Abstract
Background. The expression of CD24 has been detected in a wide variety of human malignancies. Downregulation of CD24 inhibits proliferation and induces apoptosis in tumor cells, whereas its upregulation increases tumor growth and metastasis. However, no data on CD24 protein levels in retinoblastoma are available, and the mechanism of CD24 involvement in retinoblastoma progress has not been elucidated. The aim of this study was to explore the expression profile of CD24 in the retinoblastoma tumor samples and to correlate with clinicopathological parameters. Methods. Immunohistochemistry was performed for CD24 on the archival paraffin sections of retinoblastoma and correlated with clinicopathological features. Western blotting was performed to confirm immunoreactivity results. Results. CD24 immunoreactivity was observed in 72.0% (36/50) of the retinoblastoma specimens. Among the 35 low-risk tumors, CD24 was expressed in 62.9% (22/35) tumors and among the 15 high-risk tumors, CD24 was expressed in 93.3% (14/15) tumors. High-risk tumors showed significantly increased expression of CD24 compared to tumors with low-risk (P < 0.05). Conclusions. This is the first correlation between CD24 expression and histopathology in human retinoblastoma. Our study showed increased expression of CD24 in high risk tumors compared to low risk tumors. Further functional studies are required to explore the role of CD24 in retinoblastoma.
1. Introduction
Retinoblastoma is the most common primary intraocular malignancy in children [1]. It is more frequent in some developing areas, such as Latin America, Africa, China, and India [2]. Factors that play a role in tumor invasion and metastasis include early genetic events such as increased copy number of chromosome 6p and 1q, and late events such as high levels of telomerase activity, loss of chromosome 1p, and p53 inactivation [3]. Retinoblastoma is diagnosed late, usually when extraocular dissemination has occurred and the prognosis is poor. Primary chemoreduction is used for intraocular retinoblastoma and systemic chemotherapy is used following enucleation in patients with optic nerve and deep choroidal invasion, orbital extension, and metastatic disease. New treatment modalities, such as subconjunctival injection, selective ophthalmic artery injection, and vitreous injection, are being investigated and have achieved favorable results [4]. Although many modalities are used, almost half of eyes with retinoblastoma have to be enucleated. Thus, there is an urgent need to further study the biology and molecular mechanisms of retinoblastoma and identify the specific proteins that cause tumor progression and predict prognosis in order to improve the therapeutic outcome of patients with retinoblastoma.
CD24 is a small, heavily glycosylated, phosphatidylinositol-anchored ucin-like cell surface protein [5]. Several studies have identified CD24 as an alternative ligand for P-selectin, an adhesion molecule which is expressed by activated endothelial cells and platelets [6]. Functionally, it is considered to play a critical role in the metastasis of tumor cells through P-selectin. Many tumor cell lines can bind to platelets via P-selectin, and CD24 expression might enhance the metastatic potential of tumor cells [7]. Recently, several studies have investigated CD24 overexpression in a wide variety of human malignancies lung cancer, choriocarcinoma, cholangiocarcinoma, glioma, pancreatic cancer, prostatic cancers, renal cell carcinoma, and ovarian and breast cancers [8–15]. This increased expression is usually tied with a more aggressive course of the disease. CD24 positivity was significantly related to younger patient age, higher pT stages, and higher PSA relapse rate in prostatic adenocarcinomas [10]. Wei et al. have shown that CD24 is overexpressed in pancreatic cancer cell lines in comparison to nine normal pancreatic cell lines [11]. In hepatocellular carcinomas, CD24 expression was also correlated with serum levels of HBs-Ag, elevated serum AFP levels, and p53 mutations [12]. Lee et al. noted strong CD24 staining in renal cell carcinoma irrespective of the histological tumor type [13]. A recent study by Smith et al. reported CD24 overexpression in bladder cancer compared to normal urothelium; however, no correlation with tumor stage and grade was noted. Additionally, bladder cancer patients with strong CD24 immunoreactivity tended to have a shorter disease-free survival [14]. Moreover, it has been demonstrated that the down-regulation of CD24 inhibits proliferation and induces apoptosis in tumor cells, whereas its up-regulation increases tumor growth and metastasis. Although there have been substantial advances in the understanding of the basic biology and pathogenesis of retinoblastoma, there is no data on CD24 protein levels in retinoblastoma, and the mechanism of CD24 involvement in retinoblastoma progress has not been elucidated. The purpose of this study was to investigate if CD24 is present in retinoblastoma and to evaluate the correlation between CD24 expression and the severity of the malignancy of this tumor.
2. Materials and Methods
2.1. Patients and Tissue Samples
This study was approved by the Research Ethics Committee of DaPing Hospital, Research Institute of Surgery Third Military Medical University, Chongqing, China. Written informed consent was obtained from all of the patients. All specimens were handled and made anonymous according to the ethical and legal standards.
Fifty tumors were available from 50 eyes for the present study. Among them, there were tumors from 35 males and 15 females. The age ranged from 1 month to 8 years. (median = 22 months). There were 38 unilateral retinoblastomas and 12 bilateral retinoblastomas. Ten fresh tumors were used for studying the expression of CD24.
2.2. Histopathological Information
There were 35 tumors with no massive invasion of choroid or optic nerve (lower risk to extraocular relapse) and 15 tumors with invasion of choroid/optic nerve/orbit (higher risk to extraocular relapse). Among the 15 high-risk tumors, 2 tumors had diffuse choroidal invasion, 2 tumors with postlaminar optic nerve invasion. There are 5 tumors with both diffuse choroidal and post-laminar ON invasion, 2 tumors with both diffuse choroidal and prelaminar ON invasion, 2 tumors with focal choroidal and post-laminar ON invasion, and 1 tumor with post-laminar optic nerve invasion with surgical end involved. There was 1 tumor with orbital invasion. There were 31 tumors with poorly differentiated cells, 12 tumors with well-differentiated and 7 tumors with moderately differentiated cells.
2.3. Immunohistochemistry Assay
Formalin-fixed, paraffin-embedded, sectioned tissues (5 μm thick) were immunostained using the Labelled Streptavidin Biotin 2 System (BioGenex; San Ramon, CA, USA). Following peroxidase blocking with 0.3% H2O2/methanol for 30 min, specimens were blocked with phosphate-buffered saline (PBS) containing 5% normal horse serum (Vector Laboratories Inc., Burlingame, CA, USA). All incubations with mouse monoclonal antibody CD24 (Neomarkers, Clone 24C02, Fremont, CA, USA) at 1 : 100 dilution were carried out overnight at 4°C. Then the specimens were briefly washed in PBS and incubated at room temperature with the anti-mouse antibody and avidin-biotin peroxidase (Vector Laboratories Inc., Burlingame, CA, USA). The specimens were then washed in PBS and color-developed by diaminobenzidine solution (Dako Corporation, Carpinteria, CA, USA). After washing with water, specimens were counterstained with Meyer's hematoxylin (Sigma Chemical Co., St Louis, MO, USA). For CD24-negative controls, immunohistochemistry was performed using normal mouse immunoglobin with a dilution of 1 : 100 (0.4 μg) and normal goat immunoglobin with a dilution of 1 : 100 (8 μg/mL).
Assessment of immunohistochemical staining was evaluated by two independent pathologists. The CD24-positive cells showed immunoreactivity in the cytoplasm of tumor cells. Randomly 10 vital tumor fields were scanned for protein expression and percentage of positive tumor cells was noted for each field. Then finally the average expression was calculated from the 10 values for the entire slide. Depending on the percentage of positive cells, 4 categories were established: 0, no positive cells; 1+, positive cells in less than one-third (faint); 2+, positive cells in 33~67% (heterogeneous); 3+, positive cells in more than two-thirds (positive) of total tumor cell population.
2.4. Western Blot Analysis
Retinoblastoma tissues were homogenized in lysis buffer (PBS, 1% nonidet P-40 (NP-40), 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate (SDS), 100 μg/mL aprotinin, 100 μg/mL phenylmethylsulfonyl fluoride (PMSF), sodium orthovanadate) at 4°C throughout all procedures, and sonicated for 70 s, then added to 300 μg PMSF per gram of tissue and incubated on ice for 30 min, followed by centrifugation at 15,000 rpm for 20 min at 4°C. The protein content was determined according to Bradford's method (Bradford, 1976), with bovine serum albumin used as a standard. Equal amounts of protein were separated electrophoretically on 7.5% SDS-polyacrylamide gels and transferred onto polyvinylidene difluoride membranes (Roche; Basel, Switzerland). The membranes were probed with monoclonal mouse antihuman CD24 (SC-7034, 1 : 500, Santa Cruz Biotechnology). The expression level of CD24 was determined by incubating the membranes with horseradish peroxidase-conjugated anti-mouse immunoglobulin G (1 : 3000 dilution) and enhanced chemiluminescence reagent (Pierce; Minneapolis, MN, USA), according to the manufacturers' suggested protocols. Detection of positivity was by using ECL system (Amersham Pharmacia) or the Supersignal West Femto Maximum Sensitivity Substrate (Pierce).
2.5. Statistical Analysis
All computations were carried out using the software of SPSS version13.0 for Windows (SPSS Inc, IL, USA). Data were expressed as means ± standard deviation (SD). Mann-Whitney U test was used to determine association of immunoreactivity of CD24 with tumor invasion and differentiation. For statistical purposes, moderately differentiated and well-differentiated tumors were grouped together and were compared with poorly differentiated tumors. Differences were considered statistically significant when P was less than 0.05.
3. Results
3.1. Immunohistochemical Staining of CD24 Protein in Human Retinoblastoma Tissues
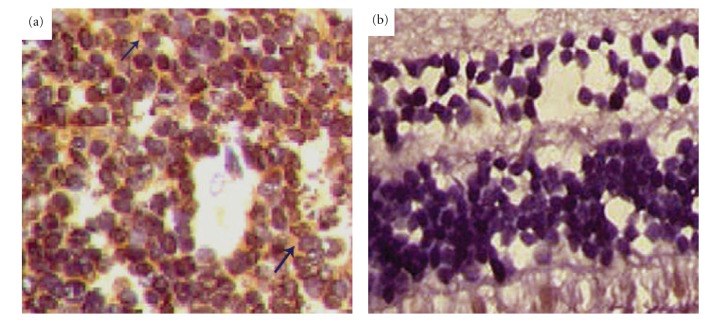
Immunohistochemistry analysis showed no positive CD24 protein expression in the nonneoplastic retina (Figure 1(a)). CD24 immunoreactivity was observed in the cytoplasm of retinoblastoma specimens with varying levels of percentage tumor staining and intensities (Figure 1(b)). CD24 immunoreactivity was observed in 72.0% (36/50) of the retinoblastoma specimens.
Figure 1.
Immunohistochemical staining for CD24 in human retinoblastoma tissues (original magnification ×200). (a) CD24 staining is negative in non-neoplastic retina; (b) CD24-positive expression was found in the cytoplasm of retinoblastoma tissues.
We observed that tumors with invasion showed significantly higher expression of CD24 compared to tumors without invasion (P < 0.05). The immunoreactivity of CD24 in high-risk and low-risk tumors is shown in Table 1. The raw data of the individual immunoscores for CD24 in the high-risk and low-risk tumors are available in Tables 2 and 3, respectively. Among the 35 low-risk tumors, CD24 was expressed in 62.9% (22/35) tumors and among the 15 high-risk tumors, CD24 was expressed in 93.3% (14/15) tumors. High-risk tumors showed significantly increased expression of CD24 compared to tumors with low risk (P < 0.05).
Table 1.
The expression of CD24 protein in high-risk and low-risk retinoblastoma.
| Group | No. of cases | CD24 immunohistochemical staining | |||
|---|---|---|---|---|---|
| Positive | Heterogeneous | Faint | Negative | ||
| Overall cohort | 50 | 19 | 11 | 6 | 14 |
| High-risk tumors | 15 | 9 | 4 | 1 | 1 |
| Low-risk tumors | 35 | 10 | 7 | 5 | 13 |
|
| |||||
| P value | <0.05 | ||||
Table 2.
The expression of CD24 protein and clinicopathological features of retinoblastoma tissues with high risk of extraocular relapse.
| Case no. | Age/sex | Clinicopathological features | CD24 positivity (%) |
|---|---|---|---|
| 1 | 3 mon/M | OS: PD, Post Lam ON Inv | 30 |
| 2 | 5/M | OD: PD, rectus orbital invasion | 80 |
| 3 | 3/M | OS: PD, Diff Ch Inv | 40 |
| 4 | 7/M | OS: PD, Post Lam ON Inv | 70 |
| 5 | 2/M | OS: PD, Diff Ch, Post Lam ON Inv | 10 |
| 6 | 20 mon/F | OS: PD, Diff Ch, Post Lam ON Inv | 30 |
| 7 | 5/M | OS: PD, Diff Ch, Pre Lam ON inv | 60 |
| 8 | 3/M | OS: PD, Diff Ch, Post Lam ON Inv | 0 |
| 9 | 1/F | OD: PD, Post Lam ON Inv with SE involved | 70 |
| 10 | 2/F | OS: PD, Diff Ch, Pre Lam ON Inv | 50 |
| 11 | 1/F | OD: PD, Diff Ch Inv | 60 |
| 12 | 4/M | OS: PD, Diff Ch Inv | 80 |
| 13 | 3/M | OD: PD, Focal Ch, Post Lam ON Inv | 90 |
| 14 | 2/F | OS: PD, Diff Ch, Post Lam ON Inv | 50 |
| 15 | 2/M | OD: PD, Focal Ch, Post Lam ON Inv | 70 |
Note: mon: months; M: male; F: female; OD: right eye; OS: left eye; PD: poorly differentiated; MD: moderately differentiated; WD: well differentiated; UL: unilateral disease; BL: bilateral disease; Diff Ch Inv: diffused choroidal invasion of tumor; Focal Ch Inv: focal invasion of tumor cells into choroids; Pre Lam ON Inv: pre-laminar invasion of optic nerve; Post Lam ON Inv: post-laminar invasion of the optic nerve.
Table 3.
The expression of CD24 protein and clinicopathological features of retinoblastoma tissues with low risk of extraocular relapse.
| Case no. | Age/sex | Clinicopathological features | CD24 positivity (%) |
|---|---|---|---|
| 1 | 22 mon/M | OD: WD | 0 |
| 2 | 3/M | OS: WD | 10 |
| 3 | 3 mon/F | OS: PD, Pre Lam ON Inv | 30 |
| 4 | 8/F | OD: PD, Pre Lam ON Inv | 60 |
| 5 | 1 mon/F | OD: PD, Pre Lam ON Inv | 60 |
| 6 | 5/F | OD: MD, Focal Ch, Pre Lam ON Inv | 90 |
| 7 | 4/F | OD: MD, Focal Ch Inv | 50 |
| 8 | 2/F | OD: WD, Pre Lam ON Inv | 70 |
| 9 | 3/F | OD: WD, Focal Ch, Pre Lam ON Inv | 50 |
| 10 | 2/M | OS: PD, Focal Ch Inv | 80 |
| 11 | 3/M | OD: WD, Focal Ch Inv | 60 |
| 12 | 3/M | OS: PD | 0 |
| 13 | 5/M | OS: PD | 0 |
| 14 | 1/F | OD: WD | 50 |
| 15 | 3/F | OS: PD | 0 |
| 16 | 4 mon/M | OS: PD | 0 |
| 17 | 2 mon/M | OS: WD | 0 |
| 18 | 2/M | OD: PD | 40 |
| 19 | 2/M | OD: MD | 10 |
| 20 | 16 mon/M | OS: PD, Pre Lam ON Inv | 70 |
| 21 | 6 mon/M | OS: PD | 30 |
| 22 | 2/M | OD: WD | 60 |
| 23 | 20 mon/M | OD: WD | 0 |
| 24 | 23 mon/M | OS: PD | 50 |
| 25 | 3 mon/M | OD: PD | 0 |
| 26 | 1/M | OS: WD | 0 |
| 27 | 5 mon/M | OD: WD | 30 |
| 28 | 1 mon/M | OS: WD | 20 |
| 29 | 3/M | OS: PD | 0 |
| 30 | 11 mon/M | OD: MD | 10 |
| 31 | 5/M | OS: MD | 0 |
| 32 | 2/F | OS: PD | 20 |
| 33 | 1/M | OS: MD | 0 |
| 34 | 4 mon/M | OD: MD | 0 |
| 35 | 5/M | OS: PD, Pre Lam ON Inv | 60 |
Note: mon: months; M: male; F: female; OD: right eye; OS: left eye; PD: poorly differentiated; MD: moderately differentiated; WD: well differentiated; UL: unilateral disease; BL: bilateral disease; Focal Ch Inv: focal invasion of tumor cells into choroids; Pre Lam ON Inv: pre-laminar invasion of optic nerve.
3.2. Western Blot Analysis on Expression Levels of CD24 Protein in Human Retinoblastoma Tissues
Consistent with the results of immunohistochemical staining, western blot analysis also showed no CD24 protein in the non-neoplastic retina (lane 0), but positive expression in the retinoblastoma tissues which showed a single band of approximately 35~45 kDa for CD24 protein in all the tumors (Figure 2). Of the 10 tumors studied, 6 tumors were of high-risk and 4 tumors were of low-risk. CD24 was strongly expressed in 5 (83.3%, lanes 1~5) and faintly expressed in 1 (16.7%, lanes 6) high-risk tumors, whereas it was strongly expressed in 2 (50.0%, lanes 7~8) and faintly expressed in 2 (50.0%, lanes 9~10) low-risk tumors, also suggesting high-risk tumors showed significantly increased expression of CD24 compared to tumors with low-risk (P < 0.05).
Figure 2.
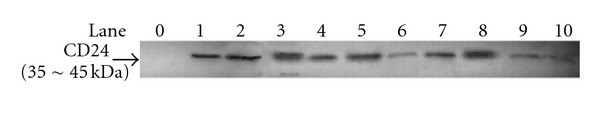
Western blot analysis for CD24 protein expression in retinoblastoma tissues, with non-neoplastic retina as control. The picture shows that CD24 was negative in non-neoplastic retina (lane 0), strongly expressed in 5 (83.3%, lanes 1~5) and faintly expressed in 1 (16.7%, lanes 6) high-risk tumors, whereas it was strongly expressed in 2 (50.0%, lanes 7~8) and faintly expressed in 2 (50.0%, lanes 9~10) low-risk tumors.
4. Discussion
To the best of our knowledge, this is the first study to demonstrate the correlation between CD24 and retinoblastoma. There are three main findings in the present study. At first, there was no CD24 expression in non-neoplastic retina; secondly, positive CD24 protein expression was observed in more than half of the retinoblastoma tissues by both immunohistochemistry and western blot analysis; and thirdly the level of CD24 was significantly higher in the retinoblastoma tissues with high risk of extraocular relapse than those with low risk of extraocular relapse. These results suggest that CD24 protein may be involved in the pathogenesis of retinoblastoma, and the level of CD24 protein expression may be associated with the severity of retinoblastoma.
CD24 was identified 30 years ago [16]. Since then, it has been extensively used as a marker for the differentiation of hematopoietic and neuronal cells, in addition to tissue and tumor stem cells [17]. CD24 has been reported to be involved in the control of cell proliferation, apoptosis, and cell adhesion. CD24 gene encodes a sialoglyco-protein that is expressed mainly on mature granulocytes, premature lymphocytes, and epithelial and neural cells. The encoded CD24 protein is anchored to the cell surface by a glycosylphosphatidylinositol and acts as a ligand for P-selectin on activated endothelial cells and platelets [18]. In lymphocytes, CD24 can modulate the capacities of early T and B lymphoid progenitor cells to proliferate and survive. Antibody-mediated cross-linking of CD24 induces apoptosis in a process involving the B-cell receptor and mitogen-activated protein kinases [19]. CD24 expression is only limited to several cell types in physiological condition. However, a large variety of tumors express CD24. For example, CD24 is broadly overexpressed on esophageal squamous cell carcinoma, small cell lung cancer, hepatocellular carcinoma, cholangiocarcinoma, pancreatic adenocarcinoma, urothelial carcinoma, prostate carcinomas, ovarian cancer, breast cancer, B-cell lymphomas, erythroleukemia, gliomas, and primary neuroendocrine carcinomas [8–15, 20]. In most cancer types, CD24 expression is significantly associated with a more aggressive course of the disease, indicating CD24 may play a role in the progression of cancer. In the pancreatic cancers, Ikenaga et al. demonstrated that higher tumor stage, nodal metastasis, and higher-grade tumors were more frequent in the CD24-positive group compared with the CD24-negative group. CD24 expression was also associated with shorter survival in univariate analysis [21]. In addition, Lee et al. reported that the non-small-cell lung carcinoma patients with CD24-high tumors tended to have a higher risk of disease progression and cancer-related death. Multivariate analysis proved CD24-high expression as independent prognostic factors of disease progression and cancer-related death [22]. Moreover, Sano group indicated that CD24 expression was associated with lymph node metastasis status, pathologic stage, number of nodal metastases, lymphatic invasion status, and venous invasion status of patients with esophageal squamous cell carcinoma. They also concluded that the overexpression of CD24 was a novel independent prognostic marker for identifying patients with poor prognosis after curative resection of esophageal squamous cell carcinoma [23]. In agreement with these previous reports, our study demonstrated an overexpression of CD24 in human retinoblastoma tissues, which also correlated significantly to the disease progression. Non-neoplastic retina failed to show any detectable immunoreactivity for CD24.
The significance and clinical implications of CD24 expression in retinoblastoma tissues are yet to be elucidated. In this study, the expression levels of CD24 protein in patients with high risk of extraocular relapse were significantly higher than in patients with lower risk of extraocular relapse. These results suggest tumor tissue CD24 may act as a biomarker for prediction of the severity of retinoblastoma and the prognosis of the patients.
In conclusion, this preliminary study has demonstrated for the first time that more than half of retinoblastoma express the CD24 protein. Tissue levels of CD24 protein are increased in high-risk tumors compared to low-risk tumors. Further functional studies are required to explore the role of CD24 in retinoblastoma.
Conflict of Interests
The authors declare no conflict of interests.
References
- 1.Mallikarjuna K, Sundaram CS, Sharma Y, et al. Comparative proteomic analysis of differentially expressed proteins in primary retinoblastoma tumors. Proteomics—Clinical Applications. 2010;4(4):449–463. doi: 10.1002/prca.200900069. [DOI] [PubMed] [Google Scholar]
- 2.Vandhana S, Deepa PR, Jayanthi U, Biswas J, Krishnakumar S. Clinico-pathological correlations of fatty acid synthase expression in retinoblastoma: an indian cohort study. Experimental and Molecular Pathology. 2011;90(1):29–37. doi: 10.1016/j.yexmp.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 3.Indovina P, Acquaviva A, De Falco G, et al. Downregulation and aberrant promoter methylation of p16ink4a: a possible novel heritable susceptibility marker to retinoblastoma. Journal of Cellular Physiology. 2010;223(1):143–150. doi: 10.1002/jcp.22019. [DOI] [PubMed] [Google Scholar]
- 4.Kase S, Parikh JG, Rao N. Expression of α-crystallin in retinoblastoma. Archives of Ophthalmology. 2009;127(2):187–192. doi: 10.1001/archophthalmol.2008.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bleckmann C, Geyer H, Lieberoth A, et al. O-glycosylation pattern of CD24 from mouse brain. Biological Chemistry. 2009;390(7):627–645. doi: 10.1515/BC.2009.044. [DOI] [PubMed] [Google Scholar]
- 6.Bleckmann C, Geyer H, Reinhold V, et al. Glycomic analysis of N-linked carbohydrate epitopes from CD24 of mouse brain. Journal of Proteome Research. 2009;8(2):567–582. doi: 10.1021/pr800729r. [DOI] [PubMed] [Google Scholar]
- 7.Cremers N, Deugnier MA, Sleeman J. Loss of CD24 expression promotes ductal branching in the murine mammary gland. Cellular and Molecular Life Sciences. 2010;67(13):2311–2322. doi: 10.1007/s00018-010-0342-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu B, Zhang Y, Liao M, et al. Clinicopathologic and prognostic significance of CD24 in gallbladder carcinoma. Pathology and Oncology Research. 2011;17(1):45–50. doi: 10.1007/s12253-010-9278-2. [DOI] [PubMed] [Google Scholar]
- 9.Riener MO, Vogetseder A, Pestalozzi BC, et al. Cell adhesion molecules P-cadherin and CD24 are markers for carcinoma and dysplasia in the biliary tract. Human Pathology. 2010;41(11):1558–1565. doi: 10.1016/j.humpath.2009.12.016. [DOI] [PubMed] [Google Scholar]
- 10.Lee JH, Kim SH, Lee ES, Kim YS. CD24 overexpression in cancer development and progression: a meta-analysis. Oncology Reports. 2009;22(5):1149–1156. doi: 10.3892/or_00000548. [DOI] [PubMed] [Google Scholar]
- 11.Wei HJ, Yin T, Zhu Z, Shi PF, Tian Y, Wang CY. Expression of CD44, CD24 and esa in pancreatic adenocarcinoma cell lines varies with local microenvironment. Hepatobiliary and Pancreatic Diseases International. 2011;10(4):428–434. doi: 10.1016/s1499-3872(11)60073-8. [DOI] [PubMed] [Google Scholar]
- 12.Yang XR, Xu Y, Yu B, et al. CD24 is a novel predictor for poor prognosis of hepatocellular carcinoma after surgery. Clinical Cancer Research. 2009;15(17):5518–5527. doi: 10.1158/1078-0432.CCR-09-0151. [DOI] [PubMed] [Google Scholar]
- 13.Lee HJ, Kim DI, Kwak C, Ku JH, Moon KC. Expression of CD24 in clear cell renal cell carcinoma and its prognostic significance. Urology. 2008;72(3):603–607. doi: 10.1016/j.urology.2008.01.061. [DOI] [PubMed] [Google Scholar]
- 14.Smith SC, Theodorescu D. The ral gtpase pathway in metastatic bladder cancer: key mediator and therapeutic target. Urologic Oncology. 2009;27(1):42–47. doi: 10.1016/j.urolonc.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bektas S, Bahadir B, Ucan BH, Ozdamar SO. CD24 and galectin-1 expressions in gastric adenocarcinoma and clinicopathologic significance. Pathology and Oncology Research. 2010;16(4):569–577. doi: 10.1007/s12253-010-9248-8. [DOI] [PubMed] [Google Scholar]
- 16.Wang Z, Shi Q, Wang Z, et al. Clinicopathologic correlation of cancer stem cell markers CD44, CD24, VEGF and HIF-1α in ductal carcinoma in situ and invasive ductal carcinoma of breast: an immunohistochemistry-based pilot study. Pathology Research and Practice. 2011;207(8):505–513. doi: 10.1016/j.prp.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 17.Overdevest JB, Thomas S, Kristiansen G, Hansel DE, Smith SC, Theodorescu D. CD24 offers a therapeutic target for control of bladder cancer metastasis based on a requirement for lung colonization. Cancer Research. 2011;71(11):3802–3811. doi: 10.1158/0008-5472.CAN-11-0519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taniuchi K, Nishimori I, Hollingsworth MA. Intracellular CD24 inhibits cell invasion by posttranscriptional regulation of bart through interaction with g3bp. Cancer Research. 2011;71(3):895–905. doi: 10.1158/0008-5472.CAN-10-2743. [DOI] [PubMed] [Google Scholar]
- 19.Kristiansen G, MacHado E, Bretz N, et al. Molecular and clinical dissection of CD24 antibody specificity by a comprehensive comparative analysis. Laboratory Investigation. 2010;90(7):1102–1116. doi: 10.1038/labinvest.2010.70. [DOI] [PubMed] [Google Scholar]
- 20.Fang X, Zheng P, Tang J, Liu Y. CD24: from A to Z. Cellular and Molecular Immunology. 2010;7(2):100–103. doi: 10.1038/cmi.2009.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ikenaga N, Ohuchida K, Mizumoto K, et al. Characterization of CD24 expression in intraductal papillary mucinous neoplasms and ductal carcinoma of the pancreas. Human Pathology. 2010;41(10):1466–1474. doi: 10.1016/j.humpath.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 22.Lee HJ, Choe G, Jheon S, Sung SW, Lee CT, Chung JH. CD24, a novel cancer biomarker, predicting disease-free survival of non-small cell lung carcinomas: a retrospective study of prognostic factor analysis from the viewpoint of forthcoming (seventh) new tnm classification. Journal of Thoracic Oncology. 2010;5(5):649–657. doi: 10.1097/JTO.0b013e3181d5e554. [DOI] [PubMed] [Google Scholar]
- 23.Sano A, Kato H, Sakurai S, et al. CD24 expression is a novel prognostic factor in esophageal squamous cell carcinoma. Annals of Surgical Oncology. 2009;16(2):506–514. doi: 10.1245/s10434-008-0252-0. [DOI] [PubMed] [Google Scholar]