated ManLAM, it was from 4.23 to 7.64, thus indicating that intrinsic ManLAM characteristics may alter the final IEF gel pH gradient. As expected, ManLAM separated into several isoforms in the IEF gel, with the most abundant isoforms located at segments 6 (pH 5.25) and 8 (pH 5.46). Contrary to our expectations, deacylated ManLAM migrated to the acidic end (pH 4.23, see segment 1, black bar), indicating that the acyl groups present in ManLAM are important for its IEF separation.
Fig. 3.
The acyl groups of ManLAM are responsible for IEF separation. Carbohydrate determination by the phenol/H2SO4 assay of the excised tube gels after ManLAM separation by charge indicates that acylated ManLAM separated in a broad pI range (4.7–6.5); however, deacylated ManLAM (dLAM) ran into the acidic region (pI 4.7). A representative experiment is shown of n = 3.
Purification of ManLAM isoforms
To determine whether ManLAM isoforms could be purified in quantities to allow for structural evaluation, two different preparative approaches were attempted, the rotofor (Ayala et al. 1998) and the whole-gel eluter (Weldingh et al. 2000), both previously successfully applied for protein purification based on their net charge by IEF. However, in the case of ManLAM, these methods commonly used to fractionate proteins by charge and/or size did not work (data not shown).
The observation that ManLAM separates in different bands in the IEF tube gels after electrofocusing led us to develop an approach that yielded unexpected optimal separation and yields. Multiple preparative IEF (tube) gels (155 mm × 6 mm i.d.) were loaded each with 1 mg of ManLAM and electrophoresed for 3 h at 1000 V. A control tube containing only 100 μg of ManLAM was stained with periodic acid silver nitrate staining (PAS) and discrete bands were visualized corresponding to their pI values. The stained gel was used as a control to identify the bands on the remaining unstained tube gels. Gels were excised per banding pattern, and each isoform recovered from each excised band. Aliquots from band extractions were examined by 2D SDS–PAGE (Figure 4). Eight isoforms were detected with pI values 4.7, 4.8, 5.1, 5.3, 5.5, 5.7, 5.8 and 6.8. Interestingly, lighter bands observed in 2D SDS–PAGE prior to performing the preparative IEF appeared to intensify after focusing. Several of the isoforms were pure and others less so as a consequence of their close pI values (Figure 4). Using this approach, sample recovery was ∼99% (Table I).
Fig. 4.
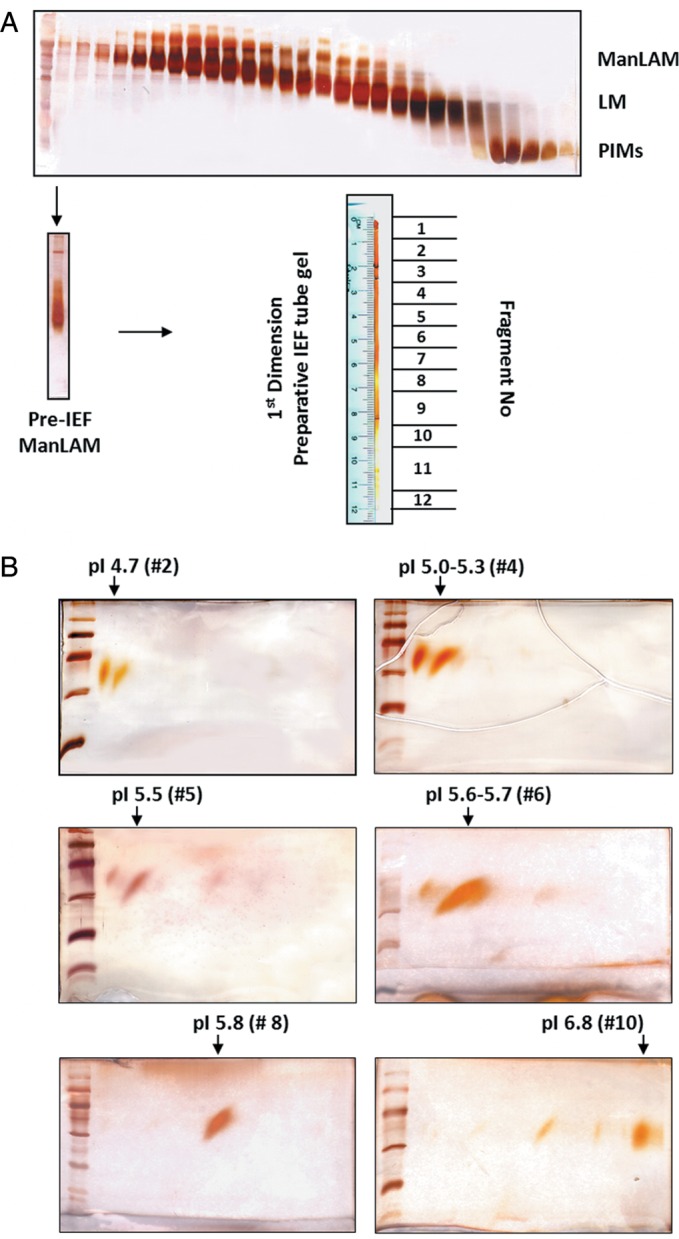
Purification of ManLAM isoforms by an unconventional approach. (A) Soluble M.tb lipoglycans were separated by size-exclusion chromatography. Fractions were analyzed by SDS–PAGE showing the purification of ManLAM, LM and PIMs. Up to 1 mg of ManLAM (pre-IEF) were run in large tube gels (total of 12 tube gels, loaded with 1 mg/each) and isoforms were excised and extracted from the tube gels as described in Materials and methods. Twelve fractions were obtained. (B) The SDS-PAGE analyses of fractions #2 (pI 4.7), #4 (pI 5.0–5.3), #5 (pI 5.5), #6 (pI 5.6–5.7), #8 (pI 5.8) and #10 (pI 6.8) corresponding to different ManLAM isoforms separated by their pI value (representative experiments of n = 3).
Table I.
ManLAM isoform weights obtained by preparative tube gel IEF
| Fraction number | Weight (mg) | pI |
|---|---|---|
| 1 | 1.120 | 4.5–4.6 |
| 2 | 0.986 | 4.7 |
| 3 | 0.835 | 4.8–4.9 |
| 4 | 0.650 | 5.0–5.3 |
| 5 | 0.615 | 5.5 |
| 6 | 0.590 | 5.6–5.7 |
| 7 | 0.418 | 5.7 |
| 8 | 0.378 | 5.8 |
| 9 | 0.201 | 5.9–6.7 |
| 10 | 0.078 | 6.8 |
| 11 | 0.054 | 6.9 |
| 12 | 0.018 | 7.0 |
| Total | 5.943 | |
| Recovery | 99.05% |
Six preparative tube gels loaded with 1 mg of ManLAM (pre-IEF) each were submitted to IEF. After focusing, tubes were excised into 12 different segments obtaining 12 different fractions, their pI calculated and ManLAM isoforms recovered and their weights obtained.
Compositional analysis of ManLAM isoforms
The sugar content of purified pre-IEF ManLAM and two of its isoforms (pI 5.6–5.7 and pI 5.8) was determined by gas chromatography (GC) analyses. Results in Table II showed a similar number of mannose for the pre-IEF and the isoforms analyzed. The ratio Ara:Man was virtually unchanged for pre-IEF and pI 5.6–5.7 ManLAMs (∼1.20:1); however, pI 5.8 ManLAM had a decrease in its Ara:Man molar ratio (0.84:1). The isoform pI 5.8 ManLAM still had more arabinose residues than LM, which had an Ara:Man molar ratio of 0.20:1, indicating that LM contained ∼4 or 5 Ara residues (Table II). Having established that ManLAM isoforms differ in their total neutral sugar content, efforts were next focused on analyzing the GPI anchor to establish if there were differences in the fatty acid profiles. Methyl ester derivatives of ManLAM fatty acyl groups were characterized by GC/mass spectrometry (GC/MS). The spectrum of the pre-IEF ManLAM yielded ions at m/z 270 (16:0, palmitic acid) and m/z 298 (18:0, stearic acid), identifying the major species present. Tuberculostearic acid (TBST) at m/z 312 and oleic acid (18:1) at m/z 296 were also present in small amounts. The ManLAM pI 5.6–5.7 isoform had an increase of 16:0, whereas the main fatty acyl constituents for ManLAM pI 5.8 were identified as 16:0 and TBST and were significantly more abundant (∼2- and ∼3-fold higher, respectively) when compared with pre-IEF ManLAM or when compared with ManLAM pI 5.6–5.7 (∼1.5- and ∼13-fold higher, respectively). Conversely, the presence of 18:0 in ManLAM pI 5.8 was significantly lower (2.4-fold lower) with respect to pre-IEF ManLAM (Table II). Importantly, ManLAM pI 5.8 also had traces of myristic acid (14:0), arachidic acid (20:0), behenic acid (22:0), erucic acid (22:1) and lignoceric acid (24:0); fatty acids that were undetectable in the other ManLAM isoforms analyzed.
Table II.
Compositional analysis of M.tb mannosylated lipoglycans
| ManLAM pre-IEF | ManLAM pI 5.6–5.7 | ManLAM pI 5.8 | LM | PIMs | |
|---|---|---|---|---|---|
| Neutral sugar analysis (residues per molecule) | |||||
| Ara | 28 | 30 | 21 | 5 | 0 |
| Man | 24 | 25 | 25 | 25 | 4 |
| myo-Inos | 1 | 1 | 1 | 1 | 1 |
| Total CHO | 53 | 56 | 47 | 31 | 5 |
| Ara:Man | 1.17:1 | 1.2:1 | 0.84:1 | 0.2:1 | N/A |
| Fatty acid analysisa (per myo-Inos) | |||||
| 16:0 | 1.34 | 1.77 | 2.66*** | 0.72 | 0.51 |
| 18:0 | 0.84 | 0.54 | 0.35** | 0.57 | 0.23 |
| 18:1 | 0.12 | 0.07 | 0.15 | 0.08 | 0.12 |
| TBST | 0.62 | 0.14 | 1.83*** | 0.55 | 0.75 |
| Succinate analysisa (per myo-Inos) | |||||
| Succinyl | 4.6 | 1 | 0.28 | 0.84 | 0 |
The sugar, fatty acyl and succinyl composition was determined by separate GC and GC/MS analyses. Shown are mean values from n = 2 performed in duplicate.
aValues for each experiment are nmol of fatty acid and succinates per nmol of myo-inositol.
**P < 0.0005.
***P < 0.0001, Student's t-test ManLAM pI 5.8 vs ManLAM Pre-IEF.
The presence of succinates was confirmed by GC/MS of their octyl ester derivatives obtained through the octanolysis of intact ManLAM (Table II). Octyl ester derivatives with a retention time of 24.6 min were co-eluted with an authentic dioctyl succinate with a mass value of 343 [M + H], and ions at m/z 213 and 157 as we have previously described (Torrelles et al. 2004). Integration of this peak with respect to neutral sugar indicated that there could be in total 4–5 succinyl residues present in the pre-IEF ManLAM. The succinyl residues in ManLAM pI 5.6–5.7 and LM were decreased to 1, and ManLAM pI 5.8 had only 0.3 succinates; the later meaning that for every three ManLAM pI 5.8 molecules, only one of these contained a succinate. Thus, the presence of succinates was detectable in all samples and inversely correlated with the isoform pI value. Conversely, lactates were not detected in any of the samples studied. A study of the phosphorous content did not reveal any difference among pre-IEF ManLAM and its isoforms with respect to the control diacylated phosphatidyl-myo-inositol dimannoside (PIM2), a molecule which is structurally defined as containing only one phosphate (data not shown). This result suggests that the difference in charge between ManLAM isoforms was not due to differences in their phosphate content. In summary, ManLAM pI 5.8 had a smaller arabinan chain, limited succinylation and more fatty acids than other ManLAM isoforms and pre-IEF ManLAM examined.
CD1b-restricted T cell proliferation induced by ManLAM isoforms
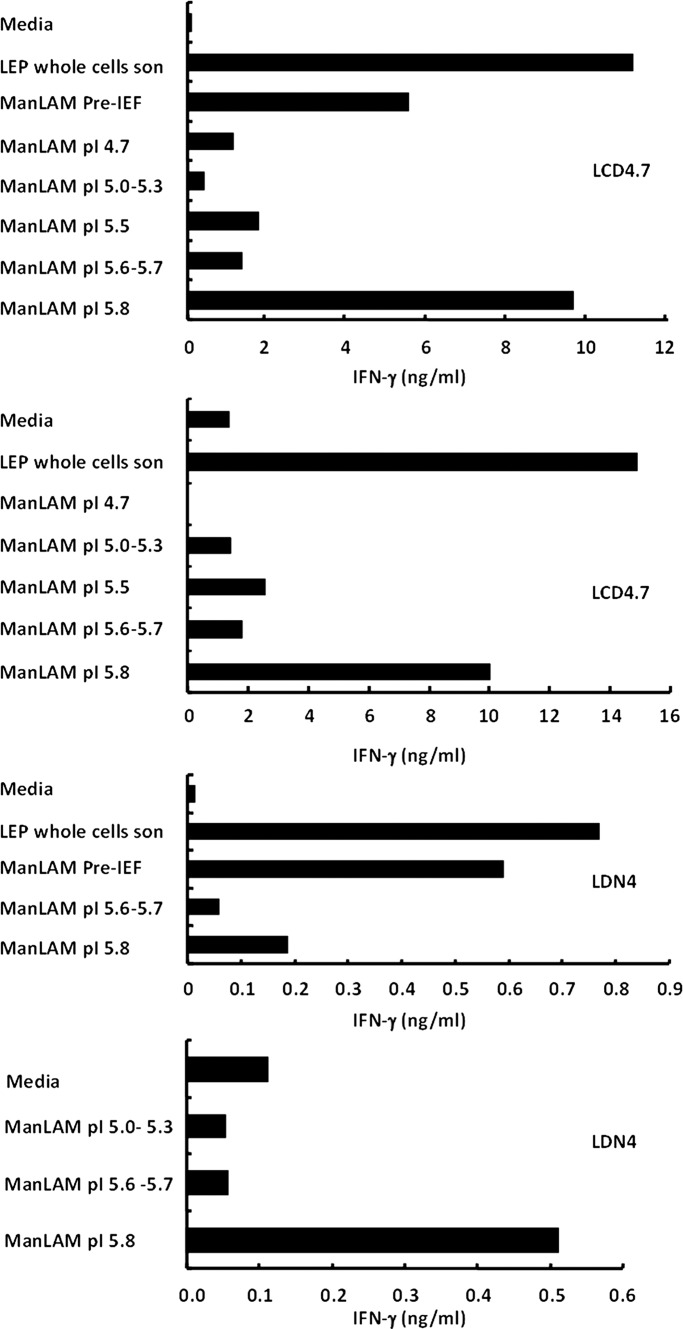
Based on their overall compositional differences, pre-IEF ManLAM, ManLAM pI 5.6–5.7 and ManLAM pI 5.8 were evaluated for differential abilities to induce CD1b-restricted T cell responses. We hypothesized that a shorter arabinan domain together with the larger number of acyl groups would be important in inducing T cell proliferation. This hypothesis is based on our previous report (Torrelles et al. 2004), demonstrating that CD1b-restricted T cell responses were specific to M. leprae ManLAM and M.tb CSU20 ManLAM (the latter being the pre-IEF ManLAM used in this study), which were characterized as containing smaller arabinans, large quantities of succinates and maximally acylated with fatty acids in their GPI anchors. Two M.tb LAM-reactive CD1b-restricted T cell clones, both obtained from patients infected with M. leprae, were studied in terms of their response to the two different ManLAM isoforms. Based on our previous dose-dependent studies using the same pre-IEF ManLAM (Torrelles et al. 2004), two different CD1b-restricted T cell clones were incubated with 0.5 μg/mL of pre-IEF ManLAM or its isoforms and assessed for their response in the terms of interferon-γ (IFN-γ) production (Figure 5). Strikingly, ManLAM pI 5.8 was a more potent inducer of T cell activation with respect to CD1b-restricted T cell responses when compared with the other ManLAM isoforms, including ManLAM pI 5.6–5.7. Specifically, for LCD4.7, ManLAM pI 5.8 was able to induce higher levels of IFN-γ secretion from CD1b reactive T cells than the pre-IEF ManLAM, reaching similar levels to our positive control M. leprae whole-cell sonicate. These results, combined with our previous results obtained with M.tb H37Rv ManLAM and M. leprae ManLAM (Torrelles et al. 2004), indicate that CD1b antigen presentation of lipoglycan antigens to T cells is regulated by the overall charge provided by the presence of specific acyl moieties. Among the acyl moieties, it is difficult to differentiate between the fatty acids in the GPI anchor and the succinyl groups, since chemical deacylation removes all acyl groups indiscriminately. Thus, the specific role of the succinates located in the arabinan domain as well as the arabinan domain size per se is uncertain. However, our comparative studies using purified species of PIMs, which structurally share a similar GPI anchor to ManLAM and LM, and importantly lack succinates (Torrelles et al. 2004), did not show any evidence that the GPI anchor alone is involved in inducing T cell activation (Torrelles et al. 2011). Thus, our results suggest that the GPI anchor of ManLAM (the same as the one present in PIMs) is not directly responsible for inducing IFN-γ by CD1b-restricted T cells. The fact that two separate CD1b-restricted T cell clones showed T cell reactivity to ManLAM pI 5.8, points to the functional significance of the overall charge present among ManLAM isoforms, where this charge is probably influenced by the overall size of its arabinan domain, the number of succinates in their arabinan and/or mannan domains and the number of fatty acids in their GPI anchor. Thus, a higher acylation together with smaller arabinan may induce/generate an overall molecular charge that results in a particular 3D structure important for CD1b/T cell interaction/recognition. All of these structural parameters, together with the degree of succinylation, could represent the prerequisite molecular charge required for the activation of T-cell responses via CD1.
Fig. 5.
The ManLAM pI 5.8 isoform is capable of stimulating the production of IFN-γ in CD1b-restricted T cell lines. Purified ManLAM isoforms (at 0.5 µg/mL) induced preferential CD1b-restricted T cell activation. The isoform with pI 5.8 exclusively activated CD1b-restricted T cells inducing IFN-γ production. Mycobacterium leprae whole-cell sonicate was used as the positive control for comparison purposes. Shown are representative experiments using two different CD1b-restricted T cell lines (i.e. LCD4.7 and LDN4; n = 4 for each cell line).
Discussion
The precise definition of the molecular structure and functionality of ManLAM remains an important challenge in the study of M.tb pathogenesis. In this regard, ManLAM biological properties are difficult to address due to the innate heterogeneity of this molecule. In this study, a method was developed that delineates the heterogeneity of the lipoglycan ManLAM by charge. This allowed us to examine the potential of this charged molecule in mediating immune responses to M.tb. We previously reported that ManLAM could be separated into eight distinct isoforms based on charge using an appropriate ampholyte mixture that allowed us to have a pI gradient between 4.0 and 8.0 (Torrelles et al. 2004). These isoforms seemed to differ only in charge and not in size as demonstrated by the 2D SDS–PAGE, where subtle size differences in their carbohydrate domain might not be reflected. Using this fact, here we assessed several IEF approaches to purify these ManLAM isoforms. Although the rotofor and electro eluter techniques have been shown to be successful for protein purification based on their pI values, this was not the case for ManLAM, and thus, an approach was developed based on our observation that ManLAM can be separated into distinct bands in the IEF tube gel. Pure and partially pure isoforms were obtained by directly excising them from the IEF tube gel. Compositional analyses of the isoforms indicated that ManLAM pI 5.8 had a smaller arabinan and more fatty acids than other ManLAM isoforms and pre-IEF ManLAM tested. However, this isoform had limited succinylation. The contribution of each domain of ManLAM as separated by IEF was examined. In this context, we previously reported that the mannose caps or the arabinan domain of ManLAM does not play a role on the separation observed by IEF, where α-mannosidase [which removes the mannose caps of ManLAM (Schlesinger et al. 1994; Khoo et al. 2001)] or endoarabinanase [which trims away the arabinan domain of ManLAM (Chatterjee et al. 1993)] treatments did not collapse the blueprint of the isoforms except for some minor size shifting (Torrelles et al. 2004). Furthermore, the pre-fractionation of ManLAM into subpopulations containing a different number of fatty acids (1–4 fatty acids) by hydrophobic interaction chromatography did not alter the IEF migration pattern observed, indicating that ManLAM heterogeneity in fatty acid composition (number and species) did not influence its IEF separation (Torrelles et al. 2004). However, by using saponified ManLAM, we demonstrated that deacylation in ManLAM prevented separation by IEF. In this regard, it is still unclear why deacylated ManLAM migrated to the acidic end after being submitted to IEF. A plausible explanation could be the presence of the negative charge provided by the phosphate group located in the GPI anchor of ManLAM.
The role of the mannan domain of ManLAM in IEF separation is unclear, as we previously showed that ManLAM with its arabinan domain trimmed by the action of an endoarabinanase still could be separated into at least six different isoforms (Torrelles et al. 2004). However, this was not the case for the structurally related LM, which only has two overlapping isoforms. Interestingly, PIMs, which are known to contain a limited number of mannose residues, separated into four distinct isoforms by IEF, thus establishing the plausible hypothesis that the overall molecular size of the M.tb mannosylated lipoglycans and glycolipids directly contribute to the net charge important for IEF separation.
In the context of T cell reactivity, the ManLAM pI 5.8 isoform preferentially stimulated CD1-restricted T cells to secrete IFN-γ. Our recent studies strongly suggest that the overall charge present in mycobacterial lipoglycans may dictate their presentation via CD1b (Torrelles et al. 2004, 2011). Thus, additional structural analyses will be necessary to further elucidate the overall charge present in the ManLAM pI 5.8 isoform and how this is influencing its presentation via CD1b.
Several reports (Sieling et al. 1995; Prigozy et al. 1997; Torrelles et al. 2004) have now shown that mycobacterial lipoglycans in the form of ManLAM and its structurally related variants LM and PIMs can be recognized by T cells in the context of CD1, although the precise epitopes involved in this recognition have not yet been delineated. In these studies, a CD4/CD8 double-negative T cell clone (LDN4) derived from a skin lesion of a leprosy patient recognized ManLAM from M. leprae, but not ManLAM from M.tb H37Rv or M.tb Erdman (Sieling et al. 1995), in the context of human CD1b molecules. This fact led us to hypothesize that compositional differences between ManLAMs from different mycobacterial species may be critical for stimulating T cells. In this regard, M.tb LMs from all the strains tested were shown to be less effective at activating this specific T cell clone, lending support to the tentative conclusion that the proliferation observed by the clone was primarily through some component of the arabinan domain of ManLAM. In contrast, another human CD1b-restricted T cell clone (BDN4), derived from the peripheral blood of a normal donor, recognized ManLAMs from all three mycobacterial strains, as well as LM and a mixture of different acylated forms of phosphatidyl-myo-inositol hexamannosides (i.e. PIM6, Ac1PIM6, Ac2PIM6) but not PIM2s (PIM2, Ac1PIM2, Ac2PIM2). Later, it was suggested that those CD1b molecules had specificity to interact with fatty acid components present in the mycobacterial mannosylated lipoglycans and glycolipids (ManLAM, LM and PIMs; Ernst et al. 1998). Recently, we found that only highly acylated mycobacterial LMs (with 3–4 fatty acids and ∼5 succinates per molecule) stimulated CD1-restricted T cells; however, tetra-acylated forms of PIMs did not (Torrelles et al. 2011). In this context, ManLAM treatment with mild alkali resulted in a decrease in its ability to suppress in vitro antigen-induced T cell proliferation, IFN-γ activation of macrophages and cytokine secretion highlighting the importance of the alkali-labile residues (Vercellone et al. 1998). To date, fatty acids and succinates are the only alkali-labile residues that have been identified in ManLAM (Hunter and Brennan 1990; Delmas et al. 1997), although other undefined acyl groups may be present (Hunter et al. 1986). Thus, the degree of acylation, the type of acylation and the overall molecular structure of the particular mannosylated lipoglycan/glycolipid that allows for the exposure of its acyl groups seem to play a role in dictating the proper spatial conformation for these antigens being loaded onto CD1 and subsequent presentation to and recognition by T cells. In this context, ManLAM loading to CD1 has been shown to occur after uptake via the macrophage MR (Schlesinger et al. 1994; Sieling et al. 1995; Venisse et al. 1995); thus, differences in T cell activation might be also linked to solely recognition of the pI 5.8 isoform by the macrophage MR. Future studies are required to determine the mechanism by which the ManLAM pI 5.8 isoform is a more potent stimulator of CD1b-restricted T cells in relation to antigen recognition and uptake (Prigozy et al. 1997), processing and binding to CD1b (Moody et al. 2002) or affinity for the T cell receptor (Moody et al. 1997).
Further precise definition of the molecular structure and functionality of ManLAM remains an important challenge in the study of tuberculosis pathogenesis. It is essential to determine a more precise structural model of ManLAM. The innate and adaptive immune events that occur through the interaction of ManLAM with the host cell are still understudied. Major interest exists in using mycobacterial lipids as adjuvants in vaccines, thus, knowing the precise structure that stimulates T cells would aid in the design of such vaccines. In this context, LAM molecules have also been shown to activate TLR2 giving it adjuvant properties; thus, it is important to know if the structure of LAM that stimulates TLR2 is the same structure that stimulates CD1b-restricted T cells. In summary, characterizing the functional groups in ManLAM that mediate its dual role in pathogenesis or protective immunity against tuberculosis is critical for providing a better understanding of the interaction between T cell receptors and antigen-presenting cells (APCs) via CD1 molecules and subsequent activation or dampening of this branch of the adaptive response against M.tb infection. Further investigations will enable a more precise ManLAM structural model which will help explain the specific immunological activities of ManLAM.
Materials and methods
Chemical reagents
All chemical reagents were of high grade from Aldrich/Fluka/Sigma (Saint Louis, MO) unless otherwise specified. Endotoxin-free sterile water was used for all chemical reactions and also for the isolation of lipoglycans and column chromatography.
Growth of M.tb and extraction of soluble lipoglycans
M.tb strains (laboratory strain H37Rv and a clinical isolate isolated from a patient from South Korea, CSU20) were grown and lipoglycans (ManLAM, LM and PIMs) purified as we described previously (Torrelles et al. 2004; Shi et al. 2008). Purified ManLAM, LM and PIMs were analyzed and recovered by SDS–PAGE followed by PAS (Shi et al. 2008). Sample concentrations were maintained at 0.5 μg/μL in a sample buffer. ManLAM deacylation was performed as described (Mikusova et al. 1995).
Isoelectric focusing
IEF tube gels were prepared in-house containing 8 M urea and ampholytes 4.5–5.4 and 3–10 (GE Healthcare Biosciences, Pittsburgh, PA). Purified ManLAM was loaded into tube gels and then electrophoresed for 3 h at 1000 V with degassed 0.1 M NaOH (pH 2.2) and 0.1 M phosphoric acid (pH 12.2) buffers (Torrelles et al. 2004). Tube gels were then transferred to SDS–PAGE (15%) and run for 1.5 h at 100 V. The minimum amount of ManLAM required to obtain separation on 2D SDS–PAGE was 10 μg. The pI value of each band was determined according to an IEF pH gradient profile with a pH-meter. Each band was identified as an isoform of ManLAM depending on its net charge. For preparative IEF electrophoresis, 1 mg of ManLAM was applied to multiple IEF tube gels (155 mm × 6 mm i.d.) and electrophoresed. One of the tube gels was stained with PAS and eight discrete bands were differentiated corresponding to their respective pI values. The stained gel was used as a gauge to locate bands on the unstained tube gels. Gels were excised per banding pattern, and each band was homogenized, sonicated and centrifuged in sterile endotoxin-free H2O to disrupt the acrylamide matrix and liberate ManLAM isoforms. Supernatants were dialyzed to eliminate salts and ampholytes, and then filtered using 0.2-μm filters to eliminate micro-acrylamide debris. Samples were lyophilized and suspended at a known concentration leaving them in an endotoxin-free physiological buffer. Aliquots of the extractions were reexamined by 2D SDS–PAGE and their reactivity to ManLAM antibodies by immunoblotting as described previously (Torrelles et al. 2004).
Analytical procedures
For sugar analysis, scyllo-inositol (55 nmol) was added as an internal standard to dried preparations of lipoglycans [ManLAM (pre-IEF and LAM isoforms), LM and PIMs]. Each sample was hydrolyzed with 2 M trifluoroacetic acid at 120°C for 2 h, and then the residue was reduced, acetylated and the resulting alditol acetates analyzed by GC as we described previously (Chatterjee et al. 1992). For fatty acid analysis, fatty acid methyl esters were obtained after lipoglycans were treated with 3 M HCl in methanol at 80°C and trimethylsilylated using TRI-SIL as described previously (Torrelles et al. 2004). Heptadecanoic acid (17:0) was used as our internal standard. GC/MS on the fatty acid methyl esters was carried out following our published procedure (Torrelles et al. 2004). To evaluate the succinate content, succinate derivatives were obtained after the octanolysis of lipoglycans using glutaric acid as an internal standard. The resulting octyl succinates were analyzed by GC/MS as described previously (Torrelles et al. 2004). Phosphate was quantified as described (Ames 1966) with some modifications. Briefly, 100 μL of the washing buffer [10 g Mg(NO3)2 in 100 mL of ethanol] was added to the samples, and the mixtures were dried using a strong flame on the Bünsen burner. Then, 300 μL of 0.5 N HCl was added to each sample and boiled for 15 min in a water bath. Finally, 600 μL of ammonium molybdate in 1 N H2SO4 and 100 μL of 10% ascorbic acid were added to each tube. After 60 min of incubation at 37°C, samples were read at 820 nm. A standard curve was created using 0, 3, 5, 7, 10, 12 and 15 nmol of NaH2PO4.
In vitro culture of CD1-expressing monocyte-derived dendritic cells (DCs)
CD1+ monocyte-derived DCs were generated in vitro with a combination of recombinant human GM-CSF (200 U/mL) and recombinant human IL-4 (100 U/mL) as described (Porcelli et al. 1992; Kasinrerk et al. 1993). Cells were harvested using incubation in phosphate-buffered saline/0.5 mM ethylenediaminetetraacetic acid (EDTA) to detach adherent cells and analyzed by flow cytometry using CD1-specific mAbs (14) or irradiated (5000 Rad) and used as APCs.
T cell lines and stimulation assays
T cell lines were derived from leprosy patients as described previously (Sieling et al. 1995). Cytokine release from T cells was measured by enzyme-linked immunosorbent assay (ELISA) after stimulation with CD1+ APCs and antigen (0.5 µg/mL) or media for 24 h. IFN-γ ELISA (Pharmingen, San Diego, CA) was performed according to the manufacturer's instructions.
Funding
This work was supported in part by National Institute of Health (AI-37139 to D.C., AI-22553 to R.L.M., NO1 AI 55262 and AI-47197 to P.J.B., NO1 AI-040091 to J.T.B., and AI-073856 to J.B.T.; and the Parker B. Francis Fellowship to J.B.T.
Conflict of interest
The authors have no financial conflict of interest.
Abbreviations
CD, cluster of differentiation; DCs, dendritic cells; EDTA, ethylenediaminetetraacetic acid; ELISA, enzyme-linked immunosorbent assay; GC, gas chromatography; GPI, glycosylphosphatidyl-myo-inositol; IEF, isoelectric focusing; IFN-γ, interferon-γ; LM, lipomannan; ManLAM, mannose-capped lipoarabinomannan; MR, mannose receptor; MS, mass spectrometry; M.tb, Mycobacterium tuberculosis; PAS, periodic acid silver nitrate staining; pI, isoelectric point; PIM2, phosphatidyl-myo-inositol dimannosides; PIM6, phosphatidyl-myo-inositol hexamannosides; PIM, phosphatidyl-myo-inositol mannoside; SDS–PAGE, sodium dodecyl sulfate–polyacrylamide gel electrophoresis; TBST, tuberculostearic acid; TLR, Toll-like receptor; TNF, tumor necrosis factor.
References
- Ames BN. Assay of inorganic phosphate, total phosphate and phosphatases. Methods Enzymol. 1966;8:115–118. doi:10.1016/0076-6879(66)08014-5. [Google Scholar]
- Ayala A, Parrado J, Machado A. Use of Rotofor preparative isoelectrofocusing cell in protein purification procedure. Appl Biochem Biotechnol. 1998;69:11–16. doi: 10.1007/BF02786017. doi:10.1007/BF02786017. [DOI] [PubMed] [Google Scholar]
- Briken V, Porcelli SA, Besra GS, Kremer L. Mycobacterial lipoarabinomannan and related lipoglycans: From biogenesis to modulation of the immune response. Mol Microbiol. 2004;53:391–403. doi: 10.1111/j.1365-2958.2004.04183.x. doi:10.1111/j.1365-2958.2004.04183.x. [DOI] [PubMed] [Google Scholar]
- Chatterjee D, Khoo K-H, McNeil MR, Dell A, Morris HR, Brennan PJ. Structural definition of the non-reducing termini of mannose-capped LAM from Mycobacterium tuberculosis through selective enzymatic degradation and fast atom bombardment-mass spectrometry. Glycobiology. 1993;3:497–506. doi: 10.1093/glycob/3.5.497. doi:10.1093/glycob/3.5.497. [DOI] [PubMed] [Google Scholar]
- Chatterjee D, Lowell K, Rivoire B, McNeil MR, Brennan PJ. Lipoarabinomannan of Mycobacterium tuberculosis. Capping with mannosyl residues in some strains. J Biol Chem. 1992;267:6234–6239. [PubMed] [Google Scholar]
- Crick DC, Brennan PJ, McNeil MR. The cell wall of Mycobacterium tuberculosis. In: Rom WM, Garay SM, editors. Tuberculosis. Philadelphia, USA: Lippincott Williams and Wilkins; 2003. pp. 115–134. [Google Scholar]
- Delmas C, Gilleron M, Brando T, Vercellone A, Gheorghui M, Riviere M, Puzo G. Comparative structural study of the mannosylated-lipoarabinomannans from Mycobacterium bovis BCG vaccine strains: Characterization and localization of succinates. Glycobiology. 1997;7:811–817. doi: 10.1093/glycob/7.6.811. doi:10.1093/glycob/7.6.811. [DOI] [PubMed] [Google Scholar]
- Ernst WA, Maher J, Cho S, Niazi KR, Chatterjee D, Moody DB, Besra GS, Watanabe Y, Jensen PE, Porcelli SA, et al. Molecular interaction of CD1b with lipoglycan antigens. Immunity. 1998;8:331–340. doi: 10.1016/s1074-7613(00)80538-5. doi:10.1016/S1074-7613(00)80538-5. [DOI] [PubMed] [Google Scholar]
- Hunter SW, Brennan PJ. Evidence for the presence of a phosphatidylinositol anchor on the lipoarabinomannan and lipomannan of Mycobacterium tuberculosis. J Biol Chem. 1990;265:9272–9279. [PubMed] [Google Scholar]
- Hunter SW, Gaylord H, Brennan PJ. Structure and antigenicity of the phosphorylated lipopolysaccharide antigens from the leprosy and tubercle bacilli. J Biol Chem. 1986;261:12345–12351. [PubMed] [Google Scholar]
- Jo EK, Yang CS, Choi CH, Harding CV. Intracellular signalling cascades regulating innate immune responses to mycobacteria: Branching out from Toll-like receptors. Cell Microbiol. 2007;9:1087–1098. doi: 10.1111/j.1462-5822.2007.00914.x. doi:10.1111/j.1462-5822.2007.00914.x. [DOI] [PubMed] [Google Scholar]
- Kang BK, Azad AK, Torrelles JB, Kaufman TM, Beharka AA, Tibesar E, DesJardin LE, Schlesinger LS. The human macrophage mannose receptor directs Mycobacterium tuberculosis lipoarabinomannan-mediated phagosome biogenesis. J Exp Med. 2005;202:987–999. doi: 10.1084/jem.20051239. doi:10.1084/jem.20051239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasinrerk W, Baumruker T, Majdic O, Knapp W, Stockinger H. CD1 molecule expression on human monocytes induced by granulocyte-macrophage colony-stimulating factor. J Immunol. 1993;150:579–584. [PubMed] [Google Scholar]
- Kaur D, Guerin ME, Skovierova H, Brennan PJ, Jackson M. Chapter 2: Biogenesis of the cell wall and other glycoconjugates of Mycobacterium tuberculosis. Adv Appl Microbiol. 2009;69:23–78. doi: 10.1016/S0065-2164(09)69002-X. doi:10.1016/S0065-2164(09)69002-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur D, Lowary TL, Vissa VD, Crick DC, Brennan PJ. Characterization of the epitope of anti-lipoarabinomannan antibodies as the terminal hexaarabinofuranosyl motif of mycobacterial arabinans. Microbiology. 2002;148:3049–3057. doi: 10.1099/00221287-148-10-3049. [DOI] [PubMed] [Google Scholar]
- Khoo K-H, Tang J-B, Chatterjee D. Variation in mannose-capped terminal arabinan motifs of lipoarabinomannans from clinical isolates of Mycobacterium tuberculosis and Mycobacterium avium complex. J Biol Chem. 2001;276:3863–3871. doi: 10.1074/jbc.M004010200. doi:10.1074/jbc.M004010200. [DOI] [PubMed] [Google Scholar]
- Kolattukudy PE, Fernandes ND, Azad AK, Fitzmaurice AM, Sirakova TD. Biochemistry and molecular genetics of cell-wall lipid biosynthesis in mycobacteria. Mol Microbiol. 1997;24:263–270. doi: 10.1046/j.1365-2958.1997.3361705.x. doi:10.1046/j.1365-2958.1997.3361705.x. [DOI] [PubMed] [Google Scholar]
- Lemassu A, Daffe M. Structural features of the exocellular polysaccharides of Mycobacterium tuberculosis. Biochem J. 1994;297(Pt 2):351–357. doi: 10.1042/bj2970351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikusova K, Slayden RA, Besra GS, Brennan PJ. Biogenesis of the mycobacterial cell wall and the site of action of ethambutol. Antimicrob Agents Chemother. 1995;39:2484–2489. doi: 10.1128/aac.39.11.2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody DB, Briken V, Cheng TY, Roura-Mir C, Guy MR, Geho DH, Tykocinski ML, Besra GS, Porcelli SA. Lipid length controls antigen entry into endosomal and nonendosomal pathways for CD1b presentation. Nat Immunol. 2002;3:435–442. doi: 10.1038/ni780. [DOI] [PubMed] [Google Scholar]
- Moody DB, Reinhold BB, Guy MR, Beckman EM, Frederique DE, Furlong S, Ye S, Reinhold VN, Sieling PA, Modlin RL, et al. Structural requirements for glycolipid antigen recognition by CD1b-restricted T cells. Science. 1997;278:283–286. doi: 10.1126/science.278.5336.283. doi:10.1126/science.278.5336.283. [DOI] [PubMed] [Google Scholar]
- Porcelli SA, Modlin RL. The CD1 system: Antigen-presenting molecules for T cell recognition of lipids and glycolipids. Annu Rev Immunol. 1999;17:297–329. doi: 10.1146/annurev.immunol.17.1.297. doi:10.1146/annurev.immunol.17.1.297. [DOI] [PubMed] [Google Scholar]
- Porcelli S, Morita CT, Brenner MB. CD1b restricts the response of human CD4–8− T lymphocytes to a microbial antigen. Nature. 1992;360:593–597. doi: 10.1038/360593a0. doi:10.1038/360593a0. [DOI] [PubMed] [Google Scholar]
- Prigozy TI, Sieling PA, Clemens D, Stewart PL, Behar SM, Porcelli SA, Brenner MB, Modlin RL, Kronenberg M. The mannose receptor delivers lipoglycan antigens to endosomes for presentation to T cells by CD1b molecules. Immunity. 1997;6:187–197. doi: 10.1016/s1074-7613(00)80425-2. doi:10.1016/S1074-7613(00)80425-2. [DOI] [PubMed] [Google Scholar]
- Quesniaux VJ, Nicolle D M, Torres D, Kremer L, Guerardel Y, Nigou J, Puzo G, Erard F, Ryffel B. Toll-like receptor 2 (TLR2)-dependent-positive and TLR2-independent-negative regulation of proinflammatory cytokines by mycobacterial lipomannans. J Immunol. 2004;172:4425–4434. doi: 10.4049/jimmunol.172.7.4425. [DOI] [PubMed] [Google Scholar]
- Rademacher C, Shoemaker GK, Kim HS, Zheng RB, Taha H, Liu C, Nacario RC, Schriemer DC, Klassen JS, Peters T, et al. Ligand specificity of CS-35, a monoclonal antibody that recognizes mycobacterial lipoarabinomannan: A model system for oligofuranoside-protein recognition. J Am Chem Soc. 2007;129:10489–10502. doi: 10.1021/ja0723380. doi:10.1021/ja0723380. [DOI] [PubMed] [Google Scholar]
- Rajaram MV, Ni B, Morris JD, Brooks MN, Carlson TK, Bakthavachalu B, Schoenberg DR, Torrelles JB, Schlesinger LS. Mycobacterium tuberculosis lipomannan blocks TNF biosynthesis by regulating macrophage MAPK-activated protein kinase 2 (MK2) and microRNA miR-125b. Proc Natl Acad Sci USA. 2011;108:17408–17413. doi: 10.1073/pnas.1112660108. doi:10.1073/pnas.1112660108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasindran SJ, Torrelles JB. Mycobacterium tuberculosis infection and inflammation: What is beneficial for the host and for the bacterium? Front Microbiol. 2011;2:1–16. doi: 10.3389/fmicb.2011.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger LS, Hull SR, Kaufman TM. Binding of the terminal mannosyl units of lipoarabinomannan from a virulent strain of Mycobacterium tuberculosis to human macrophages. J Immunol. 1994;152:4070–4079. [PubMed] [Google Scholar]
- Shi L, Torrelles JB, Chatterjee D. Lipoglycans of Mycobacterium tuberculosis: Isolation, purification, and characterization. In: Parish T, Brown NG, editors. Mycobacteria Protocols. Totowa, NJ: Humana Press; 2008. pp. 23–45. [DOI] [PubMed] [Google Scholar]
- Sieling PA, Chatterjee D, Porcelli SA, Prigozy TI, Mazzaccaro RJ, Soriano T, Bloom BR, Brenner MB, Kronenberg M, Brennan PJ, et al. CD1-restricted T cell recognition of microbial lipoglycan antigens. Science. 1995;269:227–230. doi: 10.1126/science.7542404. doi:10.1126/science.7542404. [DOI] [PubMed] [Google Scholar]
- Stenger S, Mazzaccaro RJ, Uyemura K, Cho S, Barnes PF, Rosat JP, Sette A, Brenner MB, Porcelli SA, Bloom BR, et al. Differential effects of cytolytic T cell subsets on intracellular infection. Science. 1997;276:1684–1687. doi: 10.1126/science.276.5319.1684. doi:10.1126/science.276.5319.1684. [DOI] [PubMed] [Google Scholar]
- Torrelles JB. Broadening our view about the role of Mycobacterium tuberculosis cell envelope components during infection: A battle for survival. In: Cardona PJ, editor. Understanding Tuberculosis—Analyzing the Origin of Mycobacterium tuberculosis Pathogenicity. Rijeka, Croatia: Intech; 2012. pp. 1–46. [Google Scholar]
- Torrelles JB, Azad AK, Schlesinger LS. Fine discrimination in the recognition of individual species of phosphatidyl-myo-inositol mannosides from Mycobacterium tuberculosis by C-type lectin pattern recognition receptors. J Immunol. 2006;177:1805–1816. doi: 10.4049/jimmunol.177.3.1805. [DOI] [PubMed] [Google Scholar]
- Torrelles JB, Khoo K-H, Sieling PA, Modlin RL, Zhang N, Marques AM, Treumann A, Rithner CD, Brennan PJ, Chatterjee D. Truncated structural variants of lipoarabinomannan in Mycobacterium leprae and an ethambutol-resistant strain of Mycobacterium tuberculosis. J Biol Chem. 2004;279:41227–41239. doi: 10.1074/jbc.M405180200. doi:10.1074/jbc.M405180200. [DOI] [PubMed] [Google Scholar]
- Torrelles JB, Schlesinger LS. Diversity in Mycobacterium tuberculosis mannosylated cell wall determinants impacts adaptation to the host. Tuberculosis. 2010;90:84–93. doi: 10.1016/j.tube.2010.02.003. doi:10.1016/j.tube.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrelles JB, Sieling PA, Arcos J, Knaup R, Bartling C, Rajaram MV, Stenger S, Modlin RL, Schlesinger LS. Structural differences in lipomannans from pathogenic and non-pathogenic mycobacteria that impact CD1b-restricted T cell responses. J Biol Chem. 2011;286:35438–35446. doi: 10.1074/jbc.M111.232587. doi:10.1074/jbc.M111.232587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venisse A, Fournié J-J, Puzo G. Mannosylated lipoarabinomannan interacts with phagocytes. Eur J Biochem. 1995;231:440–447. doi: 10.1111/j.1432-1033.1995.tb20717.x. doi:10.1111/j.1432-1033.1995.tb20717.x. [DOI] [PubMed] [Google Scholar]
- Vercellone A, Nigou J, Puzo G. Relationships between the structure and the roles of lipoarabinomannans and related glycoconjugates in tuberculosis pathogenesis. Front Biosci. 1998;3:e149–e163. doi: 10.2741/a372. [DOI] [PubMed] [Google Scholar]
- Vergne I, Chua J, Deretic V. Tuberculosis toxin blocking phagosome maturation inhibits a novel Ca2+/calmodulin-PI3K hVPS34 cascade. J Exp Med. 2003;198:653–659. doi: 10.1084/jem.20030527. doi:10.1084/jem.20030527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weldingh K, Hansen A, Jacobsen S, Andersen P. High resolution electroelution of polyacrylamide gels for the purification of single proteins from Mycobacterium tuberculosis culture filtrate. Scand J Immunol. 2000;51:79–86. doi: 10.1046/j.1365-3083.2000.00655.x. doi:10.1046/j.1365-3083.2000.00655.x. [DOI] [PubMed] [Google Scholar]