Executive Summary
Objective
This health technology policy assessment will answer the following questions:
When should in-room air cleaners be used?
How effective are in-room air cleaners?
Are in-room air cleaners that use combined HEPA and UVGI air cleaning technology more effective than those that use HEPA filtration alone?
What is the Plasmacluster ion air purifier in the pandemic influenza preparation plan?
The experience of severe acute respiratory syndrome (SARS) locally, nationally, and internationally underscored the importance of administrative, environmental, and personal protective infection control measures in health care facilities. In the aftermath of the SARS crisis, there was a need for a clearer understanding of Ontario’s capacity to manage suspected or confirmed cases of airborne infectious diseases. In so doing, the Walker Commission thought that more attention should be paid to the potential use of new technologies such as in-room air cleaning units. It recommended that the Medical Advisory Secretariat of the Ontario Ministry of Health and Long-Term Care evaluate the appropriate use and effectiveness of such new technologies.
Accordingly, the Ontario Health Technology Advisory Committee asked the Medical Advisory Secretariat to review the literature on the effectiveness and utility of in-room air cleaners that use high-efficiency particle air (HEPA) filters and ultraviolet germicidal irradiation (UVGI) air cleaning technology.
Additionally, the Ontario Health Technology Advisory Committee prioritized a request from the ministry’s Emergency Management Unit to investigate the possible role of the Plasmacluster ion air purifier manufactured by Sharp Electronics Corporation, in the pandemic influenza preparation plan.
Clinical Need
Airborne transmission of infectious diseases depends in part on the concentration of breathable infectious pathogens (germs) in room air. Infection control is achieved by a combination of administrative, engineering, and personal protection methods. Engineering methods that are usually carried out by the building’s heating, ventilation, and air conditioning (HVAC) system function to prevent the spread of airborne infectious pathogens by diluting (dilution ventilation) and removing (exhaust ventilation) contaminated air from a room, controlling the direction of airflow and the air flow patterns in a building. However, general wear and tear over time may compromise the HVAC system’s effectiveness to maintain adequate indoor air quality. Likewise, economic issues may curtail the completion of necessary renovations to increase its effectiveness. Therefore, when exposure to airborne infectious pathogens is a risk, the use of an in-room air cleaner to reduce the concentration of airborne pathogens and prevent the spread of airborne infectious diseases has been proposed as an alternative to renovating a HVAC system.
Airborne transmission is the spread of infectious pathogens over large distances through the air. Infectious pathogens, which may include fungi, bacteria, and viruses, vary in size and can be dispersed into the air in drops of moisture after coughing or sneezing. Small drops of moisture carrying infectious pathogens are called droplet nuclei. Droplet nuclei are about 1 to 5μm in diameter. This small size in part allows them to remain suspended in the air for several hours and be carried by air currents over considerable distances. Large drops of moisture carrying infectious pathogens are called droplets. Droplets being larger than droplet nuclei, travel shorter distances (about 1 metre) before rapidly falling out of the air to the ground. Because droplet nuclei remain airborne for longer periods than do droplets, they are more amenable to engineering infection control methods than are droplets.
Droplet nuclei are responsible for the airborne transmission of infectious diseases such as tuberculosis, chicken pox (varicella), measles (rubeola), and dessiminated herpes zoster, whereas close contact is required for the direct transmission of infectious diseases transmitted by droplets, such as influenza (the flu) and SARS.
The Technology
In-room air cleaners are supplied as portable or fixed devices. Fixed devices can be attached to either a wall or ceiling and are preferred over portable units because they have a greater degree of reliability (if installed properly) for achieving adequate room air mixing and airflow patterns, which are important for optimal effectiveness.
Through a method of air recirculation, an in-room air cleaner can be used to increase room ventilation rates and if used to exhaust air out of the room it can create a negative-pressure room for airborne infection isolation (AII) when the building’s HVAC system cannot do so. A negative-pressure room is one where clean air flows into the room but contaminated air does not flow out of it. Contaminated room air is pulled into the in-room air cleaner and cleaned by passing through a series of filters, which remove the airborne infectious pathogens. The cleaned air is either recirculated into the room or exhausted outside the building. By filtering contaminated room air and then recirculating the cleaned air into the room, an in-room air cleaner can improve the room’s ventilation. By exhausting the filtered air to the outside the unit can create a negative-pressure room. There are many types of in-room air cleaners. They vary widely in the airflow rates through the unit, the type of air cleaning technology used, and the technical design.
Crucial to maximizing the efficiency of any in-room air cleaner is its strategic placement and set-up within a room, which should be done in consultation with ventilation engineers, infection control experts, and/or industrial hygienists. A poorly positioned air cleaner may disrupt airflow patterns within the room and through the air cleaner, thereby compromising its air cleaning efficiency.
The effectiveness of an in-room air cleaner to remove airborne pathogens from room air depends on several factors, including the airflow rate through the unit’s filter and the airflow patterns in the room. Tested under a variety of conditions, in-room air cleaners, including portable or ceiling mounted units with either a HEPA or a non-HEPA filter, portable units with UVGI lights only, or ceiling mounted units with combined HEPA filtration and UVGI lights, have been estimated to be between 30% and 90%, 99% and 12% and 80% effective, respectively. However, and although their effectiveness is variable, the United States Centers for Disease Control and Prevention has acknowledged in-room air cleaners as alternative technology for increasing room ventilation when this cannot be achieved by the building’s HVAC system with preference given to fixed recirculating systems over portable ones.
Importantly, the use of an in-room air cleaner does not preclude either the need for health care workers and visitors to use personal protective equipment (N95 mask or equivalent) when entering AII rooms or health care facilities from meeting current regulatory requirements for airflow rates (ventilation rates) in buildings and airflow differentials for effective negative-pressure rooms.
The Plasmacluster ion technology, developed in 2000, is an air purification technology. Its manufacturer, Sharp Electronics Corporation, says that it can disable airborne microorganisms through the generation of both positive and negative ions. (1) The functional unit is the hydroxyl, which is a molecule comprised of one oxygen molecule and one hydrogen atom.
Plasmacluster ion air purifier uses a multilayer filter system composed of a prefilter, a carbon filter, an antibacterial filter, and a HEPA filter, combined with an ion generator to purify the air. The ion generator uses an alternating plasma discharge to split water molecules into positively and negatively charged ions. When these ions are emitted into the air, they are surrounded by water molecules and form cluster ions which are attracted to airborne particles. The cluster ion surrounds the airborne particle, and the positive and negative ions react to form hydroxyls. These hydroxyls steal the airborne particle’s hydrogen atom, which creates a hole in the particle’s outer protein membrane, thereby rendering it inactive.
Because influenza is primarily acquired by large droplets and direct and indirect contact with an infectious person, any in-room air cleaner will have little benefit in controlling and preventing its spread. Therefore, there is no role for the Plasmacluster ion air purifier or any other in-room air cleaner in the control of the spread of influenza. Accordingly, for purposes of this review, the Medical Advisory Secretariat presents no further analysis of the Plasmacluster.
Review Strategy
The objective of the systematic review was to determine the effectiveness of in-room air cleaners with built in UVGI lights and HEPA filtration compared with those using HEPA filtration only.
The Medical Advisory Secretariat searched the databases of MEDLINE, EMBASE, Cochrane Database of Systematic Reviews, INAHATA (International Network of Agencies for Health Technology Assessment), Biosis Previews, Bacteriology Abstracts, Web of Science, Dissertation Abstracts, and NIOSHTIC 2.
A meta-analysis was conducted if adequate data was available from 2 or more studies and where statistical and clinical heterogeneity among studies was not an issue. Otherwise, a qualitative review was completed. The GRADE system was used to summarize the quality of the body of evidence comprised of 1 or more studies.
Summary of Findings
There were no existing health technology assessments on air cleaning technology located during the literature review. The literature search yielded 59 citations of which none were retained. One study was retrieved from a reference list of a guidance document from the United States Centers for Disease Control and Prevention, which evaluated an in-room air cleaner with combined UVGI lights and HEPA filtration under 2 conditions: UVGI lights on and UVGI lights off. Experiments were performed using different ventilation rates and using an aerosolized pathogen comprised of Mycobaterium parafortuitum, a surrogate for the bacterium that causes tuberculosis. Effectiveness was measured as equivalent air changes per hour (eACH). This single study formed the body of evidence for our systematic review research question.
Experimental Results
The eACH rate for the HEPA-UVGI in-room air cleaner was statistically significantly greater when the UV lights were on compared with when the UV lights were off. (P < .05). However, subsequent experiments could not attribute this to the UVGI. Consequently, the results are inconclusive and an estimate of effect (benefit) is uncertain.
The study was reviewed by a scientific expert and rated moderate for quality. Further analysis determined that there was some uncertainty in the directness of the outcome measure (eACH); thus, the GRADE level for the quality of the evidence was low indicating that an estimate of effect is very uncertain.
There is uncertainty in the benefits of using in-room air cleaners with combined UVGI lights and HEPA filtration over systems that use HEPA filtration alone. However, there are no known risks to using systems with combined UVGI and HEPA technology compared with those with HEPA alone. There is an increase in the burden of cost including capital costs (cost of the device), operating costs (electricity usage), and maintenance costs (cleaning and replacement of UVGI lights) to using an in-room air cleaner with combined UVGI and HEPA technology compared with those with HEPA alone. Given the uncertainty of the estimate of benefits, an in-room air cleaner with HEPA technology only may be an equally reasonable alternative to using one with combined UVGI and HEPA technology
Conclusions
In-room air cleaners may be used to protect health care staff from air borne infectious pathogens such as tuberculosis, chicken pox, measles, and dessiminated herpes zoster. In addition, and although in-room air cleaners are not effective at protecting staff and preventing the spread of droplet-transmitted diseases such as influenza and SARS, they may be deployed in situations with a novel/emerging infectious agent whose epidemiology is not yet defined and where airborne transmission is suspected.
It is preferable that in-room air cleaners be used with a fixed and permanent room placement when ventilation requirements must be improved and the HVAC system cannot be used. However, for acute (temporary) situations where a novel/emerging infectious agent presents whose epidemiology is not yet defined and where airborne transmission is suspected it may be prudent to use the in room air cleaner as a portable device until mode of transmission is confirmed. To maximize effectiveness, consultation with an environmental engineer and infection control expert should be undertaken before using an in-room air cleaner and protocols for maintenance and monitoring of these devices should be in place.
If properly installed and maintained, in room air cleaners with HEPA or combined HEPA and UVGI air cleaning technology are effective in removing airborne pathogens. However, there is only weak evidence available at this time regarding the benefit of using an in-room air cleaner with combined HEPA and UVGI air cleaner technology instead of those with HEPA filter technology only.
Objective
This health technology policy assessment will answer the following questions:
When should in-room air cleaners be used?
How effective are in-room air cleaners?
Are in-room air cleaners that use combined HEPA and UVGI air cleaning technology more effective than those that use HEPA filtration alone?
What is the Plasmacluster ion air purifier in the pandemic influenza preparation plan?
The experience of sever acute respiratory syndrome (SARS) locally, nationally, and internationally underscored the importance of administrative, environmental, and personal protective infection control measures in health care facilities. In the aftermath of the SARS crisis, there was a need for a clearer understanding of Ontario’s capacity to manage suspected or confirmed cases of airborne infectious diseases. This included a review of methods to increase the capacity for airborne isolation within health care facilities in Ontario and to provide health care workers, patients, and visitors to these facilities with safer indoor air quality. In so doing, the Walker commission (2) thought that more attention should be paid to the potential use of new technologies, such as in-room air cleaning units, and subsequently recommended that the Medical Advisory Secretariat of the Ontario Ministry of Health and Long-term Care evaluate the appropriate use and effectiveness of such new technologies.
Therefore, The Ontario Health Technology Advisory Committee asked the Medical Advisory Secretariat to review the effectiveness and utility of in-room air cleaners that use high-efficiency particle air (HEPA) filters and ultraviolet germicidal irradiation (UVGI) air cleaning technology.
Additionally, the Ontario Health Technology Advisory Committee prioritized a request from the ministry’s Emergency Management Unit to investigate the possible role of the Plasmacluster ion air purifier, manufactured by Sharp Electronics Corporation in the pandemic influenza preparation plan.
This health technology policy assessment will answer the following questions:
When should in-room air cleaners be used?
How effective are in-room air cleaners?
Are in-room air cleaners that use combined UVGI lights and HEPA filters more effective than those that use only HEPA filters?
What is the role of the Plasmacluster ion air purifier in the pandemic influenza preparation plan?
Background
Clinical Need
Airborne transmission of infectious diseases depends in part on the concentration of breathable infectious pathogens (germs) in room air. (3) Because of this, infection control measures are needed to decrease the exposure to and risk of illness from such pathogens. Infection control is achieved by a combination of administrative, engineering, and personal protection methods. (3;4) Of these, engineering methods, which are usually carried out by the building’s heating, ventilation, and air conditioning (HVAC) system work to prevent the spread of airborne infectious pathogens by diluting (dilution ventilation) and removing (exhaust ventilation) contaminated air from a room, controlling the direction of airflow and the air flow patterns in a building. (4) However, over time, design (capacity of filters and air ducts), comfort (noise and drafts) issues, and general wear and tear on the HVAC system may limit its ability to maintain adequate indoor air quality. (5) Likewise, financial issues may limit the completion of necessary HVAC system renovations to maintain or increase its effectiveness. Therefore, when exposure to airborne infectious pathogens is a risk and increased ventilation is required, the use of an in-room air cleaner to reduce the concentration of airborne pathogens and prevent the spread of airborne infectious diseases has been proposed as an alternative to renovating the HVAC systems. (3;4;6)
Airborne transmission is the spread of infectious pathogens through the air over large distances. Infectious pathogens, which may include fungi, bacteria, and viruses, vary in size and can be dispersed into the air in drops of moisture after coughing or sneezing. (7). Small drops of moisture carrying infectious pathogens are called droplet nuclei and are about 1 to 5 micrometers (μm) in diameter. This small size in part allows them to remain suspended in the air for several hours and be carried by air currents over considerable distances. Moreover, if inhaled, they are small enough to bypass the protective mechanisms of the respiratory tract and settle in the lung where they may cause infection. (7;8) However, large drops of moisture carrying infectious pathogens are called droplets. Droplets are larger than droplet nuclei; because of this, they travel shorter distances (about 1 metre) before rapidly falling out of the air onto the ground.
Droplet nuclei are responsible for the airborne transmission of infectious diseases like tuberculosis (TB), chicken pox (varicella), measles (rubeola), and dessiminated herpes zoster, whereas direct contact (about 1 metre or less between people) is the primary route of transmission of an infectious diseases spread by droplets, such as influenza (the flu) and SARS.(9) Airborne infection isolation (AII) refers to the isolation of patients infected with diseases spread by droplet nuclei. (4;6;9)
Because droplet nuclei remain airborne for longer periods than do droplets, they are amenable to engineering infection control methods such as air cleaning more so than droplets.
Ventilation
The 3 functions of the general ventilation (HVAC) system of a building are to dilute the concentration of pathogens in room air and remove contaminated room air to the outdoors, control the direction of airflow in a building, and control the airflow patterns with in a room. (4)
Dilution and Removal of Contaminated Air
Two types of general ventilation systems are used to dilute and remove contaminated air: single-pass and recirculating air systems. With a single-pass system, 100% of the air passing through the room is exhausted (removed) to the outside. In a recirculating air system, some of the room air is filtered and recirculated back into the room. (6) With high ventilation rates, the concentration of infectious pathogens in the room air is diluted (decreased). The rate of room ventilation (ventilation rate) is equal to the amount of air moved in and out of a room and is measured as room air changes per hour (ACH). The ACH is the ratio of the volume of air entering the room per hour to the room volume. (6) For example, one room air change occurs when the volume of air entering the room equals the room volume.
Under ideal air mixing conditions, about 63% of airborne particles are removed in 1 ACH. However, given the variation in air mixing that occurs, a more realistic estimate is between 20% and 60%. (8) The number of air changes per hour will determine how quickly airborne pathogens are removed from the room air. (7) (Table 1) It has been estimated that between 12 and 15 ACH are sufficient to remove airborne pathogens and the cost of additional ACH over and above this exceeds the benefits (Personal communication, clinical expert, November 10, 2005) (7)
Table 1: Air Changes Per Hour and Time Required To Remove 99% or 99.9% of Airborne Particles From Room Air*(9).
| Air Changes | Minutes Required for Removal of Airborne Particles | |
|---|---|---|
| Per Hour | 99% Removal | 99.9% Removal |
| 2 | 138 | 207 |
| 4 | 69 | 104 |
| 6 | 46 | 69 |
| 8 | 35 | 52 |
| 10 | 28 | 41 |
| 12 | 23 | 35 |
| 15 | 18 | 28 |
| 20 | 14 | 21 |
| 50 | 6 | 8 |
From: Centers for Disease Control and Prevention. Guidelines for environmental infection control in health-care facilities: recommendations of CDC and the Healthcare Infection Control Practices Advisory Committee (HICPAC). MMWR Morb Mortal Wkly Rep [serial on the Internet] 2003; 52(RR-10): 1-48 [cited 2005 Sept. 11]. Available at: www.cdc.gov/ncidod/hip/enviro/guide.htm (7)
Recommended ventilation rates vary among different patient care areas of a hospital (9; 10) and between agency standards. (4;6;11;12) For example, The United States American Institute of Architects and Centers for Disease Control and Prevention recommends that aII rooms in existing facilities have at least 6 ACH and those in newly constructed or renovated facilities have a minimum of at least 12, whereas Health Canada states 6 to 9 ACH is adequate. However, the Health Canada recommendation may not reflect current evidence as it references the 1991 Canadian Standards Association Standard, (10) which was revised in 2001 and now supports 12 ACH for AII rooms.
When a room has no general ventilation system, the system cannot provide adequate ACH, or an increase in ventilation effectiveness is needed, in-room air cleaners may be considered to increase room ACH and thus ventilation rates of the room. (4;6)
Because in-room air cleaners recirculate room air, their effectiveness is expressed in equivalent air changes per hour (eACH), which compares airborne particle removal of the recirculated air with particle clearance from exhaust ventilation (HVAC system). (6) Equivalent ACH is determined by calculating the ratio of the airflow rate of the unit measured in cubic units per hour (e.g., cubic feet per minute [cfm]) to the room volume also expressed in cubic units (e.g., cubic feet). (13) For example an in-room air cleaner with a 400 cfm airflow rate through the unit may provide up to 13 equivalent air changes per hour in a hospital room that is 1800 cubic feet. (14) (See example calculation below.) The number of equivalent ACH provided by an in-room air cleaner will largely depend on the relative airflow rate through the device. It has been recommended that such units be designed to achieve at least 12 equivalent ACH and be compatible with the general ventilation system of a building. (6)
Example calculation of equivalent eACH:
Volume of room: 1800 cubic feet (volume = length × width × height of room)
Airflow rate of in-room air cleaner: 400 cfm × 60 minutes = 24,000 cubic feet per hour
eACH = 24,000/1800, or 13
Control of Airflow Direction and Patterns
Airflow Direction
The direction of airflow within a building can be manipulated to prevent the spread of contaminated air to uncontaminated air spaces. When the air pressure in a room is lower than that in the surrounding spaces, the room is said to be under negative pressure, and the room air is prevented from passively leaking out to the surrounding adjacent spaces. (Air always flows into the room but not out of the room.) Exhausting room air at a higher volumetric rate than the rate at which the air is entering the room achieves negative pressure. (6;15)
Negative-pressure rooms are used to isolate patients with confirmed or suspected airborne infections. Health care facility areas, including bronchoscopy suites, sputum induction rooms, selected examination and treatment rooms, autopsy suites, and clinical laboratories, have all been recommended to be in a negative-pressure environment. (6)
To achieve negative pressure, a pressure differential between the inside and outside of a room of at least 0.01 inches of water gauge (2.5 Pascals) is recommended. (6) Rooms under negative pressure must be monitored to ensure that this pressure differential exists so that air always flows in the intended direction (i.e., into the room for negative-pressure rooms). Monitoring can be undertaken with such methods as chemical aerosols (smoke tubes), differential pressure-sensing devices (manometer) and or physical indicators (flutter strips). (6) Smoke tubes generate smoke, which follows the air currents. If the smoke flows into the room from the corridor, then the room is under negative pressure (Figure 1). (6) When occupied by a patient with a suspected or confirmed airborne infectious disease, the pressure differential of a negative-pressure room should be checked daily using a smoke tube; otherwise, monthly monitoring with a smoke tube is recommended. (6)
Figure 1: Smoke tube testing to determine the direction of airflow.
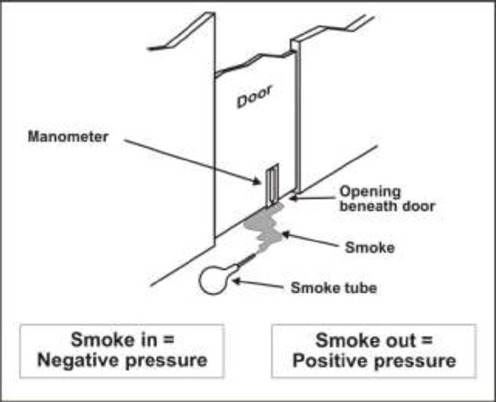
From: Centers for Disease Control and Prevention. Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care settings. MMWR Morb Mortal Wkly Rep [serial on the Internet] 2005; 54(RR17): 1-141 [cited 2005 Sept. 11]. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5417a1.htm (6)
Air sampling can be used to monitor the cleanliness of the indoor air space. By sampling room air the concentration of airborne contaminants can be determined and the risk of exposure to infectious microorganisms evaluated. Air sampling does not provide absolute assurance that an area is free of biological contamination, because microorganisms may become reaerosolized from surfaces during routine activity. Because of this, surface sampling may also be used to determine areas of contamination.
Airflow Patterns
Airflow patterns refer to the movement of the air within the room. General ventilation should prevent air from stagnating (i.e., not moving) and from short-circuiting, which occurs when air moves directly to the exhaust from the supply without first mixing in the room. (16) Airflow patterns are determined in part by the location of the air supply and exhaust as well as the configuration of the room furniture and movement of people with in the room. In general, air should flow from less contaminated areas (clean spaces) to more contaminated spaces (less clean spaces) (16) Therefore, ideally, clean air entering the room should move first to the areas of the room where the health care workers are likely to be positioned, then to the patient (infectious source), and then to the exhaust (removal area). (6).
Filtration
Mechanical Filtration
Mechanical filtration is the physical removal of particles from air by capturing them in a fibrous net-like structure more commonly called a filter. Mechanical filters may be incorporated into the ducts of the HVAC system or in-room air cleaners. (6; 17)
Filter performance is characterized in terms of its single-pass removal efficiency, which is the fraction of particles captured as air passes once through the filter. (17) The American Society of Heating, Refrigeration and Air-Conditioning Engineers (ASHRAE) standard number 52.2-1999 classifies filter efficiency by how well it removes a particle from the air (particle removal efficiency), expressed as the minimum efficiency reporting value, or MERV. The higher the MERV the more efficient the filter.(18) Sixteen performance ratings covering efficiency in 3 particle size ranges, including 0.3–1.0μm, 1.0–3.0 μm, and 3.0–10.0 μm have been classified (Appendix 1).(18)
ASHRAE standard 52.1-1992 gives parameters for 2 tests used to measure filter efficiency: the dust spot test and the weight arrestance test (Appendix 1). The dust spot test evaluates the ability of a filter to remove large airborne particles. Outdoor air or a defined dust suspension (air suspension of particles of any solid material usually ≤ 100 μm in diameter), is fed into a test duct and captured by a filter, which results in dust spots or stains being left on the filter. The darkness of the stain on the filter is evaluated and based on this an efficiency number is given to the filter. Arrestance is used to describe low and medium-efficiency filters and measures the filter’s ability to capture a mass fraction of coarse test dust. To determine the weight arrestance of a filter, a standardized synthetic dust is fed to the filter and the fraction of the dust that is removed by the filter is weighed.(18) Particles that are about 0.3μm in diameter are the most difficult to capture by mechanical filtration. (17) Disadvantages of mechanical filtration include the possibility of air leaking around the filter (bypassing the filter) and the growth of moulds on the filter surface. (16)
Managing Environmental Infection Control Methods
For optimal effectiveness, environmental infection control methods must be installed, operated, and maintained correctly.
Ongoing maintenance is critical.
The health care facility should develop standard operating procedures for delegating maintenance of and staff training for managing and caring for all types of ventilation and filtration systems responsible for air cleaning.
Emergency power must be available to avoid the disruption of all environmental infection control methods during power failures
Types of Air Cleaning Technology
In-room air cleaners use one or more types of air cleaning technologies. High-efficiency particulate air (HEPA) filters and ultraviolet germicidal irradiation (UVGI) are the 2 most commonly used. Less commonly, ion emission has also been used. Each technology will be described in turn.
High-Efficiency Particulate Air Filtration
A HEPA filter uses mechanical filtration to remove airborne particles. A true HEPA filter is standardized at a minimum 99.97% efficiency rating for removing particles greater than or equal to 0.3μm (1/83,000 of an inch) in diameter. This means that for every 10,000 particles that are 0.3μm in diameter, 3 will pass through the filter, and the rest will be trapped by the filter. (6) To be fully effective, a HEPA filtration system must be leak-proof Maintenance costs associated with HEPA filters are higher compared with those for other types of filters, but the use of in-line disposable prefilters can increase the life of the HEPA filter by about 25%. (7) A true HEPA filter efficiency has been tested with a dioctyl phthalate particle test, which challenges the filter using particles that are 0.3 μm in diameter.
The efficiency of a filter will improve as particles accumulate (are loaded) on the filter. However, with loading, the airflow rate of an air cleaner will also decrease, as will the number of eACH the unit can provide. (3)
HEPA filters can be fitted into HVAC ducts or into in-room air cleaners. (6) Rutala et al. (14) found that an in-room air cleaner with a HEPA filter operating at about 400 cubic feet per minute (cfm) could clear 90% of airborne particles greater than or equal to 0.3μm in diameter within 5 to 8 minutes, compared with 12 to 16 minutes for air cleaners without a HEPA filter.
A regularly scheduled maintenance program is required to check for leakage and filter loading. It is suggested that the dioctyl phthalate penetration test should be performed at the initial installation and every time the filter is changed. Likewise, a leakage test using a particle counter should be done every 6 to 12 months on the filter. Trained personnel should always do the filter maintenance, and only while the ventilation system of unit is not operating.
Ultraviolet Germicidal Irradiation
Upper air irradiation with ultraviolet (UV) light was first developed in 1938. (19) Research has shown that under experimental conditions ultraviolet germicidal irradiation (UVGI) is effective in reducing the transmission of tubercle bacilli, the bacteria responsible for TB, as well as reducing the transmission of other airborne infections in hospitals, military housing and classrooms. (4) The CDC cautions that UVGI is not a substitute for HEPA filtration when air from AII rooms is to be recirculated within the building. (6)
UVGI is a form of radiation encompassing wavelengths from 100 to 400 nm of the electromagnetic spectrum (Figure 2). (15) UV light has been classified into 3 wavelength bands: UV-A (long wavelengths, range 315-400mn); UV-B (midrange wavelengths, range: 280-315 nm); and UV-C (short wavelengths, range: 100-280nm). Most commercially available UV lights used for germicidal purposes are low-pressure mercury vapor lights that emit radiant energy at the UV-C wavelength of 253.7 nm. UVGI damages the deoxyribonucleic (DNA) of microorganisms, destroying their ability to replicate and thereby rendering them noninfections. (8)
Figure 2: Electromagnetic Spectrum.
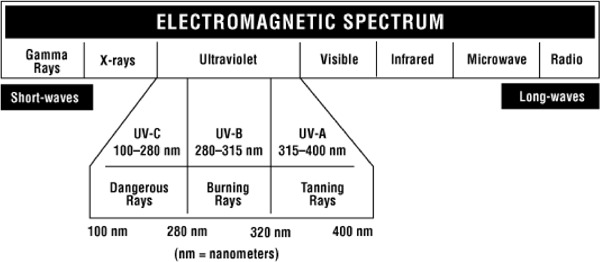
Reproduced with permission from the Canadian Centre for Occupational Health and Safety. What is ultraviolet radiation [homepage on the Internet]. CCOHS. 2005. [cited Nov. 2005]. Available from: http://www.ccohs.ca/oshanswers/phys_agents/ultravioletradiation.html (20)
There are 3 primary methods of UV air disinfection: duct irradiation, upper-room air irradiation, and in-room air cleaners. (6) With duct irradiation, UVGI lamps are placed within the ducts of the building’s HVAC system that are used to remove air from the room. When air exits the room, it passes by the UV lamps where it gets irradiated (disinfected) and is then either exhausted to the outside or recirculated into the building. With duct irradiation, human exposure to the UVGI can only occur during maintenance of the filters and UV lights in the ducts. To achieve upper-room air irradiation, the UVGI lights are mounted on either the ceiling or the upper wall of a room; contaminated lower air must be moved up toward the lights for disinfection to occur. UVGI lights are also contained in-room air cleaners. The UV lights are contained within the body of the unit, and the number of lights is model specific. The unit’s fan draws contaminated air into the air cleaner and past the UVGI lights for disinfection. (15)
The ability for UVGI to kill pathogens such as bacteria, moulds, and viruses depends on the intensity of the light, the duration of exposure of the pathogen to the UVGI, and the relative humidity of the environment. (6) Different pathogens require different light intensities and durations of exposure. (6) For example, the effective kill dose of UVGI for TB bacilli is reported at 0.01 W-sec/cm2 of ultraviolet-C radiation. Moulds require a higher kill dose than that reported for TB bacilli. Importantly, the rate of the airflow (airflow rate) through the in-room air cleaner will affect the duration of exposure of the pathogen to the UVGI light and therefore the effectiveness of the UVGI to disinfect. The effectiveness of UVGI to kill or inactivate microorganisms has been shown to decline when the relative humidity in a room exceeds 60%. (6)
There are 2 known side effects of human overexposure to UVGI lights using UV-C energy: skin reddening, called erythema, and external eye inflammation, called photokeratitis. (6) UV photokeratitis clears without complications within 24 to 48 hours after exposure. UV-C energy does not penetrate the cornea; therefore, there are no adverse effects on the eye lens or the retina. Threshold limit values for UV-C exposure is 6.0mJ/cm2 during an 8-hour period. (6;21) Exposure above this intensity during an 8-hour period may result in erythema of the skin and photokeratitis. UV exposure is only a concern with upper-room irradiation lamps, but not with in-room air cleaners, unless the UV lamps do not automatically turn off when the cabinet doors are opened.
Ion Generation and Emission
Air ions, which were discovered at the end of the 19th century, are naturally occurring particles that have a positive or negative electrical charge. (22) Research (23) has investigated the physiological benefits of positive and negative air ions, and their role in reducing the concentration of airborne dust and microorganisms in indoor environments. Ion generators that artificially manipulate the ion content in air have been developed and marketed as domestic air cleaners for removing airborne dust and smoke. (24) Ion generator air cleaners can differ by the ion polarity generated such that unipolar generators emit either positive or negative ions, while bipolar generators emit both. Mechanistically, the emission of ions is thought to charge airborne particles similarly, causing them to repel each other and migrate toward and attach themselves to indoor surfaces, thereby being removed from the air. Negative ion generators may produce excessive concentrations of ozone and nitrogen oxides. (25) Likewise, the continuous infusion of unipolar ions into an enclosed environment leads to an electrical charge accumulation on insulating surfaces, which may cause static-related problems, especially at low humidity levels. (23) The Ontario Health Technology Advisory Committee has requested a review of a specific bipolar ion generator (Plasmacluster ion air purifier). Unipolar ion generator air cleaners are beyond the scope of this review.
New Technology Being Reviewed
In-Room Air Cleaners
In-room air cleaners are supplied as portable or fixed units. Fixed units can be attached either to the wall or ceiling of a room, and are preferred over portable units for reasons which will be discussed. In-room air cleaners may be used to increase room ventilation rates in areas where there is no or insufficient ventilation, when an increase in ventilation is needed, or to manipulate the direction of the airflow to create negative-pressure environments. (Not all units can do this.) A fan within the air cleaner pulls room air into the unit, where it then passes through a series of filters that remove particles. The cleaned air can then be recirculated into the room or exhausted to the outside of the building. By filtering contaminated room air and then recirculating the cleaned room air, an in-room air cleaner can increase the number of eACH within the room thereby improving the room’s rate of ventilation. By exhausting the filtered air to the outside of the building, the unit can create a negative-pressure room. There are many types of in-room air cleaners with wide variation in airflow rates through the unit and technical designs. However, at the minimum, each device includes the following features (26):
Intake duct (where air enters the unit)
Prefilter
HEPA filter
Motor/blower assembly (also called the fan)
Control panel with at least an on/off switch, an airflow speed control, a differential pressure gauge and/or a filter change indicator such as an hour metre or timed warning light.
Exhaust duct (air exits the unit)
In-room air cleaners may use a variety of air cleaning technologies including UVGI lights and HEPA filters, either alone or in combination, as well as ion generation and emission mechanisms. (13)
Effectiveness of In-Room Air Cleaners
The effectiveness of an in-room air cleaner depends on several factors, including the single-pass efficiency of the filter, the airflow rate through the filter, the airflow patterns in the room, and the relative positions of the source of contamination (patient) and the receptor (health care worker that can be infected) to the air cleaner. (3) The effectiveness of several types of in-room air cleaners, including those with HEPA and non-HEPA filters, (3;14;27)UVGI lights,(15) or combined HEPA and UVGI technology, (5;28) has been evaluated under a variety of conditions and found to be variable (Appendix 2). Miller-Leiden (3) reported that the effectiveness of recirculating air from in-room air cleaners with HEPA and non-HEPA filters alone or in combination with HVAC ventilation (2 ACH) ranged from 30% to 90%. Green and Scarpino (15) reported that in room air cleaners with UVGI technology only were effective in inactivating greater than 99% of the concentration of airborne pathogens. Similarly, Kujundzic et al. (28) determined that the effectiveness of a ceiling-mounted in-room air cleaner with combined HEPA and UVGI technology for reducing room air bacteria concentration in a building with an indoor pool ranged from 12% to 76%. Even though the effectiveness varies widely, the CDC (6) has acknowledged in-room air cleaners as alternative technology for increasing room ventilation when this cannot be achieved by the building’s HVAC system; preference is given to fixed re-circulating systems over portable ones.
The configuration of a room as determined by the positioning of furniture and people in the room, and the placement of the air cleaner relative to the contents and layout of the room, can affect room airflow patterns, airflow rate through the air-cleaner, and the effectiveness of the unit. (29) Likewise, the location of the air supply and air exhaust registers can also affect airflow patterns, which has an impact on the air cleaner’s effectiveness. For instance, Miller-Leiden (3) reported that more effective particle removal was achieved when the ventilation exhaust was placed near the source of the contamination (the patient), supporting the CDC’s recommendations that the ventilation exhaust should be located near the more contaminated area of the room.
Because airflow patterns within a room affect the effectiveness of in-room air cleaners, fixed in-room air cleaning systems are preferable to portable ones, because they more reliably achieve adequate room air mixing and airflow patterns if installed properly. (6) Likewise, some fixed systems may also have a higher airflow capacity when compared with portable systems. For these reasons, the HVAC system is the preferred means of room ventilation. The CDC’s hierarchy of ventilation methods for a TB isolation and treatment room supports the concept of using a fixed in-room air cleaner rather than a portable one. Table 2 lists the recommended ventilation methods to achieve dilution ventilation and/or a negative-pressure environment in order of most to least desirable. (4;6)
Table 2: Hierarchy of Recommended Ventilation Methods for Tuberculosis Isolation Rooms and Treatment Rooms*.
| Reducing Concentration of Airborne Tubercle Bacilli |
Achieving Directional Airflow Using Negative Pressure |
|---|---|
|
|
From: Centers for Disease Control and Prevention. Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care settings. MMWR Morb Mortal Wkly Rep [serial on the Internet] 2005; 54(RR17): 1-141 [cited 2005 Sept. 11]. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5417a1.htm (6)
HEPA indicates high-efficiency particulate air.
Whether fixed or portable, the strategic placement and set-up of these units within a room are crucial to maximizing the efficiency of in-room air cleaners and should be done in consultation with a ventilation engineer, infection control expert, and/or an industrial hygienist. A poorly positioned in-room air cleaner risks furniture or people obstructing the airflow through the air cleaner and compromising its efficiency. If the room’s intake air supply is too close to the air cleaner’s intake duct, then inadequate air mixing will occur, which will reduce the effectiveness of the unit. Monitoring of airflow patterns within the room is required for optimal device effectiveness.
Importantly, the use of an in-room air cleaner does not preclude the need for health care workers and visitors to wear personal protective equipment (e.g., N95 mask or equivalent) when entering AII rooms. Nor does it preclude the health care facility meeting regulatory requirements for airflow rates (ACH) for adequate ventilation and airflow differentials for effective negative pressure rooms.
Safety and Performance of In-Room Air Cleaners
ECRI (29) reviewed the safety, performance, human factors design, and cost of in-room air cleaners. Results of this review are summarized below and provide guidance for the selection of in-room air cleaners.
The suggested safety features of an in-room air cleaner include the following:
It should not have any accessible sharp edges or protrusions, its accessible surfaces should not reach temperatures that could damage skin, and it should not allow access to energized or moving parts.
In-room air cleaners that use UVGI lights should not expose people to harmful UV radiation, and UVGI lights should shut off automatically when the access door is opened.
Mechanisms to prevent unauthorized or inadvertent adjustment of controls should be incorporated into the design of the air cleaner.
An in-unit alarm should be programmed to sound if the unit is turned off or unplugged.
The unit should have visible warning signs to prevent the obstruction of the intake or exhaust ducts.
The unit should be equipped with a heavy-duty cord and a hospital-grade plug.
The HEPA filters within the unit should be individually tested and certified as true HEPA filters.
There should be no leaks around filter.
The need for filter changes should be clearly indicated on the unit, and filter maintenance should be easy to perform.
The exhaust blowers should be positioned downstream (after) the HEPA filter to minimize the possibility of exposure to infectious particles.
Portable devices should be physically stable during movement or when stationary and easy to transport otherwise.
Performance issues include the airflow rate through the device and size of the room to be cleaned, the room placement of the device, the air cleaning technology used, and the maintenance needs of the device.
Airflow Rate:
In an evaluation of volumetric airflow rates of in-room air cleaners, ECRI reported that devices with a volumetric airflow greater than or equal to 600 cfm had higher eACH than those with volumetric airflow rates less than or equal to 400 cfm did.
It should be noted that as the HEPA filter becomes dirty (loaded with particulate matter), airflow and subsequently eACH will be reduced. Therefore, choosing an in-room air cleaner with a high volumetric airflow rate will allow a margin of safety for this variable.
Airflow should be verified either by the manufacturer or by an independent third party such as a ventilation consultant or an industrial hygienist. A standard HVAC flow metre may be used to measure airflow.
It has been suggested that the minimum required airflow of an in-room air cleaner be determined by calculating the product of the required airflow for at least 12 eACH and a factor of 1.5 to account for air mixing and the unit efficiency. For example, for a 60 cubic metre room, the desired airflow rate would be 720 cfm to achieve 12 eACH. Therefore, the minimum required airflow for an in-room air cleaner would be 1.5 × 720 or 1080 cfm.
Size:
The appropriate sized unit depends on the size of the room to be serviced. Larger rooms will require devices with larger volumetric airflow rates.
Room Placement:
Poor placement of the unit in a room can reduce its effectiveness. The location of the room air intake and air exhaust areas, and the placement of the in-room air cleaner and the room furniture will affect the room airflow patterns and the effectiveness of the in-room air cleaner.
In-room air cleaners should be placed so that the device’s air intake is unobstructed by furniture and the device’s exhaust can move air as far as possible before being deflected.
Optimally, in-room air cleaners should have a fixed room placement that is determined in consultation with ventilation engineers, infection control practitioners, and/or industrial hygienists.
Air Cleaning Technology:
In an evaluation of in-room air cleaners with UVGI lights, it was reported that some generate ozone when new. Therefore, when UVGI lights are incorporated into in-room air cleaners, the manufacturer should be consulted regarding the possibility that the device will produce ozone when first used.
In-room air cleaners with UVGI lights will consume more power than will those without.
Additional expense will be incurred to maintain UVGI lights in the device.
It has been suggested that adding UVGI technology to an in-room air cleaner with a HEPA filter may be advantageous in 3 ways: the UVGI will sterilize the air that passes by it as well as the inside of the unit; it will serve as a backup air cleaning technology to the HEPA filter should the filter become damaged (leak) or fail; and the UVGI acts to protect the filter maintenance staff from exposure to infectious microorganisms by inactivating microorganisms within the system. Additionally, if the UVGI lights are upstream to (before) the HEPA filter, such that air passes first by the UVGI lights and then to the HEPA filter, this may help to prolong the life of the filter.
Dust build-up on UVGI lights will reduce their effectiveness; therefore, periodic cleaning is required.
UVGI may sterilize the interior surfaces of an in-room air cleaner that are directly exposed to it but not unexposed or shadowed areas. Because of this, the shadowed areas within the cabinetry of the air cleaner may still contain viable infectious pathogens to which maintenance personnel could be exposed. Because of this, when changing filters, proper isolation precautions should be used by maintenance personnel, regardless if UVGI lights are incorporated within the system.
Maintenance:
In-room air cleaners should be used and maintained by people knowledgeable about this technology.
Maintenance, physical plant, or biomedical engineering staff should be instructed in the use of the in-room air cleaner and about infection-control precautions to be used when servicing the device.
Regardless of the use of UVGI, lights within the in-room air cleaner system and any contaminated filters should be treated as infectious material and disposed of according to local institutional policy for the management of biohazardous waste.
The HEPA filter should be adequately sealed within the in-room air cleaner and periodically inspected for damage and particle loading.
Human Factors Design:
The airflow patterns generated by the unit should not create uncomfortable drafts within the room.
The noise created by the unit should allow for conversation at normal volumes. Suggested noise levels should be less than 55dbA (the level of normal conversation) at required airflows for at least 12 equivalent ACH.
The appropriate professionals should evaluate the electrical safety of the unit before it is used.
Technical support from the manufacturer should be available.
Cost Issues
The use of an in-room air cleaner to improve room ventilation rates and/or create negative-pressure rooms may be economically superior to the costs of renovating an HVAC system. Nevertheless, there are costs incurred with using these devices, including capital expenses of purchasing the devices, operational costs, and maintenance costs, which should be evaluated before purchase. Importantly, superior economy does not mean superior effectiveness for reasons previously discussed (in the section headed Technology Being Reviewed).
Some of the ongoing costs associated with in-room air cleaners include these:
Supplying new pre-filters about every 60 days (maintenance cost)
Providing a new HEPA filter about every 2 years, (maintenance cost)
Providing new UVGI lights if applicable about every year (maintenance cost)
Energy (electricity) usage (operational cost)
Plasmacluster Ion Air Filtration
The Emergency Managment Unit of the Ministry of Health requested a review of the possible role of the Plasmacluster ion air purifier in the pandemic influenza preparation measures. A literature search reported 1 published peer-reviewed report describing Plasmacluster ion technology. Other than this, to date, information regarding the Plasmacluster ion technology can only be found on the manufacturer’s Web site. (1)
The Plasmacluster ion technology, developed in 2000, is an air purification technology. Its manufacturer, Sharp Electronics Corporation, says that it can disable airborne microorganisms through the generation of both positive and negative ions. (1) The functional unit is the hydroxyl, which is a molecule comprised of one oxygen molecule and one hydrogen atom.
Plasmacluster ion air purifier uses a multilayer filter system composed of a prefilter, a carbon filter, an antibacterial filter, and a HEPA filter, combined with an ion generator to purify the air. The ion generator uses an alternating plasma discharge to split water molecules into positively and negatively charged ions. The positive ion has one hydrogen atom (H+), and the negative ion has 2 oxygen molecules (O2). When these ions are emitted into the air, they are surrounded by water molecules and form cluster ions. These cluster ions are attracted to airborne particles because of their electrical charge. The cluster ion surrounds the airborne particle, and the positive and negative ions react to form hydroxyls. These hydroxyls steal the airborne particle’s hydrogen atom, which creates a hole in the particle’s outer protein membrane, thereby rendering it inactive. The hydroxyl molecule bonds with the stolen hydrogen atom to form water, which is returned to the air. This process generates a small amount of ozone (< 0.01 parts per million).
Because influenza is primarily acquired by large droplets and direct and indirect contact with an infectious person, any in-room air cleaner will have little benefit in controlling and preventing its spread (Personal communication, clinical infection control expert, August, 2005). Therefore, there is no role for the Plasmacluster ion air purifier or any other in-room air cleaner in the control of the spread of influenza. Accordingly, the Medical Advisory Secretariat did no further analysis of the Plasmacluster ion air purifier in this health technology review.
Regulatory Status
In-room air cleaners do not meet the definition of a medical device as stated in the Canada Food and Drugs Act; therefore, they are exempt from device classification in Canada. (30;31)
Literature Review
Objective
The objective of the systematic review was to determine the effectiveness of in-room air cleaners with combined HEPA filters and UVGI lights compared with those using only HEPA filters.
Questions Asked
What is the benefit of adding UVGI lights to an in-room air cleaner with a HEPA filter?
Are in-room air cleaners that use combined HEPA and UVGI air cleaning technology more effective than those that use HEPA filtration alone?
Methods
Inclusion Criteria:
Basic science/laboratory aerosol-chamber studies
Systematic reviews
Randomized controlled trials (RCTs)
Observational epidemiological studies
Studies that directly compare the effectiveness of portable air cleaners with HEPA and UVGI to those using HEPA filters only
Studies using aerosolized pathogens between 1-5μm in diameter.
Outcome Measures:
Quantification of colony forming units (CFU) and or particle concentration counts of pathogens
Equivalent ACH
Any reported adverse effects of in-room air cleaners
Exclusion Criteria
Studies investigating pollens, dust mites, allergens and other airborne non-infectious particles
Non-comparative studies of portable air cleaners with HEPA filters only
Duplicate publications
Air cleaning for Aspergillus species and or protection of immunocompromised patients
Literature Search Strategy
The detailed search strategy can be found in Appendix 3 and an annotated bibliography of the databases in Appendix 4.
OVID MEDLINE: 1966 to March, week 2, 2005
EMBASE: 1996 to week 12, 2005
Cochrane Database of Systematic Reviews
INAHATA (International Network of Agencies for Health Technology Assessment)
Biosis Previews (Biological Abstracts)
Bacteriology Abstracts
Web of Science
Dissertation Abstracts
NIOSHTIC 2 (National Institute of Occupational Safety and Health)
A meta-analysis was conducted when adequate data was available from 2 or more studies and where statistical and clinical heterogeneity among studies was not an issue. Otherwise, a qualitative review was completed.
GRADE Quality of the Body of Evidence
The GRADE system (32) was used to summarize the overall quality of evidence in the systematic review. This system has 4 grade levels: very low, low, moderate, and high. The criteria for assigning GRADE of evidence is as follows:
Type of evidence (initial GRADE point):
Randomized trial = high GRADE
Observational study = low GRADE level to start
Any other evidence = very low GRADE level to start
Decrease grade if:
Serious limitation to study quality (-1, reduce GRADE level by 1; for example, an initial GRADE point of high will become moderate) or very serious limitation to study quality (-2, reduce GRADE level by 2; for example, an initial GRADE point of high will become low).
Important inconsistency (-1, reduce GRADE level by 1).
Some (-1) or major (-2) uncertainty about directness.
Imprecise or sparse data (-1).
High probability of reporting bias (-1).
Increase GRADE level if:
Strong evidence of association-significant relative risk of > 2 (or < 0.5) based on consistent evidence from 2 or more observation studies, with no plausible confounders (+1, increase GRADE level by 1; for example, if the GRADE level is moderate it will become high. However, a high grade will remain high).
Very strong evidence of association-significant relative risk of > 5 (or < 0.2) based on direct evidence with no major threats to validity (+2, increase GRADE level by 2; for example, if the GRADE level is a low grade it will become a high).
Evidence of a dose response gradient (+1).
All plausible confounders would have reduced the effect (+1).
GRADE scoring definitions:
| High: | Further research is very unlikely to change our confidence in the estimate of effect. |
| Moderate: | Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. |
| Low: | Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. |
| Very low: | Any estimate of effect is very uncertain. |
Strength of Recommendations
The GRADE system (33) also offers a framework with which to grade the strength of the recommendations that may stem from the body of evidence. The system is predicated on the GRADE level of the body of the evidence (high, moderate, low or very low) and the overall balance between the benefits, risks and burdens of the technology. Briefly, if the benefits clearly outweigh the risks and burdens, then a strong recommendation is made. However, if the benefits are closely balanced with the risks and burdens, or there is uncertainty in the benefits, risks, and burdens, then the recommendation is weak.
Results of Literature Review
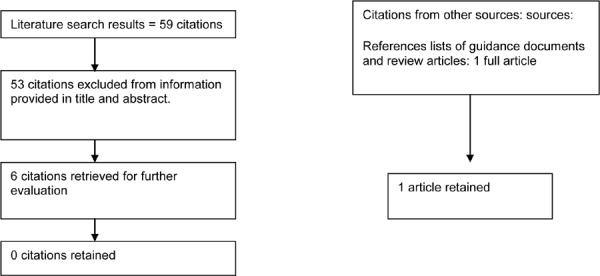
The literature search yielded 59 studies on air cleaning technologies (Figure 3). There were no existing health technology assessments. After reviewing the information in the title and abstract of the 59 citations, 6 were retained, and the full articles were retrieved for further review. After review, all 6 studies were rejected because they did not meet the inclusion criteria of comparing HEPA filtration air cleaners to those with HEPA plus UVGI. One study was retrieved from the reference list of the Guidelines for Preventing the Transmission of Mycobacterium tuberculosis in Health-Care Settings, 2005.(6). This study was retained and included in this review.
Figure 3: Summary of the Literature Review.
Study: Evaluating Portable Air Cleaner Removal Efficiencies for Bioaerosols (13)
Table 3: Quality of Evidence of Included Studies According to Study Design.
| Study Design | Level of Evidence |
Number of Eligible Studies |
|---|---|---|
| Systematic review(s) of large RCTs* | 1a | 0 |
| Large RCT | 1b | |
| Large RCT unpublished but reported to an international scientific meeting | 1(g)† | 0 |
| Small RCT | 2 | 0 |
| Small RCT unpublished but reported to an international scientific meeting | 2(g) | 0 |
| Non-RCT with contemporaneous controls | 3a | 1 |
| Non-RCT with historical controls | 3b | 0 |
| Non-RCT presented at international conference | 3(g) | 0 |
| Surveillance (database or register) | 4a | n/a |
| Case series (multisite) | 4b | n/a |
| Case series (single site) | 4c | n/a |
| Retrospective review, modeling | 4d | n/a |
| Case series presented at international conference | 4(g) | n/a |
RCT refers to randomized controlled trial. A large RCT is defined as one that has adequate power to detect differences in the primary outcome.
indicates gray literature.
Objectives
To determine effectiveness expressed as eACH of 3 in room air cleaners (also called portable air cleaners (PAC))
To determine the effectiveness expressed as eACH of an air cleaner with combined UVGI and HEPA technology with and without the use of the UV lights
To determine the effectiveness expressed as eACH of combining an in room air cleaner with an upper-room UVGI system
Methods
In a crossover design study and using a decay experiment methodology, Miller and Hernandez (13) compared the ability of 3 in-room air cleaners – a negative ion generator, an electrostatic precipitator, and a combined HEPA filtration UVGI unit (HEPA-UV) – to reduce the concentration of airborne pathogens in an 89 cubic metre (m3) test chamber, which simulated a hospital patient room. The room air concentration of airborne pathogens was measured after each in-room air cleaner was sequentially placed in the test chamber and turned on. Four experiments were carried out under 0 and 6 ACH room ventilation rates. The airborne pathogens Mycobacterium parafortuitum (M. parafortuitum) and Micrococcus luteus (M. Luteus) and a non-biological aerosol were used to challenge the in-room air cleaners.
Experiment 1: In the first experiment, the HEPA-UV in-room air cleaner was tested to determine its ability to reduce the concentration of an airborne pathogen under 2 conditions: with the HEPA filter in place and the UV lamps on (filter in/lights on) and then with the HEPA filter in place and the UV lamps removed from the device (filter in/lights removed). The experiments were performed under 0 and 6 ACH room ventilation. The M parafortuitum pathogen was used for this experiment.
Experiment 2: In a second experiment, the investigators aimed to determine if there was a change in the internal airflow through the air cleaner when the UV lights were removed from the HEPA-UV device, and which might affect the function of the HEPA filter. The eACH was determined under 3 conditions: HEPA-UV air cleaner with the internal UV lights on (ON); HEPA-UV air cleaner with internal UV lights switched off but left in the unit (OFF/IN); and HEPA-UV air cleaner with the internal UV lights removed from the unit (OUT). Each condition was tested using a non-biological aerosol (phosphate buffer solution particles) to isolate the effect of the airflow through the unit. Experiments were done under 0 and 6 ACH room ventilation.
Experiment 3: In the third experiment, the investigators aimed to determine the effectiveness of the HEPA-UV air cleaner with the UVGI lights functioning and the HEPA filter removed (UVGI only). The HEPA filter was removed, and the UV lights were left in the device and turned on. The author does not describe the methods for this experiment nor the pathogen(s) used.
Experiment 4: In a final experiment, the investigators aimed to determine the effectiveness of combining the HEPA-UV air cleaner with the UV lights functioning with an upper-room UVGI air system functioning at either 100% or 50%. One pathogen (M. parafortuitum) was used for this set of experiments conducted under 0 ACH conditions.
For experiments 1, 3, and 4, the concentration of airborne pathogens in the room was quantified using culture plates and/or direct microscopy. For study 2, an optical particle counter was used. For all studies, the rate at which aerosols were removed from room air was determined using the natural log of a completely mixed room air model, which is a log equation that determined the rate of change of the aerosol concentration with time during the decay period. The eACH for each air cleaner was estimated using a least squares linear regression equation fitted to the decay data of microorganism counts. The eACH rate is useful for determining the rate at which aerosols are removed by methods other than the general ventilation. It also allows the effectiveness of different kinds of air cleaners to be directly compared. Further details of the experimental methods are in Appendix 5.
Results of Experiments
All Experiments: There was no statistically significant difference between the eACH determined using either culturing or direct microscopy. Nor was there a statistically significant difference between the eACH rates of the in-room air cleaner under the different ventilation rates of 0 or 6 ACH.
Experiment 1: There was a statistically significant difference in the eACH rates for the HEPA-UV in-room air cleaner when the HEPA filter was left in the device and the UV lights were also left in and turned on (filter in/lights on) compared when the HEPA filter was left in and the UV lights were removed (filter in/lights removed) (P < .05) (Table 4). The investigators hypothesized that the differences in eACH between conditions (filter in/lights on vs. filter in/lights removed) might be due to a disruption in the internal airflow patterns of the air cleaner when the UV lights were removed, thereby reducing the HEPA filter’s effectiveness and the overall effectiveness of the device. Experiment 2 tested this hypothesis.
Table 4: Equivalent Air Changes Per Hour Rates With M. parafortuitum Across Groups.
| Experiment* | Mean (SE) Equivalent Air Changes Per Hour |
|---|---|
| Filter in/UV lights removed -0 ACH | 2.93 (0.96) |
| Filter in/UV lights in and on -0 ACH | 11.70 (1.20) † |
| Filter in/UV lights removed -6 ACH | 3.76 (2.32) |
| Filter in/UV lights in and on -6 ACH | 10.90 (1.20)‡ |
HEPA represents high-efficiency particulate air; ACH, air changes per hour; UV, ultraviolet.
Significantly different compared with Filter in/UV lights removed condition at 0 eACH. P < .05
Significantly different compared with Filter in/UV lights removed condition HEAP-UV at 6 eACH. P< .05
Experiment 2: There was no statistically significant difference between eACH estimated when the UV lights were left in the air cleaner but turned off (in/off) compared with having the UV lights out (OUT) (Table 5). This did not support the hypothesis that removing the UV lights disrupted the airflow patterns.
Table 5: Equivalent Air Changes per Hour Using Nonbiological Aerosol Across Groups.
| HEPA* Filter In, UV† Lights In and On, Mean (SE) |
HEPA Filter In, UV Lights In and Off, Mean (SE) |
HEPA Filter In, UV lights Out, Mean (SE) |
|---|---|---|
| 7.79 (0.45) | 4.70 (0.33) | 4.70 (0.33) |
| 5.89 (0.34) | 3.97 (0.23) | 3.97 (0.23) |
| 5.48 (0.03) |
HEPA indicates high-efficiency particulate air
UV indicates ultraviolet
Experiment 3: With the HEPA filter removed and the UV lights left in the air cleaner and turned on, the estimated eACH due to the UV lights only was approximately 1.
Experiment 4: The eACH from upper-room UVGI combined with an in-room HEPA-UVGI air cleaner is additive (Table 6).
Table 6: Equivalent Air Changes Per Hour of UVGI Upper-Room Air System plus Portable HEPA-UV Air Cleaner, and for Portable HEPA-UV Air Cleaner and UVGI Systems Only, using M. parafortuitum and 0 Air Changes Per Hour Ventilation.
| Experiment (Comparisons)* | Combined Equivalent eACH (eACH PAC + eACH Upper room UVGI), Mean (SE)* |
Equivalent ACH PAC, Mean (SE) |
Equivalent ACH Upper room UVGI, Mean (SE) |
|---|---|---|---|
| HEPA-UV + UVGI system at 100% | 27.2 (5.6) | 10.7 (3.2) | 16.5 (6.4) |
| HEPA-UV + UVGI system at 50% | 16.9 (5.7) | 9.8 (1.6) | 7.10 (6.0) |
HEPA represents high-efficiency particulate air; UV, ultraviolet; UVGI, ultraviolet germicidal irradiation; eACH, equivalent air changes per hour; PAC, portable air cleaner.
The authors concluded that more analyses should be performed on the HEPA-UV in-room air cleaning system to understand the discrepancies between the eACH measured when the UV lights where left in the air cleaner and turned on compared to when the UV lights were removed from the air cleaner. They also noted that operating an upper-room UVGI system in conjunction with an in-room HEPA-UVGI air cleaner gives a total eACH that is the sum of the individual eACH of each air cleaning system.
Table 7: GRADE Profile Question: What is the Benefit of Adding Ultraviolet Germicidal Irradiation Air Cleaning Technology to an In-Room Air Clean That Uses a HEPA Filter?
| Quality Assessment | Summary of Findings | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N (no. of experimental runs) | Effect | |||||||||
| Comparison (study) |
Design | Quality | Consistency | Directness | Other modifying factors | HEPA* only | HEPA + UVGI* | Relative Risk (95% CI) |
Quality | Outcome |
| Outcome: Equivalent Air Changes Per Hour (eACH) | ||||||||||
| (Miller and Hernandez, 2002) | Cross-over laboratory studies† | Some limitations‡ | One study | Some uncertainty§ | None | 6 | 6 | N/A | Low | Important |
| Quality GRADE | High | moderate | low | low | ||||||
HEPA indicates high-efficiency particulate air; UVGI, ultraviolet germicidal irradiation.
Author indicated that air cleaners were evaluated in no particular order.
Scientific expert had some methodological criticisms including that the investigators used glass impingers (AGI-30), whereas it might have been better to use a slit sampler to generate time-related data.
Author converted room airborne pathogen concentration counts to equivalent air changes per hour.
The body of evidence comprised 1 publication with 4 laboratory experiments investigating the value of adding UVGI lights into an in-room air cleaner with a HEPA filter. After consultation with a scientific expert (personal communication, November, 2005), the quality of the experiments were graded as moderate. The body of evidence was further downgraded because of some uncertainty in expressing the outcome as eACH instead of as the concentration of pathogens. The eACH was accepted as a surrogate outcome measure. Therefore the body evidence was given a GRADE level of low. According to GRADE, low quality is defined as “further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.”
Strength of Recommendations
There is uncertainty in the benefit of using an in room air cleaner with combined HEPA and UVGI technology compared with using one with HEPA filtration only. There is/are no known risk(s) to using an in room air cleaner with combined HEPA and UVGI air cleaning technology. There can be an increase in the burden of cost (purchasing, operation, and maintenance costs) to using an in room air cleaner with combined HEPA and UVGI air cleaning technology compared with using a HEPA only model. Given this, the strength of a recommendation to use an in room air cleaner with UVGI lights and HEPA filtration would be rated as weak, meaning alternatives such as an in room air cleaner with a HEPA filter only may be an equally reasonable alternative.
| Comparison | Benefit | Risk | Cost-burden | Quality | Strength |
|---|---|---|---|---|---|
| Air cleaners with HEPA filtration only | Uncertainty in the estimates of benefits | No known risk to using in-room air cleaner with UVGI and HEPA filtration |
Higher - Maintenance of UVGI lights - Purchase of new UVGI lights yearly - Higher energy usage |
Low An estimate of effect is uncertain. |
Weak recommenddation; alternatives may be equally reasonable. |
Cost Profile
The estimated purchase prices of an in-room air cleaner are reported in Table 8.
Table 8: Cost Profile of an In-Room Air Cleaner.
| Specifications | Airflow, Cubic Feet Per Minute |
Maximum Room Volume, Cubic Feet |
Cost (Cdn) |
|---|---|---|---|
| HEPA + UVGI* | ≈500 | Up to 2000 | $4,300-8,500 |
| HEPA + UVGI | 400 | Up to 2000 | $3,000 |
| HEPA only | 465 | Up to 2000 | $3,680 |
HEPA represents high-efficiency particulate air; UVGI, ultraviolet germicidal irradiation.
Existing Guidelines for Use of Technology
Guidelines dealing with environmental infection control from relevant provincial, national and international sources are outlined below.
Provincial Guidelines
For the Public’s Health: A Plan of Action. Final Report of the Ontario Expert Panel of SARS and Infectious Disease Control, April 2004 (2)
No single set of guidelines or standards exists in Ontario on infection control and facility design
There is a need for a uniform set of standards or guidelines for Ontario that should reflect, among other things, design changes essential to effective infection control particularly for emergency rooms in Ontario to ensure adequate and safe holding areas and assessment zones of patients who may pose an infectious disease risk.
All emergency rooms require a minimum ability to isolate suspected or actual cases of infectious disease. Ranging from separate rooms without special air handling provisions, to negative-pressure rooms with HEPA filtration, to a full negative-pressure isolation rooms with an anteroom and adjacent bath.
All facilities require some baseline capacity to deal with infectious cases. The needs of the facility should be determined based upon a graduated approach to risk.
Recommendation 42: The Ministry should immediately undertake an independent evidence-based needs assessment, reporting back to the Ministry by March 1, 2004 on the supply and distribution of negative-pressure rooms between and within hospitals. The Ministry must ensure that there is a sufficient supply of negative-pressure rooms on a regional basis.
Recommendation 43: The evidence-based needs assessment should be undertaken using standards and guidelines developed through Provincial Communicable Disease Committee. The Ministry should develop and maintain a current inventory of the number and location of all existing negative-pressure and isolation rooms in Ontario.
Recommendation 45: The Ministry, through the Ontario Health Technology Advisory Committee, the Medical Advisory Secretariat, and additional relevant external expertise, should immediately establish a process to evaluate the appropriate use and effectiveness of new technology applicable to isolation precautions, such as portable air filtration units and portable single patient isolation units.
Preventing Respiratory Illnesses Protecting Patients and Staff. (December 2003) Ontario (34) http://www.health.gov.on.ca/english/providers/program/pubhealth/sars/docs/docs3guide_fri_non_acute_031104.pdf (last accessed, November 6, 2005)
All acute care hospitals should have at least one negative-pressure room that meets Health Canada standards as outlined in the Guidelines for Preventing the Transmission of TB in Canadian Facilities. (http://www.phac-aspc.gc.ca/publicat/ccdr-rmtc/96vol22/22s1/, last accessed November 6, 2005)
The negative-pressure room can be used for special procedures that create aerosols in patients with droplet-spread infections. This room may be routinely required to provide patient care for airborne infections.
National Guidelines
Special Requirements for Heating, Ventilation, and Air Conditioning (HVAC) Systems in Health Care Facilities. Z317.2-01 A National Standard of Canada (approved February 2003) Canadian Standards Association (10)
Standard Z317.2-01 states that airborne isolation rooms should have the following:
Inward directional airflow from adjacent spaces to the room
Directional airflow within the room so that clean supply air flows first to parts of the room where workers or visitors are likely to be present, and then flows across the infection source (the patient) to the exhaust.
Nonaspirating diffusers
Low-level exhaust near the head of the patient bed
All air exhausted to the outdoors
HEPA filtration of exhaust in cases where exhaust air is not discharged clear of building openings or where a risk of recirculation exists.
Monitoring and alarm of room pressure
Monitoring of supply and exhaust system function
An exhaust fan supplied by emergency power
As well:
Air may be recirculated within individual infectious isolation rooms if HEP A filtration is used.
Recirculation-type HEPA filters may be used to increase the room air change rate.
Different health care facility service areas require different rates of ventilation (ACH)
Infection Control Guidelines: Health Canada 1999 Routine Practices and Additional Precautions for Preventing the Transmission of Infection in Health Care. (9)
-
Airborne Precautions (does not represent total precautions, but only those recommendations pertaining to engineering methods of infection control):
Negative pressure in relation to surrounding areas
A minimum of 6-9 air exchanges per hour; Health Canada considers 6-9 ACH adequate for patients with TB
Air discharged outside the building and away from intake ducts or through a HEPA filter if recirculated.
An anteroom may help to maintain inward directional airflow; however, it is not essential.
If the numbers of negative-pressure isolation rooms are limited, priority for using such a room should be set according to the impact of potential airborne transmission in that specific institution (e.g., infectious TB > measles > varicella > disseminated zoster > extensive localized zoster)
Guidelines for Preventing the Transmission of Tuberculosis in Canadian Health Care Facilities and other Institutional Settings, 1996. (11)
There is no consensus about the recommended rate of ACH in isolation rooms.
Based on the 1991 CSA guidelines, a minimum rate of 9 ACH has been suggested to provide adequate ventilation in isolation rooms.
At high rates of ACH, further increases in the number of ACH fail to provide a meaningful reduction in infectious particles, and the cost of operation at higher ventilation rates increases.
Until further information becomes available, it is recommended that newly constructed isolation rooms or areas have a minimum of 9 ACH.
Existing facilities should have at least 6 ACH.
Portable HEPA filter devices are becoming available. To date, evaluation of their effectiveness is limited.
UVGI may be considered a useful adjunct in ventilation ducts or in high-risk areas such as bronchoscopy suites, autopsy suites, or other areas where patients with undiagnosed TB may present.
Current data do not support the use of UVGI as the sole source of engineering controls.
International Guidelines
American Institute of Architects: Guidelines for Design and Construction of Hospital and Health Care Facilities.(12)
Architects, engineers, and health care professionals in the United States and other countries who are involved in the construction of new, or renovation of existing health care facilities refer to these guidelines. Below are excerpts from the DRAFT 2006 AIA Guidelines. Bold print indicates proposed changes from the April 2001 Guidelines.
AII room requirements for service areas in a health care facility should be predicated on an infection control risk assessment and on the needs of specific community and patient populations served.
At least one AII room shall be provided in the hospital.
Rooms shall have a permanently installed visual mechanism to monitor constantly the pressure status of the room when occupied by the patient with an airborne infectious disease. The mechanism shall continuously monitor the direction of the airflow.
Room with reversible airflow provisions for the purposes of switching between protective environment and AII rooms shall not be permitted.
At least one AII room shall be provided for critical care areas.
The American Society of Heating, Refrigerating and Air-Conditioning Engineers (35) (ASHRAE)
ASHRAE does not have a policy or recommendation on the use of ultraviolet light in air systems for microbial control (Personal communication with AHSRAE, September 26, 2005).
Centre for Disease Control (CDC) and Prevention. The Department of Health and Human Services, Centre for Disease Control (CDC) and Prevention, United States (US) Guidelines for Preventing the Transmission of Mycobacterium tuberculosis in Health-Care Settings, 2005 (6)
Environmental controls include local exhaust and general ventilation and air-cleaning methods including HEPA filtration and UVGI.
AII rooms in existing health care settings should have airflow of at least 6 mechanical ACH and, when feasible, should be increased to at least 12 mechanical ACH either by adjusting or modifying the ventilation system, or increased to at least 12 equivalent ACH by using air-cleaning methods such as fixed or portable room-air recirculation units or UVGI systems.
New construction or renovation of health care facilities should be designed to achieve ventilation of at least 12 mechanical ACH.
Some portable room air recirculation units are designed to create a negative-pressure room.
The pressure differential for negative-pressure AII rooms should be at least 0.01 inche of water gauge.
Settings experiencing a high prevalence of patients with TB may need to improve the existing general ventilation system or use air-cleaning technologies in waiting rooms, emergency medical service areas, and radiology suites.
-
HEPA filters must be used:
When discharging air from an AII room or other negative-pressure rooms into the general ventilation system.
-
HEPA filters can be used:
To remove infectious droplet nuclei from the air before it is recirculated to other area of a health-care setting or exhausted directly to the outside.
-
HEPA filters can be used to recirculate air in areas in which:
No general ventilation system is present
An existing system is incapable of providing sufficient mechanical ACH
Air cleaning without affecting the fresh-air supply or negative-pressure system is desired to increase the number of equivalent ACH in the room.
-
Recirculation of HEPA-filtered air can be achieved by:
Exhausting air from the room into a duct, which contains a HEPA filter.
Filtering air through HEPA re-circulation systems installed on the wall or ceiling of the room.
Filtering air through portable room-air recirculation units.
The following recommendations apply to UVGI:
UVGI is not a substitute for HEPA filtration before exhausting the air from AII rooms back into the general circulation.
Change and clean UVGI tubes according to the manufacturer’s recommendations or when irradiance measurements indicate that output is reduced below effective levels.
The use of portable room-air recirculation units in conjunction with upper-air UVGI systems may increase the overall removal of Mycobacterium tuberculosis droplet nuclei from room air. A recent study (3) showed that the equivalent ACH produced by the portable unit and that produced by the upper-air UVGI system were approximately additive.
Guidelines for Environmental Infection Control in Health-Care Facilities, Recommendations of CDC and the Healthcare infection Control Practices Advisory Committee (HICPAC), 2003 (7)
The following guidelines pertain to UVGI:
It is not recommended for air management before air recirculation form AII rooms
It is not recommended as a substitute fore HEPA filtration, local exhaust of air to the outside, or negative pressure
The use of UVGI lamps and HEPA filtration in a single unit offers only minimal infection-control benefits over those provided by the use of a HEPA filter alone.
In-duct systems with UVGI are not recommended as a substitute for HEPA filters if the air from isolation rooms must be recirculated to other areas of the health care facility.
Appraisal
Policy Considerations
Air cleaning (ventilation and filtration) is an important and necessary technology for preventing the spread of airborne transmitted infections such as TB, chickenpox, measles, and disseminated herpes zoster, as well as in situations when a novel/emerging infectious agent whose epidemiology is not yet defined and where airborne transmission is suspected.
Air cleaning technology will not be effective in preventing the spread of non-airborne infections such as influenza and SARS. Protection against acquiring these infections includes proper hand-washing, surface disinfection, and for health care workers, the use of personal protection such as a gown, mask, and gloves when working with an infectious patient.
Negative-pressure rooms are necessary to control the spread of airborne-transmitted infections in a health care facility, but they are not required for infections that are not transmitted through the air. Hospitals need to have enough (based on a risk assessment) negative-pressure rooms to manage suspected or confirmed cases of airborne-transmitted infections.
A hospital’s HVAC system is the best system to provide building ventilation. However, if ventilation requirements must be improved (e.g., to increase ventilation (ACH) or the number of negative-pressure rooms) and the HVAC system cannot be used, in-room air cleaners may be used, preferably with a fixed and permanent room placement after consultation with an environmental engineer and infection control expert. In acute (temporary) situations where a novel/emerging infectious agent presents whose epidemiology is not yet defined and where airborne transmission is suspected it may be prudent to use the in room air cleaner as a portable device until mode of transmission is confirmed (Figure 4).
Figure 4.
Results of previous experiments to determine the benefit of adding UVGI lights to an in-room air cleaner with a HEPA filter are inconclusive, and an estimate of effect is uncertain.
For any air cleaning system (HVAC or in-room), protocols for careful maintenance and monitoring must be developed by the health care facility to ensure effectiveness of the system.
Ontario Profile: Negative-Pressure Rooms
A survey conducted by the Hay Group in 2003 (36) found that of the Greater Toronto Area (GTA) hospitals having at least 100 acute care beds, between 0-12% of these acute care beds were equipped with negative pressure. The survey also reported that 3.8% of Toronto and the GTA acute care hospital beds are in single negative-pressure rooms. Furthermore, it found that 1% of Toronto and the GTA non-acute care hospital beds are in single negative-pressure rooms.
The Walker report (2) recommended that the ministry undertake an independent evidence-based needs assessment to ensure the appropriate supply and distribution of negative-pressure rooms between and within hospitals. Second, that the ministry should develop and maintain a current inventory of the number and location of all existing negative-pressure isolation rooms in Ontario.
Diffusion – International, National, Provincial
Figures 5 and 6 show data from a Canadian distributor of an in-room air cleaner that uses HEPA and UVGI technology. As of September 8, 2005, there are about 310 in-room air cleaners with HEPA and UVGI technology in health care facilities in Ontario (Figure 5). Of these, about two-thirds are used for recirculation, with the rest used for negative pressure. Emergency department and negative-pressure isolation rooms are the service areas where they are most used (Figure 6).
Figure 5: In-Room Air Cleaners in Ontario*.
Data are from one Canadian distributor; numbers may underestimate use
Figure 6: In-Room Air Cleaner Use in Ontario Hospitals by Service Area*.
Data are from one Canadian distributor; numbers may underestimate use
In Canada, hospitals in 2 provinces and 1 territory use in-room air cleaners to create negative-pressure rooms. One other province is currently negotiating the purchase of several in-room air cleaners to create negative-pressure environments in their health care facilities.
In the United States, in-room air cleaners are in use in health care facilities in 36 states. New York and Connecticut have purchased the most units (Table 9).
Table 9: Diffusion of In-Room Air Cleaners With Ultraviolet Germicidal Irradiation Plus High-Efficiency Particulate Air Filtration in the United States*.
| State | Number of In room air cleaners |
State | Number |
|---|---|---|---|
| Alabama | 10 | Minnesota | 1 |
| Arkansas | 1 | Missouri | 5 |
| Arizona | 4 | Nebraska | 8 |
| California | 73 | New Hampshire | 5 |
| Connecticut | 153 | New Jersey | 4 |
| Florida | 6 | New Mexico | 1 |
| Georgia | 7 | New York | 323 |
| Hawaii | 9 | Ohio | 13 |
| Iowa | 1 | Oklahoma | 1 |
| Illinois | 8 | Oregon | 3 |
| Indiana | 4 | Pennsylvania | 10 |
| Kansas | 4 | Rode Island | 4 |
| Kentucky | 3 | Tennessee | 14 |
| Louisiana | 30 | Texas | 43 |
| Massachusetts | 35 | Virginia | 2 |
| Maryland | 16 | Washington | 3 |
| Maine | 6 | Wisconsin | 4 |
| Michigan | 5 | West Virginia | 16 |
Data are from one distributor; therefore, numbers may underestimate use.
Internationally, China, England, and France have purchased in-room air cleaners for their health care facilities. The exact diffusion (number of units purchased) in these countries is not known.
Cost
Costs include the one-time purchase cost of the device, that to run the unit (energy expense), and the cost to service and maintain the unit.
Stakeholder analysis
Ventilation engineers, infection control practitioners, and hospital maintenance personnel must understand the issues involved in the use of these devices.
Health Care personnel must have protocols for monitoring negative pressure and airflow patterns in rooms regardless if of the use of an in room air cleaner.
Glossary
- Aerosol
Particles of respirable size generated by both human and environmental sources and that can remain viable and airborne for extended periods in indoor air.
- Aerosolization
The process of creating droplet nuclei (very small droplets of moisture) that may carry infectious microorganisms.
- Cubic feet per minute
A standard measurement of airflow; abbreviated as cfm.
- Droplets
Particles of moisture like that generated when a person coughs or sneezes, which may contain infectious microorganisms. They are usually intermediate in size between drops and droplet nuclei. They tend to quickly settle out from the air so that any risk of disease transmission is generally limited to persons in close proximity to the droplet source.
- Droplet nuclei
Sufficiently small particle (1-5μm in diameter) that can remain airborne indefinitely and cause infection when a susceptible person is exposed at or beyond 3 feet of the source of these particles.
- Ion
An atom or group of atoms that carries a positive or negative electric charge as a result of having lost or gained one or more electrons
- Air filter effectiveness
The impact of the filter on room air concentrations of pathogens.
- Irradiance
The unit of radiant power (as that from ultraviolet light) per unit area (watt/cm2).
- Micrometer
Sometimes called a micron, it is one-millionth of a metre. It is abbreviated as μm.
- Nanometer
One-billionth of a metre. It is abbreviated as nm.
- Pascal
A metric unit of measurement for pressure based on air velocity. It is abbreviated as Pa; 250 Pa equals 1 inch water gauge.
- Pathogen
Any microorganism that produces disease.
- Radiant exposure
The unit of radiant energy per unit area (joules/cm2).
- Single-pass efficiency
The likelihood that an airborne particle that passes through a filter will be removed.
- Virulence
The relative ability of a pathogen to cause disease.
Appendices
Appendix 1: ASHRAE Filtration Standards 52.1 and 52.2
| ASHRAE 52.2 | ASHRAE 52.1 | ||||||
|---|---|---|---|---|---|---|---|
| Particle Size Range | Test | Particle Size Range, μm | Applications | ||||
| MERV | 3-1 0μm, % | 1-3μm, % | 0.3-1.0μm, % | Arrestance, % | Dust spot, % | > 10.0 Residential light pollen, dust mites | |
| 1 | < 20 | n/a | n/a | < 65 | < 20 | ||
| 2 | < 20 | n/a | n/a | 65-70 | < 20 | ||
| 3 | < 20 | n/a | n/a | 70-75 | < 20 | ||
| 4 | < 20 | n/a | n/a | > 75 | < 20 | ||
| 5 | 20-35 | n/a | n/a | 80-85 | < 20 | 3.0-10.0 Industrial, dust, moulds, spores | |
| 6 | 35-50 | n/a | n/a | > 90 | < 20 | ||
| 7 | 50-70 | n/a | n/a | > 90 | 20-25 | ||
| 8 | > 70 | n/a | n/a | > 95 | 25-30 | ||
| 9 | > 85 | < 50 | n/a | > 95 | 40-45 | 1.0-3.0 Industrial, Legionella, dust | |
| 10 | > 85 | 50-65 | n/a | > 95 | 50-55 | ||
| 11 | > 85 | 65-80 | n/a | > 98 | 60-65 | ||
| 12 | > 90 | > 80 | n/a | > 98 | 70-75 | ||
| 13 | > 90 | > 90 | < 75 | > 98 | 80-85 | 0.3-1.0 Hospitals, smoke removal, bacteria | |
| 14 | > 90 | > 90 | 75-85 | > 98 | 90-95 | ||
| 15 | > 90 | > 90 | 85-95 | > 98 | ≈95 | ||
| 16 | > 95 | > 95 | > 95 | > 98 | > 95 | ||
| 17 | n/a | n/a | ≥ 99.97 | n/a | n/a | < 0.3 Clean rooms, surgery, viruses | |
| 18 | n/a | n/a | ≥ 99.99 | n/a | n/a | ||
| 19 | n/a | n/a | ≥ 99.999 | n/a | n/a | ||
| 20 | n/a | n/a | ≥ 99.9999 | n/a | n/a | ||
Department of Health and Human Services. Guidance for filtration and air-cleaning systems to protect building environments from airborne chemical, biological, or radiological attacks [report on the Internet]. DHHS (NIOSH) Publication No. 2003-136. April, 2003. National Institute of Occupational Safety and Health (NIOSH). [cited 2005 Sept. 1]. Available from: http://www.cdc.gov/niosh/docs/2003-136/pdfs/2003-136.pdf. (18)
Appendix 2: Annotated Bibliography of Studies Evaluating In-Room Air Cleaners
HEPA and Non-HEPA Filtration Units
-
Rutala et al. (14) determined the efficacy of portable in-room air cleaners to reduce the concentration of aerosolized mineral particles in the size range of M. tuberculosis in a test chamber (760 ft3) without ventilation and in a hospital room (1,824 ft3) with a ventilation rate of 6.5ACH that was provided by the building’s HVAC system.
Three portable HEPA filtration in-room air cleaners and one with 95% filter efficiency were tested. Airflow for the study ranged from 80 to 390 cfm between in-room air cleaning models. Two units had the capability for UVGI, and it is unclear from the report whether this feature was used during the testing. Mineral aerosols between 0.3μm and 0.5 μm were generated in the test chamber and hospital room. Air cleaner efficiency was calculated as the time to achieve a particle clearance of 50%, 90%, and 100%.
Results showed that all portable in-room air cleaners cleared the aerosolized mineral oil droplets rapidly in either environment. In the test chamber, the time to achieve 90% clearance of particles with a portable HEPA filtration in-room air cleaner was about 5 minutes, compared with 120 minutes without the in-room air cleaner functioning. In the hospital room, supplemental ventilation with a portable HEPA filtration in-room air cleaner with about 400 cfm airflow cleared 90% of particles more than or equal to 0.3μm within 5 to 8 minutes, compared with 12 to 16 minutes with the HVAC ventilation of 6.5 ACH alone. The time to achieve 50% clearance varied between models and was a function of air mixing. Airflow rates through the unit were thought to be the most important factor affecting the rate of particle clearance, but this could not be evaluated in this experiment. Ratuala et al. also studied the effect of placing the portable units in either the centre or corner of the hospital room, and found that the location of the unit did not affect the clearance of aerosolized particles.
-
Miller-Leiden et al.,(3) evaluated the effectiveness of in-room air cleaners, including portable and ceiling-mounted units, in combination with dilution ventilation for infection control of airborne diseases. In addition, they aimed to determine if improved effectiveness was a function of filter design, higher recirculating filter airflow rates, and/or increased ventilation rates.
Experiments were carried out in a furnished 36m3 room simulating a hospital patient room. Four portable units, 3 with HEPA filters and 1 with a 95% efficiency for particles 0.3μm in diameter, and 3 ceiling mounted air filters, 2 with HEPA filtration and 1 with a 60% efficiency filter for particles of 0.3μ in diameter, were tested. Three portable units were located near the centre of the room, and the fourth was mounted on a wall.
The test room had a ventilation rate of 6 ACH. Two test aerosols were generated: nonviable chemical particles consisting of oleic acid and viable bacterial particles from a suspension of Bacillus subtilis with a diameter of 0.7 to 0.8 μm. An electrically heated mannequin was positioned in a chair in the test room, and the aerosol was dispersed near the mannequin’s head. Air samples were obtained from 1.6 metres above the floor, which is considered the breathing zone.
Results showed no difference in effectiveness of air cleaners when tested with either the chemical or bacterial test aerosol. Similarly, there was no difference in the effectiveness of in-room air filters that used HEPA filtration compared with a non-HEPA filtration (filters less than 99.97% efficiency). The investigators hypothesized that this may be a function of airflow because the airflow rate is reduced with the HEPA filter and increased with a non-HEPA filter and because of this the overall result may be higher efficiency with the non-HEPA filter making it comparable to the HEPA filter. The core findings from the study showed that single pass ventilation plus recirculating air filtration from in-room air cleaners can decrease the concentration of droplet nuclei with effectiveness typically ranging from 30%-90%. However, the combination of single pass ventilation and in-room air cleaning was not as effective as single-pass efficiency of a HEPA filter. Finally, more effective particle removal was achieved when the ventilation exhaust was placed near the source of the contamination (the patient).
-
Mead and Johnson (27) evaluated the use of portable in-room HEPA filtration air cleaners for expedient transformation of a standard 2- or 3-patient room into single patient negative-pressure isolation zones. Such zones are anticipated as necessary areas during emergencies requiring isolation surge capacity. The patient room was sectioned off using plastic sheeting. The portable HEPA filtration unit was placed between the 2 newly created spaces. Negative-pressure airflow was verified using a smoke test. Once created, chemical aerosols 1.64μm in diameter were generated from the area of the room where the patient’s head would be positioned (the source of airborne pathogens). The effectiveness of the isolation zone was tested under different room configurations.
Under the best design, the mean respirable particle counts were up to 87% lower at the health care worker position compared to those measured at the source, the patient. No migration of respirable particles out of the isolation zone was noted.
Internal UVGI Lights only or HEPA Plus UVGI lights Units
-
In test chamber experiments, Marier et al (5) evaluated the effectiveness of an in-room air cleaner with internal UVGI lights and an ultra-low penetration air (ULPA) filter rated at 99.9995% efficiency for particles 0.12μm or larger. Airflow through the unit was set at 850 cfm.
Particle counts were recorded using a particle counter capable of measuring particles from 0.2 to 10μm in diameter. Culture plates were also used to quantify the room air concentration of pathogens. Test pathogens of Enterbacter cloacae and mycobacterium were used to challenge the in-room air cleaner. The effectiveness of the air cleaner was also tested under different airflow rates to achieve 0, 10, and 44 ACH.
Results showed that the in-room air cleaner removed 100% of all particles and airborne pathogens in the test chamber. The independent effect of the UVGI technology in the unit was not tested.
-
Green and Scarpino (15) evaluated the effectiveness of 4 in-room air cleaners with 1, 2, 4, or 8 internal UVGI (UV-C) lights and no HEPA filters. Each UV light operated at a dose of 142μWs/cm2. Five airborne pathogens, including Escherichia coli, Pseudomonas fluorescens, M. luteus, Staphylococcus aureus, and B. subtilis, were used for the experiments.
The experiments were conducted in a test chamber 0.75 m wide and 3.7 m long. Air was sampled for the pathogen concentration before it entered the UVGI air cleaner and upon exiting the unit. Control runs were completed whereby the in-room air cleaners were individually positioned in the test chamber with the UV bulbs off but the fan turned on and balanced with the test chamber exhaust. Test studies were carried out in the same manner, except the UV lights were activated along with the unit’s fan.
Results indicated that all air cleaners were efficient in inactivating more than 99% of all 5 types of test pathogens. The effectiveness of the unit was compromised when the internal UV light failed.
-
Kujundzic et al. (28) completed a controlled assessment (in a test chamber) and an in vivo (in a therapy pool) analysis of a ceiling-mounted HEPA-UV in-room air cleaner to reduce the concentration of airborne pathogens. The air cleaner tested had a large airflow rate, a HEPA filter, and 2 low-pressure mercury UV lights with a total UV irradiance of 36 watts. After mounting the in-room air cleaner on the ceiling, air is drawn into the unit from below and exhausted from the sides. Contaminated air flows first past the HEPA filter and then past the internal UV lights.
An 87 m3 test chamber was used to examine the in-room air cleaner under controlled conditions. An aerosolized pathogen of M. parafortuitum was generated for the test. An estimate of the inactivation and removal of the pathogen by the air cleaner expressed as equivalent air changes was completed. During the experiment no mechanical ventilation was supplied to the test chamber.
The ceiling in-room air cleaner was also tested in an indoor therapy pool building with a HVAC system providing a ventilation rate of 2 ACH. Air samples were taken during 2 periods: first, 3 months after the air cleaners were installed; second, after 1.5 years of operation. Effectiveness was determined by comparing pathogen counts when the air filters were turned on compared with when they were off.
Culture plating was used during the indoor therapy pool building experiment to quantify the concentration of the pathogen expressed in colony forming units. Direct microscopy was used for both test chamber and therapy pool experiments.
Results of the test chamber studies showed that the in-room air cleaner removed the pathogen at a mean equivalent ACH of 10.6 (standard deviation, 0.4). Results of the indoor therapy pool building indicated that culturable bacteria concentrations as well as total bacteria concentrations were significantly reduced when the ceiling air cleaner was turned on compared with turned off (P = .001). The effectiveness of the in-room air cleaners to reduce the concentration of culturable bacteria ranged from 69% to 80% and total bacteria from 12%-76%. However the in-room air cleaner did not significantly affect the airborne endotoxin concentrations.
Appendix 3: Final Updated Air Purifier Search Strategy
Search date: October 21, 2005
Databases searched: Databases searched: Medline, Medline In Process and Other Non-Indexed Citations, Embase, Cochrane DSR, Cochrane CENTRAL
Database: Ovid MEDLINE(R) <1966 to October Week 2 2005>
Search Strategy:
--------------------------------------------------------------------------------
plasmacluster.mp. [mp=title, original title, abstract, name of substance word, subject heading word] (0)
lifetronic$.mp. [mp=title, original title, abstract, name of substance word, subject heading word] (0)
exp Ions/ or exp Air Ionization/ (288257)
((ultraviolet adj2 irradiation) or (ultra-violet adj2 irradiation)).mp. [mp=title, original title, abstract, name of substance word, subject heading word] (3902)
(uvgi or ultraviolet rays).mp. [mp=title, original title, abstract, name of substance word, subject heading word] (44444)
exp ultraviolet rays/ or High efficiency particulate air.mp. or ion generation.mp. or ion emission.mp. [mp=title, original title, abstract, name of substance word, subject heading word] (44514)
or/1-6 (331052)
(air purif$ or air filt$ or air cleaner$).mp. [mp=title, original title, abstract, name of substance word, subject heading word] (602)
7 and 8 (101)
exp Infection Control/ or exp Disease Outbreaks/ (70395)
exp Cross Infection/ (29107)
(airborne and (pathogen$ or disease$ or bacteria$ or virus$ or microorganism$)).mp. [mp=title, original title, abstract, name of substance word, subject heading word] (2567)
exp TUBERCULOSIS/ (81071)
exp Communicable Diseases, Emerging/ (965)
exp disease transmission, patient-to-professional/ or exp disease transmission, professional-to-patient/ (2925)
or/10-15 (174484)
9 and 16 (58)
limit 17 to (humans and english language) (49)
from 18 keep 1-49 (49)
Database: EMBASE <1980 to 2005 Week 42>
Search Strategy:
--------------------------------------------------------------------------------
exp Infection Control/ or exp Disease Outbreaks/ (31866)
exp Cross Infection/ (772)
(airborne and (pathogen$ or disease$ or bacteria$ or virus$ or microorganism$)).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (2618)
exp TUBERCULOSIS/ (42750)
exp Communicable Diseases, Emerging/ (1814)
exp disease transmission, patient-to-professional/ or exp disease transmission, professional-to-patient/ (48918)
or/1-6 (117927)
[from 18 keep 1-49] (0)
[from 19 keep 1-10] (0)
(plasmacluster or lifetronic).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (0)
exp IONIZATION/ (6458)
exp Ion/ (122230)
exp Ultraviolet Irradiation/ (4991)
((ultraviolet adj2 irradiation) or (ultra-violet adj2 irradiation)).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (6921)
(uvgi or ultraviolet rays).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (161)
(High efficiency particulate air or ion generation or ion emission).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (217)
or/10-16 (134634)
(air purif$ or air filt$ or air cleaner$).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (750)
17 and 18 (87)
exp infection control/ (18169)
exp airborne infection/ or exp communicable disease/ or exp cross infection/ or exp hospital infection/ (19973)
exp Communicable Disease/ (1814)
exp Disease Transmission/ (48918)
exp TUBERCULOSIS/ (42750)
exp Epidemic/ (15794)
(airborne and (pathogen$ or disease$ or bacteria$ or virus$ or microorganism$)).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (2618)
or/20-26 (130657)
19 and 27 (31)
from 28 keep 1-31 (31)
Appendix 4: Annotated Bibliography of Databases
MEDLINE: The MEDLINE database contains citations and abstracts from more than 4,600 biomedical journals published in the United States and 70 other countries, resulting in over 11 million citations dating back to the mid 1960s. Also included are about 130,000 population-related journal citations that were added in October of 2002. While coverage is worldwide, most records are from English-language sources or have English abstracts.
EMBASE: The Excerpta Medica database (EMBASE) is a major biomedical and pharmaceutical database indexing over 3,500 international journals on drug research, pharmacology, pharmaceutics, toxicology, clinical and experimental human medicine, health policy and management, public health, occupational health, environmental health, drug dependence and abuse, psychiatry, forensic medicine, and biomedical engineering/instrumentation. EMBASE differs from MEDLINE in that it provides more coverage of European literature and has a stronger focus on pharmaceutical literature.
Cochrane Database of Systematic Reviews (DSR): The DSR is a database that contains citations of systematic reviews of health care interventions. It is published by The Cochrane Collaboration, an independent not-for-profit international organization that is dedicated to producing and disseminating accurate information about health care interventions.
INAHATA (International Network of Agencies for Health Technology Assessment): The health technology assessment database was created in 1998 and includes assessments from 41 health technology organizations from 21 countries. Included in this database are documents dating back to 1988 http://www.york.ac.uk/inst/crd/htahp.htm (accessed July 18, 2005).
BIOSIS Previews: This supplies comprehensive coverage of life science journals including those pertaining to agriculture, biomedical, molecular genetics, and zoology. It also has coverage from international meetings, review articles, books, and additional references from Biological Abstracts and Biological Abstracts/RRM.
Bacteriology Abstracts: This is a medically oriented database that includes references on bacterial immunology, vaccinations to diseases, pure bacteriology, biochemistry, and genetics.
Web of Science: This is a multidisciplinary database that contains citations covering the arts and humanities, social sciences, and sciences including health sciences and medicine. Subject, author, journal, and/or author address may be used to search specific articles. The database may also be searched for articles that cite a known author or publication.
Dissertation Abstracts: This is a definitive subject, title, and author guide to virtually every American dissertation accepted at an accredited institution since 1861. Abstracts are included for doctoral records from July 1980 (Dissertation Abstracts International, Volume 41, Number 1) to the present and for masters theses from Spring 1988 (Masters Abstracts, Volume 26, Number 1) to the present.
NIOSHTIC 2 (National Institute of Occupational Safety and Health): This is a bibliographic database of occupational safety and health publications, documents, grant reports, and other communication products supported in whole or in part by the National Institute for Occupational Safety and Health (NIOSH). (http://www2a.cdc.gov/nioshtic-2/Nioshtic2.htm (accessed July 18, 2005).
Appendix 5: Methods of Experiments
Test Chamber
The temperature of the room was maintained at 15°C–35°C and a relative humidity of 50%–90%.
The room had insulated walls, one door, and no windows.
The floor to ceiling height was 2.4 m.
There was a natural infiltration rate of 0.1–0.3 air changes per hour (ACH).
A computer-controlled ventilation system delivered a minimum of 2 ACHs and a maximum of 8 ACHs of high-efficiency particulate air (HEPA)-filtered outside air through 2 circular diffusers located in the ceiling.
The test chamber was under negative pressure (12Pa) and was continuously monitored using pressure gauges and ventilation system feedback control loops.
Two box fans were used to ensure complete mixing of room air within the test chamber.
Portable (In-Room) Air Cleaners
Portable air cleaners were located on the west wall during testing.
The 3 portable air cleaners tested included a negative ion generator, electrostatic precipitator, and one with a HEPA filter and internal ultraviolet (UV) lights. Their characteristics are shown in Table 1.
Table 1: Characteristics of Portable Air Cleaners.
| Characteristic | Negative Ion Generator |
Electrostatic Precipitator |
PAC with combined HEPA filter and UV lights* |
|---|---|---|---|
| Airflow rate as specified by manufacturer (cfm)* | Not specified | Variable (150–340) | 550 |
| Measured airflow rate (cfm) | 24 | 75-122 | 504 |
| Equivalent ACH* | 0.46 | 1.4-4.2 | 9.6 |
| Air intake position | Back | Left-right | Top |
| Air exhaust position | Top | Right-left | Bottom-front |
| Dimensions of PAC (cm) | 28 × 25 × 13 | 30 × 38 × 48 | 109 × 79 × 56 |
| Weight of PAC (kg) | 2 | 18 | 91 |
| Mounting Options | Portable | Portable/Ceiling | Mobile |
PAC indicates portable air cleaner; HEPA, high-efficiency particulate air; UV, ultraviolet; cfm, cubic feet per minute; ACH, air changes per hour.
Upper-Room Ultraviolet Germicidal Irradiation System
An ultraviolet germicidal irradiation (UVGI) system that consisted of 5 luminaries with multiple lights was used. Four luminaries were mounted in each corner of the room, and 1 was hung from the centre of the ceiling.
The centre luminary had 4 lights each of 18 watts (W) for a total of 72 watts (W). The corner luminaries each had 2 lights each of 18 watts for a total of 36 W per luminary.
Each luminary had black louvers of 1.9 cm.
Each luminary was installed so that the lower edge was located 2.2 m above the floor and the top was 3 cm below the ceiling.
The overall effect was a 30-cm band of UVGI in the upper level of the room.
The UVGI system was operated for more than 100 hours before the experiments commenced.
Test Microorganisms
Mycobacterium parafortuitum: a gram-positive organism similar in size to Mycobacterium tuberculosis.
Micrococcus luteus: a gram-positive microorganism.
The microorganisms were aerosolized using a 6-jet Collison nebulizer located outside the test room.
The aerosol generation was located in the centre of the room between the ventilation exhaust and the supply airflow.
The aerosolized pathogen was delivered into the test chamber at a rate of 12.5 liters / minute and released approximately 1.5 m above the floor.
Aerosolized pathogens were generated for 30 minutes to raise the pathogen concentration in the room. During this period the room was not ventilated and the air in the room was not mixed.
Upon reaching the desired concentration, the generation of the pathogen was stopped and the concentration was allowed to decay.
Pathogen Sampling
Airborne bacteria were sampled with AGI-30 impingers, 1.6 m above the floor, below the ventilation exhaust outlet, and at a standard location in the room
Impingers collected bacteria in 30 ml of sterile phosphate buffered solution
Pathogens were collected sequentially 5 times during the decay period, and 2 impingers were operated simultaneously to collect duplicate samples.
A nonbiological aerosol was used for experiments testing the HEPA-UV portable air cleaner with UV lights in turned on or off, and with the UV-lights removed.
Pathogen Quantification
Culturing and epifluorescent direct microscopy were used to quantify the pathogens.
Culturable plate counts quantified pathogens in terms of colony-forming units, which represented the number of cells in a sample that are capable of forming colonies on a suitable agar medium.
Direct microscopic counts, also called total counts, represent the number of alive and dead bacterial cells collected. All direct counts were reported as the mean of the number of fields.
An optical particle counter was used to quantify the amount of nonbiological aerosol for experiments testing the HEPA-UV portable air cleaner with UV lights in and turned on or off, and with UV lights removed.
Suggested Citation
This report should be cited as follows:
Medical Advisory Secretariat. Air cleaning technologies: an evidence-based analysis. Ontario Health Technology Assessment Series 2005; 5(17)
Permission Requests
All inquiries regarding permission to reproduce any content in the Ontario Health Technology Assessment Series should be directed to MASinfo@moh.gov.on.ca
How to Obtain Issues in the Ontario Health Technology Assessment Series
All reports in the Ontario Health Technology Assessment Series are freely available in PDF format at the following URL: www.health.gov.on.ca/ohtas
Print copies can be obtained by contacting MASinfo@moh.gov.on.ca
Conflict of Interest Statement
All analyses in the Ontario Health Technology Assessment Series are impartial and subject to a systematic evidence-based assessment process. There are no competing interests or conflicts of interest to declare.
Peer Review
All Medical Advisory Secretariat analyses are subject to external expert peer review. Additionally, the public consultation process is also available to individuals wishing to comment on an analysis prior to finalization. For more information, please visit http://www.health.gov.on.ca/english/providers/program/ohtac/public_engage_overview.html
Contact Information
The Medical Advisory Secretariat
Ministry of Health and Long-Term Care
20 Dundas Street West, 10th floor
Toronto, Ontario
CANADA
M5G 2N6
Email: MASinfo@moh.gov.on.ca
Telephone: 416-314-1092
ISSN 1915-7398 (Online)
ISBN 1-4249-0065-4 (PDF)
About the Medical Advisory Secretariat
The Medical Advisory Secretariat is part of the Ontario Ministry of Health and Long-Term Care. The mandate of the Medical Advisory Secretariat is to provide evidence-based policy advice on the coordinated uptake of health services and new health technologies in Ontario to the Ministry of Health and Long-Term Care and to the healthcare system. The aim is to ensure that residents of Ontario have access to the best available new health technologies that will improve patient outcomes.
The Medical Advisory Secretariat also provides a secretariat function and evidence-based health technology policy analysis for review by the Ontario Health Technology Advisory Committee (OHTAC).
The Medical Advisory Secretariat conducts systematic reviews of scientific evidence and consultations with experts in the health care services community to produce the Ontario Health Technology Assessment Series.
About the Ontario Health Technology Assessment Series
To conduct its comprehensive analyses, the Medical Advisory Secretariat systematically reviews available scientific literature, collaborates with partners across relevant government branches, and consults with clinical and other external experts and manufacturers, and solicits any necessary advice to gather information. The Medical Advisory Secretariat makes every effort to ensure that all relevant research, nationally and internationally, is included in the systematic literature reviews conducted.
The information gathered is the foundation of the evidence to determine if a technology is effective and safe for use in a particular clinical population or setting. Information is collected to understand how a new technology fits within current practice and treatment alternatives. Details of the technology’s diffusion into current practice and information from practicing medical experts and industry, adds important information to the review of the provision and delivery of the health technology in Ontario. Information concerning the health benefits; economic and human resources; and ethical, regulatory, social and legal issues relating to the technology assist policy makers to make timely and relevant decisions to maximize patient outcomes.
If you are aware of any current additional evidence to inform an existing Evidence-Based Analysis, please contact the Medical Advisory Secretariat: MASInfo@moh.gov.on.ca. The public consultation process is also available to individuals wishing to comment on an analysis prior to publication. For more information, please visit http://www.health.gov.on.ca/english/providers/program/ohtac/public_engage_overview.html
Disclaimer
This evidence-based analysis was prepared by the Medical Advisory Secretariat, Ontario Ministry of Health and Long-Term Care, for the Ontario Health Technology Advisory Committee and developed from analysis, interpretation and comparison of scientific research and/or technology assessments conducted by other organizations. It also incorporates, when available, Ontario data, and information provided by experts and applicants to the Medical Advisory Secretariat to inform the analysis. While every effort has been made to do so, this document may not fully reflect all scientific research available. Additionally, other relevant scientific findings may have been reported since completion of the review. This evidence-based analysis is current to the date of publication. This analysis may be superceded by an updated publication on the same topic. Please check the Medical Advisory Secretariat Website for a list of all evidence-based analyses: http://www.health.gov.on.ca/ohtas
Abbreviations
- ACH
Air changes per hour
- AII
Airborne infection isolation
- ASHRAE
American Society of Heating, Refrigerating and Air-Conditioning Engineers
- cfm
Cubic feet per minute.
- cfu
Colony forming units
- eACH
Equivalent air change per hour
- HEPA
High-efficiency particulate air
- MERV
Minimum efficiency reporting value
- NIOSH
National Institute for Occupational Safety and Health
- UVGI
Ultraviolet germicidal irradiation
- nm
Nanometer
- μm
Micrometer
References
- 1.Canada: 2003. [[cited Dec. 2005]]. Sharp. Plasma cluster showcase [homepage on the Internet] Available from: http://www.sharp.ca/products/ion/index.asp . [Google Scholar]
- 2.Ontario Expert Panel on SARS and Infectious Disease Control. Apr, 2004. [[cited 2005 Sept. 1]]. For the public’s health: a plan of action [report on the Internet] Available from: http://www.health.gov.on.ca/english/public/pub/ministry_reports/walker04/walker04_mn.html .
- 3.Miller-Leiden S, Lobascio C, Nazaroff WW, Macher JM. Effectiveness of in-room air filtration and dilution ventilation for tuberculosis infection control. J Air Waste Manag Assoc. 1996;46((9)):869–882. doi: 10.1080/10473289.1996.10467523. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. [[cited 2005 Sept 20]];MMWR Morb Mortal Wkly Rep [serial on the Internet] 1994 43((RR-13)):1–132. Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care facilities. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/00035909.htm . [Google Scholar]
- 5.Marier RL, Nelson T. A ventilation-filtration unit for respiratory isolation. Infect Control Hosp Epidemiol. 1993;14((12)):700–705. doi: 10.1086/646672. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. [[cited 2005 Sept. 11]];MMWR Morb Mortal Wkly Rep [serial on the Internet] 2005 54((RR17)):1–141. Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care settings. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5417a1.htm . [Google Scholar]
- 7.Centers for Disease Control and Prevention. [[cited 2005 Sept. 11]];MMWR Morb Mortal Wkly Rep [serial on the Internet] 2003 52((RR-10)):1–48. Guidelines for environmental infection control in health-care facilities: recommendations of CDC and the Healthcare Infection Control Practices Advisory Committee (HICPAC) Available at: www.cdc.gov/ncidod/hip/enviro/guide.htm . [Google Scholar]
- 8.Brickner PW, Richard LV, First M, Nardell E, Murray M, Kaufaman W. The application of ultraviolet germicidal irradiation to control transmission of airborne disease: bioterrorism countermeasure. Public Health Rep. 2003;(118):99–114. doi: 10.1016/S0033-3549(04)50225-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Infection Control Guidelines. Volume 25S4. Health Canada: Jul, 1999. [[cited 2005 Sept. 1]]. Routine practices and additional precautions for preventing the transmission of infection in health care. [report on the Internet] Available from: http://www.phac-aspc.gc.ca/publicat/ccdrrmtc/99pdf/cdr25s4e.pdf . [PubMed] [Google Scholar]
- 10.Mississauga: Canadian Standards Association; Sep, 2001. Canadian Standards Association. Special requirements for heating, ventilation, and air conditioning (HVAC) systems in health care facilities. Z317.2-01. [Google Scholar]
- 11.Public Health Agency of Canada. Can Commun Dis Rep. 1996. p. 22S1. Guidelines for preventing the transmission of tuberculosis in Canadian health care facilities and other institutional settings.
- 12.The American Institute of Architects. 2006. [[cited 2005 Dec. 1]]. Guidelines for design and construction of hospital and health care facilities (DRAFT) [homepage on the Internet] Available from: http://www.aia.org/aah_gd_hospcons .
- 13.Miller SL, Hernandez M. NIOSH contract report PO-36755-R-00077B5D; PB2003-101407. Boulder, CO: University of Colorado at Boulder; May 22, 2002. Evaluating portable air cleaner removal efficiencies for bioaerosols. [Google Scholar]
- 14.Rutala WA, Jones SM, Worthington JM, Reist PC, Weber DJ. Efficacy of portable filtration units in reducing aerosolized particles in the size range of Mycobacterium tuberculosis. Infect Control Hosp Epidemiol. 1995;16((7)):391–398. doi: 10.1086/647136. [DOI] [PubMed] [Google Scholar]
- 15.Green FC, Scarpino PV. The use of ultraviolet germicidal irradiation (UVGI) in disinfection of airborne bacteria. Environ Eng Policy. 2002;3:101–107. [Google Scholar]
- 16.ECRI. Health Devices. 1995;24((10)):370–417. Mobile high-efficiency-filter air cleaners. [Google Scholar]
- 17.Nazaroff WW. Indoor particle dynamics. Indoor Air. 2004;14((Suppl 7)):175–183. doi: 10.1111/j.1600-0668.2004.00286.x. [DOI] [PubMed] [Google Scholar]
- 18.DHHS (NIOSH) Publication No. 2003-136. National Institute of Occupational Safety and Health (NIOSH); Apr, 2003. [[cited 2005 Sept. 1]]. Department of Health and Human Services. Guidance for filtration and air-cleaning systems to protect building environments from airborne chemical, biological, or radiological attacks [report on the Internet] Available from: http://www.cdc.gov/niosh/docs/2003-136/pdfs/2003-136.pdf . [Google Scholar]
- 19.Riley RL, Permutt S. Room air disinfection by ultraviolet irradiation of upper air. Arch Environ Health. 1971;22:208–219. doi: 10.1080/00039896.1971.10665834. [DOI] [PubMed] [Google Scholar]
- 20.CCOHS; 2005. [[cited Nov. 2005]]. Canadian Centre for Occupational Health and Safety. What is ultraviolet radiation [homepage on the Internet] Available from: http://www.ccohs.ca/oshanswers/phys_agents/ultravioletradiation.html . [Google Scholar]
- 21.National Institute for Occupational Health and Safety; 1972. [[cited 2005 Sept. 5]]. United States Department of Health. Occupational exposure to ultraviolet radiation [report on the Internet]. HSM 73-11009. Available from: http://www.cdc.gov/niosh/73-11009.html . [Google Scholar]
- 22.Kreuger AP, Reed EJ. Biological impact of small air ions. Science. 1976;193((4259)):1209–1213. doi: 10.1126/science.959834. [DOI] [PubMed] [Google Scholar]
- 23.Uk Lee B, Yermakov M, Grinshpun S. Removal of fine and ultrafine particles from indoor air environments by the unipolar ion emission. Atmos Environ. 2004;38:4815–4823. doi: 10.1016/j.atmosenv.2004.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blackhall K, Appleton S, Cates CJ. Ionisers for chronic asthma. Cochrane Database Syst Rev. 2003. 2(DOI:10.1002/14651858.CD002986) [DOI] [PubMed]
- 25.Health Canada. 1999. [[cited Dec. 2005]]. Health Canada warns the public about air cleaners designed to intentionally generate ozone [homepage on the Internet] Available from: http://www.hc-sc.gc.ca/ahc-asc/media/advisories-avis/1999/1999_62_e.html .
- 26.ECRI. Health Devices. 1997;26((6)):228–245. Mobile high-efficiency-filter air cleaners. [PubMed] [Google Scholar]
- 27.Mead K, Johnson DL. An evaluation of portable high-efficiency particulate air filtration for expedient patient isolation in epidemic and emergency response. Ann Emerg Med. 2004;44((6)):635–645. doi: 10.1016/j.annemergmed.2004.07.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kujundzic E, Zander DA, Hernandez M, Angenent LT, Henderson DE, Miller SL. Effects of ceiling-mounted HEPA-UV air filters on airborne bacteria concentrations in an indoor therapy pool building. J Air Waste Manag Assoc. 2005;55((2)):210–218. doi: 10.1080/10473289.2005.10464612. [DOI] [PubMed] [Google Scholar]
- 29.ECRI. Health Devices. 1995;24((10)):368–369. The resurgence of Tuberculosis-from old problem to new menace. [PubMed] [Google Scholar]
- 30.Food and Drug Act. [[cited 2005 Sept. 21]];Canada Gazette. 1998 132((11)) Medical devices regulations. SOR/98-282 7. [serial on the Internet] Available at: http://gazetteducanada.gc.ca/partII/1998/19980527/html/sor282-e.html . [Google Scholar]
- 31.Ottawa: Industry Canada; 2005. [[cited 2005 Sept. 20]]. Industry Canada. Canadian requirements [monograph on the Internet] Available from: http://strategis.ic.gc.ca/epic/internet/inmdam.nsf/en/hi00038e.html#_1_58 . [Google Scholar]
- 32.GRADE Working Group. BMJ. 2004;328:1–8. Grading quality of evidence and strength of recommendations. [Google Scholar]
- 33.American College of Chest Physicians. 2005. [[cited Dec. 2005]]. How to develop a guideline [homepage on the Internet] Available from: http://www.chestnet.org/education/guidelines/development/q7.php .
- 34.Ontario Ministry of Health. Protecting patients and staff. Recommended infection control and surveillance standards for febrile illness (FRI) in Non-Outbreak Conditions [report on the Internet] Dec, 2003. [[cited 2005 Sept. 2]]. Preventing respiratory illnesses. Available from: http://www.health.gov.on.ca/english/providers/program/pubhealth/sars/docs/docs2/dir_122303_a cute_care_nonoutbreak.pdf .
- 35.The American Society of Heating Refrigerating and Air-Conditioning Engineers. 2006. [[cited Sept. 2005]]. ASHRAE [homepage on the Internet] Available from: http://www.ashrae.org/
- 36.National Advisory Committee on SARS and Public Health. Oct, 2003. [[cited 2005 Sept. 1]]. Learning from SARS: renewal of public health in Canada [report on the Internet] Available from: http://www.phac-aspc.gc.ca/publicat/sars-sras/naylor/


