Executive Summary
Objective
To conduct an evidence-based analysis of the effectiveness and cost-effectiveness of bariatric surgery.
Background
Obesity is defined as a body mass index (BMI) of at last 30 kg/m2.1 Morbid obesity is defined as a BMI of at least 40 kg/m2 or at least 35 kg/m2 with comorbid conditions. Comorbid conditions associated with obesity include diabetes, hypertension, dyslipidemias, obstructive sleep apnea, weight-related arthropathies, and stress urinary incontinence. It is also associated with depression, and cancers of the breast, uterus, prostate, and colon, and is an independent risk factor for cardiovascular disease.
Obesity is also associated with higher all-cause mortality at any age, even after adjusting for potential confounding factors like smoking. A person with a BMI of 30 kg/m2 has about a 50% higher risk of dying than does someone with a healthy BMI. The risk more than doubles at a BMI of 35 kg/m2. An expert estimated that about 160,000 people are morbidly obese in Ontario. In the United States, the prevalence of morbid obesity is 4.7% (1999–2000).
In Ontario, the 2004 Chief Medical Officer of Health Report said that in 2003, almost one-half of Ontario adults were overweight (BMI 25–29.9 kg/m2) or obese (BMI ≥ 30 kg/m2). About 57% of Ontario men and 42% of Ontario women were overweight or obese. The proportion of the population that was overweight or obese increased gradually from 44% in 1990 to 49% in 2000, and it appears to have stabilized at 49% in 2003. The report also noted that the tendency to be overweight and obese increases with age up to 64 years. BMI should be used cautiously for people aged 65 years and older, because the “normal” range may begin at slightly above 18.5 kg/m2 and extend into the “overweight” range.
The Chief Medical Officer of Health cautioned that these data may underestimate the true extent of the problem, because they were based on self reports, and people tend to over-report their height and under-report their weight. The actual number of Ontario adults who are overweight or obese may be higher.
Diet, exercise, and behavioural therapy are used to help people lose weight. The goals of behavioural therapy are to identify, monitor, and alter behaviour that does not help weight loss. Techniques include self-monitoring of eating habits and physical activity, stress management, stimulus control, problem solving, cognitive restructuring, contingency management, and identifying and using social support. Relapse, when people resume old, unhealthy behaviour and then regain the weight, can be problematic.
Drugs (including gastrointestinal lipase inhibitors, serotonin norepinephrine reuptake inhibitors, and appetite suppressants) may be used if behavioural interventions fail. However, estimates of efficacy may be confounded by high rates of noncompliance, in part owing to the side effects of the drugs. In addition, the drugs have not been approved for indefinite use, despite the chronic nature of obesity.
The Technology
Morbidly obese people may be eligible for bariatric surgery. Bariatric surgery for morbid obesity is considered an intervention of last resort for patients who have attempted first-line forms of medical management, such as diet, increased physical activity, behavioural modification, and drugs.
There are various bariatric surgical procedures and several different variations for each of these procedures. The surgical interventions can be divided into 2 general types: malabsorptive (bypassing parts of the gastrointestinal tract to limit the absorption of food), and restrictive (decreasing the size of the stomach so that the patient is satiated with less food). All of these may be performed as either open surgery or laparoscopically. An example of a malabsorptive technique is Roux-en-Y gastric bypass (RYGB). Examples of restrictive techniques are vertical banded gastroplasty (VBG) and adjustable gastric banding (AGB).
The Ontario Health Insurance Plan (OHIP) Schedule of Benefits for Physician Services includes fee code “S120 gastric bypass or partition, for morbid obesity” as an insured service. The term gastric bypass is a general term that encompasses a variety of surgical methods, all of which involve reconfiguring the digestive system. The term gastric bypass does not include AGB. The number of gastric bypass procedures funded and done in Ontario, and funded as actual out-of-country approvals,2 is shown below.
Number of Gastric Bypass Procedures by Fiscal Year: Ontario and Actual Out-of-Country (OOC) Approvals.
Data from Provider Services, MOHLTC
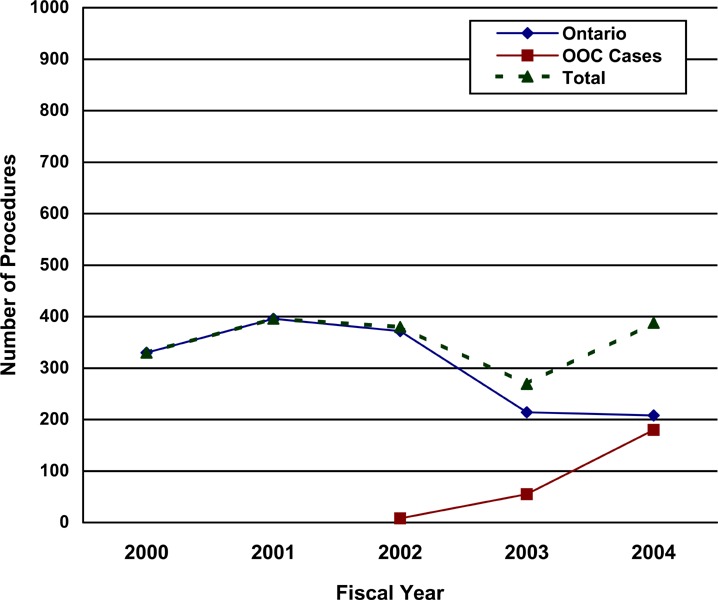
Courtesy of Provider Services, Ministry of Health and Long Term Care
Review Strategy
The Medical Advisory Secretariat reviewed the literature to assess the effectiveness, safety, and cost-effectiveness of bariatric surgery to treat morbid obesity. It used its standard search strategy to retrieve international health technology assessments and English-language journal articles from selected databases. The interventions of interest were bariatric surgery and, for the controls, either optimal conventional management or another type of bariatric procedure. The outcomes of interest were improvement in comorbid conditions (e.g., diabetes, hypertension); short- and long-term weight loss; quality of life; adverse effects; and economic analysis data. The databases yielded 15 international health technology assessments or systematic reviews on bariatric surgery.
Subsequently, the Medical Advisory Secretariat searched MEDLINE and EMBASE from April 2004 to December 2004, after the search cut-off date of April, 2004, for the most recent systematic reviews on bariatric surgery. Ten studies met the inclusion criteria. One of those 10 was the Swedish Obese Subjects study, which started as a registry and intervention study, and then published findings on people who had been enrolled for at least 2 years or at least 10 years. In addition to the literature review of economic analysis data, the Medical Advisory Secretariat also did an Ontario-based economic analysis.
Summary of Findings
Bariatric surgery generally is effective for sustained weight loss of about 16% for people with BMIs of at least 40 kg/m2 or at least 35 kg/m2 with comorbid conditions (including diabetes, high lipid levels, and hypertension). It also is effective at resolving the associated comorbid conditions. This conclusion is largely based on level 3a evidence from the prospectively designed Swedish Obese Subjects study, which recently published 10-year outcomes for patients who had bariatric surgery compared with patients who received nonsurgical treatment. (1)
Regarding specific procedures, there is evidence that malabsorptive techniques are better than other banding techniques for weight loss and resolution of comorbid illnesses. However, there are no published prospective, long-term, direct comparisons of these techniques available.
-
Surgery for morbid obesity is considered an intervention of last resort for patients who have attempted first-line forms of medical management, such as diet, increased physical activity, behavioural modification, and drugs. In the absence of direct comparisons of active nonsurgical intervention via caloric restriction with bariatric techniques, the following observations are made:
A recent systematic review examining the efficacy of major commercial and organized self-help weight loss programs in the United States concluded that the evidence to support the use of such programs was suboptimal, except for one trial on Weight Watchers. Furthermore, the programs were associated with high costs, attrition rates, and probability of regaining at least 50% of the lost weight in 1 to 2 years. (2)
A recent randomized controlled trial reported 1-year outcomes comparing weight loss and metabolic changes in severely obese patients assigned to either a low-carbohydrate diet or a conventional weight loss diet. At 1 year, weight loss was similar for patients in each group (mean, 2–5 kg). There was a favourable effect on triglyceride levels and glycemic control in the low-carbohydrate diet group. (3)
A decision-analysis model showed bariatric surgery results in increased life expectancy in morbidly obese patients when compared to diet and exercise. (4)
A cost-effectiveness model showed bariatric surgery is cost-effective relative to nonsurgical management. (5)
Extrapolating from 2003 data from the United States, Ontario would likely need to do 3,500 bariatric surgeries per year. It currently does 508 per year, including out-of-country surgeries.
Issue
To conduct an evidence-based analysis of the effectiveness and cost-effectiveness of bariatric surgery.
Background
Clinical Need – Target Population and Condition
Obesity is defined as an excessive accumulation of body fat as measured by the body mass index (BMI). BMI is calculated as body weight in kilograms (kg) divided by height in metres squared (m2): weight (kg)/[height (m)]2. People with a BMI over 30 are considered obese in most countries. (6)
Obesity is associated with the development of several diseases, including hypertension, diabetes mellitus (type 2 diabetes), hyperlipidemia, coronary artery disease, obstructive sleep apnea, depression, and cancers of the breast, uterus, prostate, and colon. (7) Obesity is also an independent risk factor for cardiovascular disease. A study from the United States found that after adjusting for age and smoking, the risks of nonfatal myocardial infarction and fatal coronary heart disease were more than 3 times as high in women with a BMI of 29 kg/m2 or more than in women with a BMI lower than 20 kg/m2. (8) Also in the United States, men with a BMI higher than 33 kg/m2 have 3 times the risk of developing coronary heart disease than do men with a BMI of less than 23 kg/m2. (9)
Obesity is also associated with higher all-cause mortality at any age, even after adjusting for potential confounding factors like smoking. (10) A person with a BMI of 30 kg/m2 has about a 50% higher risk of dying than does someone with a healthy BMI. The risk more than doubles at a BMI of 35 kg/m2. (10)
Clinically severe or morbid obesity is commonly defined as a BMI of at least 40 kg/m2, or a BMI of at least 35 kg/m2 if there are comorbid conditions, like diabetes, cardiovascular disease, arthritis, shortness of breath, gallbladder disease, back or disc disease, fatigue, or disability. (5;11) In the United States, the age-adjusted prevalence of extreme obesity (BMI ≥ 40 kg/m2) for adults aged 20 years and older has increased significantly in the population, from 2.9% (1988–1994) to 4.7% (1999–2000). (12) An expert estimated that about 160,000 people are morbidly obese in Ontario.
Health Canada’s guidelines to classify the body weight of adults (13) are shown in Table 1.
Table 1: Canadian Guidelines for Body Weight Classification in Adults, 2003* (13).
| Classification | BMI Category (kg/m2) | |
|---|---|---|
| Underweight | < 18.5 | |
| Normal weight | 18.5–24.9 | |
| Overweight | 25.0–29.9 | |
| Obese | ||
| Class I | 30.0–34.9 | |
| Class II | 35.0–39.9 | |
| Class III | ≥ 40.0 | |
This is for use with adults aged 18 and over, and is not for use with pregnant and lactating women. For people aged 65 and older, the “normal” range may begin slightly above a BMI of 18.5 kg/m2 and extend into the “overweight” range.
(Courtesy of Health Canada. Canadian Guidelines for Body Weight Classification in Adults. 2003).
In a survey study (14) of 19,841 Canadians aged 18 to 74 years across 10 provinces from 1986 to 1992, 35% of men and 27% of women had a BMI of more than 27 kg/m2. Overall, men had a higher mean BMI than did women. Mean BMI increased with age up to 55 to 64 years, and then it fell. The proportion of men and women with a healthy body weight (BMI 20–24 kg/m2) fell from 60% of men and 56% of women in the youngest age category to 26% of men and 33% of women for those aged 55 to 64 years.
Morbid obesity, defined by McDonald et al. (14) as a BMI over 35 kg/m2, was twice as likely in women (4%) as in men (2%), and it had appeared in 7% of women and 4% of men by 35 to 44 years of age.
The incidence of morbid obesity (BMI > 40 kg/m2) has not been reported in the literature.
In Ontario, the 2004 Chief Medical Officer of Health Report (15) said that in 2003, almost one-half of Ontario adults (i.e., those aged 18 and older) were overweight (BMI 25–29.9 kg/m2) or obese (BMI ≥ 30 kg/m2). About 57% of Ontario men and 42% of Ontario women were overweight or obese. The proportion of the population that was overweight or obese increased gradually from 44% in 1990 to 49% in 2000, and it appears to have stabilized at 49% in 2003.
The report also noted that the tendency to be overweight and obese increases with age up to 64 years: among Ontario adults aged 35 to 49, more than 50% are overweight or obese, compared with more than 60% of adults aged 50 to 64. BMI should be used cautiously for people aged 65 years and older, because the “normal” range may begin at slightly above 18.5 kg/m2 and extend into the “overweight” range.
The Chief Medical Officer of Health cautioned that these data may underestimate the true extent of the problem, because they were based on self reports, and people tend to over-report their height and under-report their weight. The actual number of Ontario adults who are overweight or obese may be higher. (15)
Existing Treatment Options Other Than Technology Being Reviewed
Diet, exercise, and behavioural therapy are used to help people lose weight. (11)
The goals of behavioural therapy are to identify, monitor, and alter behaviour that does not help weight loss. Techniques include self-monitoring of eating habits and physical activity, stress management, stimulus control, problem solving, cognitive restructuring, contingency management, and identifying and using social support. Behavioural therapy reportedly is particularly helpful for people with binge-eating disorders (about 30% of obese people). (16) Relapse, when people resume old, unhealthy behaviour and then regain the weight, can be problematic. Therefore, a relapse prevention strategy should include preventing and anticipating problematic situations, and regularly following-up with program staff to encourage adherence to diet, physical activity, and behavioural changes. (16)
Drugs (including gastrointestinal lipase inhibitors, serotonin norepinephrine reuptake inhibitors, and appetite suppressants) may be used if behavioural interventions fail. (11) However, estimates of efficacy may be confounded by high rates of noncompliance, in part owing to the side effects of the drugs. (17) In addition, the drugs have not been approved for indefinite use, despite the chronic nature of obesity. (17)
New Technology Being Reviewed: Bariatric Surgery
Men and women with morbid obesity may be eligible for surgical intervention. There are numerous different surgical procedures, with several different variations. (11) The procedures can be divided into 2 general types: malabsorptive (bypassing parts of the gastrointestinal tract to limit the absorption of food) and restrictive (decreasing the size of the stomach in order for the patient to feel satiated with a smaller amount food). All can be performed either as open surgery or laparoscopically.
Surgery for morbid obesity is usually considered a last resort for people who have attempted first-line medical management (e.g., diet, behaviour modification, increased physical activity, and drugs) but who have not lost weight permanently. Surgery is restricted to people with morbid obesity (BMI ≥ 40 kg/m2) or with a BMI of at least 35 kg/m2 and serious comorbid conditions. (5;11)
Surgery to treat morbid obesity may be contraindicated if candidates have shown any of the following:
Perioperative risk of cardiac complications
Poor myocardial reserve
Significant chronic obstructive airways disease or respiratory dysfunction
Noncompliance with medical treatment
Psychological disorders of a significant degree that would be considered by a psychologist or psychiatrist to worsen or interfere with the long-term management of the patient after the operation
A serious eating disorder
Severe hiatus hernia/gastroesophageal reflux
Malabsorptive Interventions
Biliopancreatic diversion
Biliopancreatic diversion (BPD) involves removing a large part of the stomach to control oral intake, followed by reconstructing the small intestine to divert the bile and pancreatic juices so they meet the ingested food closer to the middle or the end of the small intestine (Appendix 1). (5)
Roux-en-Y Gastric Bypass
Roux-en-Y gastric bypass (RYGB), or simply gastric bypass, combines restriction and malabsorption techniques and creates a small gastric pouch and an intestinal bypass (Appendix 1). (5)
A common complication resulting from malabsorptive procedures is dumping syndrome. Dumping syndrome happens when food or liquid enters the small intestine too quickly. Symptoms may include weakness, nausea, cramps, and diarrhea. (5) These symptoms can be made worse by eating highly refined, high-calorie foods (like sweets). Some researchers have hypothesized that dumping syndrome aids weight loss by conditioning people to avoid eating sweets. (5)
Restrictive Procedures
Vertical Banded Gastroplasty
Vertical banded gastroplasty (VBG) involves dividing the stomach into 2 parts. The aim is to cause the patient to feel satiated from a limited intake of food, owing to the reduced capacity of the small upper section of the stomach and the slow emptying through a small gap into the rest of the digestive system.
VBG creates a small vertical pouch in the upper stomach (Appendix 1). A band is put around the lower end of the vertical pouch to prevent stretching. (5)
Adjustable Gastric Banding
Adjustable gastric banding (AGB) limits food intake by placing a constricting ring completely around the stomach below the junction of the stomach and esophagus. Early bands were nonadjustable, but bands now have an inflatable balloon in their lining to allow the size of the hole to be adjusted to regulate food intake (Appendix 1).
The bands can be inserted laparoscopically and can be adjusted without surgery by adding or removing appropriate filler material (saline).
Number of Bariatric Procedures in Ontario
The Ontario Health Insurance Plan (OHIP) Schedule of Benefits for Physician Services includes fee code “S120 gastric bypass or partition, for morbid obesity” as an insured service. Gastric bypass is a general term that encompasses a variety of methods, all of which involve reconfiguring the digestive system. The term gastric bypass does not refer to adjustable gastric banding.
The number of gastric bypass procedures done in Ontario and funded as actual out-of-country approvals3 is presented in Figure 1 on the next page.
Figure 1. Number of Gastric Bypass Procedures by Fiscal Year: Ontario and Actual Out-of-Country (OOC) Approvals.
Data from Provider Services, MOHLTC
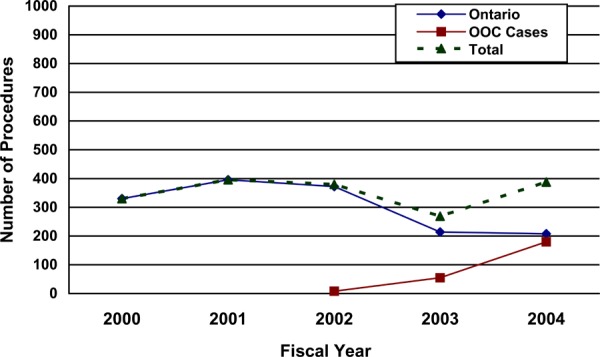
(Provided courtesy of Provider Services, Ministry of Health and Long Term Care)
Regulatory Status
Health Canada has licensed the following laparoscopic gastric banding devices:
LAP-BAND Adjustable Gastric Banding System (INAMED Health, Santa Barbara, CA) (Licence 12197, Class III)
Swedish Adjustable Gastric Band (Johnson & Johnson) (Licence 61087, Class III)
Midband Adjustable Peri Gastric Belt (Medical Innovation Developpement, France) (Licence 62577, Class III)
The United States Food and Drug Administration (FDA) did not approve the original application of the LAP-BAND system in August 2000. The full FDA summary (18) of effectiveness and safety is in Appendix 2. The FDA said that 2 years of follow-up data was inadequate. It recommended at least 3 years of follow-up data be obtained before the system is approved. The protocol for the clinical study in the United States called for 3 years of follow-up. The FDA also made recommendations about the labelling and running of a study after approval. In this study, men and women enrolled in the trial in the United States will be followed for 5 years after the LAP-BAND is implanted. This is to obtain more information on excess weight loss and adverse events, mostly esophageal dilatation and band erosion.
The FDA finally approved the LAP-BAND system in the United States in June 2001. It noted the following:
“The LAP-BAND® system is indicated for use in weight reduction for severely obese patients with a BMI of at least 40 or a BMI of at least 35 with one or more severe comorbid conditions or those who are 100lbs or more over their estimated ideal weight according to the 1983 Metropolitan Life Insurance Tables (use the midpoint for medium frame). It is indicated for use only in severely obese adult patients who have failed more conservative weight reduction alternatives such as supervised diet, exercise and behaviour modification programs. Patients who elect to have this surgery must make the commitment to accept significant changes in their eating habits for the rest of their lives.” (18)
The FDA noted the LAP BAND system is contraindicated in people with the following:
Inflammatory disease of the gastrointestinal tract
Cardiopulmonary diseases or other serious organic disease which makes them poor surgical candidates
Potential upper gastrointestinal bleeding conditions such as esophageal or gastric varices
Portal hypertension
Congenital or acquired anomalies of the gastrointestinal tract such as atresias or stenoses
Experience of an intraoperative gastric injury during the implantation procedure, such as a gastric perforation at or near the location of the intended band placement
Cirrhosis
Chronic pancreatitis
Addiction to alcohol or drugs
Pregnancy
An infection anywhere in the body or where the possibility of contamination prior to or during the surgery exists
A known diagnosis or pre-existing symptoms of autoimmune connective tissue disease (or who have family members with the diagnosis or symptoms)
Age younger than 18 years (i.e., children or adolescents)
It is also contraindicated in patients who are on chronic, long-term steroid treatment; who are unable or unwilling to comply with necessary dietary restrictions; who are known or suspected to have an allergic reaction to materials in the system; or who have exhibited a pain intolerance to implanted devices.
Surgical stapling devices are used in all bariatric surgical procedures except gastric banding. (19) The devices have been approved by the FDA for use in various general surgical procedures. The following are licensed by Health Canada:
Pre-loaded stapler units (United States Surgical, a division of Tyco Health Care Group, NJ), (Class IV, licence 11683)
Proximate stapling devices (Ethicon Endo-Surgery Inc., a Johnson & Johnson Company, Cincinnati OH), (Class III, licence 2735)
Literature Review on Effectiveness
Objective
The Medical Advisory Secretariat reviewed the literature to assess the effectiveness, safety, and cost-effectiveness of bariatric surgery to treat morbid obesity.
Questions Asked
Do patients maintain weight loss in the short and long term after bariatric surgery?
Do comorbid conditions (e.g., diabetes, hypertension) improve in the short and long term after bariatric surgery?
Is the newer adjustable gastric banding procedure more effective than other commonly used bariatric procedures?
Is any one type of bariatric surgery more effective than any other type?
What, if any, adverse effects are associated with each type of bariatric surgery?
Methods
Inclusion criteria
English-language articles (January 1996–December 2004)
Journal articles that reported primary data on the effectiveness or cost-effectiveness of data obtained in a clinical setting, or analysis of primary data maintained in registries or databases
Study design and methods that were clearly described
Systematic reviews, randomized controlled trials (RCTs), non-RCTS or cohort studies that had >100 patients, and cost-effectiveness studies
Exclusion criteria
Duplicate publications (superseded by another publication by the same investigator group, with the same objective and data)
Non-English-language articles
Non-systematic reviews, letters, and editorials
Animal and in-vitro studies
Case reports
Studies that did not examine the outcomes of interest
Interventions
Bariatric surgery
Controls underwent either optimal conventional management or another type of bariatric procedure
Literature Search
Cochrane database of systematic reviews
ACP Journal Club
DARE
INAHTA
EMBASE
MEDLINE
Reference sections from reviews and extracted articles
Outcomes of Interest
Improvement in comorbid conditions (e.g., diabetes, hypertension)
Short- and long-term weight loss
Quality of life (QoL)
Adverse effects
Economic analysis data
Results of Literature Review on Effectiveness
Summary of Existing Health Technology Assessments
The Cochrane and INAHTA databases yielded 15 international health technology assessments or systematic reviews on bariatric surgery. A summary of the results of the health technology assessments is shown in Tables 2 and 3, followed in turn by a discussion of each assessment.
Table 2: Summary of Findings on Excess Weight Loss and Resolution of Comorbid Conditions From Previous Health Technology Assessments.
| Procedure | Excess Weight Loss,* Range (%) |
Resolution† of Comorbid Conditions, Range (%) |
|
|---|---|---|---|
| Malabsorptive | |||
| Roux-en-Y gastric bypass | 60–90 | Diabetes: 74–99 | |
| Hypertension: 67–93 | |||
| Dyslipidemias: 73–-99 | |||
| Restrictive | |||
| Adjustable gastric banding | 42–60 | Diabetes: 29–92 | |
| Hypertension: 29–40 | |||
| Dyslipidemia: 24 | |||
| Vertical banded gastroplasty | 58–87 | Diabetes: 100 | |
| Hypertension: 50–60 | |||
| Dyslipidemias: 14–72 | |||
Percentage of excess weight loss = (weight loss/excess weight) × 100
(where excess weight = total preoperative weight – ideal weight).
Defined as the stopping of medication taken for comorbid condition.
Table 3: Summary of Findings on Mortality and Adverse Effects From Previous Health Technology Assessments.
| Procedure | Mortality, Range (%) | Adverse Effects, Range (%) | |
|---|---|---|---|
| Malabsorptive | |||
| Roux-en-Y gastric bypass | 0.1– 4.1 | 0.1– 70 | |
| Restrictive | |||
| Adjustable gastric banding | 0– 0.9 | 1.1– 18 | |
| Vertical banded gastroplasty | 0– 0.8 | 1–30.4 | |
Buchwald et al. (20) systematically reviewed and did a meta-analysis of studies on bariatric surgery. Their aims were as follows:
To analyze the impact of bariatric surgery on diabetes, hyperlipidemia, hypertension, and obstructive sleep apnea, as well as on health care economics and disease impact
To analyze weight reduction efficacy outcomes in the studies selected for the comorbid conditions
To summarize mortality outcomes
Methods
The inclusion criteria were as follows:
Studies reporting on surgical outcomes (e.g., efficacy and safety)
Studies containing guidelines
Studies reporting on health care economics
Studies examining the impact of disease (e.g., utilization: length of hospital stay, readmissions, and QoL)
The exclusion criteria were as follows:
Abstracts
Case reports, letters, commentaries, and reviews
Animal or in vitro studies
Studies with fewer than 10 patients
Studies with follow-up of fewer than 30 days
Studies in languages other than English
Studies where there was no surgical intervention for obesity
Studies on intragastric balloon therapy (an experimental device)
Extracted studies could have any design. All outcomes were preferentially extracted at the times for which the comorbidity outcomes were available or at the latest time available for follow-up of at least 50% of the population.
Surgical procedures were grouped into the following categories:
Gastric banding (including adjustable and nonadjustable bands)
Gastric bypass (mainly Roux-en-Y variations)
Gastroplasty (mainly VBG)
BPD or duodenal switch (including a variety of modifications)
Mixed and other (biliary intestinal bypass, ileogastrostomy, jejunoileal bypass, and unspecified bariatric)
Results were reported individually for AGB, gastric bypass, gastroplasty, and BPD or duodenal switch procedure groups. Results were also reported for the “total population,” which included gastric banding, gastric bypass, gastroplasty and BPD or duodenal switch plus mixed groups and other less common bariatric surgery procedures (biliary intestinal bypass, ileogastrostomy, jejunoileal bypass, and unspecified bariatric surgery). Outcomes of selected comorbid conditions were grouped into categories of “resolved” and “resolved or improved.”
Weight loss was reported as the mean percentage of EWL, a standard calculation in the bariatric surgery literature. The calculation is based on the following formula:
Percentage of excess weight loss = (weight loss/excess weight) × 100
(Where excess weight = total preoperative weight – ideal weight)
Changes in absolute weight (kg), BMI, and percentage of initial weight were also reported.
Data on operative mortality (≤ 30 days) were also extracted. The complication rates were difficult to record, because they varied among studies, depended on the duration of the follow-up period, and were procedure specific. They were also a function of technique: open versus laparoscopic. Therefore, Buchwald et al. did not analyze complication rates. The meta-analysis used a random-effects model.
Results
The initial literature review yielded 2738 citations. In the end, 134 fully extracted primary studies were available for meta-analysis. Included were 5 RCTs, 28 observational studies, and 101 uncontrolled case series. Most of the studies were done at single centres (n = 126). A few were multicentre studies (n = 5). At least 1 categorical outcome of interest (proportion of patients with resolution or improvement in diabetes, hyperlipidemia, hypertension, or obstructive sleep apnea), or one continuous outcome of interest (change in a laboratory or physiological measure) was reported in each.
Weight Loss
The mean BMI for 16,944 patients at baseline was 46.85 kg/m2 (range, 32.30–68.80 kg/m2).
The mean (95% CI) EWL by meta-analysis at the outcome time point when comorbid conditions were assessed was 47.5% (40.7%–54.2%) for gastric banding; 61.6% (56.7%–66.5%) for gastric bypass; 68.2% (61.5%–74.8%) for gastroplasty; and 70.1% (66.3%–73.9%) for BPD or duodenal switch. The overall EWL for 10,172 patients across all surgeries was 61.2% (58.1%–64.4%) (Table 4).
Table 4: Weight Loss After Bariatric Surgery.
| Weight Loss After Bariatric Surgery. Surgery (n) |
% Excess Weight Loss (Meta-Analytic Mean [95% CI]) |
|---|---|
| All bariatric (10,172) | 61.2 (58.1–64.4) |
| Roux-en-Y gastric bypass (4,204) | 61.6 (56.7– 66.5) |
| Vertical banded gastroplasty (506) | 68.2 (61.5– 74.8) |
| Other banding (fixed and variable; 1,848) | 47.5 (40.7– 54.2) |
(From Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K et al. Bariatric surgery: a systematic review and meta-analysis. JAMA 2004; 292:1724-1737).
Sometimes weight loss outcomes were reported as a decrease in BMI (mean change, 14.2 kg/m2 [95% CI, 13.3–15.1 kg/m2] in 8,232 patients) and a decrease in absolute weight (mean change, 39.7 kg [37.2–42.2 kg] in 7,588 patients. In most cases, weight loss outcomes did not differ significantly for assessments at 2 years or less compared with those at more than 2 years.
Operative Mortality
The rate of operative mortality was 0.1% for the purely restrictive procedures (2,297 patients receiving banding and 749 patients receiving gastroplasty), 0.5% in 5,644 patients receiving gastric bypass procedures, and 1.1% in 3,030 patients undergoing BPD or duodenal switch procedures.
Comorbidity Outcomes
The outcomes for comorbid conditions reported by Buchwald et al. are shown in Table 5.
Table 5: Outcomes for Comorbid Conditions After Bariatric Surgery.
| Type of Surgery | Resolution of Diabetes (mean% [95% CI]) (n resolved/n evaluated) |
Resolution of Hypertension (mean % [95% CI]) (n resolved/n evaluated) |
Improvement in Hyperlipidemia (mean % [95% CI]) (n improved/n evaluated) |
|---|---|---|---|
| All types of bariatric | 76.8% (70.7%–82.9%) | 61.7% (55.6% - 67.8%) | 79.3% (88.2%–90.5%) |
| surgery | (1417/1846) | (3151/4805) | (846/1019) |
| Roux-en-Y gastric | 83.7% (77.3%–90.1%) | 67.5% (58.4%–76.5%) | 96.9% (93.6%–100%) |
| bypass | (829/989) | (1594/2115) | (117/125) |
| Vertical banded | 71.6% (55.1%–88.2%) | 69.0% (59.1%–79.0%) | 73.6% (60.8%–86.3%) |
| gastroplasty | (45/66) | (277/382) | (174/215) |
| Other banding | 47.9% (29.1%–66.7%) | 43.2% (30.4%–55.9%) | 58.9% (28.2%–89.6%) |
| (fixed and variable) | (98/205) | (232/604) | (333/426) |
(From Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K et al. Bariatric surgery: a systematic review and meta-analysis. JAMA 2004; 292:1724-1737).
Diabetes
When defined as being able to discontinue all diabetes-related medications and maintain blood glucose levels within the normal range, there was strong evidence for improvement in type 2 diabetes. Impaired glucose tolerance was found across all of the surgery types.
Within the studies reporting resolution of diabetes, 1,417 of 1,846 patients experienced complete resolution (meta-analytic mean [95% CI], 76.8% [70.7%–82.9%]). Within studies reporting both resolution and improvement, or only improvement, 414 of 485 patients experienced resolution or improvement (meta-analytic mean, 86.0% [95% CI, 78.4%–93.7%].
-
Diabetes outcomes differed when analyzed according to the 4 types of procedures.
For diabetes resolution, there was a gradation of effect from 98.9% [95% CI, 96.8%–100%] for BPD or duodenal switch, to 83.7% [95% CI, 77.3%–90.1%] for gastric bypass, to 71.6% [95& CI, 55.1%–88.2%] for gastroplasty, to 47.9% [95% CI, 29.1%–66.7%] for gastric banding.
Buchwald et al. suggested that the variation from the trend solely for diabetes resolved may have been because of the far greater number of patients assessed for this variable (n = 1846) compared with the number assessed for the combined variable (n = 485) in the total population.
Hyperlipidemia
Meta-analysis and weighted means analysis showed that hyperlipidemia, hypercholesterolemia, and hypertriglyceridemia improved significantly across all procedures (including the mixed and other bariatric surgery groups).
The biggest improvements in hyperlipidemia by meta-analysis were with BPD or the duodenal switch procedure (99.1% [95% CI, 97.6%–100%]) and with gastric bypass (96.9% [95% CI, 93.6%–100%]).
Hypertension
Meta-analysis and analysis of weighted proportions showed hypertension significantly improved in the total patient population across all surgical procedures.
Across all types of surgery, hypertension resolved in 61.7% of patients [95% CI, 55.6%–67.8%].
Buchwald et al.’s conclusions:
Resolution of diabetes appeared to be more prevalent after the predominantly malabsorptive procedures (BPD or duodenal switch) and the mixed malabsorptive/restrictive gastric bypass compared with the purely restrictive gastroplasty and gastric banding procedures.
-
There appeared to be a gradation of diabetes resolution as a function of the operative procedure itself:
98.9% for BPD or duodenal switch
83.7% for gastric bypass
71.6% for gastroplasty
47.9% for gastric banding
Diabetes resolution/improvement after surgery may be related to changes in gut-related hormones.
Improvement in hyperlipidemia also seemed to be higher with the malabsorptive procedures.
The reduction in blood pressure seemed to be independent of the procedure.
The operative 30-day mortality rates of 0.1% for the restrictive procedures, 0.5% for gastric bypass, and 1.1% for BPD or duodenal switch compare favourably with the accepted operative mortality rates for other major surgical procedures.
Limitations of the meta-analysis by Buchwald et al.
As the authors commented, “The heterogeneity of the immediate postoperative and long-term morbidity data did not allow for meta-analysis.”
The postsurgical follow-up timing when outcomes of interest data were extracted varied across studies. The authors stated, “Given the emphasis on comorbidities, weight loss efficacy outcomes were preferentially extracted at time points for which comorbidity changes were reported.” Therefore, time points may have varied substantially. For example, the RCTs included by Buchwald et al. had follow-ups that ranged from 6 to 36 months.
The inclusion criteria (studies of any design, surgical outcomes, guidelines, health care economics, or disease impact) were very broadly defined.
ECRI (formerly the Emergency Care Research Institute) (September 2004)
ECRI conducted a systematic literature review of bariatric surgery (19) using several databases, including MEDLINE and EMBASE from inception to April 16, 2004. The following is a summary of its findings.
ECRI graded evidence as strong, moderate, or weak using an extension of the methods recommended by the United States Agency for Healthcare Research and Quality evidence-based practice center program. (19) Accordingly, ECRI evaluated 4 aspects of the available body of evidence: quality, quantity, consistency, and magnitude of effect. ECRI decided which category a body of evidence belonged in by using an algorithm it developed.
The ECRI algorithm was used for benefits outcomes but not for adverse effects, because for all the adverse effects that were considered, patients could only experience them if they had had surgery (for example, only patients who have received staples can have staple line disruption). Therefore, ECRI considered all adverse effects as being backed by strong evidence that surgery caused them. ECRI cautioned that in calling such evidence “strong,” it is referring to the cause-and-effect relationship of surgery and adverse effects, and it is not referring to the rate at which these adverse effects occur.
Morbidly Obese Adults
The inclusion criteria were as follows:
The study had a control group of patients who did not receive surgery.
If the study did not have a control group, then the study must have used a before-and-after design.
All patients had to be at least 18 years old, or if some were not, the data must have been reported separately for the patients that were at least 18 years old.
All patients had to have morbid obesity, or if some did not, the results must have been reported separately for the patients with morbid obesity.
The study was published in 1994 or later.
If the study enrolled patients who received different procedures, then the data must have been reported separately for each procedure.
-
The study reported on at least one of the following outcomes:
Weight at 3 years after surgery
Resolution of comorbid conditions
Survival
QoL
Adverse events
Question asked: What are the benefits and adverse effects of adjustable gastric banding?
Twenty-two studies of adjustable gastric banding comprising 6,524 patients met the inclusion criteria. Nearly one-half of the studies (41%) reported information on 3-year weight loss, and most (59%–77%) had enough patients (at least 100 patients) so that adverse effects could be analyzed. Few studies (0% to 23%) reported information on comorbid conditions. No studies reported on long-term survival.
Table 6: Summary of Findings on 3-Year Weight Loss After Adjustable Gastric Banding.
| Study (Year) |
Patients at Baseline, N | Patients at Follow-up, N |
BMI at Baseline, Mean (SD) | BMI Corresponding to 25% EWL |
BMI at 3 Years After Surgery, Mean (SD) |
Did the 3-Year BMI Suggest > 25% EWL? |
|---|---|---|---|---|---|---|
| Rubin (2003) | 250 | 25 | 44 (6.4) | 38.5 | 31.7 (4.8) | Yes |
| Zinzindohoue (2003) |
500 | 45 | 44.3 (5.8) | 37.9 | 31.9 (6.5) | Yes |
| Pontiroli (2002) |
143 | 56 | 44.9 (6.3) | 39.1 | 37 (7.2) | Yes |
| Rubenstein (2002) |
63 | 13 | 48.8 (6.4) | 42.1 | 34.4 (6.4) | Yes |
| Victorzon (2002) |
110 | 26 | 44 | 38.5 | 33 | Yes |
| Dixon (2001) | 459 | 80 | 45 (8) | 39.2 | 33.5 (6) | Yes |
| BioEnterics (2001) |
288 | 178 | 47.5 (7) | 41.1 | 38.7 (7.9) | Yes |
| Nilsell (2001) | 29 | 13 | 42.8 (5.4) | 37.6 | 29.2 (5.8) | Yes |
| Lise (1994) | 111 | 22 | 46.4 (6) | 40.3 | 31.4 (5) | Yes |
(Table reproduced with kind permission from ECRI. Bariatric Surgery for Obesity. 2004. Plymouth Meeting, PA, ECRI. Technology Assessment Report.)
Table 7: Summary of Findings on Diabetes Resolution After Adjustable Gastric Banding.
| Study (Year) |
Diabetes Definition |
Follow-up, Months | Patients With Comorbidity at Baseline, N | Resolved, % (n) | Improved, % (n) | No Change, % (n) | Worse, % (n) |
|---|---|---|---|---|---|---|---|
| Dolan (2003) | Decision to stop medication for diabetes was taken to indicate diabetes remission | 6–63 | 49 | 65 (32) | Not reported | Not reported | Not reported |
| Spivak (2003) |
Status based on changes in drug regimen | 6– 27 | 14 | 29 (4) | 36 (5) | 29 (4) | 7 (1) |
| Weiner (2003) |
Required medication for type 2 diabetes | 1–97 | 161 | 92 (148) | Not reported | Not reported | Not reported |
| Zinzindohoue (2003) | Not defined | 1–49.5 (mean, 13) |
29 | 45 (13) | 34 (10) | 17 (5) | 3 (1) |
| Dixon (2001) | Type 2 diabetes, defined by abnormal fasting plasma glucose, HbA1c, fasting insulin, and C-peptide | 12 | 50 | 64 (32) | 26 (13) | 10 (5) | 0 (0) |
(Table reproduced with kind permission from ECRI. Bariatric Surgery for Obesity. 2004. Plymouth Meeting, PA, ECRI. Technology Assessment Report.)
Table 8: Summary of Findings on Hypertension Resolution After Adjustable Gastric Banding.
| Study (Year) |
Hypertension Definition | Follow-up, Months | Patients With Comorbidity at Baseline, N | Resolved, % (n) | Improved, % (n) | No Change, % (n) | Worse, % (n) |
|---|---|---|---|---|---|---|---|
| Spivak (2003) | Based on changes in medication regimen | 6–27 | 35 | 40 (14) | 23 (8) | 37 (13) | 0 (0) |
| Weiner (2003) | Not defined | 1–97 | 415 | 50 (207) | Not reported | Not reported | Not reported |
| Zinzinodoue (2003) |
Not defined | 1–49.5 (mean, 13) |
56 | 29 (16) | 43 (24) | 27 (15) | 2 (1) |
(Table reproduced with kind permission from ECRI. Bariatric Surgery for Obesity. 2004. Plymouth Meeting, PA, ECRI. Technology Assessment Report.)
Table 9: Summary of Findings on Dyslipidemia Resolution After Adjustable Gastric Banding.
| Study (Year) |
Lipids Definition | Follow-up, Months | Patients With Comorbidity at Baseline, N |
Resolved, % (n) | Improved, % (n) | No Change, % (n) | Worse, % (n) |
|---|---|---|---|---|---|---|---|
| Zinzinodoue (2003) |
Not defined | 1–49.5 (mean, 13) |
58 | 24 (14) | 43 (25) | 28 (16) | 5 (3) |
(Table reproduced with kind permission from ECRI. Bariatric Surgery for Obesity. 2004. Plymouth Meeting, PA, ECRI. Technology Assessment Report.)
Table 10: Summary of Findings on Sleep Apnea Resolution After Adjustable Gastric Banding.
| Study (Year) |
Lipids Definition |
Follow-up, Months | Patients With Comorbidity at Baseline, N |
Resolved, % (n) | Improved, % (n) | No Change, % (n) | Worse, % (n) |
|---|---|---|---|---|---|---|---|
| Weiner (2003) |
Not defined | 1–97 | 81 | 85 (69) | Not reported | Not reported | Not reported |
| Rubenstein (2002) |
Not defined | 6– 36 | 12 | 100 (12) | 0 (0) | 0 (0) | 0 (0/) |
| Dixon (2001) |
Observed sleep apnea | 12 | 41 | 95 (39) | Not reported | Not reported | Not reported |
(Table reproduced with kind permission from ECRI. Bariatric Surgery for Obesity. 2004. Plymouth Meeting, PA, ECRI. Technology Assessment Report.)
ECRI’s conclusions on adjustable gastric banding:
Treatment results in clinically significant weight loss at 3 years after surgery.
Some patients experience resolution of diabetes, hypertension, and sleep apnea.
Adjustable gastric banding was introduced more recently than other bariatric surgical procedures, and future studies may elucidate its potential benefits and harms.
Question asked: What are the benefits and adverse effects of VBG?
Fifteen studies of VBG comprising 1,874 patients were included. No studies reported data on long-term survival outcomes.
Table 11: Summary of Findings on 3-Year Weight Loss After Vertical Banded Gastroplasty.
| Study (Year) | Patients at Baseline, N | Patients at Follow-up, N | BMI at Baseline, Mean (SD) | BMI Corresponding to 25% EWL |
BMI at 3 years After Surgery, Mean (SD) | Did the 3-Year BMI Suggest > 25% EWL? |
|---|---|---|---|---|---|---|
| Avsar (2004) | 40 | 20 | 45 (6.4) | 39.2 | 28 (1.4) | Yes |
| Kalfarentzos (2001) | 35 | 29 | 44.1 | 38.5 | 32 | Yes (weight reduction was statistically significant) |
| Melissas (2001) | 125 | 86 | 47.4 (4.4) | 41 | 32.6 (4.2) | Yes |
| Nilsell (2001) | 30 | 15 | 43.9 (3.8) | 38.4 | 31.5 (7.5) | Yes |
| Hernandez-Estefania (2000) | 67 | 32 | 47.5 (7.7) | 41.1 | 34.3 (7.7) | Yes |
| Husemann (1999) | 682 | 436 | 52 | 44.5 | 34 | Yes |
| Van Gemert (1997) | 32 | 19 | 47 (7.5) | 40.7 | 30.2 (5.6) | Yes |
| Capella (1996) | 328 | 207 | 52 (9) | 44.5 | 39 (9) | Yes |
| Howard (1995) | 22 | 10 | 47.9 (6.6) | 41.4 | 34 (6.6) | Yes |
(Table reproduced with kind permission from ECRI. Bariatric Surgery for Obesity. 2004. Plymouth Meeting, PA, ECRI. Technology Assessment Report.)
Table 12: Summary of Findings on Diabetes Resolution After Vertical Banded Gastroplasty.
| Study (Year) | Diabetes Definition |
Follow-up, Months | Patients With Comorbidity at Baseline, N | Resolved, % (n) | Improved, % (n) | No Change, % (n) | Worse, % (n) |
|---|---|---|---|---|---|---|---|
| Melissas (2001) | Based on the dose of medication needed. It was considered resolved when no medication was necessary. | 34–48 | 10 | 100 (10) | 0 (0) | 0 (0) | 0 (0) |
(Table reproduced with kind permission from ECRI. Bariatric Surgery for Obesity. 2004. Plymouth Meeting, PA, ECRI. Technology Assessment Report.)
Table 13: Summary of Findings on Hypertension Resolution After Vertical Banded Gastroplasty.
| Study (Year) | Hypertension Definition |
Follow-up, Months | Patients With Comorbidity at Baseline, N | Resolved, % (n) | Improved, % (n) | No Change, % (n) | Worse, % (n) |
|---|---|---|---|---|---|---|---|
| Melissas (2001) |
Based on the dose of medication needed. It was considered resolved when no medication was necessary. | 24–48 | 20 | 60 (12) | 40 (8) | 0 (0) | 0 (0) |
| Yashkov (1997)* | Not defined | Not reported | 20 | 50 (10) | 15 (3) | 10 (2) | Not reported |
In 5 patients who had hypertension before surgery, the status of their hypertension after surgery was unknown.
(Table reproduced with kind permission from ECRI. Bariatric Surgery for Obesity. 2004. Plymouth Meeting, PA, ECRI. Technology Assessment Report.)
Table 14: Summary of Findings on Dyslipidemia Resolution After Vertical Banded Gastroplasty.
| Study (Year) | Lipids Definition | Follow-up, Months | Patients With Comorbidity at Baseline, N | Resolved, % (n) | Improved, % (n) | No Change, % (n) | Worse, % (n) |
|---|---|---|---|---|---|---|---|
| Kalfarentzos (2001) |
LDL cholesterol >160 mg/dl or triglycerides >250 mg/dl | Mean, 48 | 14 | 14 (2) | 29 (4) | 57 (8) | 0 (0) |
| Melissas (2001) |
High cholesterol; evaluation based on laboratory values. It was considered resolved when these values were within the normal range. | 24–48 | 66 | 33 (22) | 36 (24) | 30 (20) | 0 (0) |
| Melissas (2001) |
High triglycerides; evaluation based on laboratory values. It was considered resolved when these values were within the normal range. | 24–48 | 50 | 72 (36) | 0 (0) | 28 (14) | 0 (0) |
(Table reproduced with kind permission from ECRI. Bariatric Surgery for Obesity. 2004. Plymouth Meeting, PA, ECRI. Technology Assessment Report.)
Table 15: Summary of Findings on Sleep Apnea Resolution After Vertical Banded Gastroplasty.
| Study (Year) |
Sleep Apnea Definition |
Follow-up, Months | Patients With Comorbidity at Baseline, N | Resolved, % (n) | Improved, % (n) | No Change, % (n) | Worse, % (n) |
|---|---|---|---|---|---|---|---|
| Melissas (2001) |
Evaluated by symptoms and laboratory tests. It was considered resolved when patients were asymptomatic. | 24–48 | 12 | 50 (6) | 50 (6/) | 0 (0) | 0 (0) |
(Table reproduced with kind permission from ECRI. Bariatric Surgery for Obesity. 2004. Plymouth Meeting, PA, ECRI. Technology Assessment Report.)
ECRI’s conclusion on vertical banded gastroplasty:
There is evidence that patients experience long-term weight loss after this procedure.
There is evidence that VBG helps to resolve diabetes, dyslipidemia, sleep apnea; and improves QoL.
The data are insufficient to reach definitive conclusions about hypertension and long-term survival.
Question asked: What are the benefits and adverse effects of standard-limb RYGB?
Thirteen studies comprising 3,016 patients were included.
Table 16: Summary of Findings on 3-Year Weight Loss After Standard-Limb Roux-en-Y Gastric Bypass.
| Study (Year) | Patients at Baseline, N | Patients at Follow-up, N |
BMI at Baseline, Mean (SD) | BMI Corresponding to 25% EWL |
BMI at 3 years After Surgery, Mean (SD) | Did the 3-Year BMI Suggest > 25% EWL? |
|---|---|---|---|---|---|---|
| Brolin (2002) | 99 | 74 | 56.9 (7) | 48.2 | 41 (7) | Yes |
| Wittgrove (2000) | 500 | 92 | 43.7 (6.5) | 38.2 | 27.4 (6.5) | Yes |
| Smith (1996) | 205 | 44 | 45 | 39.2 | 29.8 | Yes |
| Pories (1995) | 608 | 383 | 49.7 (8.3) | 42.7 | 32.2 (8.3) | Yes |
| Carson (1994) | 159 | 61 | 48.7 (9.9) | 42 | 34.8 (9.1) | Yes |
(Table reproduced with kind permission from ECRI. Bariatric Surgery for Obesity. 2004. Plymouth Meeting, PA, ECRI. Technology Assessment Report.)
Table 17: Summary of Findings on Diabetes Resolution After Roux-en-Y Gastric Bypass.
| Study (Year) |
Diabetes Definition | Follow-p, Months | Patients With Comorbidity at Baseline, N | Resolved, % (n) | Improved, % (n) | No Change, % (n) | Worse, % (n) |
|---|---|---|---|---|---|---|---|
| Wittgrove (2000) | Diabetes mellitus, no further definition | 3–60 | 85 | 99 (84) | 1 (1) | 0 (0) | 0 (0) |
| Cowan (1998) | Fasting blood glucose > 140 mg/dl or 7.77 mmol/l | 6–12 | 13 | 92 (12) | 8 (1) | 0 (0) | 0 (0) |
| Smith (1996) | Study did not categorize patients as having or not having diabetes, but instead determined if patients were taking insulin, oral hypoglycemics, a controlled diet, or no treatment. ECRI classed the first 3 as indicating diabetes and the last category as not indicating diabetes. | 3–84 | 118 | 74 (87) | Not reported | Not reported | Not reported |
| Pories (1995) |
Non insulin dependent diabetes, abnormal values of blood glucose and glycosylated hemoglobin | < 12–168, (mean, 108) | 165 | 83 (121/146) |
Not reported | Not reported | Not reported |
(Table reproduced with kind permission from ECRI. Bariatric Surgery for Obesity. 2004. Plymouth Meeting, PA, ECRI. Technology Assessment Report.)
Table 18: Summary of Findings on Hypertension Resolution After Roux-en-Y Gastric Bypass.
| Study (Year) | Hypertension Definition |
Follow-up, Months | Patients With Comorbidity at Baseline, N | Resolved, % (n) | Improved, % (n) | No Change, % (n) | Worse, % (n) |
|---|---|---|---|---|---|---|---|
| Wittgrove (2000) | Not defined | 3–60 | 118 | 92 (108) | Not reported | Not reported | Not reported |
| Carson (1994) | Either the patient was taking hypertensive medication, or diastolic blood pressure > 90 mm Hg | 48 | 45 | 67 (12) | 22 (4) | 11 (2) | 0 (0) |
| Kalfarentzos (1999) | Systolic blood pressure > 140 mm Hg or diastolic blood pressure > 90 mm Hg | Mean, 16.8 | 13 | 69 (9) | 15 (2) | 15 (2) | 0 (0) |
| Cowan (1998) | Systolic blood pressure >140 mm Hg, diastolic blood pressure > 90 mm Hg | 6–12 | 27 | 93 (25) | Not reported | Not reported | Not reported |
(Table reproduced with kind permission from ECRI. Bariatric Surgery for Obesity. 2004. Plymouth Meeting, PA, ECRI. Technology Assessment Report.)
Table 19: Summary of Findings on Dyslipidemia Resolution After Roux-en-Y Gastric Bypass.
| Study (Year) | Lipids Definition | Follow-up, Months | Patients With Comorbidity at Baseline, N | Resolved, % (n) | Improved, % (n) | No Change, % (n) | Worse, % (n) |
|---|---|---|---|---|---|---|---|
| Wittgrove (2000) |
High triglycerides, level not defined | 3–60 | 158 | 99 (157) | Not reported | Not reported | Not reported |
| Wittgrove (2000) |
High cholesterol, level not defined | 3–60 | 275 | 97 (267) | Not reported | Not reported | Not reported |
| Kalfarentzos (1999) |
LDL cholesterol > 160 mg/dl or triglycerides > 250 mg/dl | Mean, 16.8 | 11 | 73 (8) | 18 (2) | 9 (1) | 0 (0) |
| Cowan (1998) |
High triglycerides defined as > 150 mg/dl (1.69 mmol/l) | 6–12 | 31 | 87 (27) | Not reported | Not reported | Not reported |
| Cowan (1998) |
High cholesterol defined as > 200 mg/dl (5.17 mmol/l) | 6–12 | 33 | 85 (28) | Not reported | Not reported | Not reported |
(Table reproduced with kind permission from ECRI. Bariatric Surgery for Obesity. 2004. Plymouth Meeting, PA, ECRI. Technology Assessment Report.)
Table 20: Summary of Findings on Sleep Apnea Resolution After Roux-en-Y Gastric Bypass.
| Study (Year) | Apnea Definition | Follow-up, Months | Patients With Comorbidity at Baseline, N | Resolved, % (n) | Improved, % (n) | No Change, % (n) | Worse, % (n) |
|---|---|---|---|---|---|---|---|
| Wittgrove (2000) | Not defined | 3–60 | 225 | 98 (220) | Not reported | Not reported | Not reported |
(Table reproduced with kind permission from ECRI. Bariatric Surgery for Obesity. 2004. Plymouth Meeting, PA, ECRI. Technology Assessment Report.)
Table 21: Summary of Findings on Survival After Roux-en-Y Gastric Bypass (RYGB).
| N at Baseline | Result | ||||
|---|---|---|---|---|---|
| Study (Year) | No Surgery | RYGB | Description of Measure |
No Surgery (Mean Follow-Up, 6.2 years) | RYGB (Mean Follow-Up, 9 years) |
| MacDonald (1997) [AU: There’s only one study here?] |
78 | 154 | Number of patients who died | 22 (88%) | 14 (9%)* |
| 78 | 154 | Mortality per patient year of follow-up | 0.045 | 0.01* | |
| 34 | 105 | Mortality per patient year of follow-up for patients not taking medication for diabetes | 0.035 | 0.01 | |
| 44 | 49 | Mortality per patient year of follow-up for patients taking medication for diabetes | 0.05 | 0.0125* | |
Statistically significantly different between groups; P < .001
(Table reproduced with kind permission from ECRI. Bariatric Surgery for Obesity. 2004. Plymouth Meeting, PA, ECRI. Technology Assessment Report.)
ECRI’s conclusions on standard-limb RYGB:
Weight loss persists at 3 years after surgery
Some patients experience resolution of comorbid conditions
The data are insufficient to reach definitive conclusions about long-term survival.
None of the studies reported on the overall rate of reoperations after standard-limb RYGB.
Question asked: What are the benefits and adverse effects of long-limb RYGB?
Five studies comprising 506 patients were included.
Table 22: Summary of Findings on 3-Year Weight Loss After Long-Limb Roux-en-Y Gastric Bypass.
| Study (Year) | Patients at Baseline, N | Patients at Follow-up, N |
BMI at Baseline, Mean (SD) | BMI corresponding to 25% EWL |
BMI at 3 Years After Surgery, Mean (SD) |
Did the 3-year BMI Suggest > 25% EWL? |
|---|---|---|---|---|---|---|
| Brolin (2002) | 152 | 54 | 55.3 (7) | 47 | 34.4 (7) | Yes Follow-up data estimated by ECRI from figure in publication |
| Choban (2002) | 33 | 10 | 61 (2) | 51.2 | 44.7 (4.4) | Yes |
| Balsiger (2000) | 191 | 72 | 49 (5) | 42.2 | 33.1 (7.4) | Yes Follow-up data estimated by ECRI from figure in publication |
(Table reproduced with kind permission from ECRI. Bariatric Surgery for Obesity. 2004. Plymouth Meeting, PA, ECRI. Technology Assessment Report.)
Table 23: Summary of Findings on Diabetes Resolution After Long-Limb Roux-en-Y Gastric Bypass.
| Study (Year) | Diabetes Definition | Follow-up, Months | Patients With Comorbidity at Baseline, N |
Resolved, % (n) | Improved, % (n) | No Change, % (n) | Worse, % (n) |
|---|---|---|---|---|---|---|---|
| Reddy (2002) | Not defined | Mean, 5 | 24 | 29 (7) | 21 (5) | Not reported | Not reported |
(Table reproduced with kind permission from ECRI. Bariatric Surgery for Obesity. 2004. Plymouth Meeting, PA, ECRI. Technology Assessment Report.)
Table 24: Summary of Findings on Hypertension Resolution After Long-Limb Roux-en-Y Gastric Bypass.
| Study (Year) | Hypertension Definition | Follow-up, Months | Patients With Comorbidity at Baseline, N |
Resolved, % (n) | Improved, % (n) | No Change, % (n) | Worse, % |
|---|---|---|---|---|---|---|---|
| Reddy (2002) | Not defined | Mean, 5 | 42 | 43 (18) | 10 (4) | Not reported | Not reported |
(Table reproduced with kind permission from ECRI. Bariatric Surgery for Obesity. 2004. Plymouth Meeting, PA, ECRI. Technology Assessment Report.)
ECRI’s conclusions on long-limb RYGB:
Long-limb RYGB results in long-term weight loss.
There is of resolution of diabetes and hypertension.
The data are insufficient data to reach definitive conclusions about dyslipidemia, sleep apnea, and long-term survival.
Comparisons of Different Bariatric Procedures
Inclusion criteria for this section were as follows:
The study was a RCT that compared 2 or more procedures.
If the study was not randomized, it had to be a controlled trial that compared 2 or more procedures and was published in 1994 or later.
All patients were at least 18 years old, or if some were not, the data must have been reported separately for patients that were at least 18 years old.
All patients were morbidly obese, or if some were not, the results must have been reported separately for those with morbid obesity.
If the study enrolled patients who received different procedures, the data on the different procedures must have been reported separately.
-
The study reported data on at least 1 of the following outcomes:
Weight data at 1 year and/or 3 years after surgery; acceptable measures of weight were BMI, percentage excess body weight, and percentage ideal body weight.
Resolution of comorbid conditions: diabetes, hypertension, dyslipidemia, sleep apnea, and heart disease.
Survival.
QoL.
Perioperative mortality and adverse events.
Eight studies comprising 535 patients met the inclusion criteria. In 3 studies, adjustable gastric banding was compared with VBG; in 2 studies, VBG was compared with RYGB; and in 3 studies, RYGB was compared with long-limb RYGB.
None of the studies reported comparative data on the resolution of comorbid conditions, QoL, or long-term survival.
Table 25: Comparative Studies: Adjustable Gastric Banding, Vertical Banded Gastroplasty, and Standard- and Long-Limb Roux-en-Y Gastric Bypass.
| Comparison | Study (Year) | Procedure | Time After Surgery, Years | Patients at Baseline, N | Patients at Follow-up, N | BMI at Baseline, Mean (SD) | BMI at Follow-up, Mean (SD) | Statistically Significant? |
|---|---|---|---|---|---|---|---|---|
| AGB vs. VBG | Morino (2003) |
AGB VBG |
1 1 |
49 51 |
49 51 |
44.7 44.2 |
35.5 30.1 |
Yes |
| Nilsel (2001) |
AGB VBG |
1 1 |
29 30 |
28 25 |
42.8 (5.4) 43.9 (3.8) |
34.1 (8.6) 28.9 (7.8) |
Yes | |
| AGB VBG |
3 3 |
29 30 |
13 15 |
42.8 (5.4) 43.9 (3.8) |
29.2 (5.8) 31.5 (7.5) |
No | ||
| VBG vs. RYGB |
Sugerman (1987) |
VBG RYGB |
1 1 |
20 20 |
18 19 |
49 (9) 47 (11) |
38.5 (9.0) 30.2 (7.0) |
Yes |
| VBG RYGB |
3 3 |
20 20 |
16 18 |
49 (9) 47 (11) |
39.4 (9.6) 31 (8.1) |
Yes | ||
| RYGB vs. LLRBG |
Feng (2003) |
RYGB LLRBG |
1 1 |
45 13 |
45 13 |
43.6 (3.2) 45.3 (3.9) |
28.1 (3.2) 29 (5.4) |
No |
| Brolin (1992) |
RYGB LLRGB |
1 1 |
22 23 |
17 20 |
63.4 (9) 31.6 (9) |
44 (8) 40 (9) |
No | |
| RYGB LLRGB |
3 3 |
22 23 |
12 13 |
63.4 (10) 61.6 (9) |
45 (14) 37 (6) |
Yes |
(Table reproduced with kind permission from ECRI. Bariatric Surgery for Obesity. 2004. Plymouth Meeting, PA, ECRI. Technology Assessment Report.)
ECRI’s conclusions on AGB compared with VBG:
1 year after surgery, patients who had VBG lost more weight than patients who had AGB.
The data are insufficient to permit evidence-based conclusions about the amount of weight loss 3 years after surgery.
ECRI’s conclusions on VBG compared with RYGB:
1 and 3 years after surgery, patients who had RYGB weighed less than did patients who had VBG.
ECRI’s conclusions on standard-limb RYGB compared with long-limb RYGB:
3 years after surgery, patients who had long-limb RYGB lost more weight than did patients who had standard-limb RYGB.
Nonmorbidly Obese Adults
Question asked: What are the benefits and adverse effects of bariatric surgery for nonmorbidly obese adults?
Standard indications for bariatric surgery require that patients be morbidly obese, as defined by a BMI of at least 40 kg/m2, or a BMI of at least 35 kg/m2 and a serious comorbid illness. According to ECRI, however, some patients who have had bariatric surgery are obese, but not morbidly. These people have a BMI from 30 to 35 kg/m2, or a BMI between 35 and 40 kg/m2 and serious comorbid conditions.
The inclusion criteria were as follows:
The study had a control group of patients who did not receive surgery.
If the study did not have a control group, the study had to have used a before-and-after design.
All patients were at least 18 years old, or if some were not, the data must have been reported separately for patients at least 18 years old.
All patients were obese, but not morbidly, or if some were not, the results must have been reported separately for the patients who were not morbidly obese.
One study comprising 50 patients met the inclusion criteria. The procedure used was the transected Silastic ring vertical gastric bypass. All 50 patients had BMIs between 32 and 40 kg/m2, and none had life-threatening comorbid illnesses. Some patients had conditions like diabetes (4%) and dyslipidemia (20%), but the authors noted that these were not life-threatening and that all of the patients were nonmorbidly obese. All had already attempted unsuccessfully to lose weight without surgery. The authors reported data on weight loss, perioperative mortality, and adverse events. They did not report data on resolution of comorbidities, QoL, or long-term survival.
Table 26: Weight Data for Nonmorbidly Obese Patients From ECRI.
| Study (Year) | Patients at Baseline, N | Follow-up (Months) | Patients at Follow-up, N |
BMI at Baseline, Mean (SD) | BMI Corresponding to 25% EWL |
BMI at Follow-up, Mean (SD) | Did the Postsurgical BMI Suggest ≥ 25% EWL? |
|---|---|---|---|---|---|---|---|
| Fobi (2002) |
50 | 3 | 50 | 37.9 | 33.9 | 30.0 (2.5) | Yes |
| 6 | 50 | (2.2) | 26.8 (2.5) | Yes | |||
| 12 | 47 | 24.4 (2.3) | Yes | ||||
| 18 | 47 | 23.4 (2.4) | Yes | ||||
| 24 | 38 | 24.6 (2.2) | Yes | ||||
| 27 | 15 | 23.4 (2.3) | Yes |
(Table reproduced with kind permission from ECRI. Bariatric Surgery for Obesity. 2004. Plymouth Meeting, PA, ECRI. Technology Assessment Report.)
ECRI’s conclusions on bariatric surgery for nonmorbidly obese adults:
27 months after the surgery, patients lost a clinically significant amount of weight.
-
Serious adverse events can occur after transected Silastic ring vertical gastric bypass for people who are nonmorbidly obese.
5 patients (10%) had perioperative complications; 2 had deep vein thrombosis.
There is insufficient evidence to determine the rates of adverse events in this population.
In general, the evidence on surgery in nonmorbidly obese patients is minimal.
Morbidly Obese Adolescents
Question asked: What are the benefits and adverse effects of bariatric surgery for morbidly obese adolescents?
Adolescents comprise about 1% of patients who have received bariatric surgery for morbid obesity. However, there is concern on the appropriate use of this surgery in adolescents. For example, surgery may potentially interfere with physical growth or sexual maturation. It is important to analyze such outcomes in adolescents who receive bariatric surgery.
The inclusion criteria were as follows:
The study had a control group of patients who did not receive surgery.
If the study did not have a control group, the study must have used a before-and-after design.
All patients were aged 21 years or younger, or if some were not, the data were reported separately for the patients aged 21 years or younger. Using a cut-off age of 21 instead of 18 enabled the authors to include studies that defined adolescence as lasting up to 21 years of age.
All of the patients were morbidly obese, or if some were not, the results had to be reported separately for morbidly obese patients.
Studies could have reported on more than one procedure.
Five studies comprising 87 patients met the inclusion criteria. Data on comorbid conditions from one study were excluded owing to multiple inconsistencies. In 2 studies, the surgical procedure was adjustable gastric band bypass. The other 3 studies combined data on different procedures. More than one-half of the patients were female (63%), the mean age was 15.9 years, and the mean presurgical BMI was 53.6 kg/m2.
All of the studies reported data on weight and adverse effects, and 2 studies reported data on maturation outcomes. None of the studies reported enough data on comorbid conditions, QoL, or long-term survival.
Table 27: Summary of Findings on Weight Loss From Studies of Adolescents With Morbid Obesity From ECRI.
| Study (Year) |
Patients at Baseline, N | Follow-up, Years | Patients at Follow-up, N |
BMI at Baseline, Mean (SD) |
BMI Corresponding to 25% EWL |
BMI at 3 Years After Surgery, Mean (SD) |
Did the 3-year BMI Suggest > 25% EWL? |
|---|---|---|---|---|---|---|---|
| Abu-Abeid (2003) |
11 | Mean, 1.9 | 11 | 46.6 | 40.4 | 32 | Yes |
| Dolan (2003) |
11 | 1 | 11 | 47.7 (8.7) | 40.7 | 35.8 (6.6) | Yes |
| Dolan (2003) |
11 | Mean, 1.7 | 11 | 47.7 (8.7) | 40.7 | 32.1 (8.2) | Yes |
| Sugerman (2003) |
33 | 1 | 31 | 52 (11) | 44.5 | 36 (10) | Yes |
| 5 | 20 | 52 (11) | 44.5 | 33 (11) | Yes | ||
| 10 | 14 | 52 (10) | 44.5 | 34 (8) | Yes | ||
| Strauss (2001) |
10 | Mean, 5.8 | 10 | 52 (10) | 44.5 | 36 (10) | Yes |
| Breaux (1995) |
22 | Mean, 4.2 | 22 | 63 | 52.7 | 40 | Yes |
(Table reproduced with kind permission from ECRI. Bariatric Surgery for Obesity. 2004. Plymouth Meeting, PA, ECRI. Technology Assessment Report.)
ECRI’s conclusions on bariatric surgery for morbidly obese adolescents:
-
After having adjustable gastric banding, adolescents with morbid obesity lost a clinically significant amount of weight.
The mean decrease in BMI ranged from 14.6 to 15.6 kg/m2.
The length of follow-up in the 2 studies ranged from 1.7 to 1.9 years.
-
Serious adverse events can occur after adjustable gastric banding for adolescents.
However, the evidence is insufficient to draw evidence-based conclusions about the rates of adverse effects in this patient population.
-
The evidence is also insufficient to make evidence-based conclusions on the effect of adjustable gastric banding on the growth or sexual maturation of adolescents with morbid obesity.
2 studies (N = 44) each reported that none of the patients experienced altered growth or sexual maturation. However, the low patient enrollment precludes making evidence-based conclusions about physical growth or sexual maturation.
Agency for Healthcare Research and Quality (July 2004)
The literature search by the Agency for Healthcare Research and Quality (AHRQ) for controlled studies of surgical treatments of obesity began on October 16, 2002.(21) Updates were done in 2003 on May 22, June 2, June 12, and July 3.
Studies were included if they were RCTs, non-RCTs, or case series. Case series were included because a brief scan of the literature showed that RCTs would be few in number. The AHRQ report acknowledged that inferences about efficacy cannot easily be made from case series; however, it concluded that such studies provide useful information in the absence of RCTs and are useful to assess complications and adverse events. A threshold of 10 or more patients per case series was set for inclusion.
The main outcome of interest was weight loss. Weight loss can be measured in several ways including kilograms of weight lost, “excess” weight loss, or percentage of excess weight loss (EWL). Among these, the most commonly reported outcome was kilograms of weight lost.
Among the 111 surgical studies reporting on weight loss that were reviewed, 43 reported weight loss only in terms of kilograms or pounds, 17 reported only excess weight loss or some variant, 46 reported both of these outcomes, and 5 reported neither. The authors chose weight loss (in kilograms or pounds) as the main outcome measure, because it allowed them to maximize the number of studies included in the analysis and was the only way to compare the effectiveness of surgical therapies across studies.(21)
Weight-related comorbid conditions, like diabetes, were also examined. Studies that made within-study comparisons and case series were examined.
After discussion with 3 expert bariatric surgeons, the bariatric procedures were categorized as follows:
By type
By if the procedure was laparoscopic or open
By more specific surgical details, such as length of Roux limb, or if the band was adjustable or nonadjustable
To allow for comparisons in the analysis, it was necessary to combine certain procedures that were judged clinically similar. The categories used in the AHRQ analysis are shown in Table 28 on the next page.
Table 28: Surgical Procedure Categories in the AHRQ Analysis (21).
| Upper-level category | Lower-level category | Subcategory |
|---|---|---|
| Gastroplasty^ | Open Gastroplasty | NA |
| Laparoscopic Gastroplasty | NA | |
| Jejunal-ileal bypass | NA | NA |
| BPD/Duodenal switch | NA | NA |
| Gastric Bypass | Open RYGB^^ | Open RYGB, standard limb Open RYGB, long limb |
| Open loop gastric bypass | NA | |
| Laparoscopic RYGB^^ | Laparoscopic RYGB, standard limb Laparoscopic RYGB, long limb |
|
| Band | Open Band | Open adjustable Band Open nonadjustable Band |
| Laparoscopic Band | Laparoscopic adjustable Band Laparoscopic nonadjustable Band |
|
| VBG** | Open VBG | NA |
| Laparoscopic VBG | NA |
VBG includes: Vertical Banded Gastroplasty, VBG with Marlex: VBG with Dacron: gastric restriction, 4.5 gastroplasty. 5.0 gastroplasty, and Silastic Ring Vertical Gastroplasty.
Gastroplasty includes: Horizontal Banded Gastroplasty. Gastric Portioning, Gastrogastrostomy, Gastric Portioning.
RYGB bypass includes: Roux-en-Y Gastric Bypass and RYGBs that also have ring placement (i.e.. Fobi).
(Table reproduced courtesy of the Agency for Healthcare Research and Quality (AHRQ): Shekelle PG, Morton SC, Maglione MA, Suttorp M, Tu W, Li Z et al. Pharmacological and surgical treatment of obesity. Evidence Report/Technology Assessment No. 103. AHRQ Publication No. 04-E028-2. 2004. Santa Monica, CA, Prepared by the Southern California - RAND Evidence-BAsed Practice Center).
VBG is the only “gastroplasty” procedure that is done in the United States now; therefore, the other “gastroplasty” procedures were placed into a separate single category to be used for historic comparisons as needed.
For RCTs that did within-study comparisons of 2 procedures, a mean difference was calculated (mean weight loss in procedure A minus mean weight loss in procedure B). A positive mean difference indicated that patients in Procedure A lost more weight on average than did patients in Procedure B. A negative mean difference indicated that patients in Procedure A lost less weight on average than did patients in Procedure B. Mean differences were pooled using a random-effects model and 95% confidence interval (CI). For all studies, randomized or not, a pooled mean weight loss for each procedure was estimated using a random-effects model, and an associated 95% CI was calculated.
Results
Shekelle et al.(21) summarized the updated Cochrane review of literature on surgery for obesity (February 2003). Inclusion criteria for the review were RCTs and non-RCTs that compared surgery with nonsurgical treatment for morbid obesity, and RCTs comparing surgical procedures. The review was restricted to adults aged 18 years or older with morbid obesity, defined as a BMI of more than 40 kg/m2, or a BMI of more than 35 kg/m2 with a serious comorbid condition. Studies had to report data on at least 12 months of follow-up. Due to the heterogeneity of the data, the authors said a meta-analysis was not justified; therefore, they summarized their data narratively. The authors identified 2707 citations, of which they retrieved 99 for detailed examination. Eighteen trials met the inclusion criteria.
The authors concluded that there is limited evidence to suggest that surgery supports greater long-term weight loss (maintained to at least 8 years) than do conventional treatments for morbid obesity. Additionally, surgery is associated with adverse effects and the risk of postoperative mortality. The reviewers added that the data were too limited to draw any conclusions on differences in efficacy or safety among surgical procedures. Of note, the conclusions were based primarily on papers that compared diet alone with horizontal unbanded gastroplasty, a surgical procedure that has not been used frequently for more than 20 years.(21)
Shekelle et al.(21) extended the Cochrane review by including case series (those with at least 10 cases) in addition to RCTs, and by assessing benefits (weight loss and improvement in serious medical conditions) and risks (adverse events). One hundred and forty-two studies were considered for the analysis. The Swedish obese subjects (SOS) study is discussed separately below. Shekelle et al. identified 28 RCTs or controlled trials of surgery (all but 2 compared one surgical procedure with another) and 113 case series.
Benefits - Weight Loss and Maintenance
The authors found 2 RCTs that compared patients who had received bariatric surgery with those treated without surgery. However, these studies were done more than 20 years ago, and the procedures assessed are not relevant to modern bariatric surgery. This is because improvements in procedures and techniques have been associated with significantly greater long-term weight loss compared with horizontal gastroplasty, and fewer major complications compared with the jejunoileal bypass.
In addition to the 2 RCTs, a number of papers from the large, observational, SOS study were published.
Benefits – Comorbid Conditions
A series of reports from the SOS study offers some evidence that surgery is more effective than medical therapy to reduce or prevent comorbid conditions in obesity.
The SOS study was the only trial that Shekelle et al. identified that compared comorbid conditions between surgically treated patients and a concurrent control group receiving nonsurgical treatment.
-
At 24 months after surgery, among 845 surgically treated patients and 845 matched controls, the incidences of diabetes, lipid abnormalities, and hypertension were lower in those who had surgery:
Diabetes: adjusted odds ratio [OR], 0.02; 95% CI, 0.00–0.16
Lipid abnormalities: adjusted OR, 0.10; 95% CI, 0.04–0.25
Hypertension: adjusted OR, 0.38; 95% CI, 0.22–0.65
At 8 years of follow-up, the effect of surgery on the reduction in diabetes risk yielded an OR of 0.16 (95% CI, 0.07–0.36). The effect on reduction in risk for hypertension did not persist (OR 1.01; 95% CI, 0.61–1.67).
Shekelle et al. also assessed surgery case series reports for data on 4 comorbid conditions: diabetes, hypertension, sleep apnea, and hyperlipidemia.
Diabetes
Of 114 case series publications, 21 papers reported quantitative data on diabetes. The proportion of patients with preoperative diabetes who showed improvement or resolution of their diabetes after surgery ranged from 69% to 100% (median, 100%).
Hypertension
Eighteen papers reported results showing hypertension was improved or resolved in 25% to 100% (median, 89%) of patients after surgery.
Sleep Apnea
Across 14 studies, an improvement in sleep apnea after surgery was reported in 95% to 100% of patients, with a median of 100% of patients reporting improvement or resolution of sleep apnea.
Hyperlipidemia
Ten studies reported results showing hyperlipidemia was improved or resolved in 60% to 100% (median, 88%) of patients after surgery.
Shekelle et al. concluded that overall, the improvements in comorbid conditions were substantial and suggested that bariatric surgery helps to relieve these conditions in severely obese patients. Shekelle et al. stated that these results are consistent with the statistically significant improvements reported by the SOS study for diabetes, hypertension (in the RYGB subset), and sleep apnea, although the magnitude of benefit reported in the SOS study was smaller than that reported in the case series.
Benefits – Comparison of Surgical Procedures
Shekelle et al. also identified RCTs and case series that compared weight loss outcomes among surgical procedures.
Five RCTs compared similar surgical procedures and reported enough data in enough detail to pool. In 2 studies comparing RYGB with VBG (N = 231), the pooled weight loss outcomes for both procedures were substantial (at least 30 kg at 36 months for both). Results favoured RYGB at 12 and 36 months (8–9 kg more weight loss at 36 months for both studies).
These pooled results are supported by pooled results from all studies combined (RCTs and case series) that reported data on about 2,000 patients for each procedure. These combined data showed that RYGB patients reported about 10 kg more weight loss than patients treated with VBG at both 12 and 36 months.
Several RCTs compared RYGB and other gastric bypass procedures with either VBG or other gastric partitioning procedures. However, these results could not be included in the pooled analysis because either the results were not reported in kilograms of weight lost, or they lacked sufficient statistical detail. However, the results of all the studies supported the conclusion that gastric bypass is associated with superior weight loss compared with gastroplasty.
In 2 RCTs, the weight lost with VGB compared with laparoscopic adjustable gastric banding (LAGB) was 14 kg more at 12 months follow-up but only about 3 kg more at 36 months follow-up. No difference in net weight loss was seen in the pooled results when all of the studies were combined.
One RCT compared open RYGB with laparoscopic RYGB. The weight loss for each was substantial, and no significant differences between them were found (greater than 30 kg for both at 12 months). This result was supported by the “all studies” pooled analysis at 12 months and 36 months. Because the final anatomic reconfiguration is the same for laparoscopic and open RYGB, weight loss and comorbidity outcomes should be identical; however, these procedures involve different techniques that result in different types and rates of complications.
Benefits – Summary of Findings by Shekelle et al.
-
Across studies, surgical treatment resulted in greater weight loss (20 kg–30 kg) than did medical treatment in obese people (BMI > 40 kg/m2), and this loss was maintained for up to 8 years. Surgery also was associated with significant improvements in several comorbid conditions.
However, according to the SOS inclusion criteria, patients who were not morbidly obese (BMI > 40 kg/m2) were included in the SOS.
Patients in the SOS study were self-selected.
-
For patients with a BMI between 35 and 40 kg/m2, although the data strongly supported the superiority of surgery, the findings cannot be considered conclusive in the absence of a study with a concurrent comparison group.
Similarly, the evidence supported, but did not prove an effect of, the ability of surgery to improve many weight-related comorbid conditions.
-
Weight loss reported in surgical studies was greater than weight loss reported in drug or diet studies of obesity (20 kg–40 kg at 1 or 2 years in studies on surgery versus 2 kg–5 kg studies on drugs), although the studies could not be compared directly, because the patient populations are different.
Studies on surgery had only patients who were severely obese, whereas the mean BMI in the medical weight loss studies was about 33.
It was rare for a study on surgery to report data beyond 12 months. Those that did tended to report that patients regained most of their initial weight loss.
RCTs and observational studies showed that RYGB results in more weight loss than VBG does.
All 3 procedures for which Shekelle et al. found data (RYGB, VBG, and AGB) reported long-term weight loss (defined as up to 36 months).
Mortality – Summary of Findings by Shekelle et al.
Surgical procedures were divided into 4 categories: RYGB, BPD, adjustable band procedures, and VGB. Early deaths were defined as having happened up to 30 days after the procedure, or were defined simply as “early” in the original paper. Late deaths were defined as having happened more than 30 days after the procedure, or were defined simply as late in the original paper.
No clear pattern of differential mortality among the techniques emerged. There also was no clear pattern for higher or lower early death rates in RCTs compared with case series. Early mortality after bariatric surgery was less than 1%.
A presentation in 2003 at the Clinical Congress of the American College of Surgeons reported that for over 62,000 procedures done in Washington between 1987 and 2001, the 30-day mortality rate was 1.9% (assessed using administrative data). There was a strong association between a surgeon’s experience and mortality. The 30-day mortality rate for surgeons who had done 20 or fewer procedures was almost 5%.
Shekelle et al. made the following observations:
Only 4 RCTs/observational studies were identified, and 3 of these compared RYGB with VBG. In most cases, only a few hundred patients were studied for each comparison.
None of the comparisons of complications between these procedures showed statistically significant differences, and the 95% confidence intervals were very wide. Therefore, it is not possible to conclude that clinically important differences do or do not exist.
The absolute rates of some complications are substantial, but many may be minor in the degree of severity. For example, the proportion of patients receiving VBG who had gastrointestinal complications was 15.2% in the RCT/observational study data and 17.8% in the case series data. The proportion of patients receiving RYGB who experienced nutritional deficiencies was 26.8% in the case series. (Shekelle et al. noted that many of these nutritional deficiencies were mild.)
Some of the differences between the procedures in the proportions of patients with different complications or adverse events are compatible with the anatomic changes caused by the procedure.
Overall Conclusions From the AHRQ Review by Shekelle et al.
Bariatric surgical treatment results in greater sustained weight loss than nonsurgical treatments in very obese individuals (BMI > 40 kg/m2), thereby resulting in improved health outcomes (e.g., for diabetes, sleep apnea, and QoL). Note, Shekelle et al. did not comment on the poor quality of the evidence on comorbid conditions. Much of the evidence came from case series and the SOS study, which included self-selected patients who were not all morbidly obese.
Case series data suggest greater sustained weight loss for bariatric surgical treatment than for nonsurgical treatment in patients with a BMI between 35 and 40 kg/m2.
RYGB results in greater weight loss than does VBG in severely obese patients.
Postoperative mortality rates of less than 1% have been achieved by a number of surgeons and bariatric surgical centres. The postoperative mortality rate in other settings may be higher.
Few clinical trials have compared outcomes among different types of bariatric surgery. The data suggest there may be clinically important differences in the proportion of patients reporting various complications and adverse events among those treated with RYGB, VBG, and AGB procedures.
Laparoscopic procedures cause fewer wound complications or incisional hernias than open procedures do.
-
Future studies should address the balance between benefits and risk in relevant patient subgroups. For example, a person’s age, weight, and severity of existing medical conditions may influence the net benefit of surgery compared with nonsurgical intervention. Subgroups could be identified based on BMI; age could be stratified as below or above 55 years old, and comorbid conditions like diabetes could be stratified by measures such as hemoglobin A1c or the presence of end organ damage.
Few patients would need to be studied to assess if bariatric surgery provides greatly superior outcomes to nonsurgical treatment. Shekelle et al. estimated that 215 patients would need to be in each group to provide 80% power to detect an effect of bariatric surgery on reducing diabetes equal to half that reported in the SOS trial.
If the eligibility criteria for bariatric surgery were relaxed to allow inclusion of people with a BMI of 30–32 kg/m2, then it becomes justifiable to require an RCT to assess the relative health benefits and risks of surgical versus nonsurgical treatment. According to Shekelle et al., a precedent exists for mounting such studies even when the surgical therapy is already disseminated.
RCTs would be useful to compare the effectiveness and safety of surgical procedures (adjustable band procedures versus RYGB).
Doing an RCT of bariatric surgery in the adolescent population is still feasible, given the paucity of data regarding either drug or surgical treatment of adolescent and pediatric patients. To the extent that current data on adults are deemed inapplicable to adolescents or children, new studies will be needed.
Blue Cross Blue Shield Association Technology Evaluation Center
The objectives of the review by Blue Cross Blue Shield in September 2003 (22) were as follows:
To compare weight loss achieved with surgery versus nonsurgical treatment
To examine evidence on morbidity, mortality, and QoL
To examine if there is a threshold amount of weight loss needed to improve health outcomes
Blue Cross Blue Shield reached the following conclusions:
There is sufficient evidence to conclude that surgery improves health outcomes for patients with morbid obesity, compared with nonsurgical treatment. The best evidence comes from the SOS intervention trial, which reported on several hundred patients in each group with up to 8 years of follow-up. The trial showed that surgery resulted in large amounts of weight loss compared with usual care (16% decrease in total body weight at 6 years versus an increase of 0.8% for usual care).
The SOS trial also showed that some comorbid conditions and QoL improve after surgery. It reported a large reduction in diabetes over a 5½-year mean follow-up for the surgery group (3.6% versus 18.5%, P = .0001). A decrease in the proportion of patients with hypertension was found 2 years after surgery but was not sustained with longer follow-up.
The evidence provides some qualitative information into the relationship between amount of weight loss and change in health outcome measures and the clinical significance of these changes. However, it is not possible to draw conclusions about the relation of increment of weight loss to increment of improvement in health outcome measures. It is also not possible to identify a weight loss threshold for success of a surgical procedure.
Blue Cross Blue Shield Association Technology Evaluation Center (September 2003)
The authors of the review (23) aimed to determine if newer, less invasive procedures (e.g., laparoscopic gastric bypass and laparoscopic gastric banding) improve patient outcomes compared with open gastric bypass. They also aimed to determine if newer variations on gastric bypass (e.g., BPD and long-limb gastric bypass) improve outcomes for patients with super obesity (BMI > 50 kg/m2).
For the following points, Blue Cross Blue Shield reached the following conclusions:
Scientific evidence must permit conclusions concerning the effect of the technology on health outcomes.
The evidence is not sufficient to form conclusions about the relative efficacy and morbidity of less invasive approaches to bariatric surgery specifically laparoscopic gastric bypass and laparoscopic gastric banding. Too few high-quality clinical trials have directly compared these procedures with open gastric bypass procedures. The evidence consists largely of clinical series of individual procedures. This makes comparing the outcomes of different procedures difficult because of the variability in patient populations, surgeons, and hospitals. There is a lack of consistency in reporting outcomes, especially for adverse events, and only a relatively few patients have been included at longer-term follow-ups (i.e., 3 years or longer). The evidence allows for crude comparisons of weight loss outcomes between procedures at 1 year, but it is not sufficiently robust to make meaningful comparisons at longer intervals. The evidence is also not adequate to determine comparative rates of adverse events.
The evidence suggests that weight loss outcomes for laparoscopic gastric bypass and open gastric bypass are similar at 1 year. Because of the technical complexity of the laparoscopic procedure, short-term complications such as anastomotic leaks are of concern. Leakage of intestinal contents may cause peritonitis, which can be life threatening and usually requires reoperation. The comparative rates of these adverse events cannot be determined from the data, which leaves uncertainty as to the relative safety of laparoscopic bypass, and information is lacking on outcomes longer than 1 year.
For laparoscopic gastric banding, the evidence suggests that weight loss at 1 year is less than that achieved with gastric bypass. Limited evidence on 3-year weight loss suggests that this difference in weight loss may narrow over time. Early adverse event rates are low after laparoscopic gastric banding and are probably lower than gastric bypass. There is a higher rate of long-term adverse events, and there are several potentially serious long-term adverse events such as band slippage or erosion. The incidence of slippage of the device from its intended location or erosion through the gastric wall increases over time and can result in visceral organ damage, abdominal pain, and intestinal obstruction. The data are not sufficient to determine the rates of these longer-term adverse events confidently.
There are limited data on outcomes of BPD and/or long-limb gastric bypass for patients with super obesity. There are no high-quality comparative trials. There are only limited clinical series data for these indications. These limited data do not establish that these or other variants (e.g., duodenal switch) have any additional benefit for patients with super obesity as compared with gastric bypass.
Technology must improve net health outcome and must be as beneficial as any established alternatives.
There is insufficient evidence to conclude that these procedures (i.e., laparoscopic gastric bypass, laparoscopic gastric banding, BPD, long-limb gastric bypass) either improve the net health outcome or are as beneficial as the current established surgery: open gastric bypass with Roux-en-Y anastomosis.
Improvement must be attainable outside the investigational setting.
It has not been shown that these procedures (i.e., laparoscopic gastric bypass, laparoscopic gastric banding, BPD, long-limb gastric bypass) improve health outcomes in the investigational setting.
Based on these criteria, laparoscopic gastric bypass, laparoscopic gastric banding, BPD, and long-limb gastric bypass do not meet the Blue Cross Blue Shield Technology Evaluation Center criteria. (23)
Australian Medical Services Advisory Committee (2003)
The questions in the Australian Medical Services Advisory Committee (MSAC) report addressed the use of LAGB in patients with morbid obesity. (24) The authors of the report asked the following questions:
What is the value of LAGB in the treatment of morbidly obese patients (BMI ≥ 35 kg/m2) who have failed to lose weight through nonsurgical means compared with VBG?
What is the value of LAGB in the treatment of morbidly obese patients (BMI ≥ 35 kg/m2) who have failed to lose weight through nonsurgical means compared with open RYGB?
Methods
MSAC used 2 search strategies. The first identified RCTs of LAGB, open RYGB, and open VBG; and nonrandomized studies comparing LAGB with open RYGB or open VBG. These studies were used as primary evidence in the review. Previous reviews of gastric surgery have found a lack of high-level evidence for LAGB. Therefore, a second search was done to identify non-comparative studies of LAGB. The results of these studies were used as supportive evidence of the safety of LAGB and as longer-term evidence of the effectiveness of LAGB.
The authors searched the literature up to July 2002.
Table 29: Levels of Evidence Used by the Australian Medical Services Advisory Committee.
| Level of evidence | Study design |
|---|---|
| I | Evidence obtained from a systematic review of all relevant randomised controlled trials |
| II | Evidence obtained from at least one properly-designed randomised controlled trial |
| III-1 | Evidence obtained from well-designed pseudo-randomised controlled trials (alternate allocation or some oilier method) |
| III-2 | Evidence obtained from comparative studies (including systematic reviews of such studies) with concurrent controls and allocation not randomised, cohort studies, case-control studies, or interrupted time series with a control group |
| III-3 | Evidence obtained from comparative studies with historical control, two or more single arm studies, or interrupted time series without a parallel control group |
| IV | Evidence obtained from case series, either post-test or pre-tesf/post-test |
Modified From NHMRC. 1999.
(Reproduced with kind permission from the Medical Services Advisory Committee (MSAC). Laparoscopic adjustable gastric banding for morbid obesity. 2003. Commonwealth of Australia)
Results
Primary Studies
No RCTs directly comparing LAGB with either VBG or RYGB were identified. The search identified non-RCTs that compared LAGB with VBG or RYGB (level III-2 and III-3). To supplement the nonrandomized comparative studies, the authors decided to include data from RCTs that included an open RYGB, open VBG, or LAGB arm. The data collected from a single arm of an RCT is classified as level IV evidence, but it was hoped that the more formal design of the RCT would remove some of the methodological biases normally associated with a case series.
There were 27 articles describing 19 RCTs that included an open RYGB, open VBG, or LAGB arm.
Supportive Studies
Of 136 articles describing case series identified by the literature search, 106 reports with fewer than 200 patients were excluded. The review of level IV evidence was restricted to case series with more than 200 patients to reduce the effect of the “learning curve” on the results of the studies. In addition, because many of the complications of bariatric surgery occur in less than 1% of patients, the authors decided that including small case series would not reflect the true incidence of complications.
The eligible articles described the results of case series from 14 centres. The number of patients in the case series ranged from 207 to 950. Follow-up ranged from 12 months to 7 years. Studies that reported data on patient follow-up showed that few patients were lost to follow-up. Eight reports stated that they were analyses of the first series of patients operated on by their institution. Therefore, the data in these reports incorporated the learning curve. Four reports stated that the surgeons had prior experience or that the patients who had at least a minimum length of follow-up were selected from a case series. The remaining reports supplied no information on the experience of the surgeons.
The eligibility criteria for all of the studies were similar. The main criterion was that patients had to have a minimum BMI of 35 kg/m2.
Safety
Primary results
The data in this section were based on the 7 studies that compared LAGB to either open RYGB, open VBG, or both; the 17 RCTs that included an open VBG and/or an open RYGB arm; and the 2 RCTs that included a LAGB arm. When a study had multiple publications, only the most up-to-date results for each outcome were used.
Supportive results
The data for this section came from 28 articles identified by the literature search describing case series of LAGB from 14 centres. Due to the small incidences of many of the adverse events associated with LAGB and the effect of the learning curve on surgical outcomes, only case series with more than 200 patients were included. When a study had multiple publications, only the most up-to-date results for each outcome were used.
Conversion from laparoscopic procedure to an open procedure during surgery
In the MSAC study, conversion is only relevant to the LAGB arms of the studies.
Conversion rates ranged from 0% to 10.5%.
All of the studies that reported on more than 200 patients had a conversion rate of ≤ 3%.
High conversion rates seen in case series of < 200 patients may reflect the learning curve for the procedure as well as the small sample size (Figure 2).
Figure 2: Conversion Rates Within Individual LAGB Consecutive Case Series in a Review by the Medical Services Advisory Committee.
(Reproduced with kind permission from the Medical Services Advisory Committee (MSAC). Laparoscopic adjustable gastric banding for morbid obesity. 2003. Commonwealth of Australia)
Reoperation rates
-
Reoperation rates in primary studies of LAGB patients ranged from 0% to 22.4%; in open VBG, they ranged from 0% to 66.6%; in open RYGB, from 0% to 47.4%.
When the primary results were combined, the rates of reoperation were 9.9% for LAGB, 11.6% for RYGB, and 16.6% for VBG.
The follow-up for LAGB studies was shorter than in the RYGB and VBG studies; therefore, the differences in reoperation rates may have been due to these differences in follow-up.
-
Supportive studies included patients who were followed-up for up to 7 years.
The combined reoperation rate in these studies was 7.8%.
Although it is possible that the reoperation rates for LAGB were slightly lower than for VBG and open RYGB, no definitive conclusion on the rates for each procedure could be reached.
Mortality
LAGB did not seem to be associated with a higher risk of death than either RYGB or VBG.
Morbidity
Port complications were the most common complication arising from gastric banding. The rate of port complications in the primary studies was 28%; however, this was based on 25 patients. Although port complications were still one of the most common complications reported in the supportive studies, the rate based upon 3,003 patients was much lower (5.5%).
The most commonly reported complications after RYGB were dumping (20%) and ulcers (12.1%). The most commonly reported complications after VBG were herniation (15.8%) and stenosis (9.3%). These figures must be treated with caution, because they are based on small sample sizes.
Stenosis was reported in all 3 procedures. This complication was rectified in the LAGB patients, by a band adjustment. Correction of stenosis in VBG and RYGB patients however, usually involves surgery or endoscopy.
The rates on nonspecific morbidity in LAGB patients were slightly lower or comparable to the rates in patients who had received RYGB or VBG. One of the LAGB supportive studies reported a high rate of food intolerance (11.7%) only in early patients.
Effectiveness
Primary results
The data were based on 7 studies that compared LAGB to either open RYGB, open VBG, or both; the 17 RCTs that included an open VBG and/or an open RYGB arm; and the 2 RCTs that included a LAGB arm.
Supportive results
The data were based on 27 articles describing case series of LAGB from 14 centres. Only case series with more than 200 patients were included.
Weight Loss
Comparative studies indicated patients who had RYGB lost significantly more weight than patients who had LAGB (Table 30). Patients who had VBG lost more weight than did LAGB patients immediately after surgery, but this difference disappeared by 2 years after surgery (Tables 31 to 33).
The RCT arms indicated that RYGB patients lost weight for 1 to 2 years before maintaining a weight at a lower level. In contrast, VBG patients appeared to regain weight after 12 to 18 months, but they did not return to their preoperative weight.
The LAGB arms of RCTs and the supportive case series evidence indicated that LAGB patients lost weight over the first few years and this weight loss seemed to be sustained up to 7 years later. However, fewer patients were available to follow-up for more than 1 year.
In summary, it appears that LAGB was less effective than RYGB but the weight loss achieved in LAGB patients was comparable to that in patients who received VGB.
Table 30: Weight loss in LAGB and RYGB Non-Randomized Comparative Studies in a Review by the Medical Services Advisory Committee.
| N | One year | Two years | Three + years | |
|---|---|---|---|---|
| Weight loss (kd) | ||||
| Korankov et al (2002)* | ||||
| LAGB | 30 | 47.7±13.0 | --- | --- |
| RYGB | 20 | 63.6±21.5 | --- | --- |
| Statistically different? | Yes (p = 0.0063) | --- | --- | |
| Excess weight loss (%) | ||||
| Hel et al (2000) | ||||
| LAGB | 30 | --- | --- | -60% |
| RYGB | 30 | --- | --- | 90% |
| Statistically different? | --- | --- | Yes (p < 0.05) |
a) At baseline the mean weight of patients undergoing RYGB was significantly greater than the mean weight of the LAGB patients.
(Reproduced with kind permission from the Medical Services Advisory Committee (MSAC). Laparoscopic adjustable gastric banding for morbid obesity. 2003. Commonwealth of Australia)
Table 31: Weight Loss (kg) in LAGB and VBG Non-Randomized Comparative Studies in a Review by the Medical Services Advisory Committee.
| N | Baseline | Two years | |
|---|---|---|---|
| Freid, Petrous and kasalicky (1997) | |||
| VBG | 52 | 135.5 | 40.5 |
| LAGB | 15 | 140.2 | 37.2 |
| Statistically different? | Not assessable | No |
(Reproduced with kind permission from the Medical Services Advisory Committee (MSAC). Laparoscopic adjustable gastric banding for morbid obesity. 2003. Commonwealth of Australia)
Table 33: Excess Weight Loss (%) in LAGB and VBG Non-Randomized Comparative Studies in a Review by the Medical Services Advisory Committee.
| N | Six months | Nine months | One year | 18 months | Two years | Three years | |
|---|---|---|---|---|---|---|---|
| Ashy and Merdad (1998) | |||||||
| VBG | 30 | 87 | |||||
| LAGB | 30 | 50 | |||||
| Statistically different? | Not assessable | ||||||
| Hel et al. (2000) | |||||||
| LAGB | 30 | -60 (n=30) | |||||
| LAGB | 30 | -60 (n=30) | |||||
| Statistically different? | No | ||||||
| Suter et al. (1999) | |||||||
| VBG | 197 | 58(n=154) | 65(n=110) | 65(n=133) | 65(n=84) | 65(n=84) | |
| LAGB | 76 | 36(n=64) | 48(n=51) | 56(n=47) | 59(n=23) | 59(n=19) | |
| Statistically different? | Yes | Yes | Yes | No | No | ||
(Reproduced with kind permission from the Medical Services Advisory Committee (MSAC). Laparoscopic adjustable gastric banding for morbid obesity. 2003. Commonwealth of Australia)
Table 32: Mean BMI (kg/m2) in LAGB and VBG Non-Randomized Comparative Studies in a Review by the Medical Services Advisory Committee.
| N | Baseline | Six months | Nine months |
One year | 18 months | Two years | |
|---|---|---|---|---|---|---|---|
| Ashy and Merded (1998) | |||||||
| VBG | 30 | 53.53 | 33.33 | ||||
| LAGB | 30 | 48.63 | 38.46 | ||||
| Statistically different? | Not assessable | Not assessable | |||||
| Suter et al. (1999) | |||||||
| VBG | 197 | 42.7 (n=197) | 31.3 (n=154) | 29 (n=110) | 29 (n=133) | 28.7 (n=84) | 29 (n=84) |
| LAGB | 76 | 45.5 (n=76) | 37 (n=64) | 337 (n=51) | 32 (n=47) | 31 (n=23) | 31 (n=19) |
| Statistically different? | Yes | Yes | Yes | Yes | Yes | Yes | |
(Reproduced with kind permission from the Medical Services Advisory Committee (MSAC). Laparoscopic adjustable gastric banding for morbid obesity. 2003. Commonwealth of Australia)
Length of Stay
For LAGB, the mean/median length of stay ranged from same-day discharge to 42 days, with most values falling around 1 to 5 days.
For VBG, the mean/median ranged from 3 to 45 days.
For RYGB, the mean/median ranged from 4 to 29 days.
Because there is substantial variation among the studies, probably reflecting the different techniques and experience of surgeons performing the procedure, the results suggest that the procedure with the longest length of stay was RYGB. This is expected, given the complexity and extent of the RYGB operation compared to that of LAGB and VBG.
Length of Procedure
For LAGB, the mean length of procedure ranged from 57 to 140 minutes in patients with previous gastroplasty, with most values falling around 60 to 90 minutes. The shortest reported time was 20 minutes, while the longest was 340 min.
For VBG, the mean was 60 to 135 minutes with a range of 40 to 125 minutes.
For RYGB, the mean ranged from 48 to 195 minutes. Most values fell between 100 and 150. The shortest reported time was 48 minutes, and the longest was 310 minutes.
Because there is substantial variation between studies, probably reflecting the different techniques and experience of surgeons performing the procedure, the results suggest that the longest procedure is RYGB. This is expected, considering the complexity of the operation compared to LAGB and VBG.
Quality of Life
Very few of the studies reported on QoL. Two of the comparative studies and 1 of the LAGB consecutive case series reported on QoL.
Most patients who had any bariatric procedure reported improved QoL. There did not appear to be any significant difference between QoL measures in patients with VGB or LAGB.
Band Adjustments
The number of band adjustments ranged from 0 to 6.
Adjustments usually were done to regulate weight loss or resolve postoperative complications.
MSAC Safety and Effectiveness Summary
Safety
Conclusions pertaining to the safety of LAGB were based on MSAC level III and level IV evidence.
Based on the available evidence, LAGB seems to be at least as safe as the comparators, VBG and open RYGB. LAGB appears to have a lower rate of mortality and reoperations than does VBG and RYGB procedures, but this could be an artefact of the shorter follow-up period for the LAGB patients.
Effectiveness
Conclusions pertaining to the effectiveness of LAGB are based upon level III and level IV evidence. No RCTs of LAGB versus open RYGB or VBG were identified.
LAGB is less effective that RYGB for weight loss. There is some evidence that RYGB patients may be happier with their procedure than LAGB patients. Length of hospital stay and length of procedure appear to be shorter in LAGB patients.
Based on the evidence, it appears that LAGB is as effective as VBG for weight loss. There is some preliminary evidence that weight loss lasts longer in patients who have LAGB, compared with VBG. There do not appear to be any significant differences on QoL measures, length of procedure, or length of hospital stay between VBG and LAGB.
There is no evidence that any 1 of the 3 procedures is significantly better at resolving obesity-related comorbid conditions than any of the other procedures.
Follow-up information on a very limited number of patients included in level IV articles indicates that weight loss can last up to 7 years after an LAGB procedure.
United States Centers for Medicare and Medicaid Services (CMS) (November, 2004)
The Centers for Medicare and Medicaid Services (CMS) in the United States has a national coverage determination that covers bariatric surgery if the surgery is done to correct an illness that caused the obesity or was aggravated by the obesity. CMS asked the Medicare Coverage Advisory Committee to make recommendations on the adequacy of the evidence for bariatric surgery in Medicare beneficiaries who have comorbid conditions (currently covered) and in Medicare beneficiaries who are obese, but who do not have comorbid conditions.
In a review of bariatric surgery, CMS concluded the following:
In general, for the outcomes of short- and long-term mortality, comorbid conditions, sustained weight loss, and complications from surgery, there is little or no data that would enable people with at least 1 comorbid condition to be compared to those with no such conditions.
In the general population, sustained weight loss may be an attainable goal. Combination or malabsorptive procedures lead to greater weight loss than restrictive procedures, which in turn lead to much more weight loss than no surgery. Sustained and sufficient weight loss may improve or resolve comorbid conditions. Short-term mortality is low, and the rates of short-term mortality are lower for experienced surgeons than for inexperienced surgeons. Longevity is shorter in persons with high BMIs and longer in patients who have had bariatric surgery if they survive to 1 year after the surgery. Laparoscopic procedures may have fewer complications than open ones.
CMS could not find any significant amount of data to apply these results to the Medicare population that is aged 65 or older.
CMS stated that there is a need for more high-quality studies on clinically important gaps in the scientific evidence. In particular, evidence is needed on short-term mortality, long-term survival, comorbid conditions, sustained weight loss, complications, and people older than 65. CMS also noted that a registry to track bariatric surgery patients warrants consideration.
United Kingdom National Health Service Research and Development Health Technology Assessment Program (NHS R&D HTA Programme) (2002)
Clegg et al. (5) systematically reviewed the clinical effectiveness and cost-effectiveness of surgery to manage morbid obesity. They developed a cost-effectiveness model using the best available evidence to determine cost-effectiveness in the United Kingdom. The following is a summary of the review.
Methods
Clegg and colleagues searched 16 databases from inception to October 2001. Studies were included if they fulfilled the following criteria:
Interventions: surgical procedures, performed either as open procedures or laparoscopically, including restrictive procedures such as gastroplasty (vertically banded or gastric banding), and malabsorptive procedures such as BPD, RYGB or jejunoileal bypass. The review concentrated on the clinical effectiveness of the different surgical interventions when compared with each other or with nonsurgical interventions (e.g., drugs, exercise).
Participants: patients diagnosed as morbidly obese, defined as a BMI > 40 kg/m2 or with BMI > 35 kg/m2 with a serious comorbid disease, in whom previous nonsurgical interventions had failed.
Outcomes: measures of weight change, fat content, fat distribution, QoL; perioperative and postoperative mortality and morbidity, revision rates and obesity-related comorbid conditions were primary outcomes at baseline and follow-up (minimum of 12 months).
-
Design:
Clinical effectiveness: systematic reviews of RCTS and RCTs comparing the surgical interventions with each other and with nonsurgical interventions; systematic reviews of prospective cohort studies; and prospective cohort studies comparing surgical procedures with nonsurgical treatment.
Cost-effectiveness: economic evaluations of surgery for people with morbid obesity that included a comparator (i.e., usual care) and both the costs and outcomes of treatment.
Results
Seventeen RCTs and 1 non-RCT were included. Two RCTs and the non-RCT compared surgical interventions with nonsurgical treatment. The remaining 15 RCTs compared different types of surgery. The quality of the included studies varied (Table 34).
Table 34: The Methods and Quality of Studies Included in the Assessment of Clinical Effectiveness.
| Study | Random assignment | Proper sampling | Sample size | Objective outcomes | Blind assessment | Eligibility criteria | Attrition reported | Comparable groups | Generalizable results | |
|---|---|---|---|---|---|---|---|---|---|---|
| Surgery vs nonsurgical interventions | ||||||||||
| Andersen et al (1988)18,19 | NR | ✓ | ✓ | ✓ | × | ✓ | ✓ | ✓ | ✓ | |
| Danish Obesity Project20-22 | NR | NR | NR | ✓ | NR | ✓ | ✓ | Sub | ✓ | |
| Swedish obese subjects23-31 | × | × | NR | ✓ | n/a | ✓ | ✓ | × | ✓ | |
| Comparison of different surgical procedures | ||||||||||
| Gastric bypass vs gastroplasty | ||||||||||
| Hall et al (1990)53 | ✓ | ✓ | ✓ | ✓ | NR | ✓ | ✓ | ✓ | ✓ | |
| Howard et al (1995)23 | NR | NR | NR | ✓ | NR | ✓ | ✓ | ✓ | ✓ | |
| Laws et al (1981)46 | ✓ | ✓ | NR | ✓ | NR | Sub | × | Sub | ✓ | |
| Lechner et al (1981)34 | NR | NR | NR | ✓ | NR | ✓ | × | ✓ | ✓ | |
| MacLean et al (1995)35,36 | NR | NR | NR | ✓ | NR | × | ✓ | ✓ | ✓ | |
| Naslund et al (1988)47-52 | ✓ | ✓ | NR | ✓ | NR | ✓ | ✓ | ✓ | ✓ | |
| Pories et al (1982)41 | ✓ | NR | NR | ✓ | ✓ | Sub | ✓ | ✓ | ✓ | |
| Sugerman et al (1987)40 | ✓ | NR | NR | ✓ | NR | ✓ | ✓ | ✓ | ✓ | |
| Gastric bypass vs jejunoileostomy | ||||||||||
| Buckwater et al (1980)39,43,44 | ✓ | NR | NR | ✓ | NR | ✓ | × | ✓ | ✓ | |
| Griffen et al (1977)32 | Sub | NR | NR | ✓ | NR | ✓ | × | ✓ | ✓ | |
| Vertical banded gastroplasty vs horizontal gastroplasty | ||||||||||
| Andersen et al (1987)45 | ✓ | ✓ | NR | ✓ | NR | Sub | × | Sub | Uncertain | |
| Vertical banded gastroplasty vs adjustable gastric banding | ||||||||||
| Nilsell et al (2001)38 | ✓ | NR | NR | ✓ | × | ✓ | ✓ | ✓ | ✓ | |
| Open vs laporoscopic gastric bypass | ||||||||||
| Nguyen et al (2001)34 | ✓ | ✓ | ✓ | ✓ | × | ✓ | ✓ | ✓ | ✓ | |
| Westling et al (2001)37 | ✓ | Sub | NR | ✓ | Sub | ✓ | ✓ | × | ✓ | |
| Open vs laparoscopic adjustable silicone gastric banding | ||||||||||
| De Wit et al (1999)42 | ✓ | NR | ✓ | ✓ | NR | ✓ | ✓ | ✓ | ✓ | |
✓ = yes; × = no; NR = not reported; sub = substandard or incomplete.
(Table reproduced with kind permission from the National Coordinating Centre for Health Technology Assessment: Clegg A, Colquitt J, Sidhu M, Royle P, Walker A. Clinical and cost effectiveness of surgery for morbid obesity: a systematic review and economic evaluation. Health Technol Assess 2002; 6(12).
Of the original 18 studies, 7 lacked an adequate description of the method of allocation, 12 either did not discuss or reported inappropriate sampling methods, and 14 did not provide a sample size or power calculation.(5) All 18 studies had objective outcome measures; however, only 1 study adequately blinded their assessment. In total, 14 studies used eligibility criteria to include patients, and 13 studies had comparable patient groups at the baseline assessment. Attrition was adequately reported in 13 studies, and results were “thought” to be generalizable in 17 studies. (5)
Clinical Effectiveness of Surgery Compared with Nonsurgical Management
Three studies comparing surgery with nonsurgical management assessed different interventions: horizontal gastroplasty and diet compared with a very low calorie diet; jejunoileal bypass with medical management; and either VBG, gastric banding, or gastric bypass with nonsurgical management (Table 35). After 2 years, patients in all of the studies had statistically significant weight loss after surgery, compared with those who did not have surgery. They lost between 23 and 37 kg more weight. Two of the studies assessed weight loss beyond 2 years, and one found a statistically significant 21 kg weight loss had been maintained to 8 years after the surgery.
Table 35: Surgical Versus Nonsurgical Treatment for Morbid Obesity: A Summary of the Evidence on Effectiveness.
| Author, year and study details | Weight Change | QoL/comorbidities | Complications |
|---|---|---|---|
| Andersen et al., (1984, 1988)34,30 | Net weight change: | Not assessed. | Re-operations: |
| 12 months VLCD 18kg; GP 23kg(p=ns) | None of GP patients were re-operated. | ||
| 18 months VLCD 10.5kg; GP 18.5kg | Perloperative complications (GP only, n–27): subphrenic abscess 7%; atelectasis/pneumonia 4%; wound infection 4%. | ||
| Design: | (p=ns). | ||
| RCT (Single centre | 24 months VLCD 9kg; GP 32kg; p<05). | ||
|
Intervention: Gastroplasty (GP) (Gomez horizontal) + diet (500keal, 34g protein daily)(n–27) Very low calorie diet (VLCD): cycles of 8 weeks (341 keal) and 2 weeks (900 keal)(n–30) Patients: at least 60% overweight(N–57) |
Less than 40% overweight: 24 months: GP 58% (95%CI: 28, 85%), VLCD 17% (95%CI: 0, 34% p<0.05). Success defined as a net weight loss of ≥10kg (60 months): GP 30% (95%CI:25, 50%), VLCD 17% (95%CI:6,35%). Median (range) weight loss of patients with ‖success‗ 60 months: GP 18.2Kg(14.2-50.3Kg), VLCD 26.8kg (13.0-38.2kg)p=ns. Cumulated success rate (5-6 years): GP (n=8) 16% (95%CI: 11, 21%) VLCD (n=8) 2% (95%CI: 1,3%)p<0.05. |
Later complications (GP (n–27) vs VLCD (n–30)) thrombophebitis (4% vs 0%); nausea (15% vs 7%); heartbum (11% vs 0%); ruetus (1% vs 0%); pain projected to left shoulder (15% vs 0%); epigastric pain (22% vs 10%); outlet obstruction (4% vs 0%); vomiting (52% vs 0%, p<0.05); cholesystectomy (7% vs 0%); obstipation (26% vs13%); orthostatic hypotension (7% vs 27%); dizziness (7% vs 17%); transient loss of hair (15% vs 10%); head ache (11% vs 17%); fatigue (30% vs 53%); irritability and low spirits (0% vs 33%, p<0.05); gout (0% vs 3%); staple line rupture (4% vs 0%), ventral hernia (4% vs 0%) 2(6%) VLCD patients had GP elsewhere having regained all weight lost on diet |
|
| Danish Obesity Project Stokholm et al., (1982)58 Backer et al., (1979)59 Quaade et al., (1977)60 Design: RCT (14 centres) Intervention: Medical management (n–66) Jejunoileostomy (end-to-side)(n-130) Patients: at least 80% overweight (N–196) |
Medium weight loss(range) 24 months: Medical 5.9kg(-11.9-40.4) Surgical 42.9Kg (20.5-108.5)p<0.001. Body weight at maximal body weight change (MBW)(at median 24 months, range 12-48), median and 5%-95% percentiles: Surgical: baseline 124.0kg (104.2-164.9) MBW 81.2kg (64.0-103.9)p<0.0001. Medical: Baseline 129.0kg (104.6-166.3) MBW 199.0kg(74.3-159.0)p<0.0005. |
Quality of Life (> 15 months post randomisation)(Medical vs Surgical): Somatic symptoms: dyspnoea 42% v 14%a, precordial pain 21% v 7%, heartburn 38% v 14%a, abdominal pain 54% v 87%a, flatulence 40% v 93%a, anal complaints 17% v 40%b, low back pain 63% v 41%b, pain hips/knees/ankles 67% v 22%a, excessive sweating 54% v 15%a, heat intolerance 69% v 22a, cold intolerance 6% v 39%a, dermal irritation/rashes 77% v 16%a. Psychological symptom: excessive fatigue 69% v 41%a, periodic depression 62% v 36%b, periodic irritability 71% v 41%, insecurity 65% v 40%b, inferiority/insufficiency 65% v 37%a, isolation 35% v 14%b, exposure to contempt 69% v 21%a. Social factor: exercise daily 35% v 55%c, participates in organised sport 12% v 26%c, normal sex life 52% v 78%b, wear ready made clothes 46% v 96%b, socially satisfied 52% v 76%b, sexually satisfied 48% v 82%b. a p=0.00; b p=0.01; c p=0.06 BP at MBW, median and 5%. 95% percentiles: Surgical Systolic: baseline 140mmHg (116-180), MBZ 120mmHg (105-150)o<0.0001. Diastolic: baseline 85mmHg (70-109) MBW 80mmHg (60-99) p<0.0001. Medical Systolic: baseline 140mmHg(118-197), MBW 140mmHg (110-187p=ns.) Diastolic: baseline 90mmHg (67-122), MBW 90mmHg (70-100)p=ns. |
No surgical deaths (95% CI 0-2.7%). 2 deaths in medical (1 complications of liver biopsy, 1 after bypass surgery 4 years after medical treatment). Surgical: 3% pulmonary complications, 6% wound infection or dehiscence. 1.5% severe but transient hepatic dysfunction. Other complications encountered but not reported. Intestinal continuity re-established in 0.7%. |
| Swedish Obese Subjects 1. Sjostrom et al., (2001)70 2. Sjostrom(2000)60 3. Karason (2000)65 4. Sjostrom (1999)68 5. Karason (1999)65 6. Narbro (1999)67 7. Karason (1999)64 8. Karisson (1997)66 9. Karason (1997)62 10. Torgerson (2001) (overview)71 Design: Multi-centre (25 surgical and 480 non-surgical) Cohort study with matched controls. Intervention: Surgical: a. Vertical banded gastroplasty (VBG) b. GaStric banding (GB) c. Gastric banding (GB) Controls: conventional treatment. Patients: BMI ≥38kg/m2 women,≥34kg/m2 men |
Weight (kg), surgical (n=1210) vs control (n=1099): Baseline: difference 7kg (9.5%)CI 5.7, 8.3). 24 months: difference –21kg (95% CI–23, –19). Weight loss after 24 months: surgical: 28 kg (23%), Control: unchanged, p<0.001. Weight changes at 8 years: (surgical n=232, control n=251) Baseline: surgical 120.4kg (SD 16.0), control 114.7kg (SD 17.8), control 115.4 (SD 19.2). Difference in weight change between groups at 8 years: 20.7kg (p<0.001). Relative weight change at 8 years: surgical=-16.3% (SD 12.3%), control=0.9%(SD 10.8%). Weight at 8 years: GBP 92kg vs VBG 100kg (p=ns) vs GB 103kg (p<0.05). All had a larger weight reduction than controls (p<0.01). |
Health Related Quality of Life (HRQoL): Current health perception GHRI/CH (mean; 95%CI): Baseline–surgery 26.9 (26.1,27.7); control 29.4 (28.5, 30.2). 2 years – surgery 34.3 (33.4, 35.1); control 30.2 (29.4, 31.1). Psychosocial functioning OP change by 2yrs (mean, 95% CI): surgery–males–1.01(-1.14, -0.87), females–1.10(-1.19,-1.00); control–males–0.07(-0.17,0.03)(p=0.001); females–0.16(-0.22, -0.09)(p=0.001). SIP/SI change by 2 yrs (mean, 95% CI): surgery–males–3.3 (-5.0, -1.5), females –5.2 (-6.5, -4.0); control–males 1.5 (0.2, 3.2)(p=0.001): females 1.2 (0.2, 2.2)(p=0.0001). Mental well-being scales MACL change by 2 yrs (mean, 95% CI): Pleasantness/unpleastness Surgery 0.21 (0.16,0.26); control –0.04 (-0.09,0.01) (p=0.001); Calmness/tension Surgery 0.20(0.15,0.26); control –0.01(-0.06,0.04) (p=0.001). HAD change by 2 yrs (mean, 95% CI): Anxiety Surgery –1.7(-2.0, -1.4); control –0.6(-0.9,-0.2) (p=0.0001); Depression Surgery –2.2 (-2.5-1.9); control -0.4(-0.6, -0.1) (p=0.0001); At 24 months: Improvement in surgical vs. controls on all HQRL measures (p<0.0001). Changes in all HRQoL measures significantly related to magnitude of weight loss. Diabetes: 2-year unadjusted incidence: controls 4.7%, surgical 0.0% (p=0.0012). 8-year unadjusted incidence: controls 18.5%, surgical 3.6% (p=0.0001). Adjusted odds ratios of developing diabetes, 8 years: Completers (n=437)0.17(95% CI 0.08-0.38). All(ITT)(n=611)0.16(95% CI 0.07-0.36). Hypertension: 2-year unadjusted incidence: controls 9.9%, surgical 3.2% (p=0.032). 8 year unadjusted incidence controls 25.8%, surgical 26.4% (p=0.91). Adjusted odds ratios of developing hypertension, 24 months: Completers(n=257)0.27(95% CI 0.07, 0.99). All(ITT)(n=377)0.27(95% CI 0.09, 0.76) Adjusted odds ratios of developing hypertension, 8 years: Completers(n=257) 1.05 (95% CI 0.58-1.89). All (ITT) (n=377) 1.01 (95% CI 0.61-1.67). Lipids: Adjusted odds ratios at 24 months (95% CI): Hypertrigly ceridaemia 0.10 (95% CI 0.04, 0.25)p<0.001. Hypo HDL-cholesterolemia 0.28 (95% CI 0.16, 0.49) p<0.001. Hypercholesterolemia 1.24 (95% CI 0.84, 1.8)p-ns. Relative risks for recovery from disease: Hyperinsulinaemia (n=221) 1.4 (95% CI 1.2, 1.7)p‼0.00001. Hypertrigly ceridaemia (n=314) 1.9(95% CI 1.5,2.4) p<0.00001. Hypo HDL-cholesterolaemia (n=216) 1.7 (95% CI 1.4, 2.1) p<0.00001. Hypercholesterolaemia (n=531) 1.2 (95% CI 0.95, 1.5) P=ns. |
Postoperative mortality, 4(0.2%) deaths, 3 due to leakage detected too late and 1 due to a technical laparoseopic mistake. Pertoperative complications: 13% experienced complications. Bleeding 0.9%, thrombo-embolic events 0.8%, wound complications 1.8%, abdominal infection 2.1%, pulmonary symptoms 6.2%, miscellaneous 4.8%. Re-operation: 2.2%. |
(Table reproduced with kind permission from the National Coordinating Centre for Health Technology Assessment: Clegg A, Colquitt J, Sidhu M, Royle P, Walker A. Clinical and cost effectiveness of surgery for morbid obesity: a systematic review and economic evaluation. Health Technol Assess 2002; 6(12).
Two of the 3 studies assessed the effects of surgery and nonsurgical management on QoL and comorbid conditions. QoL improved significantly after surgery, compared with no surgery, on many somatic symptoms, psychological symptoms, and social factors at 15 months (P < .05), and on all health-related measures at 2 years. The effects on diabetes were maintained at 8 years. No deaths were reported. Complications from surgery were wound infection and subphrenic abscess. Vomiting was a side effect. Some patients required another operation or a reversal of the procedure (Table 35, next page).
Clinical Effectiveness of Different Surgical Procedures
Gastric Bypass Versus Gastroplasty
Eight RCTs compared gastric bypass with different types of gastroplasty: 3 with VBG, 4 with horizontal gastroplasty, and 1 with vertical gastroplasty and gastrogastrostomy. Seven of the 8 RCTs showed that gastric bypass led to significantly greater weight loss than from gastroplasty. On average, those who had the procedure lost an additional 6 to 12 kg (Table 35). In 4 RCTs, the differences in weight loss remained significant to 3 and 5 years.
None of the RCTs assessed the effect of surgery on QoL. Three assessed the effect of surgery on comorbid conditions at either 2- or 3-year follow-up, and found improvements in diabetes, hypertension, joint pain, and asthma. None of the RCTs reported data on perioperative deaths; however, 3 RCTs reported 5 postoperative deaths after gastric bypass and 1 death after horizontal gastroplasty. Complications were common after all of the surgical procedures; however, dumping syndrome and heartburn were reported more often after gastric bypass than after gastroplasty. Revisions, reoperations, and/or conversions were more common after gastroplasty (VBG, 2%–53% of patients) than after gastric bypass (0%–39% of patients).
Vertical Banded Gastroplasty Versus Adjustable Gastric Banding
One RCT was identified. At 5 years, there was no significant difference in weight loss between the procedures. QoL and comorbid conditions were not assessed. One postoperative death was reported after both VBG and adjustable gastric banding. There were few differences in complications between procedures. About one-third of patients who had VBG had another operation because of staple line disruption or strictures of the stoma. Ten percent of patients who had adjustable gastric banding had another operation because of gastric pouch dilation.
Open Versus Laparoscopic Gastric Bypass
Two RCTs were identified. Neither found a statistically significant difference in weight loss between the procedures. Early differences in QoL were assessed using the Short Form Health Survey (SF-36) at 1 month and the Moorehead-Ardelt questionnaire at 3 months. Results favoured laparoscopic gastric bypass, but these differences disappeared at later follow-up (3 and 6 months respectively).
There was 1 postoperative death after laparoscopic gastric bypass surgery. There were limited differences between the procedures on major, minor, and late complications. Reoperations were more common after laparoscopic procedures. Laparoscopic procedures took longer; however, they caused significantly less blood loss, required shorter intensive care unit stay, shorter hospital stay, and shorter time to return to activities of daily living and work (Table 35).
Open Surgery Versus Laparoscopic Adjustable Silicone Gastric Banding
One RCT was identified. Both procedures resulted in statistically significant weight loss at 1-year follow-up (about 35 kg); however, there was no statistically significant difference between the procedures. Readmissions and overall length of stay were significantly higher for people who had open, rather than laparoscopic, surgery. A few laparoscopic patients were converted to open procedures (Table 35).
Summary of Benefits
Bariatric Surgery Versus Nonsurgical Treatment
Compared with nonsurgical treatment, surgery resulted in significantly more weight loss (23–37 kg more weight), which was maintained at 8 years. QoL and comorbid conditions improved (Table 35).
Comparison of Different Types of Bariatric Surgery
Gastric bypass seemed more beneficial with greater weight loss (6–14 kg more) and/or improvements in comorbid conditions and complications compared with either gastroplasty or jejunoileal bypass.
Open Surgery Versus Laparoscopic Surgery
Assessment of open versus laparoscopic gastric bypass and adjustable silicone gastric banding showed fewer serious complications with laparoscopic surgery. Laparoscopic surgery took longer, but resulted in less blood loss, fewer patients requiring intensive care unit stay, shorter length of hospital stay, and fewer days to return to the activities of daily living and to work.
Recommendations for Further Research
Clegg et al. (5) noted the following:
There is little good-quality evidence on the epidemiology of morbid obesity or on assessing the clinical effectiveness and cost-effectiveness of surgery for people with morbid obesity.
Few trials compared surgery with nonsurgical interventions or different restrictive procedures with malabsorptive procedures. This may be because different centres specialize in particular procedures and are restricted by financial and other resource constraints.
More epidemiological studies on morbid obesity, as well as RCTs and “quasi-experimental studies” of good quality that compare different operative techniques, are needed.
The outcomes of surgery generally were assessed over relatively short periods, usually up to 12 months. Only 1 study considered the long-term effects of surgery compared with conventional treatment beyond 5 years; therefore, there is a need for good-quality, long-term RCTs and non-RCTs.
Three studies assessed the effects of surgery on the QoL of patients. More studies are needed.
Four economic evaluations that assessed the cost-effectiveness of surgery for morbid obesity were identified. More good-quality economic evaluations are needed. This means good-quality costing data and information on the epidemiology of comorbid conditions needs to be compiled.
Australian Safety and Efficacy Register of New Interventional Procedures-Surgical (2002)
The aim of the systematic review by the Australian Safety and Efficacy Register of New Interventional Procedures-Surgical (ASERNIP-S) was to assess the safety and efficacy of LAGB, compared with the more established operations of VBG and gastric bypass.(25)
Methods
Databases were searched from 1988 to August 2001.
The inclusion criteria were as follows:
Patients with morbid obesity (BMI > 35 kg/m2)
Intervention was LAGB and included the Lap-band or the Swedish Gastric Band
Comparator intervention was RYGB and/or VBG
RCTs, controlled clinical trials, and prospective case series; for VBG, case series were only considered if they were part of multicentre trials or if patients were followed-up for more than 5 years and/or there were more than 500 patients.
Data must have been reported on at least 1 of the following outcomes of the intervention: Weight loss
Kilograms/pounds lost or gained
Change in BMI
Change in excess weight
Sustainability of weight loss over 5 years
Complications including, but not limited to, these:
Displacement of adjustable band
Vomiting
Erosion of band into adjacent tissue
Infection of band or its reservoir
Systemic infection
Respiratory complications
Pulmonary embolism
Psychosocial effects
Change in comorbid conditions
Revision rates
Mortality rates
Cost-effectiveness
Results
Six studies reported comparative results for laparoscopic gastric banding and other surgical procedures. One reported comparative results for all 3 procedures. This study was of moderate quality. In total, 64 studies reported results for LAGB, and 57 studies reported results on the comparative procedures. LAGB was associated with a mean short-term mortality rate of 0.05% and an overall median morbidity rate of 11.3%, compared with 0.50% and 23.6% for gastric bypass, and 0.31% and 25.7% for VBG.
The comparative studies suggested that gastric bypass produced superior weight loss outcomes than either of the other 2 procedures, at least up to 2 years. Beyond 2 years, gastric bypass still had superior weight loss outcomes to VBG.
Table 36: Studies Comparing Laparoscopic Adjustable Band Surgery With Vertical Banded Gastroplasty and/or Roux-en-Y Gastric Bypass.
| Study | LoE | Procedures | N | Follow up | Weight loss data | Statistics |
|---|---|---|---|---|---|---|
| Ashy & Merdad2 | II | LAGB VBG |
30 30 |
6 months | BMI 38.46; EWL: 50% BMI 33.33: EWL: 87% |
None |
| Hell er al.4 | III-2 | LAGB VBG RYGB |
30 30 30 |
39.7±7.6 months 40.2±8.3 months 60±8.2 months |
BAROS weight loss points 1.5 BAROS weight loss points: 1.6 BAROS weight points 2.7 |
RYGB v LAGB and VBG, p<0.05. |
| Wolf et al.6 | III-2 | LAGB VBG RYGB |
50 115 |
20 months | *88|54|18|6 *87|65|32|11 |
NS^^ |
| Fried et al.11 | III-3 | SAGB VBG |
15 52 |
24 months | Weight lost: 37.2 kg Weight lost: 40.5kg |
NS |
| Sutcr et al.15 | III-3 | LAGB VBG |
76 197 |
24 months | BMI: higher; EWL: loss; %IW: more BMI: lower; EWL: more, %IW: less |
All NS at 24 months.** |
| Toppino et al.16 | III-3 | LAGB VBG |
361 120 |
12 months | EWL; 41,9% EWL: 58% |
None |
LoE = Level of Evidence; BMI = body mass index; EWL = excess weight lost; IW = Ideal weight; * = % losing 25% | 50% | 75% | 100% of excess weight; NS = no significant statistical difference; ** = but significant differences were found in favour of VBG at earlier time periods; ^^ = statistical tests were not performed in the original study, but were performed post hoc as part of this review (LAGB v VBG, Fisher’s P, 2 tailed, p=1.000 for 25% excess weight lost, p=0.222 for 50% excess weight lost, p=0.088 for 75% excess weight lost, p=0.396 for 100% excess weight lost).
(Table reproduced with kind permission from Australian Safety and Efficacy Register of New Interventional Procedures-Surgical (ASERNIP-S): Chapman A et al. Systematic review of laparoscopic adjustable gastric banding for the treatment of obesity: Update and re-appraisal. ASERNIP-S Report No. 31, Second Edition. Adelaide, South Australia: ASERNIP-S, June 2002).
All 3 procedures resulted in considerable weight loss up to 4 years after LAGB (the maximum follow-up available at the time of the review), and more than 10 years after the comparator procedures.
The authors concluded that the evidence base is of average quality up to 4 years for LAGB. Laparoscopic gastric banding is safer than VBG and RYGB, in terms of short-term mortality rates.
Laparoscopic gastric banding is effective at least up to 4 years, as are VBG and RYGB. Up to 2 years, the laparoscopic gastric band results in less weight loss than does RYGB. From 2 to 4 years, there is insufficient evidence to conclude that RYGB remains more effective than laparoscopic gastric banding.
The long-term effectiveness of laparoscopic gastric banding is unproven. The ASERNIP-S Review Group recommended more evaluation by RCT to define its merits relative to VGB and RYGB.
Alberta Heritage Foundation for Medical Research (2000)
The Alberta Heritage Foundation for Medical Research (AHFMR) examined the evidence from the published scientific literature regarding the safety, efficacy, and effectiveness of LAGB.
According to AHFMR:
Gastric bypass (RYGB) is considered the gold standard to treat morbid obesity.
VBG is attractive because it preserves gastroduodenal continuity and avoids potential micronutrient deficiency. However, it tends to fail in “sweet” eaters that adapt to their reduced intake by snacking frequently on soft or liquid foods high in sugar.
-
Future research should address the following questions:
Is there a subgroup of obese patients in which this method can be used as an alternative to the gold standard of care?
If there is such a subgroup, can these people be identified preoperatively?
AHFMR concluded the following:
The clinical studies evaluated for this report were done in hospitals. It is not possible right now to determine if LAGB can be offered to the morbidly obese population outside a hospital setting..
Early attempts at LAGB show a high rate of complications and reoperations.
Only well-designed studies that follow-up people for more than 5 years will determine if LAGB will replace the current standard of care or become part of mainstream treatment for morbid obesity.
Technology Assessment Unit of the McGill University Health Centre (2004)
Chen and McGregor (26) systematically reviewed the safety and efficacy of LAGB compared with laparoscopic RYGB. They said they chose laparoscopic RYGB as the comparator because it is the preferred procedure for bariatric surgery in North America and at the McGill University Health Centre.
The authors used the ASERNIP-S report of the LAGB procedure and updated the Australian report by searching the literature between May 2001 and February 2004. They found 19 studies.
Results
No RCTs of these 2 procedures were available. The evidence came from cohort follow-up studies of varying quality and duration and with variable results. The outcomes of interest were as follows:
Weight loss
Conversion rate
Surgical mortality rate
Surgical morbidity rate
Comorbid conditions
QoL
Chen and McGregor concluded that there is sufficient evidence to indicate that LAGB is an effective procedure with an adequate safety record of up to 5 years. However, they then noted, “There are no randomized comparisons of the two procedures and there is insufficient evidence on which to decide whether LAGB is a superior procedure or not. However according to expert opinion there are some occasions on which it would be a significantly safer procedure than laparoscopic RYGB. Accordingly, it should become an accepted bariatric option within the MUHC, and the Quebec Healthcare system.” (26)
It is unclear how the authors arrived at this conclusion from their systematic review. Furthermore, there is lack of detail regarding the occasions when LAGB would be safer than laparoscopic RYGB according to the expert reviewer used for the review by Chen and McGregor.
Canadian Coordinating Office for Health Technology Assessment (2003)
In a preassessment of LAGB for clinically severe obesity, the Canadian Coordinating Office for Health Technology Assessment (CCOHTA) (11) concluded the following:
Long-term outcomes data on the effectiveness and safety of LAGB are needed.
Several health technology assessment agencies are reviewing the technology, and several have recently published assessments on surgical interventions for morbid obesity; therefore, CCOHTA will not review this topic now.
National Agency for Accreditation and Evaluation in Health (France; 2002)
The National Agency for Accreditation and Evaluation in Health (ANAES) reviewed the 3 main procedures performed in France: insertion of adjustable gastroplasty rings, VBG, and gastric bypass.
ANAES concluded the following:
“The gastroplasty ring technique I the simplest which gives it advantages in relation to the surgery itself. It also has the advantages of being closer to the normal physiological situation and of being reversible. However, it seems to be slightly less effective in terms of weight loss than VBG and gastric bypass. In addition, lack of follow-up means that its long-term benefits have not yet been evaluated.
All 3 techniques may involve complications common to any type of surgery, together with complications specific to the procedure. These are rarely severe but often require revision surgery.
In view of the inadequate long term evaluation of either efficacy or inherent risk of gastroplasty rings (notably risks relating to how the prosthetic material is tolerated, and risk of migration of the ring into the stomach), the working group was concerned about the extensive and unevaluated diffusion of this technique which is currently taking place.”
Furthermore, ANAES suggested future investigation:
“Evaluation of surgical techniques for obesity needs to be continued, and in particular a more precise evaluation of adjustable gastroplasty rings needs to be conducted.
Trials should:
Be long term. It is important to have a follow-up period equivalent to that reported in some trials of gastric bypass (10–15 years).
Be controlled prospective trials. This should include comparison of insertion of gastroplasty rings with older techniques (VBG, gastric bypass).
Ideally compare laparoscopic procedures in view of their increasing use in this type of surgery.
Include an economic arm which would address direct and indirect costs incurred by the management of obesity and the issue of comorbidities.
The incidence of complications has to be known and needs to be better evaluated from exhaustive recording of complications. It should be possible to reduce their incidence.
QoL should also be better evaluated as such an evaluation is a fundamental criterion entering into the assessment of both surgery and medical treatment.
The insertion of gastroplasty rings is increasing in France. The indications for their insertion need to be complied with and the procedure to be used for followup of surgery needs to be defined. Information derived from medical device vigilance reporting may also be useful in documenting the safety of these devices.”
Institute for Clinical Systems Improvement (2000)
Based on its review (27) of the recent evidence of bariatric surgery, the Institute for Clinical Systems Improvement’s (ICSI) technology assessment committee in the United States came to several conclusions. The committee determined the conclusion grades based on the following definitions:
Grade 1: The evidence consists of results from studies of strong design for answering the questions addressed. The results are clinically important and consistent with minor exceptions at most. The results are free of serious doubts about generalizability bias and flaws in research design. Studies with negative results have sufficiently large samples to have adequate statistical power.
Grade 2: The evidence consists of results from studies of strong design for answering the question addressed, but there is uncertainty attached to the conclusion because of inconsistencies among the results from different studies or because of doubts about generalizability, bias, research design flaws, or adequacy of sample size. Alternatively, the evidence consists solely of results from weaker designs for the question addressed, but the results have been confirmed in separate studies and are consistent with minor exceptions at most.
Conclusions:
Gastric surgery may be considered for patients aged 18 years or older with a BMI > 40 kg/m2 or a BMI > 35 kg/m2 with comorbid conditions and who have failed medical therapy.
VBG and RYGB are the surgeries being done in the United States. Both are generally safe (mortality < 1.5% at experienced centres).
There is evidence that both procedures result in weight loss that may be sustained for 7 years or longer. (Grade 2)
While many of the studies are case series, there are also prospective cohort studies.
There are clinical trials showing improvements in glucose tolerance, forms of hyperlipidemia, hypertension, and arthritis. (Grade 1)
There remains a need for long-term trials to demonstrate long-term survival benefit and long-term maintenance of weight loss and reversal of comorbidities.
Although some reports suggest that RYGB may be more durable than VBG to maintain weight loss and reverse comorbid conditions, there are insufficient data to support strongly that suggestion.
Patients who have VBG or RYGB should be followed-up by multidisciplinary teams with lifelong medical surveillance for nutrient deficiencies and medical complications.
Conseil d’Evaluation des Technologies de la Sante du Quebec (1998)
Conseil d’Evaluation des Technologies de la Sante du Quebec (CETS) examined the effectiveness of surgery to reduce weight in severely obese people (translation from French).
Four main types of surgical treatments were used in Quebec: RYGB, VBG BPD with distal gastrectomy, and BPD with parietal gastrectomy.
RYGB and VBG were considered “accepted” techniques.
BPD with distal gastrectomy was favoured less than the RYGB and given the status of an “innovative” technology, because there was limited information on the technique. The procedure caused significant loss of excess weight; however, the risks of protein, vitamin, and mineral deficiencies were considerable.
CETS concluded that it appears justified to support surgery as long as it is well delineated to reduce risks and optimize chances for success. Therefore, the treatment must be done in a specialized centre with an infrastructure that allows various measures such as these to be applied:
Implementing a rigorous selection process (e.g., patients whose BMI is > 40 or 35 kg/m2 with comorbid conditions where the risk of the operation is acceptable; patients who are motivated and informed of the risks and the need to be followed-up for the rest of their lives) and a priority system for those waiting
Requiring experienced surgeons (those that do it regularly) do the operation and are supported by a multidisciplinary team with medical, surgical, psychological, and nutritional expertise
Requiring specific long-term follow-up of patients (e.g., rigorous dietary follow-up and keeping a registry of outcomes). The latter is especially helpful to allow comparisons to be made.
CETS concluded that RYGB and VBG do not need to be subject to any criteria other than those specified above. However, BPD with distal gastrectomy should be restricted to certain hospitals, those with the necessary resources and knowledge to allow for the collection and analysis of information.
Summary of Medical Advisory Secretariat Review
The Medical Advisory Secretariat searched MEDLINE and EMBASE from April 2004 to September 2004, after the search cut-off date of April, 2004, for the most recent systematic reviews on bariatric surgery. Ten studies met the inclusion criteria. The quality of the included articles is shown in Table 37.
Table 37: Quality of Evidence of Studies in the Medical Advisory Secretariat’s Review of Bariatric Surgery.
| Study Design | Level of Evidence | Number of Eligible Studies |
|---|---|---|
| Large RCT, * systematic reviews of RCT | 1 | |
| Large RCT unpublished but reported to an international scientific meeting | 1(g)* | |
| Small RCT | 2 | |
| Small RCT unpublished but reported to an international scientific meeting | 2(g) | |
| Non–RCT with contemporaneous controls | 3a | 1 |
| Non–RCT with historical controls | 3b | |
| Nonrandomized study presented at international conference | 3(g) | |
| Surveillance (database or register) | 4a | 1 |
| Case series (multisite) | 4b | |
| Case series (single site) | 4c | 8 |
| Retrospective review, modeling | 4d | |
| Case series presented at international conference | 4(g) |
RCT refers to randomized controlled trial; g, grey literature
Summary of Findings
Swedish Obese Subjects Registry and Intervention Study: 10-Year Outcomes
The SOS study (1) started in 1991 as a registry and an intervention study of obese patients in Sweden (level 3a evidence). Sjostrom et al. reported follow-up data for patients who had been enrolled for at least 2 years (4,047 patients) or 10 years (1,703 patients).
Registry Study
Seven to ten thousand obese people participated in a health examination at about 750 primary health care centres in Sweden. The aims of the registry study were to do the following:
Trace obese people and offer them examination and treatment
Describe the body composition, adipose tissue distribution, metabolic aberrations, food habits, psychological and socioeconomic variables of these obese people
Determine the extent to which obesity is explained by genetic and by cultural factors
Create a recruitment base for the intervention study
The inclusion criteria for the registry study were as follows:
Age 37 to 57 years
Completed questionnaires on medical history
BMI of ≥ 34 kg/m2 for men and ≥ 38 kg/m2 for women
Intervention Study
The surgical techniques were gastric banding, VBG, and gastric bypass. The surgical group was recruited from the registry study and from pre-existing waiting lists at participating surgical departments
For each surgical patient, a computerized matching procedure selected the optimal control patient from the registry after considering 18 variables. All surgical and nonsurgical patients enrolled in the SOS study returned for complete medical examinations at 3 and 6 months and at 1, 2, 3, 4, 6, 8, and 10 years after surgery or inclusion.
The primary aim of the intervention study was to determine if the 10-year mortality and morbidity rates among a surgically treated group of obese patients differed from those of a conventionally treated group that was not expected to have sustained weight loss. Myocardial infarction, cerebrovascular disease, diabetes, and gall bladder disease were of interest in the analyses of morbidity and cause-specific mortality. If they found that weight reduction after surgery was associated with improved longevity and health, the secondary aim was to estimate how much weight must be lost to achieve this effect.
The inclusion criteria for the intervention study were as follows:
Age 37 to 60 years
BMI ≥ 34 kg/m2 for men, and ≥ 38 kg/m2 for women
The exclusion criteria were as follows:
Previous weight reduction surgery
Previous gastric operations, or a gastric or duodenal ulcer in the last 6 months
Active malignancy in the last 5 years
Myocardial infarction in the last 6 months
Bulimic eating pattern
Abuse of alcohol or drugs
Psychological problems suspected to result in poor cooperation
Regular use of cortisone or nonsteroidal antiinflammatory drugs
“Other severe illnesses”
At least 10 years before the analysis, 851 surgically treated patients had been enrolled in the SOS study. These patients were contemporaneously matched with 852 obese control patients. At the time of matching, compared with control patients, the surgically treated patients:
Were younger (46.1 years versus 47.4 years; P = .005)
Were heavier (119.2 kg, versus 116.1 kg; P < .001)
Had a higher mean plasma insulin level (22.8 mU per litre, versus 20.9 mU per litre; P = .009)
Weight Change
Weight change was greatest after 6 months for control patients (-1%, SD, 6%) and after 1 year for patients in the 3 surgical subgroups (gastric bypass: -38%, SD, 7%; VBG: -26%, SD, 9%; and banding: -21%, SD, 10%). After 2 years, weight had increased by 0.1% in the control group and had decreased by 23.4% in the surgical group (P < .001). After 10 years, the weight of the control patients had risen by 1.6% (SD, 12%) from inclusion weight. The maintained weight change was -25% (SD, 11%) in the gastric bypass subgroup; -16.5% (SD, 11%) in the VBG subgroup; and -13.2% (SD, 13%) in the banding subgroup.
Changes in weight among the patients followed-up for 10 years are shown in Figure 3 on the next page.
Figure 3: Weight Changes among Patients in the SOS Study over a 10-Year Period.
All data are for patients who completed 10 years of the study. The mean weight change in the group of surgically treated patients was almost identical to that in the subgroup of patients who had vertical banded gastroplasty. The vertical bars show the 95% confidence intervals.
(Copyright © 2004 Massachusetts Medical Society. All rights reserved. Sjostrom L, Kindroos AK, Peltonen M, Torgerson J, Bouchard C, Carlsson B et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. New Eng J Med 2004; 351:2683-2693).
Risk Factors
Table 38 shows the changes in weight and risk factors for the patients in the surgery and control groups. Glucose and insulin levels increased in the control group but significantly decreased in the surgically treated group after 2 and 10 years. Similarly, triglycerides and diastolic blood pressure were significantly lower in the surgery group at 2 and 10 years.
Table 38: Percentage Changes in Weight, Anthropometric Variables, Risk Factors, and Energy Intake at 2 and 10 Years.
(Copyright © 2004 Massachusetts Medical Society. All rights reserved. Sjostrom L, Kindroos AK, Peltonen M, Torgerson J, Bouchard C, Carlsson B et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. New Eng J Med 2004; 351:2683-2693).
| Variable | Charges at 2 yr† | Charges at 10 yr† | Charges at 10 yr† in Surgery Subgroups | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Control Group (N=1660) |
Surgery Group (N=1845) |
Difference (95%CI) | Control Group (N=627) |
Surgery Group (N=641) |
Difference (95%CI) | Banding (N=156) |
Vertical Banded Gastroplasty† (N=451) |
Gastric Bypass† (N=34) |
|
| percent | percent | percent | |||||||
| Weight | 0.1 | –23.4 | 22.2 (21.6 to 22.8)§ | 1.6 | –16.1 | 16.3 (14.9 to 17.6)§ | –13.2 | –16.5¶ | –25.0§ |
| Height | –0.01 | –0.06 | 0.06 (0.02 to 0.10)§ | –0.3 | –0.3 | –0.01 (–0.12 to 0.10) | –0.2 | –0.3 | –0.8§ |
| BMI | 0.1 | –23.3 | 22.1 (21.5 to 22.7)§ | 2.3 | –15.7 | 16.5 (15.1 to 17.8)§ | –12.8 | –16.0¶ | –23.8§ |
| Waist | 0.2 | –16.9 | 16.0 (15.4 to 16.5)§ | 2.8 | –10.1 | 11.3 (10.3 to 12.4)§ | –7.6 | –10.2¶ | –19.3§ |
| Systolic blood pressure | 0.5 | –4.4 | 2.8 (2.1 to 3.6)§ | 4.4 | 0.5 | 1.1 (–0.3 to 2.6) | 2.1 | 0.4 | –4.7 |
| Diastolic blood pressure | 0.3 | –5.2 | 3.2 (2.4 to 3.9)§ | –2.0 | –2.6 | –2.3 (3.5 to 1.0)§ | –1.4 | –2.5 | –10.4∥ |
| Pulse pressure | 3.2 | 0.6 | –0.5 (-2.3 to 1.3) | 18.0 | 10.8 | 3.5 (0.1 to 6.9)§ | 13.8 | 10.1 | 6.3 |
| Glucose | 5.1 | –13.6 | 16.6 (15.0 to 18.3)§ | 18.7 | –2.5 | 18.4 (14.7 to 22.1)§ | –0.8 | –2.5 | –10.0 |
| Insulin | 10.3 | –46.2 | 51.4 (48.0 to 54.8)§ | 12.3 | –28.2 | 30.3 (23.9 to 36.6)§ | –25.3 | –27.2 | –24.0§ |
| Uric acid | –0.4 | –14.9 | 13.5 (12.5 to 14.6)§ | 3.9 | –6.2 | 8.8 (6.4 to 11.1)§ | –5.2 | –6.1 | –12.3 |
| Triglycerides | 6.3 | –27.2 | 29.9 (27.4 to 32.5)§ | 2.2 | –16.3 | 14.8 (10.4 to 19.1)§ | –18.0 | –14.9 | –28.0§ |
| HDL cholesterol | 3.5 | 22.0 | –18.7 (–20.1 to 17.3)§ | 10.8 | 24.0 | –13.6 (–16.5 to 10.6)§ | 20.4 | 23.5 | 47.5¶ |
| Total cholesterol | 0.1 | –2.9 | 1.0 (0.1 to 1.9)¶ | –6.0 | –5.4 | –2.0 (–0.2 to –3.8)¶ | –5.0 | –5.0 | –12.6§ |
| Energy intake | –2.8 | –28.6 | 19.1 (16.0 to 22.2)§ | –1.0 | –20.7 | 11.6 (8.1 to 15.0)§ | –19.7 | –21.6 | –12.6 |
Data are for all subjects who completed 2 and 10 years of the study and are independent of diagnosis and medications at or after baseline. The changes with in each treatment group are unadjusted, whereas the differences between the group in the changes have been adjusted for sex, age, body-mass index (MBI), and the baseline level of the respective variable. CI de-noted confidence interval, and HDL high-density lipoprotein.
For values with in each group, minus signs denote decreases; for differences between the groups, minus signs denote smaller reductions or (in the case of HDL cholesterol) lager increases in the surgical group than in the control group.
P values are for the comparison with the bending subgroup.
P<0.001.
P<0.05.
P<0.10.
The 10-year changes in weight and BMI were significantly larger for patients who had VBG or gastric bypass compared with those who had banding (adjustable and nonadjustable). Insulin, triglycerides, HDL cholesterol, and total cholesterol levels were significantly better for patients who had gastric bypass, compared with those who had banding.
Lifestyle and Energy Changes
The baseline-adjusted energy intake was significantly lower in the surgery group than in the control group over the 10 years (Figure 4). Similarly, more patients in the surgery group were physically active during leisure time over the 10 years.
Figure 4: Lifestyle Changes Among the Subjects in the SOS Study Over 10 Years.
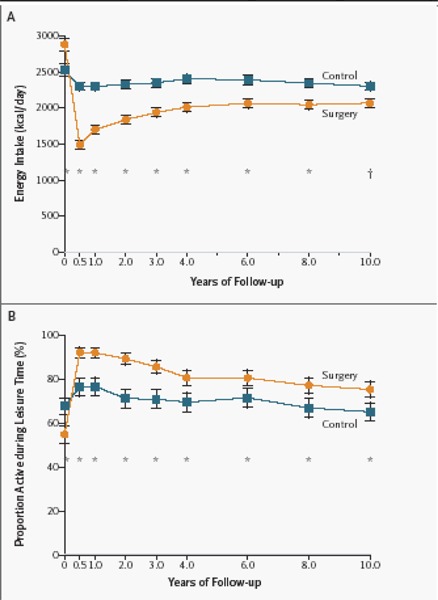
Panel A shows mean energy intake (in kilocalories per day). Panel B shows the percentage of people who were physically active during leisure time. Energy intake and the proportion of active subjects at baseline (year 0) are unadjusted values, whereas the values for the follow-up have been adjusted for sex, age, body-mass index, and energy intake or physical activity at baseline.
Data are from people who completed 10 years of the study. The numbers of subjects at each time point are the same as those in Figure 1. Asterisks denote P < .01; daggers, P < 0.05 for the between-group comparison (by tests for equality). Vertical bars show 95% confidence intervals.
(Copyright © 2004 Massachusetts Medical Society. All rights reserved. Sjostrom L, Kindroos AK, Peltonen M, Torgerson J, Bouchard C, Carlsson B et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. New Eng J Med 2004; 351:2683-2693).
Incidence of Risk Conditions
The incidences of hypertriglyceridemia and diabetes were significantly lower (P < .001 and P < .03) in the surgically treated group compared to the control group at 2 and 10 years (Figure 5). The incidences of hypertension and hypercholesterolemia were not significantly different between the control and surgery groups at 2 and 10 years.
Figure 5: Incidence of Diabetes, Lipid Disturbances, Hypertension, and Hyperuricemia Among Subjects in the SOS Study at 2 and 10 Years. From Sjostrom et al. (1).
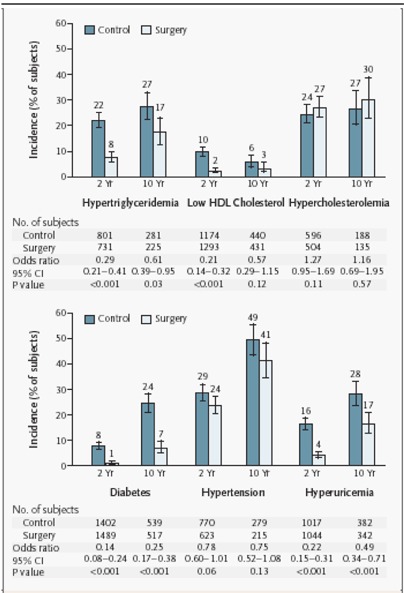
Data are for patients who completed 2 years and 10 years of the study. The bars and the values above the bars indicate unadjusted incidence rates; vertical bars show the corresponding 95% confidence intervals. The odds ratios, confidence intervals, and P values have been adjusted for sex, age, and BMI at the time of inclusion in the intervention study
(Copyright © 2004 Massachusetts Medical Society. All rights reserved. Sjostrom L, Kindroos AK, Peltonen M, Torgerson J, Bouchard C, Carlsson B et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. New Eng J Med 2004; 351:2683-2693.)
Recovery of Risk Conditions
Recovery from hypertension, diabetes, hypertriglyceridemia, hyperuricemia, and low HDL cholesterol were significantly improved in the surgical group after 2 and 10 years (Figure 6).
Figure 6: Recovery From Diabetes, Lipid Disturbances, Hypertension, and Hyperuricemia at 2 and 10 Years in Surgically Treated Patients and Obese Controls.
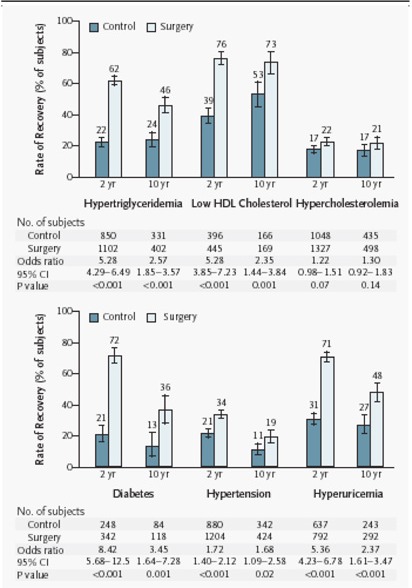
Data are for patients who completed 2 years and 10 years of the study. The bars and the values above the bars indicate unadjusted rates of recovery; vertical bars represent the corresponding 95% confidence intervals. The odds ratios, confidence intervals, and P values have been adjusted for sex, age, and body-mass index at the time of inclusion in the intervention study.
(Copyright © 2004 Massachusetts Medical Society. All rights reserved. Sjostrom L, Kindroos AK, Peltonen M, Torgerson J, Bouchard C, Carlsson B et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. New Eng J Med 2004; 351:2683-2693).
Death and Adverse Effects
Five (0.25%) of 2010 patients who had surgery died postoperatively. Postoperative complications occurred in 151/1164 patients (13.0%):
0.5% bleeding
0.8% embolism or thrombosis
1.8% wound complications
2.1% deep infections (leakage or abscess)
6.1% pulmonary complications
4.8% other complications
In 26 (2.2%) patients, complications were serious enough to require reoperation.
Sjostrom et al. stated that they are continuing to analyze mortality and the incidence of myocardial infarction, stroke, and cancer.
Limitations of the SOS study are as follows:
By definition, the inclusion criteria were not specific to “morbidly obese” patients (BMI ≥ 40 or ≥ 35 with comorbid illnesses). However, the baseline BMI was 41 kg/m2.
Patients were not randomized to groups. The research ethics boards of the participating centres considered the high mortality rate initially observed in the 1980s (1%–5%) as cause to preclude randomization.
The nonsurgical treatment was not standardized and ranged from sophisticated lifestyle intervention, to behaviour modification, to no treatment. However, this study can be considered pragmatic and reflective of “real life.”
It is unknown if risk factors return to baseline in the long term (10–20 years).
There were no data on the primary endpoint of overall mortality.
Conventional Nonsurgical Treatment for Obesity
The results of the SOS study showed that the patients who received nonsurgical weight loss treatment experienced a 1.6% increase in weight over 10 years. Therefore, the Medical Advisory Secretariat decided to review the literature to see if there were any studies that compared nonsurgical weight loss treatment with bariatric surgery for morbidly obesity.
To date, no RCTs have directly compared nonsurgical treatment with bariatric surgery for morbid obesity.
Tsai and Wadden (2) systematically reviewed major commercial and organized self-help weight loss programs in the United States. They found that the evidence supporting such programs was suboptimal, except for one trial on Weight Watchers. The programs were associated with high costs, attrition rates, and probability of regaining at least 50% of the lost weight in 1 to 2 years. Low income would be a barrier for an individual to participate in these programs.
In 2004, Stern et al. (3) reported the 1-year outcomes from an RCT of weight loss and metabolic changes in severely obese adults randomly assigned to either a low-carbohydrate diet or a conventional weight loss diet. Included were 132 adult patients with a BMI of at least 35 kg/m2; of these, 83% had diabetes or a metabolic syndrome. Patients were counselled either to restrict carbohydrate intake to less than 30 grams per day (low-carbohydrate diet) or to restrict caloric intake by 500 calories per day with less than 30% of the calories coming from fat (conventional diet).
Of the 132 enrolled patients, 79 were followed-up at 6 months, and 87 were followed-up at 1 year. By 1 year, the mean weight change for patients on the low-carbohydrate diet was -5.1 kg (SD, 8.7 kg) compared with -3.1 kg (SD, 8.4 kg) for patients on the conventional diet. The differences between the groups were not significant (-1.9 kg [95% CI, -4.9–1.0 kg], P = .20). Triglyceride levels decreased more in patients on the low-carbohydrate diet (P = .044). High-density lipoprotein cholesterol levels decreased less for this same group (P = .025).
In the group of patients with diabetes (n = 54), after adjusting for covariates, hemoglobin A1c levels improved more for patients on the low-carbohydrate diet: mean difference -0.7 (95% CI, -1.6%–0.2%). These more favourable metabolic responses to a low-carbohydrate diet remained significant after adjusting for weight loss differences (adjusted P = .019). Changes in other lipids or insulin sensitivity did not differ between groups.
Of note, weight loss in this study was modest, and the overall dropout rate was high. In addition, many patients did not meet their dietary targets.
In 2003, Patterson et al. (4) used a decision-analysis model to simulate a trial comparing diet and exercise with laparoscopic RYGB to determine which approach resulted in longer life expectancy. The model showed that RYGB resulted in an increased life expectancy of morbidly obese patients; for example, a morbidly obese 45-year-old woman could expect to gain more than 2 years of life if she had RYGB, regardless of obesity-related comorbidities.
Other Update Studies Examining Outcomes After Bariatric Surgery
Table 39 shows a summary of the patient outcomes after bariatric surgery as reported in the update studies. Each study is discussed in turn below.
Table 39: Summary of Patient Outcomes After Bariatric Surgery as Reported in the Update Studies.
| Weight Loss (Range %EWL) |
Resolution of Comorbidities (Range, %) |
Mortality (Range, %) |
Adverse Effects (Range, %) |
||
|---|---|---|---|---|---|
| Malabsorptive | |||||
| Roux-en-Y gastric bypass | 66.3– 67.1 | -* | 0.68–0.8 | 23–31 | |
| Purely restrictive | |||||
| Vertical banded gastroplasty | - | - | - | - | |
| Adjustable gastric banding | 36– 44.5 | Diabetes: 20– 66 | 0–1.6 | 8.7–16 | |
| Hypertension: 13–59 Dyslipidemia: 28 |
|||||
No data were reported for this variable in the update studies.
Roux-en-Y Gastric Bypass
Smith et al. (28) retrospectively compared open (n = 451) and laparoscopic (n = 328) RYGB using a database of 779 patients who had had the procedures between 2000 and 2002 (level 4 evidence).
Questionnaires were mailed to patients. Follow-up was 5 to 29 months. Smith et al. found that the mean preoperative BMI for the laparoscopic RYGB group was 46.7 kg/m2 (range 35–62 kg/m2). The 6-month postoperative BMI was 33.1 kg/m2, and the 12-month postoperative BMI was 29.5 kg/m2. For the open RYGB group, the preoperative BMI was 49.5 kg/m2 (range 35—83 kg/m2). The 6-month postoperative BMI was 35.3 kg/m2, and the 12-month BMI was 30.3 kg/m2. Total time in the operating room during the last year of the study averaged 155 minutes for laparoscopic RYGB and 119 minutes for open RYGB.
Overall, 89 (27%) of 328 patients had complications after laparoscopic RYGB. After open RYGB, 162 (35%) of 451 patients had complications (P = .01). However, the authors did not say if the complications were directly attributable to the surgical procedure. They listed the following complications for patients who received RYGB:
Hit in head by trapeze
Suicide
Voice change after intubation
Gilbert’s disease (genetic disorder characterized by low-grade chronic hyperbilirubinemia with daily fluctuations of the bilirubin level)
Guillain-Barré syndrome (acute infective polyneuritis that results in a form of peripheral neuropathy with temporary loss of movement and sensation due to inflammation of multiple nerves and loss of myelin)
Beriberi (common in alcoholics; it is unknown if the patient had a history of alcoholism)
A limitation of the study by Smith et al. is that it used a retrospective database design.
Christou et al. (29) did a retrospective database study to examine the morbidity and mortality of morbidly obese patients treated with bariatric surgery (n = 1,035) (level 4 evidence). These people had been treated at the McGill University Health Centre between 1986 and 2002 (level 4 evidence). Another group had gender- and age-matched patients who had not been treated surgically (n = 5,746) that were identified from the Quebec provincial health insurance database. A maximum of 6 controls were identified for each bariatric surgery recipient. The 2 groups were followed-up for 5 years at most.
For the bariatric surgery group, the exclusion criterion was admission to hospital for a chronic condition 6 months before surgery. The inclusion criteria for the controls were a diagnosis of morbid obesity according to the ICD-9 codes (278.00, 278.01) for treatment in a hospital, treatment by a physician, or as an indication for a prescription; and never having had surgery for severe obesity (44.31, 44.39). Potential participants were excluded from the control group if they had been hospitalized for a chronic condition within 6 months before the surgery date of their matched bariatric patient.
The surgical techniques used were RYGB (open and laparoscopic) and open VBG.
Data on weight loss parameters for the surgically treated patients were extracted from the institution’s bariatric surgery patient registry. Direct health care costs were expressed in 1996 Canadian dollars. For each patient, the total direct health care cost was estimated based on information in the Regie de l’assurance maladie due Quebec (RAMQ). This included the cost for hospitalization, physician’s visit, and prescription medication. The cost for the surgery and subsequent care was included in the total cost estimates of the bariatric surgery cohort.
Seven surgeons affiliated with the institution treated the 1,035 bariatric patients across 16.4 years. Most of the procedures were open RYGB (n = 820 [79.2%]), followed by VBG (n = 194 [18.7%]), and laparoscopic RYGB (n = 21 [2.2%]). Fifty-six percent of the patients were in the BMI range of 38 to 49 kg/m2; 32% were in the BMI range of 50 to 59 kg/m2; 8% were in the range of 60 to 69 kg/m2; and the rest had a BMI greater than 70 kg/m2. (The highest was 98 kg/m2.) Thirty-five percent of the patients who had VBG were subsequently converted to open RYGB because of complications comprising outlet obstruction (58%), failure to lose weight (33%), and “miscellaneous reasons” (9%).
For baseline patient demographics, there were no significant differences reported for gender, age, or length of follow-up.
For patients who had bariatric surgery, neither weight loss nor BMI was reported for the patients in the control group. There were significant reductions in the initial mean EWL (67.1%, P < .001) and in the percent change in BMI (34.6%, P < .001). The initial mean EWL was significantly higher in patients who had an open RYGB (68.7% [SD, 23.1%]) compared with those who had VBG, especially for the VBG patients who were not converted to RYGB (57.3% [SD, 24.8%], “P = .0000”). VBG patients who subsequently received RYGB achieved an EWL of 66.3% (SD 22.6), similar to de novo RYGB.
Christou and colleagues reported that the duration of follow-up to determine the adequacy of the weight loss was “good to 11 years” but fell off dramatically during the next 6 years as patients relocated without leaving forwarding addresses or other contact information. Patient follow-up was 72% during the 16 years of the study.
Based on the BMI ranges, the authors stated that 83% of morbidly obese patients had a successful outcome, and 73% of super obese patients had a successful outcome.
Patients in the bariatric surgery group had significantly lower incidences of all of the chronic conditions listed, except those related to blood and blood-forming organs (within a maximum of 5 years). Lower risk was observed in malignancies; cardiovascular and circulatory conditions, including hypertension; endocrinologic conditions, including type 2 diabetes; infectious diseases; and respiratory conditions. The surgery cohort had a higher risk of digestive disorders.
The mortality rate for patients in the bariatric surgery cohort was 0.68%, compared with 6.17% for controls (relative risk 0.11; 95% CI, 0.04–0.27). Mortality in the surgical group included perioperative deaths (0.4%). A Kaplan-Meier survival analysis found that the mortality rate in the bariatric surgery group was lower than that in the control group (P < .001). Maximum 5-year data were not reported for specific comorbid illnesses (diabetes, hypertension, and hyperlipidemia) in either group.
The mean number of hospitalizations and total in-hospital days were significantly lower in the bariatric surgery group. These patients also visited physicians less often in the 5-year follow-up period, which included the planned yearly follow-up of the surgery group.
Patients in the bariatric surgery group had fewer hospitalizations overall, but they had significantly more hospitalizations for digestive conditions than did patients in the control group.
Christou et al. reported that the mean total direct health care costs were significantly higher for the control group across all diagnostic categories (no data provided). However, the mean costs for digestive disorders were 68% higher for patients who had bariatric surgery (no more data provided). On average, the total direct health care cost was 45% higher for the controls (no more data provided).
The limitations of the study by Christou et al. include the following:
In an editorial from the New England Journal of Medicine, (17) Solomon and Dluhy commented that the control group was not well matched and confounding was likely.
Weight loss data was not reported for the control patients, nor was there any discussion about what treatment these patients were receiving.
The BMI of the control group was not reported. Therefore, it is possible that the baseline BMIs between the patient groups were unbalanced.
There were no data on specific comorbidity outcomes for diabetes, hypertension, anf dyslipidemias.
The authors said that matching patients, excluding patients with a history of the outcomes of interest, and selecting patients based on their exposure “make the current study an excellent simulation of a prospective cohort study and a valid representation of a ‘real life’ situation.” The study was not prospectively designed. It is a retrospective study.
There was no discussion or justification as to why 6 controls were used for each surgery treatment.
Patients who had had surgical procedures as far back as 1986 were included. Differences in evolving techniques may have occurred over that period.
There was no discussion of an a priori sample size calculation.
Indirect costs were not examined.
Mortality and morbidity data were examined only up to 5 years.
Biliopancreatic Diversion With or Without Duodenal Switch
In a single-centre Australian study, Dolan et al. (30) studied BPD with (n = 73) or without (n = 61) duodenal switch (level 4 evidence). They hypothesized that the risk of malnutrition and diarrhea would be reduced by pyloric preservation with BPD-duodenal switch. This was the first bariatric procedure for 70 of the patients. It was a secondary procedure for 64 patients: after failed LAGB for 50 patients and after failed VBG for 14 patients. Patients in the failed LAGB group had lost weight but could not tolerate the dietary restrictions of the band, or they had a complication that necessitated removal of the band, most commonly recurrent slippage. Further data were not reported by the authors.
At a clinic visit, patients filled in a questionnaire abut weight loss, dietary history, gastrointestinal symptoms, obesity-related comorbid conditions, and medication (including dietary supplements). They also had a serum nutritional screen.
One hundred and one (75.4%) patients were female. The median age was 44 years (range, 23–68 years). The median preoperative BMI was 44.8 kg/m2 (range, 25.5–83.7 kg/m2). Patients were followed-up for a median of 28 months (range, 6 –50 months) after surgery. Laparoscopic procedures were performed late in the series: 14 of the 73 BPD and 30 of the 61 BPD-duodenal switch procedures were done laparoscopically.
One person (0.7%) died in hospital 21 days after having laparoscopic BPD-duodenal switch due to necrotizing pancreatitis. Three other patients died later: 2 due to myocardial infarction 7 and 9 months after surgery; and 1 due to a pulmonary embolism more than 2 years after surgery.
Complications included the following:
Wound infection: 16 patients (11.9%).
Dehiscence: 14 patients (10.4%); 12 were superficial.
Anastomotic leak: 7 patients (5.2%); 1 had a further leak form the gastrojejunostomy and developed a gastrocutaneous fistula that failed to heal.
Postoperative bowel obstruction: 3 patients.
Bleeding from a staple line: 2 patients.
Deep vein thrombosis: 1 patient.
Postoperative pneumonia: 1 patient.
The BPD and BPD-duodenal switch groups did not differ on age, sex, BMI, or morbidity. The median EWL at 12, 24, and 36 months was 64.1%, 71.0%, and 72.1%, respectively. Mean BMI at 12, 24, and 36 months was 33.1 kg/m2, 31.5 kg/m2, and 31.5 kg/m2, respectively. There were no significant differences between the BPD and BPD-duodenal switch groups.
There were no significant differences between BPD and BPD-duodenal switch in treating obesity-related comorbid conditions (diabetes, hypertension, and sleep apnea; all P values > .22).
There were also no significant differences between BPD and BPD-duodenal switch in meal size, fat score, nausea, vomiting, diarrhea, or nutritional parameters (all P values > .22).
Eighty-three patients (61.9%) had a complete nutritional screen at their last clinic visit. The blood results were from a median of 37 months after BPD and 23 months after BPD-duodenal switch. There were no significant differences between BPD and BPD-duodenal switch in any of the measured serum levels.
The limitations of the study by Dolan et al. are as follows:
There was no explicit objective or specific statement about what was meant to be statistically tested
They did not describe clearly how they chose the patients or from what pool.
They used a retrospective design.
Laparoscopic Adjustable Banding
Ponce et al.(31) did a retrospective review to evaluate the effect of weight loss induced by AGB on morbidly obese patients who were taking medication for type 2 diabetes or hypertension in a centre in the United States. Of 840 patients who received an adjustable band, data were available for 402 out of 413 patients who completed at least 1 year of follow-up (the authors named them the “cohort group”).
The inclusion criteria to receive an adjustable band were as proposed by the NIH Consensus Development Panel report of 1991: adults with a BMI over 35 with comorbid conditions, or a BMI over 40, with or without the conditions.
Follow-up weights were obtained from postoperative clinic visits. However, in an unknown number of patients who were unavailable for follow-up, weights were obtained from physicians’ office scales, telephone interviews, or electronic mail questioning.
Diabetes
The inclusion criteria for people with diabetes were a diagnosis of type 2 diabetes and use of diabetic medication before surgery. Patients were divided into 2 groups based on duration of diabetes: less than 5 years and more than 5 years. People with diabetes who were not taking any medications were excluded.
Diabetes was considered resolved in patients who achieved a normal HbA1c and who required no medication for diabetes after surgery. Improvement was defined as a reduction of HbA1c and or reduction in diabetes medication or dose. Diabetes was considered unchanged if no resolution or improvement criteria were identified.
Hypertension
The inclusion criterion for people with hypertension was use of antihypertensive medication at the baseline visit. Only patients with clinical indications for hypertension were included.
Hypertension was considered resolved if the patient became normotensive without medication. Improvement was defined as normotensive with a decrease in antihypertensive agent use or dose. If the patient was taking the same or equivalent medication, then hypertension was considered unchanged.
The authors reported that data were available in 97.3% of patients at 1 year (n = 402), 94.8% at 2 years (n = 91), and 88.9% at 3 years (n = 24). It is unclear how the last 2 percentages were calculated. In total, 53 patients (12.8%) met the criteria for type 2 diabetes with medication, and 189 patients (45.7%) were taking antihypertensive agents. Of patients with diabetes, 66% (n = 35) also had hypertension, and of patients with hypertension, 18.5% had diabetes.
Complications: Diabetes
No mortalities occurred.
In the group of patients with diabetes, there were 4 access port infections (7.5% of 53 patients with diabetes versus 0.97% of all 413 patients, P < .001). One person required band removal secondary to intractable infection and on case of gastric prolapse.
Complications: Hypertension
Three patients (2 of whom also had diabetes) had access port infections (1.6% of 189 patients with hypertension). Five gastric prolapses occurred (2.7%). One band erosion occurred (0.5%), which presented as a delayed access port infection 4 months postoperatively and required removal. One patient had postoperative bleeding from one of the trocar sites that required re-exploration.
Weight Loss
The mean EWL for patients with diabetes was 39.2% at 12 months, 46.7% at 18 months, and 52.6% at 24 months after surgery. The mean EWL for patients in the “cohort group” was 41.2% at 12 months, 54.2% at 18 months, and 63.3% at 24 months. Patients with recent-onset diabetes (defined as < 5 years) lost more weight than those who had had diabetes for longer: the mean EWL was 42.5% versus 32.3% at 1 year (P = .033), and 59.0% versus 36.2% at 18 months (P = .014) for the recent-onset and longer-duration groups, respectively. Data for longer than 5 years were not reported. No other P values were reported.
For patients with hypertension, the mean EWL after surgery was 41.2% at 12 months, 52.7% at 18 months, 64.5% at 24 months, and 68.4% at 36 months. The cohort group had mean EWLs of 40.8% at 12 months, 53.1% at 18 months, 58% at 24 months, and 53.1% at 36 months. At 24 months, people with hypertension had a higher percent EWL (P = .012). No other P values were reported.
Impact on Diabetes
Of 53 patients with diabetes and at least 1-year follow-up, 66% were off all diabetic medications at 12 months, 70.6% at 18 months, and 80% at 24 months (Table 40). Three patients with diabetes completed at least 36 months follow-up, and all 3 showed complete resolution. Overall, the mean HbA1c changed from 7.25 (range, 5.6–11.0) preoperatively to 5.87 (range, 5.0–7.3) at 12 months, 5.68 (range, 4.4–6.8) at 18 months, 5.58 (range, 5.1–6.2) at 24 months, and 5.33 (range, 5.1–5.8) at 36 months.
Table 40: Outcomes for Patients With Type 2 Diabetes.
| Resolution | Non-Resolution | |||||
|---|---|---|---|---|---|---|
| 12 Mons (n=35) |
18 Mons (n=34) |
24 Mons (n=12) |
12 Mons (n=18) |
18 Mons (n=10) |
24 Mons (n=3) |
|
| Age (years) | 43.4 | 43.8 | 44.9 | 47.6 | 46.7 | 45.4 |
| Gender (% F) | 66% | 54% | 50% | 72% | 70% | 68% |
| Preop BMI (kg/m2) | 47.1 | 46.8* | 52.5 | 51.8 | 57* | 7.2 |
| Preop HbA1c(%) | 7.1 | 7.0* | 7.4 | 7.6 | 8.1* | 7.2 |
| Duration(% <5 years) | 82.8%* | 83.3%* | 58.3% | 33.3%* | 20%* | 66.6% |
| %EWL | 45.0%* | 53.9%* | 59.1%* | 27.0%* | 27.4%* | 26.5% |
P≥0.005 for resolution vs non-resolution.
(Copyright © FD-Communications. Reproduced with kind permission from Obesity Surgery. Ponce J, Haynes B, Paynter S, Fromm R, Lindsey B, Shafer A et al. Effect of Lap Band induced weight loss on type 2 diabetes mellitus and hypertension. Obesity Surgery 2004; 14:1335-1342)
Clinical resolution or improvement in diabetes occurred in all patients over 12, 18, and 24 months after surgery; however, there was no statistically significant difference between patients who had diabetes for less than 5 years and those who had had it for more than 5 years (P values not provided).
Patients whose onset of diabetes was more recent appeared to have improved more. However, at 24 months, 83% of patients who had had diabetes for more than 5 years had resolved their diabetes, compared with 78% of patients who had diabetes for fewer than 5 years.
Patients whose diabetes did not resolve had a significantly lower percent EWL than those with resolution. The mean EWL in the first group was 27.0% at 12 months, 27.4% at 18 months, and 26.5% at 24 months, compared with 45% at 12 months, 53.9% at 18 months and 59.1% at 24 months in the patients whose diabetes did resolve (P < .001 at 12 and 18 months, P = .005 at 24 months).
The authors stated that weight loss (percent EWL) and duration of diabetes were independently predictive of resolution at 12 months using a multivariate logistic model.
Impact on Hypertension
Of 189 patients with hypertension at least 1 year after follow-up, 59.8% (n = 113) had stopped all antihypertensive medications at 12 months; 68.8% (n = 66) at 18 months; and 74.0% (n = 37) at 24 months (Table 41). Hypertension improved in 38.0% (n = 72) at 12 months, 28.1% (n = 27) at 18 months, and 22.0% (n = 11) at 24 months. Hypertension remained unchanged in 2.1% (n = 4) at 12months, 3.1% (n = 3) at 18 months, and 4.0% (n = 2) at 24 months.
Table 41: Outcomes for Patients with Hypertension.
| Resolution | Non-Resolution | |||||
|---|---|---|---|---|---|---|
| 12 Mons (n=113) |
18 Mons (n=66) |
24 Mons (n=37) |
12 Mons (n=76) |
18 Mons (n=30) |
24 Mons (n=13) |
|
| Age (years) | 43.1 | 42 | 42.9 | 45.1 | 44.2 | 45.6 |
| Gender (% F) | 86% | 83% | 84% | 83% | 73% | 85% |
| Preop BMI (kg/m2) | 45.1* | 46.6† | 47.1 | 50.9* | 50.0† | 48.6 |
| %EWL | 46.4%* | 57.8%* | 69%* | 33.2%* | 41.3%* | 51.8%* |
P<0.005 for resolution vs non-resolution.
P<0.05 for resolution vs non-resolution.
(Copyright © FD-Communications. Reproduced with kind permission from Obesity Surgery. Ponce J, Haynes B, Paynter S, Fromm R, Lindsey B, Shafer A et al. Effect of Lap Band induced weight loss on type 2 diabetes mellitus and hypertension. Obesity Surgery 2004; 14:1335-1342)
Patients with resolved hypertension had significantly higher EWL compared with the group without complete resolution: at 12 months, 46.4% versus 33.2% (P < .001); at 18 months, 57.8% versus 41.3% (P < .001); and at 24 months, 69.0% versus 51.8% (P < .005).
Limitations of the study by Ponce et al. are as follows:
The design of the study was retrospective.
Follow-up data were not retrieved in the same way for all patients (telephone and e-mail).
Some patients may have been diagnosed with early diabetes or mild hypertension that was controlled by diet without medication. The data for these patients were not included.
Martikainen et al. (32) did a retrospective review of AGB in 123 Finnish patients. The mean BMI of these patients was 49 kg/m2 (range 34–85 kg/m2), and mean EBW was 67 kg (range 23-162 kg). None of the patients had had surgery for obesity. Two hundred and thirty-six comorbid conditions were recorded in 106 (86%) patients (Table 42).
Table 42: Comorbid Conditions in Patients.
| Male n (%) |
Female n (%) |
All n (%) |
|
|---|---|---|---|
| Type 2 diabetes | 15 (40) | 25 (29) | 40 (33) |
| Hypertension | 26 (68) | 29 (34) | 55 (45) |
| Dyslipidemia | 21 (55) | 50 (59) | 71 (58) |
| Sleep apnea | 21 (55) | 14 (17) | 25 (29) |
| Osteoarthrosis | 2 (5) | 14 (17) | 16 (13) |
| Asthma | 5 (13) | 15 (18) | 20 (16) |
| Psychiatric diagnosis | 4 (11) | 12 (14) | 16 (13) |
| Hypothyroid (treated) | 1 (3) | 9 (11) | 10 (8) |
(Copyright © FD-Communications. Reproduced with kind permission from Obesity Surgery. Martikainen T, Pirinen E, Alhava E, Poikolainen E, Paakkonen M, Uusitupa M et al. Long-term results, late complications and quality of life in a series of adjustable gastric banding. Obesity Surgery 2004; 14(5):648-654).
All patients had participated in long-term nonsurgical weight reduction programs before having surgery. They were evaluated preoperatively by a multidisciplinary team that included a surgeon, an endocrinologist, and a clinical nutritionist. The criteria for bariatric surgery were as follows:
Age older than 18 years
BMI > 40 or > 35 with serious comorbid conditions
Being obese for ≥ 5 years
No acute psychiatric or eating disorder
All patients were asked to attend the outpatient clinic 6 and 12 months postoperatively and thereafter annually or every second year for a check-up and nutritional counselling. At each visit, patients were assessed for the percent EWL, complication and reoperation rates, and changes in comorbidity such that the disorder was “recovered” when controlled without medication, and “improved” when controlled by reduced doses of medication.
The evaluation period ranged from 8 to 108 months (mean, 55 months). Ten people died. Two deaths occurred 30 days after the procedure: 1 from gastric perforation and 1 from respiratory insufficiency. According to the authors, the remaining 8 deaths were not related to surgery. They were due to myocardial infarction, stroke, traffic accident, breast cancer, or alcohol and drug abuse.
Forty bands (33%) were removed, which left 73 patients the end of the follow-up period with a functioning band. BMI and percent EWL were reported only for patients with functioning bands. Data were obtained during follow-up as shown in Table 43.
Table 43: Patient Follow-up Data. From Martikainen et al. (32).
| Patients Followed-Up, N | Years After Surgery |
|---|---|
| 120 | 1 |
| 108 | 2 |
| 91 | 3 |
| 86 | 4 |
| 63 | 5 |
| 44 | 6 |
| 32 | 7 |
| 16 | 8 |
| 3 | 9 |
(From Martikainen T, Pirinen E, Alhava E, Poikolainen E, Paakkonen M, Uusitupa M et al. Long-term results, late complications and quality of life in a series of adjustable gastric banding. Obesity Surgery 2004; 14(5):648-654).
Five patients were lost to follow-up.
Weight Loss
The postoperative reduction in BMI after 1 year was 8.1 kg/m2 (SD, 5.4 kg/m2); after 2 years, it was 8.7 kg/m2 (SD, 6.8 kg/m2); after 3 years, 7.5 kg/m2 (SD, 5.8 kg/m2); and after 9 years, 4.7 kg/m2 (1.1 kg/m2).
Mean weight losses and gains are shown in Table 44.
Table 44: Weight Losses and Gains in 120 Patients After Gastric Banding (Follow-Up ≥1 Year).
| Preoperative Value |
Lowest Postoperative Value |
Most Recent Value | |
|---|---|---|---|
| Mean (SD) body weight, kg range | 138 (26.0) 90.5–229.0 |
108.3 (24.8)* 57.2–179.7* |
118 (29.7)* 67.1–234.0* |
| Mean (SD) BMI, kg/m2 range | 48.8 (8.6) 33.6–85.1 |
38.3 (8.3)* 21.3–64.7* |
42.0 (10.3)* 26.2–87.0* |
| Mean (SD) excess weight, kg range | 67.2 (24.2) 23.3– 161.8 |
37.6 ±; 23.3* -10.0–106.8* |
47.7 (28.7)* 3.1–166.8* |
P < .001 from preoperative value.
(Copyright © FD-Communications. Reproduced with kind permission from Obesity Surgery. Martikainen T, Pirinen E, Alhava E, Poikolainen E, Paakkonen M, Uusitupa M et al. Long-term results, late complications and quality of life in a series of adjustable gastric banding. Obesity Surgery 2004; 14(5):648-654).
The largest mean weight loss achieved during the follow-up period was 30 kg, representing a 44.6 % EWL. However, after 1 to 3 years, most patients started to regain the weight, and the final mean weight decrease from baseline was 19.5 kg. Postoperatively, EWL varied from 36% (SD, 24%) at 1 year to 21% (SD, 5%) at 9 years. At the end of the evaluation, 11 (9%) patients had achieved a 75% EWL, 16 (13%) had a 50% to 75% EWL, 36 (29%) had a 25% to 50% EWL, and 49 (40%) had a 0% to 25% EWL. Eleven (9%) patients gained weight despite a functioning band.
In 2 patients, the operation was converted to a gastric bypass, and the further weight loss was reported as “excellent” (no data provided).
Comorbid Conditions
The outcomes of comorbid conditions, except asthma and arthritis for which data were missing, in patients who were followed-up for more than 1 year are shown in Table 45. There were 199 comorbid conditions in 106 patients preoperatively. Across all of these conditions, 75 (38%) patients had improved or recovered after 1 year, but 87 (44%) did not change. In 34 (17%) patients, the conditions worsened.
Table 45: Outcome of Comorbid Conditions Over 1 Year.
| Condition | Preoperative Comorbid Conditions, n |
Recovered † n (%) |
Improved‡ n (%) |
Stable n (%) |
Worsened n (%) |
|---|---|---|---|---|---|
| Diabetes | 40 | 8 (20) | 4 (10) | 13 (33) | 15 (38) |
| Hypertension | 55 | 7 (13) | 12 (22) | 23 (42) | 13 (24) |
| Dyslipidemia | 71 | 20 (28) | 10 (14) | 30 (42) | 6 (8) |
| Sleep apnea | 35 | 5 (14) | 9 (26) | 21 (60) | – |
| All diagnoses | 200 | 40 (20) | 35 (18) | 87 (44) | 34 (17) |
n = 106.
Controlled without medication.
Controlled with reduced doses of medication.
(Copyright © FD-Communications. Reproduced with kind permission from Obesity Surgery. Martikainen T, Pirinen E, Alhava E, Poikolainen E, Paakkonen M, Uusitupa M et al. Long-term results, late complications and quality of life in a series of adjustable gastric banding. Obesity Surgery 2004; 14(5):648-654).
Complications
During follow-up, 20 (16%) patients had early postoperative complications: gastric perforation (n = 4), pneumonia (n = 4), pulmonary emboli (n = 2), wound infections (n = 5), intraabdominal bleeding (n = 1), peripheral deep venous thrombosis (n = 1), respiratory insufficiency (n = 1), and dehiscence (n = 2).
Reoperations
The band was removed due to slippage or pouch dilatation or obstructive problems in 24 patients. Other reasons for band removals were erosion (n = 11), problems with band function (n = 2), and infection (n = 3). In 27 (22%) of the 123 patients, the band had to be repositioned, and in 5 (4%), it had to be replaced. Access port problems (displacement or rupture) occurred in 23 (19%) patients.
The authors recommended that patients be followed-up with annual gastroscopies owing to the high rate of erosions. They also noted that more long-term studies are needed to evaluate the procedure’s efficacy.
The limitations of the study by Martikainen et al. are as follows:
The design was retrospective.
There was inconsistent/unclear reporting of data.
It combined open and laparoscopic procedures.
Shen et al. (33) examined the impact of patient follow-up on weight loss after 1 year in patients who had received LAGB (n = 186) or RYGB (n = 115) (level 4 evidence). All patients who had undergone the procedures were retrospectively reviewed from a database.
LAGB patients who returned up to 6 times after surgery (Group A) were compared with those who returned more than 6 times (Group B). RYGB patients who returned up to 3 times (Group C) after surgery were compared with those who returned more than 3 times (Group D). Visits about obstruction after excessive band adjustment (typically 1 to 3 days after over-tightening); complications such as wound infection or anastomotic structure; and extraneous complaints, such as biliary colic or non-routine visits, were not counted.
At 1 year after LAGB surgery, the EWL was 44.5% (SD, 1.4%); it ranged from 0% to 118%. The mean number of visits per patient was 5.7 (SD, 2.6) in the first year. In Group A, 130 patients (70%) returned for 6 or fewer visits and had a mean EWL of 42%. In contrast, 56 patients (30%) in Group B returned for more than 6 visits and had a mean EWL of 50% (P < .05). There was no significant difference between Groups A and B in how much fluid was in the band after 1 year.
There were 115 RYGB patients available at 1 year (83%); 17% were lost to follow-up. The mean BMI was 48 kg/m2 (range 35–64.1 kg/m2). At 1 year, the mean EWL was 66.3% (SD, 1.9%); it ranged from 14.7% to 120.4%. In Group C, 53 (46%) of patients had a mean EWL of 66.1%. In Group D, 62 patients (54%) had a mean EWL of 67.6% (P value not significant).
The authors concluded that follow-up is essential after either laparoscopic RYGB or LAGB, but it may have a greater impact on weight loss after LAGB. The limitations of this study are as follows:
It was an observational database study.
There was no a priori statistical consideration.
Dolan et al. (34) examined the use of LAGB versus BPD in super obese patients (BMI > 50 kg/m2) (level 4 evidence). BPD was the primary bariatric procedure in 23 super obese patients who were sex-matched to 23 of 1,319 LAGB patients. The first 11 BPD (47.8%) procedures were done by a laparotomy, and the remaining 12 were done laparoscopically. Patients were seen in the outpatient clinic at 3 monthly intervals. Those with inadequate feelings of satiety or inadequate weight loss at any clinic visit had the band expanded with 1 ml of saline and returned to the clinic 6 weeks later. This procedure was repeated twice if required, until 3 ml were injected, and any further additions to the band were made in 0.5 ml increments to a maximum of 4 ml.
Weight loss was measured as a reduction in BMI and as percent EWL.
Dolan et al. found that weight loss after BPD was significantly greater than after LAGB at 3, 6, 12, and 24 months after surgery. BPD reduced BMI by 17.6 kg/m2 at 12 months and by 22.3 kg/m2 at 24 months. BMI fell 12.3 kg/m2 and 17.0 kg/m2 at 12 and 24 months. The median EWL 24 months after BPD was 64.4% (range, 36% to 96.5%). After LAGB, it was 48.4% (range, 26.7% to 80.2%). A summary of the results is shown in Table 46.
Table 46: Summary of Results for Super Obese Patients.
| Biliopancreatic Diversion | LAGB | P | |
|---|---|---|---|
| Patients, n | 23 | 23 | |
| Females, n (%) | 16 (69.6) | 16 (69.6) | |
| Age, years (range) | 41 (23–67) | 39 (26–58) | .31 |
| Complications, n (%) | 13 (56.5) | 2 (8.7) | .001 |
| Reoperations, n (%) | 7 (30.4) | 2 (8.7) | .06 |
| Hospital stay, days (range) | 8 (4–146) | 1 (1–2) | < .001 |
| Follow-up, months (range) | 30 (21–49) | 34 (12–59) | .18 |
| BMI preoperatively (range) | 56.9 (50.8–83.7) | 55.9 (50.7–90.6) | .89 |
| BMI at 3 months (range) | 49.3 (41.2–57.8) | 49.7 (43.9–87.4) | .34 |
| BMI at 6 months (range) | 42.7 (32.0–55.0) | 46.8 (38.4–-87.5) | .003 |
| BMI at 12 months (range) | 39.1 (31.3–50.9) | 43.6 (33.9–85.2) | .03 |
| BMI at 24 months (range) | 34.6 (26.4–48.1) | 38.9 (30.2–49.5) | .04 |
| % EWL at 3 months (range) | 23.2 (10.5–48.3) | 17.3 (4.7–35.2) | .01 |
| % EWL at 6 months (range) | 39.8 (23.3–72.7) | 29.5 (4.3–48.4) | .001 |
| % EWL at 12 months (range) | 57.5 (36.4–82.1) | 37.0 (7.8–66.0) | .001 |
| % EWL at 24 months (range) | 64.4 (36.4–96.5) | 48.4 (26.7–80.2) | .02 |
| Resolution of obstructive sleep apnea | 4 of 5 | 2 of 3 | .64 |
| Resolution of hypertension | 4 of 6 | 4 of 6 | .60 |
| Resolution of diabetes | 2 of 2 | 2 of 3 | .65 |
(Copyright © FD-Communications. Reproduced with kind permission from Obesity Surgery. Dolan K, Hatzifotis M, Newbury L, Fielding G. A comparison of laparoscopic adjustable gastric banding and biliopancreatic diversion in superobesity.[see comment]. Obesity Surgery 2004; 14(2):165-169).
When results were broken down according to laparoscopic BPD (n = 12) or LAGB (n = 12), the authors found that weight loss was significantly greater after the former (P values ranged from .05 to .001). The median hospital stay after LAGB was 1 day. After open BPD, it was 9 days. After laparoscopic BPD, it was 8 days (P < .001).
The complications are shown in Table 47.
Table 47: Complications in Super Obese Patients. From Dolan et al. (34).
| Biliopancreatic Diversion (n = 11) |
Laparoscopic Biliopancreatic Diversion (n = 11) |
LAGB (n = 23) |
|
|---|---|---|---|
| Wound infection | 3 | 2 | 0 |
| Wound dehiscence | 3 | 0 | 0 |
| Anastomotic leak | 1 | 1 | 0 |
| Postoperative bleeding | 0 | 1 | 0 |
| Incisional hernia | 2 | 0 | 0 |
| Slippage | 0 | 0 | 1 |
| Port site leak | 0 | 0 | 1 |
(Copyright © FD-Communications. Reproduced with kind permission from Obesity Surgery. Dolan K, Hatzifotis M, Newbury L, Fielding G. A comparison of laparoscopic adjustable gastric banding and biliopancreatic diversion in superobesity.[see comment]. Obesity Surgery 2004; 14(2):165-169).
The limitations of the study by Dolan et al. are as follows:
The study design was retrospective.
It is unclear how the 23/1319 patients were chosen (e.g., consecutively).
The sample size was small.
Gastric Bypass Versus Biliopancreatic Diversion With Duodenal Switch
In a single-centre study from the United States, Deveney et al. (35) compared weight loss (expressed as percent of excess body weight) after 1 and 2 years in patients who had open and laparoscopic RYGB or BPD-duodenal switch (level 4 evidence).
Deveney et al. extracted data from their bariatric patient database. Inclusion criteria consisted of either RYGB or BPD-duodenal switch. Patients who had undergone previous failed bariatric procedures were excluded. The authors calculated mean length of stay, and rates of wound infection, anastomotic leak, and mortality for each operation.
The demographic variables of the groups are shown in Table 48. Patients who had BPD-duodenal switch had significantly higher BMIs and higher rates of hypertension, diabetes, and sleep apnea at the time of surgery.
Table 48: Demographic Data of Patients.
| Roux-en-Y Gastric Bypass (n = 237) |
Biliopancreatic Diversion With Duodenal Switch (n = 113) |
|
|---|---|---|
| Age in years, mean (SD) | 44 (SD, 11) | 46 (SD, 10) |
| BMI,* mean (SD) | 55 (SD, 11) | 59 (SD, 11) † |
| Female, % | 78 | 78 |
| Diabetes, % | 30 | 41‡ |
| Sleep Apnea, % | 52 | 63 |
| Hypertension, % | 56 | 67‡ |
BMI represents body mass index.
P < .005.
P < .05.
Reprinted from American Journal of Surgery, Vol. 187(5), Deveney CW, MacCabee D, Marlink K, Welker K, Davis J, McConnell DB. Roux-en-Y divided gastric bypass results in the same weight loss as duodenal switch for morbid obesity, 655-659. Copyright 2004 Exerpta Medica Inc.
Perioperation end points from the study are shown in Table 49. Patients who had BPD-duodenal switch stayed in hospital significantly longer.
Table 49: Perioperation End Points.
| Roux-en-Y Gastric Bypass (n = 237) |
Biliopancreatic Diversion With Duodenal Switch (n = 113) |
|
|---|---|---|
| Days in hospital, mean | 5.9 | 8.7* |
| Wound infection, n (%) | 47 (20) | 25 (22) |
| Postoperative anastomotic leak, n (%) | 8 (3) | 7 (6) |
| Mortality, n (%) | 2 (0.8) | 1 (0.9) |
P < .05
Reprinted from American Journal of Surgery, Vol. 187(5), Deveney CW, MacCabee D, Marlink K, Welker K, Davis J, McConnell DB. Roux-en-Y divided gastric bypass results in the same weight loss as duodenal switch for morbid obesity, 655-659. Copyright 2004 Exerpta Medica Inc.
Patient follow-up data from 1 or 2 years after the surgeries is presented in Table 50. Weight loss was expressed as a proportion of excess body weight.
Table 50: Follow-Up Weight Loss.
| Roux-en-Y Gastric Bypass (n = 57) |
Biliopancreatic Diversion With Duodenal Switch (n = 36) |
|
|---|---|---|
| Age of patients (M/F), mean years | 45 (12/45) | 44 (8/28) |
| BMI,* mean (SD) kg/m2 | 59 (10.9) | 64 (9.5) † |
| EBWL* at 12 months, mean (SD) | 54 (16) | 53 (11) |
| EBWL at 24 months, mean (SD) | 67 (18) | 63 (21) |
BMI represents body mass index; EBWL, excess body weight lost
P < .005
(Reprinted from American Journal of Surgery, Vol. 187(5), Deveney CW, MacCabee D, Marlink K, Welker K, Davis J, McConnell DB. Roux-en-Y divided gastric bypass results in the same weight loss as duodenal switch for morbid obesity, 655-659. Copyright 2004 Exerpta Medica Inc.)
A limitation of the study by Deveney et al. (35) is that it used a retrospective database design.
Flum et al. (36) retrospectively evaluated the short- and long-term mortality rates of patients with morbid obesity who had bariatric surgery and those that did not have surgery using the Washington State Comprehensive Hospital Abstract Reporting System database and the Vital Statistics database (level 4 evidence).
The study included all patients aged 19 to 65 years from 1987 to 2001 who had gastric bypass and an ICD-9 diagnostic code for obesity. The comparator group included patients of a similar age with a diagnosis of obesity or morbid obesity who did not have bariatric surgery.
Of the 66,109 obese patients examined, 3,328 patients had a bariatric procedure over the 15-year study period. The incidence of the procedure increased from 0.7 to 10.6 per 100,000 people from 1987 to 2001, with a 2.5-fold increase in incidence rate of the procedure after 1997, the in which year laparoscopic gastric bypass was first reported (incidence rate ratio 2.5; 95% CI, 2.4–2.7).
Thirty-four (1.02%) patients who had surgery died while in hospital. The 30-day mortality rate was 1.9% (n = 64), which indicates that about one-half of all of the early deaths occurred after discharge. The yearly rate of 30-day mortality did not significantly rise over time (incidence risk ratio 0.9; 95% CI, 0.9–1.0). The rate was 3.3% in the prelaparoscopic era, versus 1.8% in 1997 or later (P = .2). Using a multivariable logistic regression analysis, only surgical experience and the advanced Charlson comorbidity index were associated with an increase in the 30-day mortality rate. The association between the number of surgical procedures done by any surgeon and predicted probability of 30-day mortality is shown in Figure 7.
Figure 7: Analysis of Surgeon Experience and 30-Day Mortality Rate After Bariatric Surgery.*.
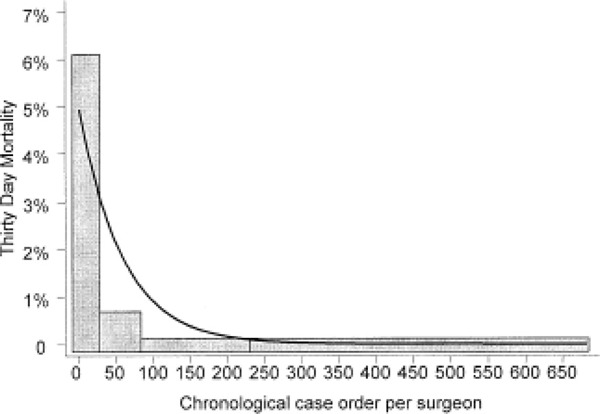
Predicted probability of 30-day mortality derived using logistic regression model with outcomes 30-day mortality and predictor variable case order, P = .001. The gray bars show quartiles of surgical experience and actual rates of 30-day mortality. Quartile 1 = case order 1 to 19, 30-day mortality rate 6.2%; Quartile 2 = case order 20 to 85, 30-day mortality rate, 0.73%; Quartile 3 = case order 86 to 220, 30-day mortality rate, 0.37%; Quartile 4 = case order 221 to 650, 30-day mortality rate, 0.34%.
(Reprinted from the Journal of the American College of Surgeons, Vol. 199, Flum DR, Dellinger EP. Impact of gastric bypass operation on survival: a population based analysis, 543-551 Copyright 2004 Exerpta Medica Inc.).
Eighty-one percent of all the cases in which a patient died within 30 days after the procedure were among the surgeon’s first 19 bariatric operations. In this dataset, 19% of surgeons performed fewer than 20 procedures in total.
Ten-year survival after surgery was high (91.2%), and the survival curves (adjusted for gender) and Charlson comorbidity index showed significant comparative benefit in survival for patients who had the surgery (P = .004). When the authors compared patient survival between groups starting at 1 year after hospitalization, they found that the hazard for death was significantly lower for those who had the surgery (hazard ratio 0.67; 95% CI, 0.54–0.85) after adjusting for age, gender, and the comorbidity index. The estimated reduction in mortality risk was not significant for patients who were younger than 40 (adjusted for gender and comorbidity) (hazard ratio 0.83; 95% CI, 0.49–1.44).
The limitations of the study by Flum et al. are as follows:
Patients defined as “obese” and “morbidly obese” were included. Bariatric procedures were defined using ICD-9 diagnostic codes for “morbid obesity” (developed in 1995) or “obesity” (before 1995) and excluded patients who had any other gastrointestinal diagnosis. BMI data were not reported.
It is unknown if hospital factors were associated with the 30-day mortality rate independently of surgeon factors.
Baseline characteristics differed between the groups.
Patients that died while in hospital were excluded from the comparator group. The authors stated this was done “to improve the chance that the comparator group was as healthy if not healthier than the operated patients.” Therefore, the comparison of 30-day mortality is not valid. Furthermore, the authors also stated, “We were more interested in comparing long-term survival in these populations.”
Case order was used as a proxy for surgeon experience generated by cases started after 1987 rather than as a true reflection of surgical case order.
The degree of obesity was not controlled in the 2 groups.
Comorbidity adjustment does not adequately deal with differences in comorbid disease severity.
Economic Analysis
Literature Review
Medical Services Advisory Committee
MSAC compared the cost of LAGB and open VBG. (24) Excluding revisions and complications, it estimated that LAGB is $3,665 more costly per patient treated in Australia. The incremental cost is due to the adjustable gastric band’s adjustment procedures and higher prosthetic, intensive care unit, and operating room costs. It is not offset by the shorter stay for patients who have LAGB. However, MSAC also found that lower rates of revision and complications in LAGB had a net clinical benefit when compared with open VBG. A maximum incremental cost-effectiveness ratio of $26,178 per quality-adjusted life year (QALY) can be inferred for LAGB compared with VBG. The authors cautioned that this estimate must be interpreted with caution. The health utility weights used to generate the QALY estimated in the NICE review, upon which the above estimate is based, were private industry data; therefore, they may not be applicable to the Australian setting.
In a cost comparison between LAGB and open RYGB, excluding revisions and complications, LAGB was estimated to be $912 more costly per patient treated in Australia. The incremental cost was due to LAGB adjustment procedures and greater prosthetic and theatre costs. It was not offset by the shorter stay or lower intensive care use in patients who have LAGB. Given the efficacy evidence presented by MSAC, LAGB was suggested to be weakly dominated (equivalent effectiveness and lower expected costs) by RYGB in the Australian setting.
National Health Service Research and Development Health Technology Assessment Program
Clegg et al.(5) in 2002 identified 4 economic evaluations: 2 from the United States, 1 from the Netherlands, and 1 from Sweden. When these studies were assessed on standard criteria of internal and external validity, all were considered poor quality.
Table 51 shows the results of the cost-effectiveness analysis by Clegg et al. All types of bariatric surgery were cost-effective when compared with nonsurgical management. AGB was cost-effective when VBG was considered as the standard treatment. AGB was not cost-effective when gastric bypass was considered as the standard treatment
Table 51: Net Cost per Quality-Adjusted Life Year (QALY) Gained for Each Intervention.
| Comparison | Additional QALYs |
Additional Cost (£) |
Net Cost per QALY Gained (£) |
|---|---|---|---|
| Vertical banding vs. nonsurgical management | 26 | 266,725 | 10,237 |
| Adjustable gastric banding vs. nonsurgical management | 45 | 383,102 | 8,527 |
| Adjustable gastric banding vs. vertical banding | 19 | 116,826 | 6,176 |
| Gastric bypass vs. nonsurgical management | 45 | 280,020 | 6,289 |
| Gastric bypass vs. vertical banding | 19 | 13,745 | 742 |
| Adjustable gastric banding vs. gastric bypass | 0.4 | 103,082 | 256,856 |
(Table reproduced with kind permission from the National Coordinating Centre for Health Technology Assessment: Clegg A, Colquitt J, Sidhu M, Royle P, Walker A. Clinical and cost effectiveness of surgery for morbid obesity: a systematic review and economic evaluation. Health Technol Assess 2002; 6(12).
Clegg et al. noted there were several limitations of the cost-effectiveness analysis:
Published studies often use different cut-off points to define obesity.
There is a continuous relationship between BMI and the risk of a disease, such as diabetes, that is not considered when examining the risk of disease according to BMI ranges.
Most studies looking at the impact of weight loss in obese people are short-term. However, long-term studies show these people have difficulty maintaining the weight loss. They should also distinguish intentional from unintentional weight loss. Unintentional weight loss may indicate disease-driven weight loss and is often associated with increased mortality and morbidity.
Neither the cost of plastic surgery nor the bariatric operating room equipment was included in Clegg’s economic analysis.
Gallagher et al. (37) examined the cost of RYGB in the Veteran’s Administration health care system. They reviewed the records of 25 patients who had RYGB from 1999 to 2000. They calculated all obesity-related health care costs, including hospitalizations and outpatient visits, medications, and home health devices, at 12 months before and after the RYGB. All figures are in US currency.
The mean age of the patients was 52 years (SD, 2 years). The mean preoperative BMI was 52 kg/m2 (SD, 2 kg/m2). Mean follow-up was 18 months. The total cost of preoperative care was $10,778 (SD, $2,460) per patient ($5,476 [SD, $682] for outpatient visits; $12,221 [SD, $6,062] for hospital admissions; and $1,383 [SD, $349] for home health devices). The postoperative length of stay was 8 days (SD, 0.5days).
The cost of the gastric bypass was $8,976 (SD, $497) per patient ($1,900 per patient for the operating room and $7,076 [SD, $497] for intensive care unit and ward care).
For the first postoperative year, 6 patients had 12 admissions, but routine outpatient visits fell significantly from 55 (SD, 6) to 18 (SD, 2) postoperatively (P < .001). The cost of all care, excluding perioperative charges for 1 year after RYGB, was $2,840 (SD, $622) per patient (P = .005, compared with preoperative charges).
A limitation of the economic analyses by Gallagher et al. is that it did not include the costs of plastic surgery or the bariatric operating room equipment.
In the United States, Craig et al. (38) estimated the cost-effectiveness (in 2001 United States currency) of gastric bypass versus no treatment from the payer perspective using a deterministic model (Figure 8). The target group of patients were men and women aged 35 to 55 years with a BMI from 40 to 50 kg/m2, who did not have cardiovascular disease and in whom conservative bariatric therapies had been unsuccessful. QALYs, life years, and cost were discounted during the patient’s lifetime.
Figure 8: Deterministic Decision Model Comparing Lifetime Expected Costs and Outcomes of Gastric Bypass and No Treatment of Severe Obesity From the Payer Perspective.
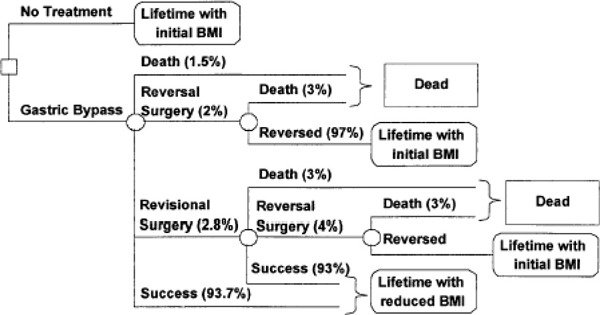
(Reprinted from the American Journal of Medicine, Vol. 113(6), Craig BM, Tseng DS. Cost-effectiveness of gastric bypass for severe obesity, 491-498. Copyright 2002 Exerpta Medica Inc.).
The base case cost-effectiveness ratios ranged from $5,000 to $16,000 (US) per QALY for women and from $10,000 to $35,600 (US) per QALY for men depending on age and initial BMI. These results suggest that gastric bypass is more cost-effective for women and those with a higher initial BMI (Figure 9). However, because the reduction in lifetime medical costs was not greater than the cost of treatment in any risk subgroup, the analysis did not show that gastric bypass saved costs.
Figure 9: Analysis of 4 Risk Subgroups Representing the Upper and Lower Bounds of the Cost-Effectiveness Ratios.
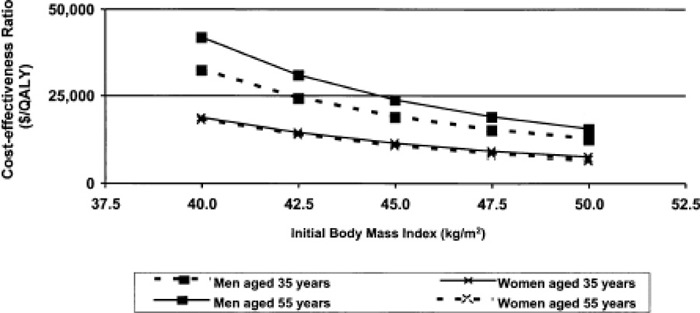
(Reprinted from the American Journal of Medicine, Vol. 113(6), Craig BM, Tseng DS. Cost-effectiveness of gastric bypass for severe obesity, 491-498. Copyright 2002 Exerpta Medica Inc.).
Craig et al. noted that because estimates of treatment effectiveness were based on case series and subject to patient selection bias, the authors set the lower bound of percentage loss of excess weight to 38%, more than one-third less than the base case estimate. Therefore, the cost-effectiveness ratio for a 45-year-old man with a BMI of 40 kg/m2 was $57,200 (US) per QALY. For a woman of the same age and BMI, it was $28,000 (US) per QALY. Further analysis suggested that the 38% estimate increased the cost-effectiveness ratio beyond $50,000 (US) per QALY for a few subgroups of older, less obese men.
Craig et al. concluded that gastric bypass does not save costs from the payer’s perspective. However, the cost-effectiveness ratio estimates compare favourably with those of other accepted interventions and appear robust to parameter variation, especially among women and younger, more obese men. Compared with no treatment, gastric bypass is cost-effective.
Limitations of the study by Craig et al. are as follows:
The sample consisted of patients who were severely obese but who did not have chronic medical conditions typically associated with obesity (i.e., diabetes, heart disease, and hypertension). Results may have been different if patients with comorbid conditions were included.
Included were patients who had been repeatedly unsuccessful at conservative interventions, which the authors acknowledge is in agreement with clinical guidelines (diet, exercise, and behaviour therapy). Of note, the authors stated that failure of pharmacotherapy is not a requirement, although it is common practice to attempt all conservative treatments before undergoing invasive procedures.
Several obesity-related costs were excluded because of insufficient evidence. Nonmedical costs were excluded. These included decreased productivity, lost wages, and other indirect costs associated with comorbid conditions.
Cooney et al. (39) examined the impact of a standardized care regimen or clinical pathway on hospital length of stay, resource use, and postoperative complications after gastric bypass surgery.
The clinical pathway was developed by examining conventional management, reviewing the literature, and discussing proposed changes in care with a multidisciplinary team of health care providers. Each phase of care was evaluated to determine if it should be included in the pathway (standardized approach to patient care). The authors stated that the process of recovery from the gastric bypass procedure was described as various phases or steps of care (postoperative pain control, fluid and electrolyte balance, pulmonary function, gastrointestinal function, deep vein thrombosis, prophylaxis mobility, etc.). Patients were compared before the pathway (n = 16) and after the pathway (n = 12).
The mean hospital length of stay was 3 days shorter in the postpathway group (P < .001) (Figure 10).
Figure 10: Impact of Gastric Bypass Pathway on Hospital Length of Stay.
The mean hospital length of stay (days) for patients undergoing gastric bypass surgery before initiating a clinical pathway (Pre) was compared with postpathway patients (Post). * P < .001.
(Reprinted from the Journal of Surgical Research, Vol. 98(2), Cooney RN, Bryant P, Haluck R, Rodgers M, Lowery M. The impact of a clinical pathway for gastric bypass surgery on resource utilization, 97-101. Copyright 2001; Exerpta Medica Inc.).
Total hospital costs were lower in the postpathway group. Postpathway savings were greatest for room and board (34%), supplies (41%), and laboratory and radiology costs (50%) (Table 52). An increase in operating room costs (22%) was observed in the postpathway group (Table 53). This was due to an increase in anesthesia time (epidural catheter placement) and equipment costs (ultrasonic shears).
Table 52: Impact of Gastric Bypass Clinical Pathway on Resource Use.
| Group | Pre | Post | Difference |
P value |
|---|---|---|---|---|
| Room$ | $3,641 ± 398.35 | $2,389 ± 346.82 | $–1,252 | 0.013 |
| OR$ | $3,467 ± 253.31 | $4,251 ± 152.19 | $+784 | 0.02 |
| Sup$ | $1.152 ± 194.06 | $679 ± 106.89 | $–473 | 0.06 |
| Lab/Rad$ | $629 ± 84.34 | $312 ± 112.29 | $–317 | 0.03 |
| Other costs | $1,098 ± 121.74 | $878 ± 179.34 | $–220 | 0.30 |
| Total$ | $1,0176 ± 788.71 | $8,511 ± 762.60 | $–1,665 | 0.15 |
Note. Room$, hospital room charge for length of stay: OR$, operating room costs: Sup$, cost of supplies for patient during the in hospital stay: Lab$/Rad$, cost of in-house laboratory studies in addition to the radiology exams performed; Misc$, miscellaneous cost which are those costs not contained in the above.
(Reprinted from the Journal of Surgical Research, Vol. 98(2), Cooney RN, Bryant P, Haluck R, Rodgers M, Lowery M. The impact of a clinical pathway for gastric bypass surgery on resource utilization, 97-101. Copyright 2001; Exerpta Medica Inc.).
Table 53: Operating Room Use by Gastric Bypass Patients.
| Group | Pre (min) | Post (min) | P value |
|---|---|---|---|
| AT (min) | 39 ± 5.0 | 51 ± 5.9 | 0.08 |
| PrepT (min) | 16 ± 1.23 | 22 ± 2.7 | 0.42 |
| SurgT (min) | 238 ± 11.61 | 215 ± 13.71 | 0.175 |
| WakeT (min) | 13 ± 1.39 | 15 ± 2.12 | 0.87 |
| ORT (min) | 306 ± 15.79 | 303 ± 12.72 | 0.55 |
Note. AT, anesthesia time (time in the door till beginning of prep time): PrepT, prep time including positioning and skin preparation: SurgT, total surgery time (from incision to dressing); WakeT, time from dressing to postanesthesia recovery; ORT, operating room time total.
(Reprinted from the Journal of Surgical Research, Vol. 98(2), Cooney RN, Bryant P, Haluck R, Rodgers M, Lowery M. The impact of a clinical pathway for gastric bypass surgery on resource utilization, 97-101. Copyright 2001; Exerpta Medica Inc.).
Limitations of the study by Cooney et al. are as follows:
There was a lack of long-term postoperative details (patient management after the patient leaves the hospital) for the postpathway plan (e.g., resolution of comorbid conditions).
The sample size was small.
In a follow-up study, (40) Cooney et al. reanalyzed hospital costs after identifying a 16% incidence of “cost outliers” after implementation of a clinical pathway. Medical records and costs for 91 gastric bypass patients were reviewed. Patients with costs more than 1 SD above the total mean hospital cost comprised the cost outlier group (n = 15). The other patients formed the normal cost group (n = 76).
Patient demographics were similar in both groups. Diabetes and severe medical comorbid conditions, especially sleep apnea and degenerative joint disease, were more common in the cost outlier group (60% versus 9.2%, P < .05). The incidence of major complications (33% versus 8%) was significantly higher in the cost outlier group (P < .05).
The authors concluded that despite utilization of a clinical pathway for gastric bypass surgery, 16% of patients were cost outliers. Factors associated with increased hospital costs after gastric bypass included severe comorbid conditions and the occurrence of major postoperative complications.
The updated study by Cooney et al. had all of the limitations of the original study. (39)
Huerta et al. (41) also examined the effect of a bariatric surgery (RYGB) clinical pathway on hospital cost and patient length of stay after its implementation in an academic health centre. The medical records of 182 consecutive patients that had RYGB were retrospectively reviewed before implementation of the pathway (Group 1). Data on length of stay, intensive care unit length of stay, standard variable cost, readmission rate, and rate of return to the operating room were collected. This information was compared with the data collected prospectively from 182 patients after implementing the pathway (Group 2).
The authors stated that the clinical pathway was developed by a committee of nurses, attending surgeons, internists, intensive care physicians, nutritionists, quality assurance specialists, and administrators to develop a uniformly agreed-upon approach to managing bariatric surgery patients.
Hospital cost per admission was 40% lower in Group 2 (P < .02). The mean length of stay fell from 4.05 days in Group 1 to 3.17 days in Group 2 (P < .033). The overall readmission rate fell from 4.2% in Group 1 to 3.2 % in Group 2 (P < .05). There were no between-group differences in morbidities. The authors concluded that implementation of a clinical pathway for bariatric surgery reduces costs and improves quality of care in an academic institution.
Postoperative management was bimonthly meetings with internists, nutritionists, surgeons, psychiatrists, and nurses. Patients were encouraged to exercise regularly, and there was an online support group.
The study by Huerta et al. did not provide long-term postoperative details (e.g., resolution of diabetes).
Using data from the SOS study, Narbro et al. (42) did a cross-sectional comparison of the use of prescribed pharmaceuticals in 1286 obese people in the SOS intervention study and 958 randomly selected reference patients. The SOS study consists of 2 parts: a cross-sectional registry study and a controlled prospective intervention study that began in 1987. In addition, a cross-sectional population study of randomly selected people (1752 men and women aged 37 to 60) from the general population during 1994 to 1999 was examined. Medication changes for 6 years after bariatric surgery were evaluated in 510 surgically and 455 conventionally treated SOS study patients. The inclusion criteria for the SOS intervention study were age 37 to 60 years and a BMI of at least 34 kg/m2 for men and at least 38 kg/m2 for women. The SOS reference study is a population study.
The authors did a cross-sectional investigation comparing baseline data on the use and cost of medications from the first 1,294 consecutive patients (surgically and conventionally treated patients combined) in the SOS intervention study with corresponding data from baseline examinations in the reference study. Data on medications were available for 958 (54.7%) patients in the reference population and 1286 (99.4%) patients in the SOS intervention study.
To estimate the effect of surgical treatment and weight reduction on the use and cost of medications for 6 years, a longitudinal comparison was undertaken on the first 647 surgically treated patients and the first 647 conventionally treated patients from the SOS intervention study. These 1294 patients included between 1987 and 1992 were compared with the reference study (1994–1997). Six-year follow-up data were unavailable for 137 (21.2%) patients in the surgical group and 192 (29.7%) patients in the conventionally treated group.
Information on prescribed medications, including dosage, was collected from questionnaires filled out by all participants at inclusion in the SOS reference and intervention studies. The same questionnaires were used at the 6-month and 1, 2, 3, 4, and 6-year follow-up visits in the SOS intervention study. Temporary medications were excluded, and 1 person in the intervention study was excluded from the calculations because of extreme costs for cancer medications. The daily costs were calculated for each drug using the 1997 official price list of the National Corporation of Swedish Pharmacies.
Participants were asked about drug use during the previous 3 months. Assuming use of medications was the same for the whole period covered by the questionnaire, the daily costs were summed for each drug and individual to estimate the drug-specific medication cost for each period. To estimate the mean yearly cost during the 6-year follow-up after obesity treatment, a weighted mean of the period costs was calculated. All costs were reported in Swedish kronor (SEK).
Compared with the reference group, obese patients were significantly more often taking diabetes, cardiovascular disease, nonsteroidal anti-inflammatory and pain, and asthma medications (risk ratios ranging from 2.3–9.2). The mean annual costs for all medications were 1,387 (SEK; US $140) in obese patients and 783 (SEK; US $80) in the reference population (P < .001). The mean yearly medication costs during follow-up were 1849 (SEK; US $185) in surgically treated patients (weight change -16%) and 1,905 (SEK; US $190) in weight-stable conventionally treated patients (P = .87). The surgical group had lower costs for diabetes mellitus (difference -94 SEK/year [-US $9]) and cardiovascular disease medications (difference: 186 SEK/year [-US $19]) but higher costs for gastrointestinal tract disorder (difference: +135 SEK/year [US $13]), and anemia and vitamin deficiency medications (difference: +50 SEK/year [US $ 5]).
The authors concluded that the use and cost of medications are higher in obese versus reference populations. Surgical treatment for obesity lowers the cost of medications for diabetes mellitus and cardiovascular disease, but it increases other medication costs, thereby resulting in similar total costs for surgically and conventionally treated obese individuals for 6 years.
Limitations of the study by Narbro et al. are as follows:
Baseline data for the intervention study population and the medication data for the general reference population were collected during different periods. Therefore, the introduction and use of new drugs may have affected the results.
Data on medication use were available for approximately 55% of the reference population, and the health status of the nonrespondents was unknown.
The SOS intervention study was based on self-selected patients who were recruited by advertisements in public media.
Information on the use of medications was self-reported and was collected from questionnaires. This may have affected the reliability of the data.
The study was not restricted to patients with morbid obesity: Inclusion criteria did not use the commonly accepted cut-off of a BMI of 40 or of 35 and comorbid conditions.
Nguyen et al. (43) compared the costs of laparoscopic RYGB (n = 79) with open RYGB (n = 76) in a single-centre RCT conducted in the United States. The patient outcomes of this study have been included in the literature searches of previous health technology assessments.
Direct operative costs were higher for laparoscopic versus open RYGB ($4,992 [SD, $1927] vs. $3,591 [SD, $1000], P < .01). Laparoscopic RYGB required more Endo GIA (stapler) reloads than did open RYGB (13.4 [SD, 5.7] vs. 5.6 [SD, 2.7], P < .01). Hospital service costs were lower in the laparoscopic RYGB group ($2,519 [SD, $1712] vs. $3,742 [SD, $3978], P = .02). Nursing costs were lower for laparoscopic RYGB ($1,201 [SD, $821] vs. $1,975 [SD, $2773], P = .03). There were no significant differences in direct, indirect, or total costs between groups.
Nguyen et al. did not provide any data on the amount of postoperative pain, functional and social disability, or lost productivity.
Monk et al. (44) retrospectively studied the first 100 patients who had undergone RYGB at a community teaching hospital in the United States. Sixty-four patients had adequate follow-up data available. The mean BMI was 57 kg/m2 (range 36.6–85.4 kg/m2). The mean monthly medication expenditure fell from $317 (US) (SEM, $47.25; range $23.12–$1,801.19) preoperatively to $135 (SEM, $35.35; range $0.00–$1,122.72) postoperatively (P < .01).
A limitation of the study by Monk et al. is that is was a retrospective single-centre case series.
Potteiger et al., (45) using retrospective data from the electronic database of 51 consecutive patients who had RYGB, reviewed prescription records preoperatively and at 3 and 9 months postoperatively.
The prevalence of diabetes and hypertension was 55.7% (29/53) and 44.3% (24/53), respectively; 34% (18/53) of patients had both. Preoperatively, patients were taking a mean 2.44 (SD, 1.86) medications that cost $187.24 (SD, $237.41) per month. Postoperatively, the mean number of medications fell to 0.56 (SD, 0.81; P < .001) at a monthly cost of $42.53 (SD, $116.60; P < .001).
Limitations of the study by Potteiger et al. include these:
It was a retrospective case series
There were no long-term data.
Sampalis et al. (46) did an observational 2-cohort study comparing patients who had bariatric surgery between 1986 to 2002 to a control group of age- and gender-matched obese patients who had not undergone weight reduction surgery from the Quebec health insurance database. This study used data analyzed by Christou et al. (29) already reviewed in this report. The cohorts were followed-up for up to 5 years. The primary outcome measure was overall direct health care costs. Secondary outcomes included cost analysis by diagnostic category for the treatment of new medical conditions after cohort inception.
Sampalis et al. found significant reductions in the mean EWL (67.1%, P < .001) and in the percent change in BMI (34.6%, P < .001) in the bariatric surgery cohort. Similar data were not reported for the control group.
The mean total cost per 1,000 patients for the 5 years following cohort inception are shown Table 54. After 5 years, the total cost per 1,000 patients for hospitalizations in the control cohort was 29% higher than for the bariatric patients (absolute difference = $5.7 million in 1996 Canadian currency in favour of weight reduction surgery).
Table 54: Mean Total Cost per 1,000 Patients for the 5 Years After Cohort Inception. From: Sampalis et al. (46).
| Year of follow-up | Cost for Bariatric Patients (Cdn) |
Cost for Control Patients (Cdn) |
Absolute Difference in Cost (Cdn) |
Cost ratio; Control/Bariatric |
|---|---|---|---|---|
| 1 | $12,461,938 | $3,609,680 | $8,852,258 | 0.29 |
| 2 | $3,398,835 | $4,846,794 | $1,447,959 | 1.43 |
| 3 | $1,362,408 | $5,831,456 | $4,469,048 | 4.28 |
| 4 | $1,318,323 | $5,895,988 | $4,577,666 | 4.47 |
| 5 | $975,163 | $5,080,690 | $4,105,526 | 5.21 |
| Total | $19,516,667 | $25,264,608 | $5,747,941 | 1.29 |
(Copyright © FD-Communications. Reproduced with kind permission from Obesity Surgery. Sampalis JS, Liberman M, Auger S, Christou NV. The impact of weight reduction surgery on health-care costs in morbidly obese patients. Obesity Surgery 2004; 14(7):939-947.
Sampalis et al. (46) said after 3.5 years, the initial investment for the weight-reduction surgery and related hospital care was compensated by a reduction in total costs, which corresponded to an expected amortization period of 3.5 years of the initial investment for bariatric surgery.
Limitations of the study by Sampalis et al. (46) are as follows:
All of the limitations of Christou et al. (29)
The design of the study was retrospective.
Agren et al. (47) looked at hospitalization length and costs for 962 surgically and conventionally treated obese patients from the SOS intervention study who were followed-up for 6 years.
After 6 years, weight change was -16.7% in the surgical group and +0.9% in the control group (P < .0001). The cumulative hospital stay over 6 years was 23.4 days in the surgical group and 6.9 days in the control group (P < .0001). The mean hospital cost for the surgical intervention was $4,300 (US). Incremental costs that could be attributable to obesity surgery averaged $1,200 (US) per year. After excluding hospitalizations for the surgical intervention and conditions common after bariatric surgery, there were no significant group differences in number of hospital days or hospitalization costs.
Agren and colleagues concluded that the mean weight reductions of 16% did not lower the hospitalization costs over 6 years. The lag time between improved cardiovascular risk factors induced by weight loss and an assumed lower incidence of cardiovascular disease that could decrease inpatient care is unknown.
Limitations of the study by Agren et al. are as follows:
All of the limitations of the SOS study.
It is possible that hospitalization costs would be reduced in a 10- to 20-year perspective.
In the United States, DeMaria et al. (48) compared the outcomes of patients who had hand-assisted laparoscopic gastric bypass (n = 25) with those who had the open procedure (n = 62). Some surgeons use hand-assisted procedures to treat obesity because it is easier to do than the total laparoscopic procedure.
Preoperatively, hand-assisted gastric surgery patients did not differ significantly from open gastric bypass patients on age, sex, BMI, weight, or comorbid conditions. Length of hospital stay did not differ significantly between the groups (hand-assisted: 3.6 days (SD, 1.3); open: 4.2 days (SD, 4.6). However, total hospital costs were higher for the hand-assisted procedure: $14,725 (SD, $3089; US) versus $10,281 (SD, $3687; US; P < .01).
Follow-up showed that the risk of postoperative complications was similar for the hand-assisted and open surgery groups. This included marginal ulcer (16% versus 14.5%), stomal stenosis (24% versus 23%), and incisional hernia (20% versus 27%). There were no major wound infections or deaths in either group. One patient in each group developed a postoperative small bowel obstruction. Loss of excess weight in hand-assisted patients at 12 months postoperatively was 66% (SD, 14%). In the patients who had the open procedure, it was 77% (SD, 14%). The difference was not statistically significant.
A limitation of the study by De Maria et al. is that disability and time and return to work were not quantified.
Ontario-Based Economic Analysis
Hospitalization Costs
A total of 283 hospital separations were isolated from the discharge abstracts database in fiscal year 2003 with the most responsible ICD-10/CA diagnosis codes and ICD-10/CA CCI procedure codes contained in Appendix 4. Of this total, 270 were funded by the province of Ontario, 10 were self-pay, and 3 were funded by the Department of Veteran Affairs.4 To determine the cost per case, data from the Ontario Case Costing Initiative (OCCI) was utilized rather than the normal practice of converting PAC-10 weights to dollars due to the overly broad range of patients in the case-mix group of which bariatric surgery patients are included and upon which the weights are calculated. The OCCI is based on a subsample of hospital facilities (n = 11) at sixteen sites in Ontario, and it provides cost data by hospital functional centre from each of these facilities. We applied the same ICD-10 CA diagnosis codes and ICD-10 CA CCI procedure codes in Appendix 4 producing a total of 5 hospital separations from the 11 OCCP facilities in FY 2003 and 40 in FY 2002. Given the small sample obtained for FY 2003, it was decided to use the FY 2002 averages. The average cost for the 40 cases during FY 2002 was $13,160 with a range of $4,504 to $107,814.
In addition, approximately 29% of patients who received gastric bypass procedures in-province during the period FY 1997 – FY 2001 eventually required a panniculectomy – on average about 2 years following the bariatric surgery procedure - the per-case expected cost being $843 (= 29% x $2,908 avg. OCCI cost for Panniculectomy5 during FY 2003).
Assuming that all of these cost figures are generalizable to the 283 hospital separations identified in the discharge abstracts database during FY 2003, the total cost of hospitalizations associated with bariatric bypass surgery including panniculectomy is estimated to have been $4.0 M.6
Professional (OHIP) Costs
Based on analyses provided by the Provider Services Branch, MOHLTC, the following OHIP costs were identified for those treated for morbid obesity:
$366 = pre-operative consultation and visits - surgeon, anaesthesia, cardiology etc.
$314 = pre-operative testing (dependent on health of patient) abdominal x-rays, ECG, ultrasound, etc. (professional and technical fees).
$2,065 = day of surgery - surgical, anaesthetist, surgical assistant, premium codes for obesity, age, etc.
$612= post-operative care or services rendered during 8 days post-surgery
$3,356 = Total OHIP costs7
Therefore, the total cost (hospitalization + OHIP costs) per case is $17,350. The current annual total cost for this procedure for the 270 cases funded by the province is approximately $4.7 million. Given that Ontario spent $8.2 million (Cdn) on 225 out-of-country bariatric procedures in fiscal year 20048, the total budget for this procedure is currently about $12.9 million per year. This represents approximately a 3-fold increase in expenditures over out-of-country cases performed in FY 2002. If out-of-country procedures were performed in Ontario, the difference in costs of approximately $20,100 per case9—a total annual difference of $4.5 million (Cdn)—could be reallocated to create new surgical units or to expand existing capacity.10 By repatriating the current annual load of 225 out-of-country patients to Ontario, the net difference could be used to fund an additional 250 procedures annually for a total of approximately 760 (≈ 283 + 225 + 250) annual procedures. (This analysis assumes that both the provincial and out-of-country expenditures represent hospital, physician and lab costs for pre-operative consultations, the peri-operative period, and post-operative follow-up including the treatment of complications.)
If AGB became an officially recognized insured service, the cost would likely be greater, given that AGB is estimated to cost $912 AU (about $800 Cdn) more than current bypass procedures. (MSAC, 2003). However, given that hospitals and the wider health care system will likely be able to capture any savings associated with lap-banding, the likely incremental cost for Ontario will be the added cost of the device (approximately $3,000). Approval of this procedure would provide added clinical choice that in some cases would be better than currently insured bariatric procedures; however, there likely would be a tradeoff with cost to the health system.
Capitalization Costs
This analysis does not include one-time capitalization costs for start-up of new bariatric surgery centres in the Province of Ontario. Given the lack of interchangability of much of the needed technology needed for morbidly obese patients (operating rooms, specialized beds, lifts, etc.), the capitalization costs could be substantial to the point that the amortized value of these costs over time could potentially impact upon the decision as to whether to continue to send patient out-of-country rather than increase capacity for such surgery in Ontario.
Diffusion Pressure
Given that there are between 160,000 and 180,000 morbidly obese residents of Ontario, a figure that is growing, there could be substantial demand for such procedures. There were 110,000 bariatric surgeries performed in the United States in 2003. This is equivalent to about 4,200 bariatric procedures in Ontario (3.8% of the United States population × 110,000 procedures), if Ontario were to achieve the levels of the United States. Supposing that it set a goal slightly below the American level of 3,500 annual procedures, Ontario would need to fund an additional 2,740 procedures at approximately $17,350 or $47.5 million in additional annual outlays.11 Furthermore, if AGB were to replace bypass procedures eventually, the additional annual outlay could exceed $58.0 million due to the incremental cost of the $3,000 device. Given the prevalence in Ontario of morbid obesity at 160,000, the goal of 3,500 annual procedures would mean that approximately 2.2% of this population would be serviced per year and approximately 11% would be serviced over five years assuming that prevalence figures remain relatively steady. Given some rise in incidence of this condition, it might be safer to say that approximately 10% of the population would be serviced over the next five years. Table 55 shows a summary of the additional cost associated with increasing use and replacement of bypass by AGB. It assumes that all procedures would be performed in the province.
Table 55: Additional Costs Associated with Increasing Use and Replacement of Gastric Bypass by Adjustable Gastric Banding (in Millions).
| Incremental Annual Budget Impact | ||||||
|---|---|---|---|---|---|---|
| Annual Number of Bariatric Surgeries in Ontario | ||||||
| 760 | 2500 | 3000 | 3500 | |||
| 0% | $0 | $30.2 M | $38.9 M | $47.5 M | ||
| % | 10% | $0.2 M | $30.9 M | $39.8 M | $48.6 M | |
| Adjustable gastric banding | 25% | $0.6 M | $32.1 M | $41.1 M | $50.2 M | |
| 50% | $1.2 M | $33.9 M | $43.4 M | $52.8 M | ||
| 100% | $2.3 M | $37.7 M | $47.9 M | $58.0 M | ||
This figure assumes that 250 more procedures over current numbers (508) could be funded from savings achieved by repatriating to Ontario the 225 annual cases now done out-of-country and funded by the Ontario government. All figures are in millions.
Downstream Cost Savings
Bariatric surgery procedures are known to lead to the resolution of several comorbid illnesses, including diabetes, hypertension, and dyslipidemias, that are very expensive to treat. The annual health care costs of treating morbidly obese patients and their comorbid conditions often exceed $10,000 per annum; therefore, the downstream cost savings associated with bariatric surgery may be substantial.
Disclaimer: This economic analysis represents an estimate only, based on assumptions and costing methodologies that have been explicitly stated. These estimates will change if different assumptions and costing methodologies are applied for the purpose of developing implementation plans for the technology.
Existing Guidelines for Use of Technology
United States Centers for Medicare and Medicaid Services
The policy of the United States Centers for Medicare and Medicaid Services (CMS; 2004) (49) for the treatment of obesity alone has been and continues to be that it does not cover it. In 2001, the Centers for Disease Control contacted CMS and asked that the language in the National Coverage Determination Manual be revised to reflect current social and clinical concerns about obesity. On July 15, 2004, CMS announced it would remove “obesity itself cannot be considered an illness.” By doing so, it removed barriers to covering obesity interventions if scientific and medical evidence show they are effective.
CMS does not believe it is appropriate to address the definition of illness in the manual, because the manual is intended to address the coverage of particular care and services rather than the definition of illness. Medicare does reimburse for treatments of diseases that cause, or are made worse by, obesity, particularly those affecting morbidly obese people.
The policy on obesity in the National Coverage Determination Manual, Section 40.5, Treatment of Obesity is as follows: (49)
“Obesity may be caused by medical conditions such as hypothyroidism, Cushing’s disease, and hypothalamic lesions or can aggravate a number of cardiac or respiratory disease as well as diabetes and hypertension. Services in connection with the treatment of obesity are covered services when such services are an integral and necessary part of a course of treatment for one of these medical conditions. However, a program payment may not b made for treatment of obesity unrelated to such a medical condition since treatment in this context has not been determined reasonable and necessary.”
Gastric bypass surgery for extreme obesity is covered by the program under these conditions:
It is medically appropriate for the person to have such surgery, and
The surgery is performed to correct an illness that caused or was aggravated by the obesity.
Other bariatric surgical procedures may be covered under contractor discretion if they meet the requirements of the local contractors and are consistent with the national policy on obesity.
American Society for Bariatric Surgery/Society of American Gastrointestinal Endoscopic Surgeons Guidelines for Laparoscopic and Conventional Surgical Treatment of Morbid Obesity
Summary (May 2000):
“Morbid obesity is a significant health concern. Medical management fails to sustain weight loss and management of the comorbidities is expensive and often ineffective. Bariatric surgery currently provides the only significant sustained weight loss. Laparoscopic techniques based on their open counterparts are available. When performed by appropriately trained surgeons, laparoscopic approaches appear to hasten the patient’s recovery and return to normal function. Experience and training in bariatric surgery, advanced laparoscopic surgery skills and a commitment to long-term patient management are required.” (50)
National Institutes of Health Consensus Conference on Surgical Treatment of Morbid Obesity
Evidence Statement (September, 1998):
“Surgical interventions in adults with a BMI > 40 or a BMI > 35 with comorbid conditions resulting a substantial weight loss. Evidence Category B.” (51)
Recommendation:
“Surgical intervention is an option for carefully selected patients with clinically severe obesity (BMI > 40 or > 35 with comorbid conditions) when less invasive methods of weight loss have failed and the patient is at high risk for obesity-associated morbidity and mortality. Evidence Category B.” (51)
Definition of Evidence Category B:
RCTs (limited body of data)
“Evidence is from endpoints of intervention studies that include only a limited number of RCTs, post hoc or subgroup analysis of RCTs, or meta-analysis of RCTs. In general, Category B pertains when few randomized trials exist, they are small in size, and the trial results are somewhat inconsistent, or the trials were undertaken in a population that differs from the target population of the recommendation.” (51)
United States: Blue Cross/Blue Shield
Blue Cross/Blue Shield (April 2004) states that bariatric surgery is considered medically necessary if the medical appropriateness criteria are met. (52)
Any device utilized for this procedure must have FDA approval specific to the indication; otherwise, it will be considered investigational. (52)
Medical Appropriateness
Bariatric surgery for the treatment of morbidly obese individuals 18 years or older is medically appropriate if all of the following criteria are met:
1. The person has a diagnosis of morbid obesity that has persisted for at least 5 years and is defined as either:
More than 45 kg (100 pounds) over the ideal weight, or at least twice the ideal weight. The ideal body weight can be determined from the Metropolitan Life Health and Weight table; or
BMI is greater than 40 kg/m2; or
-
BMI is greater than or equal to 35 kg/m2 in conjunction with any of the following obesity related comorbid medical conditions that will reduce a person’s life expectancy:
Coronary heart disease; or
Type 2 diabetes; or
Obstructive sleep apnea; or
3 or more of the following cardiac risk factors:
Hypertension (BP greater than 140 mm Hg systolic and/or 90 mm Hg diastolic); or
High density lipoprotein less than 40 mg/dL; or
Low density lipoprotein greater than 100 mg/dL; or
Impaired glucose tolerance (2-hour blood glucose greater than 140 mg/dL on an oral glucose tolerance test); or
Family history of early cardiovascular disease in first- degree relative (myocardial infarction at 50 years or younger in a male relative or at 65 years or younger in a female relative); and
2. There must be documentation of medical evaluations with a history of medical/dietary therapy failures (e.g., low calorie diet, increased physical activity, and behavioural reinforcement). The provider must submit the following:
Evidence that the attempt at conservative management was within 2 years prior to the planned surgery. An attending physician who is managing the care and weight loss of the individual recommends the bariatric surgery and documents the failure of conservative management. The physician must be someone other than the operating surgeon and his/her associates.
Documentation of the person’s willingness to comply with both the preoperative and postoperative treatment plans recommended by a licensed mental health provider.
United States: Aetna Clinical Policy Bulletin
Roux-en-Y Gastric Bypass
Aetna (February, 2004) (53) considers open or laparoscopic gastric bypass medical necessary when the selection criteria listed below are met.
1. Presence of severe obesity that has persisted for at least 5 years defined as either:
BMI over 40 or
-
BMI over than 35 with any of the following comorbid conditions:
Coronary heart disease; or
Type 2 diabetes; or
Clinically significant obstructive sleep apnea (i.e., patient meets the criteria for treatment of obstructive sleep apnea set forth in Aetna’s Clinical Policy Bulletin for Obstructive Sleep Apnea: Diagnosis and Treatment); or
Medically refractory hypertension (blood pressure >140 mm Hg systolic and/or 90 mm Hg diastolic despite optimal medical management);
and
2. Patient has completed growth (18 years of age or documentation of completion of bone growth); and
3. Member has attempted weight loss in the past without successful long-term weight reduction; and
4. Member must meet either criterion a (physician-supervised nutrition and exercise program) or criterion b (multidisciplinary surgical preparatory regimen):
a) Physician-supervised nutrition and exercise program: Member has participated in a physician supervised nutrition and exercise program (including dietician consultation, low calorie diet, increased physical activity, and behavioural modification), documented in the medical record. This physician-supervised nutrition and exercise program must meet all of the following criteria:
Nutrition and exercise program must be supervised and monitored by a physician working in cooperation with dieticians and or nutritionists; and
Nutrition and exercise program(s) must be for a cumulative total of 6 months or longer in duration, with participation in one program of at least 3 consecutive months prior to surgery. (Precertification may be made prior to completion of nutrition and exercise program as long as a cumulative of 6 months participation in nutrition and exercise program(s) will be completed prior to the date of surgery.); and
Nutrition and exercise program must occur within the 2 years prior to surgery; and
Member’s participation in a physician supervised nutrition and exercise program must be documented in the medical record by an attending physician who supervised the member’s participation. The nutrition and exercise program may be administered as part of the surgical preparative regimen, and participation in the nutrition and exercise program may be supervised by the surgeon who will perform eth surgery or by some other physician. Note: a physician’s summary letter is not sufficient documentation. Documentation should include medical records of physician’s contemporaneous assessment of patient’s progress throughout the course of the nutrition and exercise program. For members who participate in a physician administered nutrition and exercise program, program records documenting the member’s participation and progress may substitute for physician medical records.
or
b) Multidisciplinary surgical preparatory regimen: Proximate to the time of surgery, member must participate in an organized multidisciplinary surgical preparatory regimen of at least 3 months and meet all of the following criteria, to improve surgical outcomes, reduce the potential for surgical complications, and establish the member’s ability to comply with postoperative medical care and dietary restrictions:
Consultation with a dietician or nutritionist; and
Reduced calorie diet program supervised by dietician or nutritionist; and
Exercise regimen (unless contraindicated) to improve pulmonary reserve prior to surgery, supervised by exercise therapist or other qualified professional; and
Behaviour modification program supervised by qualified professional; and
Documentation in the medical record of the member’s participation in the multidisciplinary surgical preparatory regimen. (A physician’s summary letter, without evidence of contemporaneous oversight, is not sufficient documentation. Documentation should include medical records of the physician’s initial assessment of the member and an assessment of the member’s progress at the completion of the multidisciplinary surgical preparatory regimen).
and
5. For members who have a history of severe psychiatric disturbance (schizophrenia, borderline personality disorder, suicidal ideation, severe depression), or who are currently under the care of a psychologist/psychiatrist, or who are on a psychotropic medications, a preoperative psychological evaluation and clearance is necessary to exclude members who cannot provide informed consent or who are unable to comply with the preoperative and postoperative regimen. Note: the presence of depression due to obesity is not normally considered a complication to obesity surgery.
Vertical Banded Gastroplasty and Laparoscopic Adjustable Silicone Gastric Banding (LASGB or Lap-Band)
Aetna considers open or laparoscopic VBG or LASGB, Lap-Band medically necessary for people who meet the selection criteria for obesity surgery and who are at increased risk of adverse consequences of a RYGB due to the presence of any of the following comorbid medical conditions:
Hepatic cirrhosis with elevated liver function tests; or
Inflammatory bowel disease (Crohn’s disease or ulcerative colitis); or
Radiation enteritis
Demonstrated complications from extensive adhesions involving the intestines from prior major abdominal surgery, multiple minor surgeries or major trauma; or
Poorly controlled systemic disease (American Society of Anesthesiology, Class IV).
Repeat Bariatric Surgery
Aetna considers medically necessary surgery to correct complications from bariatric surgery, such as obstruction or stricture. Furthermore, Aetna considers repeat bariatric surgery medically necessary for members whose initial bariatric surgery was medically necessary (i.e., those who met medical necessity criteria for their initial bariatric surgery), and who meet either of the following medical necessity criteria:
Conversion to a RYGB may be considered medically necessary for members who have not had adequate success (defined as a loss of more than 50% of EWL), 2 years following the primary bariatric surgery procedure and the member has been compliant with a prescribed nutrition and exercise program following the procedure; or
Revision of a primary bariatric surgery procedure that has failed due to dilation of the gastric pouch, is considered medically necessary if the primary procedure was successful in inducing weight loss prior to the pouch dilation, and the member has been compliant with a prescribed nutrition and exercise program following the procedure.
Bariatric Surgical Procedures That Are Not Covered
Aetna does not consider any of the following procedures established because the peer-reviewed medical literature shows them to be either unsafe or inadequately studied:
Loop gastric bypass
Gastroplasty, more commonly know as “stomach stapling”
Duodenal switch operation
Biliopancreatic bypass (Scopinaro procedure)
Mini gastric bypass
LASGB, except in limited circumstances noted above
VBG except in limited circumstances noted above
Note on Cholecystectomy:
As a high incidence of gallbladder disease (28%) has been documented after surgery for morbid obesity, Aetna considers routine cholecystectomy medically necessary when done with elective bariatric surgery.
United Kingdom: National Institute for Clinical Excellence
For the purposes of the guidance by NICE (July 2002), people are defined as having morbid obesity if they have a BMI either equal to or greater than 40 kg/m2, or from 35 to 40 kg/m2 with serious comorbid conditions that could be improved by weight loss.
People with morbid obesity who are considering surgery to aid weight reduction should discuss in detail with the clinician responsible for their treatment (that is, the hospital specialist and/or bariatric surgeon) the potential benefits and longer-term implications of surgery, as well as the associated risks, including complications and postoperative mortality.
Surgery is recommended as a treatment option for people with morbid obesity providing all of the following criteria are fulfilled:
This type of surgery should be considered only for people who have been receiving intensive management in a specialized hospital obesity clinic.
People should be aged 18 years or over.
There should be evidence that surgical candidates have tried all appropriate and available nonsurgical measures, but have not been able to maintain weight loss.
There should be no specific clinical or psychological contraindications to this type of surgery.
People should be generally fit for anesthesia and surgery.
People should understand the need for long-term follow-up.
Surgery should be undertaken only after comprehensive, multidisciplinary assessment. In addition, arrangements should be made for appropriate health care professionals to provide preoperative and postoperative counselling and support to individuals being considered for surgery.
Given the uncertainty of the evidence on the relative safety and effectiveness of different surgical interventions, it is not possible to distinguish between them on grounds of cost-effectiveness. The choice of surgical intervention should therefore be made jointly by the person and the clinician after considering the best available evidence, the facilities and equipment available, and the experience of the surgeon who would perform the operation.
Databases should be established by hospitals wanting to develop their service, to enable the outcomes and complications of different procedures, including their impact on QoL, to be monitored in the short and long term.
It is important that services and skills to support surgery for people with morbid obesity are developed in a planned and coordinated way. Existing collective mechanisms for specialist commissioning groups may offer an appropriate way for the development of detailed implementation strategies. Each group, in discussion with other groups, should decide how best to develop and expand the service, and the number of sites from which it should be provided.
Appraisal
Policy Considerations
Ontario Data
The Ontario Health Insurance Plan (OHIP) Schedule of Benefits for Physician Services includes fee code “S120 gastric bypass or partition, for morbid obesity” as an insured service. Gastric bypass is a general term that encompasses a variety of methods, all of which involve a reconfiguration of the digestive system. The term gastric bypass does not include adjustable gastric banding.
The number of gastric bypasses done in Ontario and as actual out-of-country cases12 is shown in Figure 11. The number of actual out-of-country gastric bypasses, and the amount paid by the Ministry of Health and Long-Term Care, is shown in Table 56.
Figure 11: Number of Gastric Bypass Procedures by Fiscal Year: Ontario and Actual Out-of-Country Cases.
2004/05: projection for last quarter
Data from Provider Services, MOHLTC
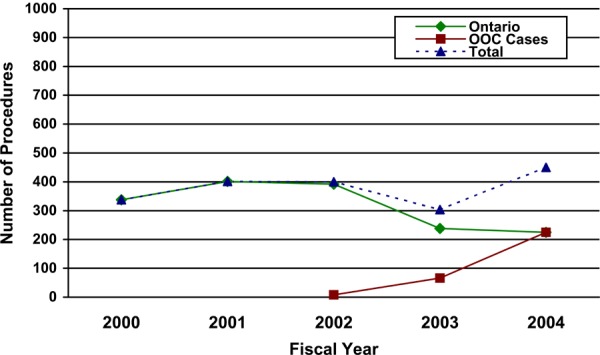
(Data for Figure 11 provided courtesy of Provider Services, Ministry of Health and Long Term Care
Table 56: Number of Actual Out-of-Country Gastric Bypasses Cases and Amount Paid By the Ministry of Health and Long-Term Care. (54).
| 2002/2003 | 2003/2004 | 2004/2005 Year-to-Date | |
|---|---|---|---|
| Number of Services | 8 | 66 | 225 |
| Amount Paid | $320,000 | $3,092,325 | $8,232,370 |
(Data for Table 56 provided courtesy of Provider Services, Ministry of Health and Long Term Care)
Note: The data for FY 2004 is current as of May 18, 2005, but may not be complete due to the fact that providers will continue to submit claims for services rendered within this FY for some time after the FY ends.
Note: The data for FY 2004 is current as of May 18, 2005, but may not be complete due to the fact that providers will continue to submit claims for services rendered within this FY for some time after the FY ends.
Why might Ontario hospitals be inclined to discourage gastric bypass surgery?
It is complex surgery that ties up operating rooms.
Hospital stays usually exceed one week even when there are no complications.
The patients require special equipment (e.g., beds, operating tables, toilets, lifts, etc.) to accommodate their extreme weight.
There is a high risk of back injury to nurses who work with these patients.
There may be a perception that gastric bypass surgery is not urgent.
An expert estimated there are about 160,000 people in Ontario who are morbidly obese.
OHIP considers requests for funding cosmetic surgery that may be required following significant weight loss (for any reason, not just bariatric surgery) on a case by case basis. These services are generally only covered in situations where there is significant symptomatology such as pain, ulceration or functional impairment.
Diffusion
In the United States, the number of bariatric surgeries done annually for severe obesity rose from about 16,000 in the early 1990s to about 103,000 in 2003 (Figure 12). (55) Steinbrook (55) suggested this has been fuelled by a rise in the number of people who are extremely obese; the failure of diets, exercise, and medical therapy; and the advent of laparoscopic procedures. The number of practising surgeons who are members of the American Society for Bariatric Surgery went from 258 in 1998 to 1070 in 2003.
Figure 12: Estimated Number of Bariatric Operations Performed in the United States, 1992–2003. Data Are From the American Society for Bariatric Surgery. (55).
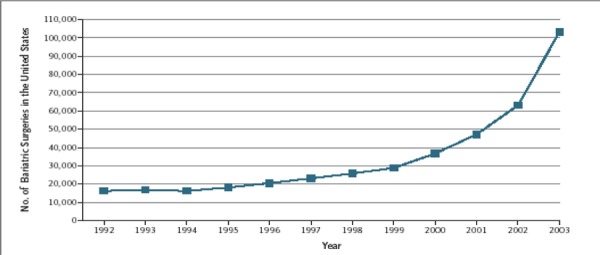
(Copyright © 2004 Massachusetts Medical Society. All rights reserved. Steinbrook R. Surgery for severe obesity. New England Journal of Medicine 2004; 350:1075-1079).
In Australia, the first LAGB procedures were offered in 1994. MSAC (24) stated that since then, the number of claims processed by the Health Insurance Commission under item 30511 has increased, while item 30512 has remained relatively steady (Figure 13). Item 30511 covers reimbursements for both LAGB and VBG procedures. Item 30512 covers gastric bypass procedures. The exact numbers of LAGB procedures done each year cannot be accurately determined. According to MSAC, the data suggest that most of the increase likely can be attributed to LAGB, which is a less invasive procedure and may be more acceptable to patients. MSAC also stated that the trend is expected to continue in the future.
Figure 13: Number of Claims Processed Under Items 30511 and 30512 by the Australian Health Insurance Commission: January 1994 to December 2002.
(Reproduced with kind permission from the Medical Services Advisory Committee (MSAC).
Laparoscopic adjustable gastric banding for morbid obesity. 2003. Commonwealth of Australia)
Stakeholder Analysis
Web sites post names of surgeons in Canada who do bariatric procedures. The Association for Morbid Obesity Support lists 13 such physicians in Ontario who can provide bariatric surgery (Table 57): (www.obesityhelp.com/morbidobesity/show_nonus_docs.phtml?Country=Canada; accessed Nov. 2004).
Table 57: Physicians Providing Bariatric Surgery in Ontario.
| City | Type of Surgery Offered | Number of Surgeons |
|---|---|---|
| Toronto | RNY/VBG/AGB RNY/VBG ? |
3 |
| Guelph | RNY | 2 |
| Ajax | ? | 1 |
| Scarborough | RNY RNY/VBG/AGB |
2 |
| Etobicoke | AGB/? | 1 |
| Bowmanville | VBG | 1 |
| Sault Ste Marie | RNY | 1 |
| Ottawa | RNY | 1 |
Cost and System Pressures
A health care facility that decides to provide bariatric surgery will require physical renovation to do the following (56):
Enlarge outside entrances and exits and internal doorways
Install larger higher capacity elevators for patient transport
Install mounted patient lifts
Reinforce patient grab bars
Replace or reinforce wall-mounted toilets
Reinforce floors to accommodate weight fluctuations
Other considerations include these (56):
Examination tables must be assessed to determine if they are wide enough, rated to accommodate the weight of an extremely obese patient, and fastened to the floor or wall to guarantee they will not tip when a patient sits on the end.
Waiting rooms, offices and lobbies will require oversized chairs.
Larger patient gowns, toilets, showers, wheelchairs, walkers, blood pressure cuffs, operating rooms, X-ray and/imaging tables, and recovery beds will be needed.
Staff must be provided with mechanical and personnel assistance and ergonomic training to prevent injury from moving patients.
Surgical instruments such as forceps, needle holders, retractors, trocar cannulae, and endoscopes are needed to accommodate larger body masses.
Downstream Cost Savings
Bariatric surgery procedures are known to lead to the resolution of several comorbid illnesses, including diabetes, hypertension, and dyslipidemias, hat are very expensive to treat. The annual health care costs of treating morbidly obese patients and their comorbid conditions often exceed $10,000 per annum; therefore, the downstream cost savings associated with bariatric surgery may be substantial.
Conclusions
Bariatric surgery generally is effective for sustained weight loss of about 16% for people with BMIs of at least 40 kg/m2 or at least 35 kg/m2 with comorbid conditions (including diabetes, high lipid levels, and hypertension). It also is effective at resolving the associated comorbid conditions. This conclusion is largely based on level 3a evidence from the prospectively designed SOS study, which recently published 10-year outcomes for patients who had bariatric surgery compared with patients who received nonsurgical treatment. (1)
Regarding specific procedures, there is evidence that malabsorptive techniques are better than other banding techniques for weight loss and resolution of comorbid conditions. However, there are no published prospective, long-term, direct comparisons available between these techniques.
-
Surgery for morbid obesity is considered an intervention of last resort for people who have previously tried first-line forms of medical management (i.e., diet, increased physical activity, behavioural modification, and drugs). In the absence of direct comparisons of active nonsurgical intervention through caloric restriction with bariatric techniques, the following observations are made:
A recent systematic review (2) of the efficacy of major commercial and organized self-help weight loss programs in the United States concluded that evidence to support the use of such programs was suboptimal, except for one trial on Weight Watchers. The programs were associated with high costs, attrition rates, and probability of regaining at least 50% of the lost weight in 1 to 2 years.
A recent RCT (3) reported 1-year outcomes on weight loss and metabolic changes in severely obese people assigned to either a low-carbohydrate diet or a conventional weight loss diet. At 1 year, weight loss was similar for both groups (mean, 2–5 kg). There was a favourable effect on triglyceride levels and glycemic control in the low-carbohydrate diet group.
A decision-analysis model showed bariatric surgery results in increased life expectancy in morbidly obese patients when compared with diet and exercise. (4)
A cost-effectiveness model showed bariatric surgery is cost-effective compared with nonsurgical management. (5)
Extrapolating from 2003 data from the United States, Ontario would likely need to do 3,500 bariatric surgeries per year. It currently does 508 per year, including out-of-country surgeries.
Glossary
- Body mass index:
Body weight expressed in kilograms (kg) divided by height expressed in square metres (m2).
- Dehiscence:
Separation of previously surgically closed wounds.
- Dumping Syndrome:
A group of symptoms that occur when food or liquid enters the small intestine too rapidly. These symptoms include cramps, nausea, diarrhea and dizziness.
- Excess weight loss:
Percentage of excess weight loss = (weight loss/excess weight) × 100 (Where excess weight = total preoperative weight – ideal weight)
- Morbid obesity:
Body mass index greater than 40 kg/m2 or 35 kg/m2 with serious comorbid conditions.
- Obesity:
Body mass index greater than 30 kg/m2.
- Super obese:
Body mass index greater than 50 kg/m2.
- Super super obese:
Body mass index greater than 60 kg/m2.
Appendices
Appendix 1
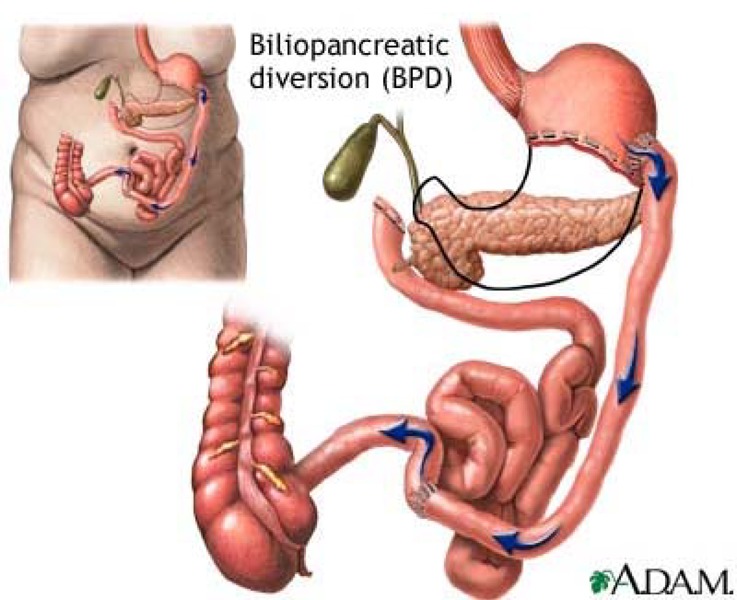
From ADAM Encyclopedia: http://adam.about.com/encyclopedia/19499.htm (accessed August 2005)
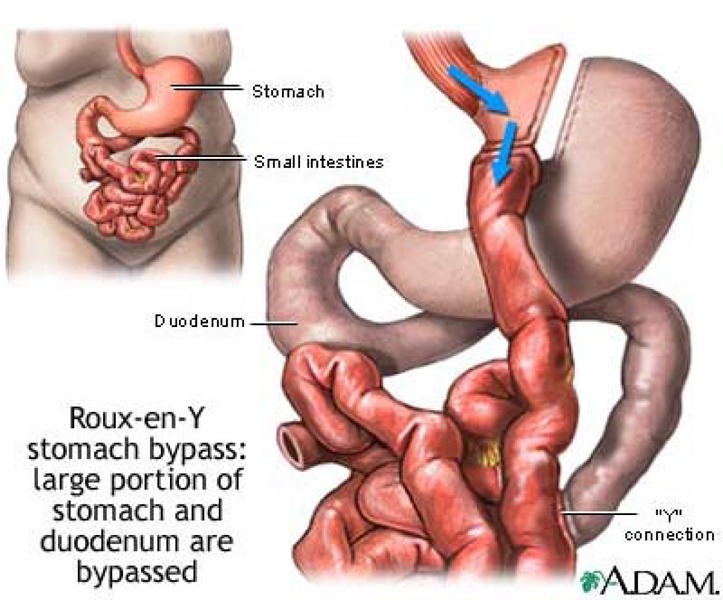
From ADAM Encyclopedia: http://adam.about.com/encyclopedia/19268.htm (accessed August 2005)
Vertical Banded Gastroplasty.
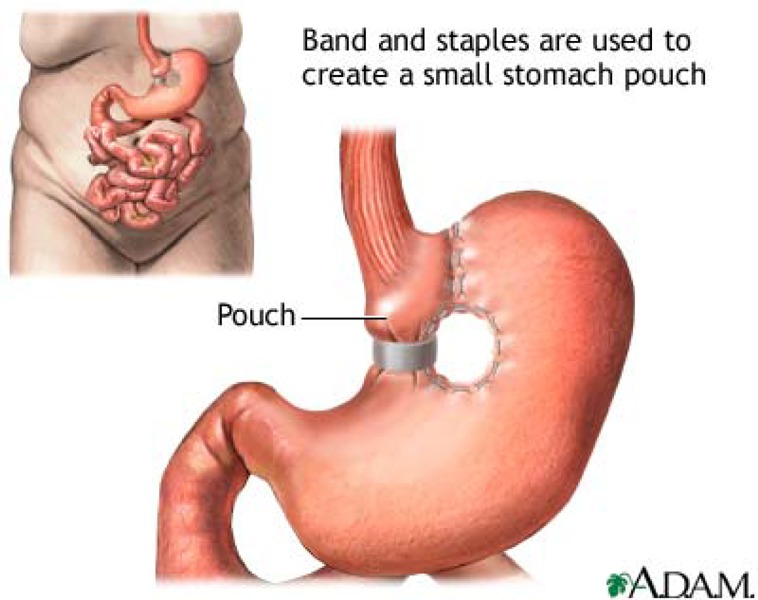
From ADAM Encyclopedia: http://adam.about.com/encyclopedia/19498.htm (accessed December 2004)
Adjustable Gastric Banding.
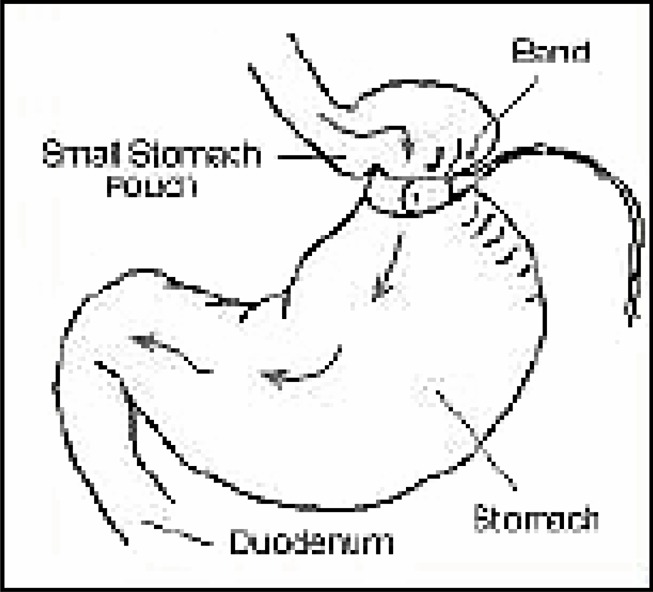
(Reproduced with kind permission from the Medical Services Advisory Committee (MSAC).
Laparoscopic adjustable gastric banding for morbid obesity. 2003. Commonwealth of Australia)
Appendix 2
United States Food and Drug Administration Summary of Safety and Effectiveness Data for the Lap-Band Adjustable Gastric Banding System® (June 5, 2001)
A clinical study was conducted in the United States under a significant risk Investigation Device Exemption to assess the safety and effectiveness of the LAP-BAND system to treat severe obesity. The study was a prospective, multicentre, single-arm trial in which each subject served as his or her own control. The study was based on a 36-month evaluation of the following clinical end points:
Effectiveness
Percent EWL
Absolute weight loss
Change in excess weight
Change in BMI
Change in QoL (based on Beck Depression Index, the Multi-Dimensional Body-Self Relations Questionnaire (MBSR) questionnaire, and the RAND SF-36 questionnaire)
Safety
Incidence and severity of complications (device-related and non-device-related)
Inclusion criteria
Aged 18–55 years
Male or female
BMI > 40 or at least 100 pounds above ideal weight according to the 1983 Metropolitan Life Insurance Tables (medium frame)
Willingness to comply with the substantial lifelong dietary restrictions required by the procedure
History of obesity for at least 5 years
History of failure with non-surgical weight loss methods
Willingness to follow protocol requirements, including signed informed consent, routine follow-up schedule, completing quality of life questionnaires, completing laboratory tests, completing diet and behaviour modification counselling
Residing within a reasonable distance from the investigator’s office and able to travel to the investigator to complete all routine follow-up visits
Exclusion criteria
Surgery or treatment represents an unreasonable risk to the subject
Family or patient history of inflammatory disease of the gastrointestinal tract (including ulceration, duodenal ulceration, Grade 2–4 esophagitis, or specific inflammation such as Crohn’s disease or ulcerative colitis)
Severe cardiopulmonary disease or other serious organic disease
Severe coagulopathy, upper gastro-intestinal bleeding conditions such as esophageal or gastric varices, congenital or acquired intestinal telangiectasia
Congenital or acquired anomalies of the GI tract such as atresias or stenoses
Severe hiatal hernia
Pregnant or has the intention of becoming pregnant in the next 12 months
Alcohol or drug addiction
Mentally retardation or emotionally instability, or showing psychological characteristics which, in the opinion of the investigator, makes the subject a poor candidate for gastric band surgery
Previous bariatric surgery (except adjustable silicone gastric band), intestinal obstruction, or adhesive peritonitis
Infection anywhere in the body at the time of surgery
Family or patient history of a known diagnosis or pre-existing symptoms of systemic lupus erythematosus, scleroderma, or other autoimmune connective tissue disorder
Participating in another ongoing clinical trial in which concomitant diagnosis or therapeutic intervention would adversely affect the integrity of the LAGB clinical trial
A total of 247 women (85%) and 45 men (15%) met the inclusion criteria and received the LAP-BAND device. Other comorbid conditions were gallbladder disease (26%), gastrointestinal diseases (24%), asthma (16%), and diabetes mellitus (16%). The mean age at time of surgery was 38.8 years (range, 18–56 years). The mean weight at baseline was 293 lbs (range, 193–475 lbs), and the mean BMI at baseline was 47.4 kg/m2 (range, 36.6–74.3 kg/m2). Thirty subjects had a BMI over 50 kg/m2 at entry. Patients had gained an average of 54 lbs in the 5 years before enrollment.
Two hundred and ninety-nine patients were enrolled. Follow-up data at 36 months were available for 178 patients. The remaining 121 patients were subdivided into 4 groups. Seven patients had previously had the Adjustable Silicone Gastric Band (ASGB) implanted and were included only in the safety analysis; 46 patients had the device explanted; 12 were lost to follow-up; and 56 patients either missed or had not yet reached the 36-month follow-up visit.
Effectiveness
The primary endpoint was percentage of EWL. The percentage of EWL increased between 3 weeks and 18 months and then remained relatively stable between 18 and 36 months. Patients who reached 36 months of follow-up were able to lose, on average, 36% of their excess body weight. It should be noted that by 3 weeks after implantation, before the first postoperative band adjustment, patients had lost an average of 10% of their excess body weight. The reason for this is not known. No significant differences in the percentage of EWL were noted based on age or gender.
For those patients completing 36 months of follow-up, the mean body weight decreased by 53 lbs, or 18% (293.5– 240.6 lbs). Maximum mean weight loss (59 lbs), however, occurred at the 24-month point with a subsequent slight gain in weight between 24 and 36 months.
Patients enrolled in the study must have had a BMI of at least 40 kg/m2 to be eligible for implantation. The mean BMI at baseline was 47.5 kg/m2. The mean BMI decreased to 38.1 kg/m2 at 24 months and 38.7 kg/m2 at 36 months for those completing follow-up. This represents a 19% reduction in BMI for those patients by study’s end.
For patients completing QoL questionnaires at 36 months, there was an improvement in mean scores for all categories measured (statistical significance not reported).
Safety and Adverse Events:
During the study, 266 (89%) of the participants reported at least one adverse event, which 34% reported as being severe. Although signs and symptoms were recorded separately as individual adverse events, many of the events were associated with a syndrome (a collection of symptoms). For example, patients with band slippage/pouch dilatation may also have reported nausea, vomiting, and stoma obstruction.
Perioperative adverse events were reported by 44% of the subjects. The most commonly reported were abdominal pain (10%), nausea and/or vomiting (9%), asthenia (5%), and incisional infection (5%). Other perioperative adverse events occurred in less than 5% of the subjects.
Eighty-two percent of subjects reported having one or more adverse events postoperatively. The most commonly reported were nausea and/or vomiting (42%), gastroesophageal reflux (32%), band slippage/pouch dilatation (24%), abdominal pain (18%), stoma obstruction (14%), dysphagia (8%), alopecia (7%), esophageal dilatation (7%), diarrhea (6%), port site pain (6%), constipation (5%), port displacement (5%), infection (5%), hernia (5%), and constipation (5%). Other postoperative adverse events occurred in less than 5% of the subjects.
Some of the commonly reported events associated with the device necessitated revision in some subjects. Surgery may have been required to revise, replace, or remove any or all components of the device. The most commonly reported events are discussed below.
Band slippage and/or pouch dilatation: 72 (24%) subjects reported 78 events of band slippage/pouch dilatation. Band slippage refers to slippage of the stomach up through the band rather than slippage of the band down lower on the stomach. This results in an increase in the proximal pouch and a difference in the relative position of the band from where it was implanted. Slippage most commonly involves the posterior gastric wall, but can include any portion of the stomach.
Slippage and pouch dilatation are reported together because pouch dilatation can result from band slippage or it can happen independently, and there is no standard for distinguishing between the events. The consequences of band slippage varied from subject to subject. In some cases, slippage was documented after radiological inspection of the band position. If subjects showed no symptoms of slippage, often no intervention was deemed necessary, or adjusting the stoma was sufficient to resolve these asymptomatic findings. Signs and symptoms associated with band slippage were abdominal pain, nausea and/or vomiting, gastroesophageal reflux, dysphagia, and gastric obstruction. For the 56 events reported as “resolved,” 52% of the subjects recovered after stoma adjustment or no intervention, while 48% underwent reoperation – either revision, replacement, or explantation of the device.
Stoma obstruction: Forty-one (14%) subjects experienced 52 events of stoma obstruction. Ten patients reported multiple episodes. Signs and symptoms associated with stoma obstruction were band slippage, asthenia, epigastric pain, nausea and vomiting, gastroesophageal reflux, dehydration and hypokalemia (secondary to vomiting). Fifty-six percent (23/41) of the subjects recovered after stoma adjustment or no intervention; 39% (16/41) of the events required surgery (7% revision or replacement and 32% band removal); and in 5% (2/41), the method of resolution was not reported. Stoma obstruction and the band slippage/pouch dilatation that may be associated with it were the most common causes of LAP-BAND System reoperations.
Abdominal pain: Eighty (27%) subjects reported 102 events of abdominal pain. Abdominal pain often accompanied other events, such as dysphagia, gastroesophageal reflux, or nausea and vomiting.
Dysphagia: – was reported in 26 (9%) of subjects. It was most commonly associated with, or related to, stomach/band slippage, stoma obstruction, or nausea and/or vomiting.
Esophageal dilatation/dysmotility: Twenty-nine (10%) subjects reported 32 events of either esophageal dilation (21), dysmotility (8), or both (3). Esophageal dilatation may be a consequence of incorrect band placement, over-restriction, stoma obstruction, or excess vomiting. Twenty of the events occurred at one site and were believed to be related to band over-inflation. Although most events appeared to resolve with deflation of the band, the long-term consequences of this event are not known.
Port site pain: Twenty-six subjects (9%) reported port site pain. Seven were reported in the peri-operative period, 18 in the post-operative period, and one in both the peri- and post-operative periods.
Port displacement: – was reported in 18 subjects (6%). In several of the events the port was suspected on x-ray of being displaced but was found not to have been displaced on further examination.
Serious adverse events reported with a frequency of less the 5% are presented below. Except for the 2 reported deaths, these events always required surgery to remove, replace or revise the device.
Deaths: Two deaths occurred during the study. One person died from “mixed drug intoxication” 1 week after explantation. A second patient died 1 day after explantation of the LAP-BAND and conversion to a RYGB. Death was due to multiple pulmonary emboli. Neither death is believed to have been device related.
Erosions: Four (1%) subjects experienced an erosion of the band into the gastric lumen. Two of the erosions were considered to have been secondary to intraoperative gastric perforations. All erosions resolved with explantation of the device.
Mechanical malfunctions: Eight (3%) subjects reported port leakage. The leakage was associated with either cracking of the kink-resistant tubing or disruption of the tubing connection from the port to the band. All of the events resolved after the port and/or port tubing was replaced. In addition, 2 (0.7%) subjects reported band leakage. One was due to a leak from a puncture through the thickness of the shell and the other was cause by 2 small holes in the shell. In addition, there were 2 reports of malfunction (irregular inflation) of the calibration tube and 1 report of breakage of the luer-lock connector end of the calibration tube.
Reoperations: Revisions, Replacements, and Explants: Twenty-six (9%) subjects had 27 surgical revisions involving the gastric band; 1 subject had 2 separate procedures. In 9 of the 27 procedures the band was removed and replaced during a single surgical procedure. Two subjects were given a new band during a separate surgical procedure. In 16 of the 27 revision procedures, the gastric band was not removed. Most revisions were to correct band slippage/pouch dilatation. Thirteen (48%) of the procedures were completed laparoscopically.
There were 26 revisions involving the access port. Thirteen access ports were removed and replaced due to tubing leaks at or near the tubing connections to the port (8/13), port displacement (1/13), or infection (4/13). Nine of these were replaced during the same surgical procedure. The 4 access ports explanted due to infection were reimplanted later. An additional 13 access ports required revision but did not require removal of the port. The port was repositioned and/or resutured in place to correct either misalignment or movement that had resulted in an inability to access the port (9/13), pain associated with movement (3/13), or associated infection (1/13).
Forty-six (15%) patients had 48 device explantations within 3 years of implantation. Another 27 (9%) subjects had the band explanted after the 3-year study period. Fifty-one (68%) of the 75 explants were due to complications, primarily band slippage/pouch dilatation and/or obstruction 32% (24/75). Other adverse events cited for explantation of the band were erosion 5% (4/75), infection 4% (3/75), gastroesophageal reflux and/or dysphagia 11% (8/75), system leaks 4% (3/75), and esophageal dilatation or dysmotility 7% (5/75). In the other 24 subjects (32%), insufficient weight loss was cited as the reason for explantation. In 45 (60%) patients the explant procedure was performed laparoscopically. About one-half (37/75) of the patients who had explants were converted to a gastric bypass, 3 were converted to a vertical banded gastroplasty, and 35 subjects had the system removed with no other obesity surgery. Nineteen of the subjects had their obesity surgical procedure (e.g., gastric bypass) performed at the same time that the study device was removed.
Adhesions: Forty-two percent of those patients undergoing a revision procedure were reported to have developed adhesions involving the stomach. The exact incidence of adhesions is not known, as many of the implanted patients did not undergo reoperation.
FDA Panel Recommendation
The Gastroenterology and Urology Devices Panel met on June 19, 2000, to consider the safety and effectiveness of the BioEnterics Corporation LAP-BAND Adjustable Gastric Banding System. The Panel voted 6 to 4to recommend that the Center for Devices and Radiological Health (CDRH) not approve the premarket approval for the LAP-BAND System. They believed that 2 years of follow-up data was not adequate and they recommended at least 3 years of follow- up before the device was approved. The protocol for the clinical study called for 3 years of patient follow-up.
Center for Device and Radiological Health (CDRH) Decision
CDRH agreed with the advisory panel recommendation and sent a major deficiency letter on August 7, 2000, detailing the data and information that were still needed. In addition to the 3 years of follow-up, there were recommendations on labeling and a postapproval study.
The applicant continued its clinical study and on December 26, 2000, amended the premarket approval. After reviewing the additional information, CDRH determined that there was enough data on safety and effectiveness to approve the LAP-BAND Adjustable Gastric Banding System. The sponsor submitted revised labeling and an outline for the postapproval study via e-mail and fax. In the postapproval study, the subjects enrolled in the clinical trial will continue to be followed-up for 5 years after surgery. The purpose of the postapproval study is to obtain more information on excess weight loss and adverse events, primarily esophageal dilatation and band erosion.
Appendix 3
Adverse Effects after Adjustable Gastric Banding.
| Study (Year) |
Timing of Event |
Description | Patients, % (n/N) | Comments |
|---|---|---|---|---|
| Chevalier (2004) |
Perioperative | Conversion to open surgery | 1.2% (12/1000) | |
| Bleeding hepatic wound | 0.4% (4/1000) | |||
| Mortality | 0% (0/1000) | |||
| Gastric perforation | 0.4% (4/1000) | |||
| Early | Minor atelectasis | 1.1%(11/1000) | ||
| Minor slippage | 0.3% (3/1000) | |||
| Major acute respiratory distress syndrome | 0.2% (2/1000) | |||
| Major perforation | 0.4% (4/1000) | |||
| Pulmonary embolism | 0.2% (2/1000) | |||
| Any complications | 2.2% (22/1000) | |||
| Late | Band migration | 0.3% (3/1000) | ||
| Esophageal dilatation | 0.5% (5/1000) | |||
| Minor incisional hernia | 0.4% (4/1000) | |||
| minor dysfunctional port | 5.7% (57/1000) | |||
| Reoperation | 11% (111/1000) | |||
| Minor slippage | 10.1% (101/1000) | |||
| Minor disruption of tube | 1.1% (11/1000) | |||
| Major gastric necrosis | 0.1% (1/1000) | |||
| Any | Mortality | 0% (0/1000) | ||
| Sarker (2004) | Perioperative | Conversion to open surgery | 0% (0/154) | |
| Early | Mortality | 0.6% (1/154) | ||
| Pulmonary embolism | 0.6% (1/154) | |||
| Major band erosion | 0.6% (1/154) | |||
| Readmission due to dehydration | 3.9% (6/154) | |||
| Late | Reoperation | 9% (14/154) | Mean follow-up was 33 weeks | |
| Port revision | 5% (7/154) | |||
| Symptomatic cholelithiasis | 0.6% (1/154) | |||
| Dukhno (2003) | Perioperative | Conversion to open surgery | 4% (10/250) | |
| Mortality | 0% (0/250) | |||
| Late | Band slippage | 5% (13/250) | ||
| Band erosion | 1.2% (3/250) | |||
| Port disconnection | 2% (5/250) | |||
| Balloon aneurysm | 0.4% (1/250) | |||
| Acute obstruction | 18% (45/250) | |||
| Reoperation | 8.8% (22/250) | Reasons: -Band slippage (13) -Band erosion (3) -Tube disconnect (5) -Balloon aneurysm (1) Mean follow-up was 12 months |
||
| Any | Mortality | 0% (0/250) | ||
| Mittermair (2003) | Perioperative | Mortality | 0% (0/454) | |
| Conversion to open surgery | 0% (0/454) | |||
| Early | Port disconnection | 6.2% (28/454) | ||
| Band leakage | 2.4% (11/454) | |||
| Pouch dilatation | 0.7% (3/454) | |||
| Port infection | 1.1% (5/454) | |||
| Late | Pouch dilation | 1.3% (6/454) | ||
| Cholethiasis | 2.2% (7/320) | |||
| Reoperation | 8.6% (39/454) | Reasons: -Leak (11) -Early pouch dilatation (3) -Late pouch dilatation (6) -Pouch hemorrhage (1) -Stomach perforation (4) -Intragastric band migration (14) Mean follow-up was 30 months |
||
| Band migration | 3.1% (14/454) | |||
| Stomach perforation | 0.9% (4/454) | |||
| Pouch hemorrhage | 0.2% (1/454) | |||
| Any | Mortality | 0% (0/454) | ||
| Rubin (2003) | Perioperative | Aborted procedure | 0.8% (2/250) | |
| Late | Gastric/esophageal dilation | 2.6% (7/250) | ||
| Port twist | 4% (12/250) | |||
| Tonsil injury (tube related) | 0.8% (2/250) | |||
| Respiratory failure | 0.4% (1/250) | |||
| Port site hernia | 0.4% (1/250) | |||
| Port breaks | 4% (13/250) | |||
| Dysphagia | 1% (3/250) | |||
| Band slippage | 1.6% (4/250) | |||
| Band leakage | 0.4% (1/250) | |||
| Port infection | 0.4% (1/250) | |||
| Band erosion | 0% (0/250) | |||
| Trocar gastric wall injury | 0.4% (1/250) | |||
| Reoperation | 12% (30/250) | Reasons: -Broken/twisted port (25) -Band slippage (4) -Trocar gastric wall injury (1) Mean follow-up was 24 months |
||
| Spivak (2003) |
Perioperative | Mortality | 0% (0/271) | |
| Conversion to open surgery | 1.1% (3/271) | |||
| Any | Band slippage | 1.8% (5/271) | ||
| Acute stoma obstruction | 1.8% (5/271) | |||
| Reoperation | 10% (28/271) | Reasons: -Port problems (20) -Band slippage (5) -Stoma obstruction (3) Mean follow-up was 12 months |
||
| Bleeding trocar site | 0.4% (1/271) | |||
| Port problems | 7.3% (20/271) | |||
| Pneumonia | 0.8% (2/271) | |||
| Pulmonary embolism | 0.4% (1/271) | |||
| Mortality | 0% (0/271) | |||
| Band removal | 0% (0/271) | |||
| Pouch dilatation | 6.6% (18/271) | |||
| Weiner (2003) |
Perioperative | Mortality | 0% (0/984) | |
| Conversion to open surgery | 0% (0/984) | |||
| Early | Gastric perforation | 0.1% (1/984) | ||
| Band slippage | 0.1% (1/984) | |||
| Late | Port rotations | 1.4% (1/984) | ||
| Band slippage | 3.3% (32/984) | |||
| Port penetrations | 0.2% (2/984) | |||
| Port breaks | 0.3% (3/984) | |||
| Band migration | 0.3% (3/984) | |||
| Mild esophageal dilatation | 2.4% (24/984) | |||
| Port infection | 0.6% (6/984) | |||
| Any | Reoperation | 8% (77/984) | ||
| Ponson (2002) | Perioperative | Conversion to open surgery | 5% (5/101) | |
| Any | Band removal | 4% (4/101) | ||
| Band repositioning | 3% (101) | |||
| Balloon leakage | 5% (5/101) | |||
| Any complications | 0% (0/101) | |||
| Reoperation | 12% (12/101) | Reasons: -Leak (5) -Repositioning (3) -Band removal (4) Mean follow-up was 9.9 months |
||
| Pontiroli (2002) | Perioperative | Conversion to open surgery | 2.8% (4/143) | |
| Sepsis | 0.7% (1/143) | |||
| Late | Gastric slippage | 5.6% (8/143) | ||
| Reoperation | 5.6% (8/143) | Reasons: -Gastric slippage (8) Mean follow-up was 28.2 months (estimated by ECRI) |
||
| Port disconnection | 2.8% (4/143) | |||
| Stoma regulation | 61.5% (88/143) | |||
| Szold (2002) | Perioperative | Reoperation | 0.8% (6/715) | |
| Band malpositioning and outlet obstruction | 0.7% (5/715) | |||
| Conversion to open surgery | 0.8% (6/715) | |||
| Bleeding | 0.6% (4/715) | |||
| Pneumothorax | 0.1% (1/715) | |||
| Stomach injury | 0.1% (1/715) | |||
| Bleeding trocar site | 0.1% (1/715) | |||
| Mortality | 0% (0/715) | |||
| Late | Reoperation | 10% (75/715) | Reasons: -Band related (57) -Port problems (18) Mean follow-up was 17 months |
|
| Major infected splenic hematoma | 0.1% (1/715) | |||
| Major trocar site hernia | 0.1% (1/715) | |||
| Major band erosion | 0.4% (3/715) | |||
| Minor wound infection | 0.4% (3/715) | |||
| Painful port site | 1.3% (9/715) | |||
| Band dislodgement or pouch dilation | 7.4% (53/715) | |||
| Major subphrenic abscess | 0.3% (2/715) | |||
| Mortality | 0% (0/715) | |||
| Victorzon (2002) | Perioperative | Mortality | 0% (0/110) | |
| Conversion to open surgery | 3.6% (4/110) | |||
| Early | Fever | 2.7% (3/110) | ||
| Obstipation | 0.9% (1/110) | |||
| Pneumonia | 1.8% (2/210) | |||
| Unclear hypotonia | 0.9% (1/110) | |||
| Wound infection | 0.9% (1/110) | |||
| Urinary infection | 0.9% (1/110) | |||
| Hemorrhage | 0.9% (1/110) | |||
| Late | Band or port leak | 4.5% (5/110) | ||
| Band erosion | 1.8% (2/110) | |||
| Infection | 0.9% (1/110) | |||
| Band slippage | 2.7% (3/110) | |||
| Reoperation | 10% (11/110) | Reasons: -Band slippage (3) -Leaking band or port (5) -Band erosion (2) -Infection (1) Mean follow-up was 21.6 months |
||
| Dixon (2001) | Perioperative | Conversion to open surgery | 0.3% (1/313) | |
| Late | Band erosion | 4% (20/459) | ||
| Band slippage | 18.3% (84/459) | |||
| Reoperation | 23% (104/459) | Reasons: -Prolapse (84%) -Band erosion (20) Mean follow-up was 33 months |
||
| BioEnterics (2001) | Perioperative | Asthenia | 5% (15/299) | |
| Mortality | 0% (0/299) | |||
| Nausea/vomiting | 9% (27/299) | |||
| Abdominal pain | 10% (30/299) | |||
| Incisional infection | 5% (15/299) | |||
| Any complications | 44% (132/299) | |||
| Any | Dysphagia | 8% (24/299) | ||
| Band slippage/pouch dilatation | 24% (72/299) | |||
| Abdominal pain | 18% (54/299) | |||
| Stoma obstruction | 14% (42/299) | |||
| Alopecia | 7% (21/299) | |||
| Diarrhea | 6% (18/299) | |||
| Severe diarrhea | 0.3% (1/299) | |||
| Reoperation | 24% (73/299) | Reasons: -Band removal (73) Follow-up ranged from 12 to 36 months |
||
| Esophageal dilation/dysmotility | 7% (21/299) | |||
| Gastroesophageal reflux | 32% (96/299) | |||
| Mild abnormal stool | 6% (18/299) | |||
| Moderate abdominal pain | 7.7% (23/299) | |||
| Moderate gastroesophageal reflux | 13% (39/299) | |||
| Moderate nausea/vomiting | 10.7% (32/299) | |||
| Mild infection | 3% (9/299) | |||
| Mild incision infection | 3.7% (11/299) | |||
| Mild port site pain | 6.4% (19/299) | |||
| Mild abdominal pain | 18.4% (55/299) | |||
| Mild esophageal dilation/dysmotility | 2.3% (7/299) | |||
| Moderate dysphagia | 3.7% (11/299) | |||
| Mild alopecia | 6.7% (20/299) | |||
| Mild constipation | 6.7% (20/299) | |||
| Mild diarrhea | 7.7% (23/299) | |||
| Mild dysphagia | 4.7% (14/299) | |||
| Mild stoma obstruction | 2% (6/299) | |||
| Mild band slippage/pouch dilation | 6.4% (19/299) | |||
| Mild port displacement | 1.7% (5/299) | |||
| Moderate port site pain | 2% (6/299) | |||
| Severe dysphagia | 1% (3/299) | |||
| Severe stomal obstruction | 5.7% (17/299) | |||
| Severe band slippage/pouch dilation | 8.4% (25/299) | |||
| Moderate incision infection | 2.3% (7/299) | |||
| Nausea/vomiting | 42% (126/299) | |||
| Moderate port displacement | 3% (9/299) | |||
| Moderate esophageal dilation/dysmotility | 2.7% (8/299) | |||
| Moderate abnormal stool | 0% (0/299) | |||
| Moderate alopecia | 0.3% (1/299) | |||
| Moderate constipation | 2% (6/299) | |||
| Port site pain | 6% (18/299) | |||
| Moderate stoma obstruction | 6% (18/299) | |||
| Moderate infection | 3.3% (10/299) | |||
| Severe infection | 1% (3/299) | |||
| Mortality | 0.7% (2/299) | |||
| Mild gastroesophageal reflux | 24.1% (72/299) | |||
| Constipation | 5% (15/299) | |||
| Moderate diarrhea | 1% (3/299) | |||
| Serious band erosion | 1% (4/299) | |||
| Serious band leakage | 0.7% (2/299) | |||
| Port revision | 8.7% (26/299) | |||
| Band problems | 9% (26/299) | |||
| Infection | 5% (15/299) | |||
| Any complications | 82% (245/299) | |||
| Port displacement | 5% (15/299) | |||
| Hernia | 5% (15/299) | |||
| Severe incision infection | 1.3% (4/299) | |||
| Severe port site pain | 0.3% (1/299) | |||
| Severe port displacement | 0.7% (2/299) | |||
| Severe esophageal dilation/dysmotility | 2.7% (8/299) | |||
| Severe abnormal stool | 0% (0/299) | |||
| Severe alopecia | 0% (0/299) | |||
| Severe constipation | 0% (0/299) | |||
| Any complications | 89% (266/299) | |||
| Mild nausea/vomiting | 42.8% (128/299) | |||
| Serious port leakage | 3% (8/299) | |||
| Mortele (2001) |
Early | Esophagitis | 5% (11/218) | |
| Decrease of stoma size | 8.7% (19/218) | |||
| Port inversion | 1.4% (3/218) | |||
| Band slippage | 7.8% (17/218) | |||
| Food obstruction | 1.8% (4/218) | |||
| Misplaced band | 2.3% (5/218) | |||
| Pouch dilation | 3.7% (8/218) | |||
| Band leakage | 0.9% (2/218) | |||
| Reoperation | 10% (22/218) | Reasons: -Band slippage (5) -Band misplacement (12) -Port adjustment (3) -Leak (2) All patients were followed-up for 1 month |
||
| Suter (2000) | Perioperative | Conversion to open surgery | 5.3% (8/150) | |
| Gastric perforation | 2% (3/150) | |||
| Severe bronchospasm | 0.7% (1/150) | |||
| Early | Mortality | 0.6% (1/150) | ||
| Pulmonary embolism | 1.3% (2/150) | |||
| Septicemia | 0.7% (1/150) | |||
| Arterial embolism | 0.7% (1/150) | |||
| Urinary tract infection | 1.3% (2/150) | |||
| Severe hypertension | 0.7% (1/150) | |||
| Bronchopneumonia | 0.7% (1/150) | |||
| Seroma | 2% (3/150) | |||
| Hematoma | 0.7% (1/150) | |||
| Prolonged apnea | 0.7% (1/150) | |||
| Any major complications | 4 (6/150) | |||
| Late | Psychological intolerance | 0.3% (1/150) | ||
| Band erosion | 2% (3/150) | |||
| Port infection | 0.6% (1/150) | |||
| Leak | 3.3% (5/150) | |||
| Dilation and/or slippage | 10.6% (16/150) | |||
| Incisional hernia | 0.3% 1/150) | |||
| Any complications | 16% (24/150) | |||
| Reoperation | 1406% (22/150) | Reasons: -Band repositioning (10) -Band removal (7) -Other (5) Mean follow-up was 17 months |
||
| Miller (1999) | Perioperative | Wound infection | 0.6% (1/156) | |
| Conversion to open surgery | 2% (3/158) | |||
| Mortality | 0% (0/156) | |||
| Gastric wall perforation | 0.6 (1/156) | |||
| Trocar site hematoma | 0.6% (1/156) | |||
| Liver hypertrophy | 0.6% (1/156) | |||
| Reoperation | 1.3% (2/156) | |||
| Pouch dilation | 1.3% (2/156) | |||
| Dysmotility of esophagus | 0.6% (1/156) | |||
| Band erosion | 0.6% (1/156) | |||
| Band leakage | 1.9% (3/156) | |||
| Port infection | 0.6% (1/156) | |||
| Any | Reoperation | 7% (11/156) | Reasons: -Pouch dilation (2) -Posterior gastric wall perforation (1) -Band leakage (3) -Hematoma of trocar site (1) -Port infection (2) -Dysmotility of esophagus and psychological problems (1) -Band erosion (1) Mean follow-up was 28 |
|
| months | ||||
| Mortality | 0% (0/156) | |||
| Lise (1994) | Perioperative | Incidental splenectomy | 0.9% (1/111) | |
| Wound infection | 2.7% (3/111) | |||
| Mortality | 0% (0/111) | |||
| Pneumonia | 4.5% (5/111) | |||
| Port stenosis | 0.9% (1/111) | |||
| Reservoir infection | 1.8% (2/111) | |||
| Band erosion | 0.9% (1/111) | |||
| Reoperation | 9% (10/111) | Reasons: -Outlet stenosis (7) -Slippage (2) -Erosion (1) Mean follow-up was 18.8 months |
||
| Outlet stenosis and pouch dilations | 6.3% (7/111) | |||
| Band slippage | 1.8% (2/111) | |||
| Mortality | 0.9% (1/111) | |||
| Reservoir leakage | 2.7% (3/111) |
(Table reproduced with kind permission from ECRI. Bariatric Surgery for Obesity. 2004. Plymouth Meeting, PA, ECRI. Technology Assessment Report.)
Adverse Effects After Vertical Banded Gastroplasty. From ECRI (19).
| Study (Year) | Timing of Event | Description | Patients, % (n/N) |
|---|---|---|---|
| Lee (2001) | Perioperative | Any complications | 3% (3/100) |
| Conversion to open surgery | 1% (1/100) | ||
| Mortality | 0% (0/100) | ||
| Early | Transient bleeding | 2% (2/100) | |
| Acute gout attack | 1% (1/100) | ||
| Gastric stasis | 2% (2/100) | ||
| Gastric perforation | 1% (1/100) | ||
| Late | Staple line disruption | 1% (1/100) | |
| Symptomatic cholelithiasis | 1% (1/100) | ||
| Gastric outlet stenosis | 21% (1/100) | ||
| Melissas (2001) | Perioperative | Mortality | 0.8% (1/125) |
| Perioperative | Gastric leak | 1.6% (2/125) | |
| Early | Pleural effusion | 12.8% (16/125) | |
| Respiratory failure | 3.2% (4/125) | ||
| Pneumonia | 4.8% (6/125) | ||
| Atelectasis | 30.4% (38/125) | ||
| Splenic injury | 0.8% (1/125) | ||
| Wound infection | 9.6% (12/125) | ||
| Husemann (1999) | Late | Staple line disruption | 7% (46/655) |
| Late | Reoperation | 5.2% (34/655) | |
| Suter (1999) | Perioperative | Intraoperative complications | 4.6% (9/197) |
| Early | Mortality | 0% (0/197) | |
| Thrombosis/embolism | 6.1% (12/197) | ||
| Pneumonia | 3.6% (7/197) | ||
| Wound infection | 7.6% (15/197) | ||
| Sepsis | 0.5% (1/197) | ||
| Stroke | 0.5% (1/197) | ||
| Prolonged apnea | 0% (0/197) | ||
| “miscellaneous” | 6.1% (12/197) | ||
| Late | Stenosis | 10.2% (20/197) | |
| Incisional hernia | 2.5% (5/197) | ||
| Band erosion | 1.5% (3/197) | ||
| Staple line disruption | 1.5% (3/197) | ||
| Catheter disruption/disconnection | 0.5% (1/197) | ||
| Port infection | 0.5% (1/197) | ||
| Reoperation | 13.7% (27/197) | ||
| Pouch dilation | 0% (0/197) | ||
| Capella (1996) | Perioperative | Mortality | 0.3% (1/329) |
| Early | Early reoperation | 0% (0/329) | |
| Late | Reversal to normal anatomy | 0.3% (1/329) | |
| Staple line disruption | 30% 99/329) |
(Table reproduced with kind permission from ECRI. Bariatric Surgery for Obesity. 2004. Plymouth Meeting, PA, ECRI. Technology Assessment Report.)
Adverse Effects After Roux-en-Y Gastric Bypass. From ECRI (19).
| Study (Year) | Timing of Event | Description | Patients, % (n/N) |
|---|---|---|---|
| Smith (2004) | Perioperative | Mortality | 0.1% (1/779)* |
| Any | Mortality | 0.1% (1/779) | |
| Gastrojejunal perforation | 1.2% (9/779) | ||
| Gastrojejunal stenosis | 7.6% (59/779) | ||
| Ventral incisional hernia | 5.8% (45/779) | ||
| Cholecystitis | 1.3% (10/779) | ||
| Gastrointestinal hemorrhage | 2.8% (22/779) | ||
| Abdominal wall hematoma | 0.6% (5/779) | ||
| Wound infection | 4.1% (32/779) | ||
| Deep vein thrombosis | 0.5% (4/779) | ||
| Pulmonary embolus | 0.5% (4/779) | ||
| Dehydration | 6.4% (50/779) | ||
| Myocardial infarction | 0.1% (1/779) | ||
| Small bowel obstruction | 4.4% (34/779) | ||
| Voice change post-intubation | 0.1% (1/779) | ||
| Gastrojejunal ulcer – no bleeding | 0.3% (2/779) | ||
| Splenectomy | 0.3% (2/779) | ||
| Appendicitis | 0.5% (4/779) | ||
| Subphrenic abscess without perforation | 0.3% (2/779) | ||
| Pneumonia | 1.2% (9/779) | ||
| Jejunojejunostomy perforation | 0.1% (1/779) | ||
| Superior mesenteric vein thrombosis | 0.1% (1/779) | ||
| Guillain barre syndrome | 0.1% (1/779) | ||
| Suicide | 0.1% (1/779) | ||
| Gilbert’s disease | 0.1% (1/779) | ||
| Nephrolithiasis | 0.9% (7/779) | ||
| Pseudomembranous colitis | 0.8% (6/779) | ||
| Decubitus ulcer | 0.3% (2/79) | ||
| Pelvic inflammatory disease | 0.1% (1/779) | ||
| Diverticulitis | 0.1% (1/779) | ||
| Dehiscence/evisceration | 0.1% (1/779) | ||
| Severe colonic inertia | 0.1% (1/779) | ||
| Clinical dumping syndrome | 0.1% (1/779) | ||
| Stroke cerebral aneurysm | 0.1% (1/779) | ||
| Beriberi | 0.1% (1/779) | ||
| Arrhythmia | 0.4% (3/779) | ||
| Heparin induced thrombocytopenia | 0.1% (1/779) | ||
| Any | 32.2% (251/779) | ||
| Perugini (2003) | Perioperative | Conversion to open surgery | 1.1% (2/188) |
| Any | Gastrojejunal leak | 1.6% (3/188) | |
| Mortality | 1.1% (2/188) | ||
| Stenosis at gastrojejunal anastomosis | 14.4% (27/188) | ||
| Staple line hemorrhage | 3.2% (6/188) | ||
| Bowel obstruction | 3.2% (6/188) | ||
| Roux limb obstruction at jejunostomy | 1.6% (3/188) | ||
| Nausea | 1.1% (2/188) | ||
| Infected hematoma | 1.1% (2/188) | ||
| Wound infection | 0.5% (1/188) | ||
| Left upper quadrant pain, unspecified | 0.5% (1/188) | ||
| Upper gastrointestinal hemorrhage | 2.1% (4/188) | ||
| Gastric perforation | 0.5% (1/188) | ||
| Incarcerated hernia | 0.5% (1/188) | ||
| Gastric outlet obstruction/bezoar | 0.5% (1/188) | ||
| Gastrogastric fistula | 0.5% (1/188) | ||
| Bile leak | 0.5% (1/188) | ||
| OR bleeding splenectomy | 0.5% (1/188) | ||
| Wittgrove (2000) |
Perioperative | Mortality | 0% (0/500) |
| Any | Stenosis | 1.6% (8/500) | |
| Hemorrhage requiring reoperation | 0.8%(4/500) | ||
| Mortality | 0% (0/500) | ||
| Major infection | 0.8% (4/500) | ||
| Minor infection | 4.8% (24/500) | ||
| Small bowel obstruction | 0.6% (3/500) | ||
| Reoperation due to leak | 2.2% (11/500) | ||
| Leak | 2.2% (11/500) | ||
| Pyelonephritis | 0.2% (1/500) | ||
| Respiratory | 1.4% (7/500) | ||
| Stroke | 0.2% (1/500) | ||
| Incisional hernia | 0% (0/500) | ||
| Spaulding (1997) |
Late | Marginal ulcer | 9.3% (14/150) |
| Stomal stenosis | 24% (36/150) | ||
| Smith (1996) | Early | Mortality | 0.5% (1/205) |
| Late | Major complications | 6.8% (14/205) | |
| Major hemorrhage | 2.4% (5/205) | ||
| Major wound infection | 1% (2/205) | ||
| Major gastrojejunostomy stenosis | 0.5% (1/205) | ||
| Major anastomic leak | 1.5% (3/205) | ||
| Major life threatening ketoacidosis | 0.5% (1/205) | ||
| Mortality | 0.5% (1/205) | ||
| Major nonfatal pulmonary embolism | 1% (2/205) | ||
| Pories (1995) | Perioperative | Mortality | 1.5% (9/608) |
| Early | Reoperation | 2.8% (17/608) | |
| Anastomotic stenosis | 3% (18/608)† | ||
| Minor wound infection | 8.7% (53/608) | ||
| Severe wound infection | 3% (18/608) | ||
| Splenic tears | 2.5% (15/608) | ||
| Minor wound seroma | 5.8% (35/608) | ||
| Subphrenic abscess | 2.5% (15/608) | ||
| Depression | 23.4% (142/608) | ||
| Bile reflux | 8.7% (53/608) | ||
| Gastritis | 13.2% (80/608) | ||
| Incisional hernia | 23.8% (145/608) | ||
| Cholethiasis | 11.3% (69/608) | ||
| Nutritional deficiency Vit B12 | 40% (243/608) | ||
| Nutritional deficiency anemia | 39% (237/608) | ||
| Staple line disruption | 15.1% (92/608) | ||
| Hospital readmission | 38.2% (232/608) | ||
| Dumping syndrome | 70.6% (429/608) | ||
| Mortality | 4.1% (25/608) |
Combined laparoscopic and open groups.
Calculated by ECRI.
(Table reproduced with kind permission from ECRI. Bariatric Surgery for Obesity. 2004. Plymouth Meeting, PA, ECRI. Technology Assessment Report.)
Adverse Effects: Comparisons of Procedures. From ECRI (19).
| Comparison | Study (Year) |
Procedure | Timing of Event |
No. of Patients |
Description | Patients at Risk, % (n/N)* |
|---|---|---|---|---|---|---|
| AGB vs. VBG |
Morino (2003) |
AGB VBG |
Perioperative | 49 51 |
Conversion to open | 0% (0/49) 0% (0/51) |
| Mortality | 0% (0/49) 0% (0/51) |
|||||
| Early | Any complications | 6% (3/39)^ 10% (5/51) |
||||
| Band slippage | 2% (1/49) 0% (0/51) |
|||||
| Fistula at staple line | 0% (0/49) 2% (1/51) |
|||||
| Port site hematoma | 2% (1/49) 0% (0/51) |
|||||
| Port site infection | 2% (1/49) 0% (0/51) |
|||||
| Prolonged pyrexia | 0% (0/49) 4% (2/51) |
|||||
| Respiratory failure (not pulmonary embolism) | 0% (0/49)^ 4% (2/51) |
|||||
| Late | Any complications | 33% (16/49)†^ 14% (7/50) |
||||
| 49 50 |
Asymptomatic pouch to fundus fistula | 0% (0/49) 2% (1/50) |
||||
| Band slippage | 18% (9/49)† | |||||
| Food intolerance | 2% (1/49) 0% (0/50) |
|||||
| Gastric bezoar | 0% (0/49) 2% (1/50) |
|||||
| Poor compliance | 2% (1/49) 0% (0/50) |
|||||
| Port infection | 2% (1/49) 0% (0/50) |
|||||
| Port twisted | 2% (1/49) 0% (0/50) |
|||||
| Pouch dilation | 0% (0/49) 2% (1/50) |
|||||
| Symptomatic reflux disease | 6% (3/49) 8% (4/50) |
|||||
| Reoperation | 25% (12/49)† 0% (0/50) |
|||||
| Nilsell (2001) |
AGB VBG |
Early | 29 30 |
Anastomotic leak | 0% (0/29) 3% (1/30 |
|
| Mortality | 0% (0/29) 0% (0/30) |
|||||
| Late | Mortality | 3%(1/29) 3% (1/30) |
||||
| Staple line disruption | 0% (0/29)† 17% (5/30) |
|||||
| Reoperation | 10% (3/2)† 37% (11/30) |
|||||
| Ashy (1998) |
AGB VBG |
Early | 30 30 |
Any complications | 0% (0/30) 0% (0/30) |
|
| Mortality | 0% (0/30) 0% (0/30) |
|||||
| Late | Any complications | 0% (0/30) | ||||
| 0% (0/30) | ||||||
| Mortality | 0% (0/30) 0% (0/30) |
|||||
| VBG vs. RYGBP |
MacLean (1995) |
VBG RYGBP |
Perioperative | 54 52 |
Mortality | 0% (0/54) 0% (0/52) |
| Early | Abscess | 2% (1/54) 0% (0/52) |
||||
| Enlarged orifice | 13% (7/54)† 0% (0/52) |
|||||
| Mortality | 0% (0/54) 0% (0/52) |
|||||
| Staple line disruption | 4% (2/54)† 23% (12/52) |
|||||
| Stenosis | 20% (11/54)† 0% (0/52) |
|||||
| Stomal ulcer | 0% (0/54)† 13% (7/52) |
|||||
| Late | Mortality | 0% (0/54) 0% (0/52) |
||||
| Reoperation | 43% (23/54)† 23% (12/52) |
|||||
| Sugerman (1987) |
Early | 20 20 |
Mortality | 0% (0/20) 5% (1/20) |
||
| Late | 16 18 |
Abnormal electrolytes | 0% (0/16) 0% (0/18) |
|||
| Abnormal liver function | 0% (0/16) 0% (0/18) |
|||||
| Abnormal renal function | 0% (0/16) 0% (0/18) |
|||||
| Conversion | 5% (1/20) 0% (0/20) |
|||||
| Marginal ulcer | 0% (0/17) 6% (1/18) |
|||||
| Mortality | 5% (1/20) 5% (1/20) | |||||
| Deficiency in albumin | 0% (0/17) 0% (0/18) |
|||||
| Deficiency in folic acid | 0% (0/17) 0% (0/18) |
|||||
| Deficiency in hemoglobin | 0% (0/17) 0% (0/18) |
|||||
| Deficiency in magnesium | 0% (0/17) 0% (0/18) |
|||||
| Deficiency in serum iron | 0% (0/17) 0% (0/18) |
|||||
| Deficiency in calcium | 0% (0/17) 0% (0/18) |
|||||
| Deficiency in transferrin | 0% (0/17) 0% (0/18) |
|||||
| Deficiency in Vitamin B1 | 0% (0/17) 0% (0/18) |
|||||
| Deficiency in Vitamin B12 | 18% (3/17) 39% (7/18) |
|||||
| Deficiency in vitamin B6 | 0% (0/17) 0% (0/18) |
|||||
| Deficiency in vitamin C | 0% (0/17) 0% (0/18) |
|||||
| Deficiency in zinc | 0% (0/17) | |||||
| 0% (0/18) | ||||||
| Staple line disruption | 6% (1/17) 0% (0/18) |
|||||
| Stomal erosion | 6% (1/17) 0% (0/18) |
|||||
| Stomal stenosis | 0% (0/17)† 28% (5/18) |
|||||
| RYBG vs. long-limb RYBG (LLRYGB) |
Feng (2003) |
RYGB LLRYGB |
Perioperative | 45 13 |
Any complications | 7% (3/45) 0% (0/13) |
| Leak at gastrojejunostomy |
2% (1/45) 0% (0/13) |
|||||
| Wound infection | 4% (2/45) 0% (0/13) |
|||||
| Choban (2002) |
RYGB LLRYGB |
Early | 35 34 |
Cirrhosis | 0% (0/35) 0% (0/34) |
|
| Leak and fistula | 3% (1/35) 0% (0/34) |
|||||
| Mortality | 3% (1/35) 0% (0/34) |
|||||
| Late | Mortality | 0% (0/35) 0% (0/34) |
||||
| Brolin | RYGB LLRYGB |
Perioperative | 22 23 |
Mortality | 0% (0/22) 0% (0/23) |
|
| Late | Mortality | 5% (1/22) 0% (0/23) |
||||
| Any | Vitamin B12 deficiency | 23% (5/22) 22% (5/23) |
||||
| Diarrhea | 0% (0/22) 0% (0/23) |
|||||
| Folate deficiency | 14% (3/22) 13% (3/23) |
|||||
| Hepatic dysfunction | 0% (0/22) 0% (0/23) |
|||||
| Iron deficiency | 50% (11/22) 57% (13/23) |
|||||
| Protein deficiency | 0% (0/22 0% (0/23) |
|||||
| Vitamin/mineral deficiency | 73% (16/22) 74% (17/23) |
ECRI did all statistical analyses, except where noted by ^
Statistically significant.
(Table reproduced with kind permission from ECRI. Bariatric Surgery for Obesity. 2004. Plymouth Meeting, PA, ECRI. Technology Assessment Report.)
Adverse Effects: Data on Adolescents With Morbid Obesity. From ECRI. (19).
| Study (Year) |
Perioperative, % (n/N) |
Early (< 30 days after surgery), % (n/N) |
Late (> 30 days after surgery), % (n/N) |
Reoperations |
|---|---|---|---|---|
| Abu-Abeid (2003) |
Any complications: 0% (0/11) Perioperative mortality: 0% (0/11) |
Not reported | Any complications: 0% (0/11) Cholelithiasis: 0% (0/11) Port complications: 0% (0/11) Hospital admission due to poor compliance with dietary changes: 0% (0/11) |
Not reported |
| Dolan (2003) |
Not reported | Not reported | Band slipped: 9% (1/11) Port leak: 9% (1/11) |
Not reported |
| Sugerman (2003) |
Perioperative mortality: 0% (0/33) Anastomotic leaks: 0% (0/33) |
Pulmonary embolism: 3% (1/33) Major wound infection: 3% (1/34) Minor wound infections: 12% (4/33) Stomal stenoses: 9% (3/33) Marginal ulcer: 12% (4/33) |
Small bowel obstruction: 3% (1/33) Incisional hernias: 18% (6/33) Mortality: 6% (2/33) |
6% (2/33) |
| Strauss (2001) |
Any complications: 0% (0/10) Perioperative mortality: 0% (0/10) |
None | Incisional hernia: 1% (1/10) Cholecystectomy: 20% (2/10) Small bowel obstruction: 10% (1/10) Protein calorie malnutrition: 10% (1/10) Minor iron deficiency: 50% (5/10) Minor folic acid deficiency: 30% (3/10) Minor vit D deficiency: 20% (2/10) Vit B12 deficiency: 0% (0/10) |
Not reported |
| Breaux (1995) |
Not reported | Vitamins A and D deficiency: 5% (1/22) Folic acid deficiency: 5% (1/22) Protein deficiency: 14% (3/22) Gallstone development: 5% (1/22) Kidney stone: 5% (1/22) Laryngeal edema: 5% (1/22) Incisional hernia: 5% (1/22) |
Mortality: 9% (2/22) | 5% (1/22) |
(Table reproduced with kind permission from ECRI. Bariatric Surgery for Obesity. 2004. Plymouth Meeting, PA, ECRI. Technology Assessment Report.)
Appendix 4
ICD-10 CA diagnosis codes:
| E66.0 | Obesity due to excess calories |
| E66.1 | Drug-induced obesity |
| E66.2 | Extreme obesity with alveolar hypoventilation |
| E66.8 | Other obesity |
| E66.9 | Obesity, unspecified |
ICD-10 CA CCI procedure codes:
1NF78SH
1NF78SJ
1NF78XP
Suggested Citation
This report should be cited as follows:
Medical Advisory Secretariat. Bariatric surgery: an evidence-based analysis. Ontario Health Technology Assessment Series 2005; 5(1)
Permission Requests
All inquiries regarding permission to reproduce any content in the Ontario Health Technology Assessment Series should be directed to MASinfo@moh.gov.on.ca
How to Obtain Issues in the Ontario Health Technology Assessment Series
All reports in the Ontario Health Technology Assessment Series are freely available in PDF format at the following URL: www.health.gov.on.ca/ohtas
Print copies can be obtained by contacting MASinfo@moh.gov.on.ca
Conflict of Interest Statement
All analyses in the Ontario Health Technology Assessment Series are impartial and subject to a systematic evidence-based assessment process. There are no competing interests or conflicts of interest to declare.
Peer Review
All Medical Advisory Secretariat analyses are subject to external expert peer review. Additionally, the public consultation process is also available to individuals wishing to comment on an analysis prior to finalization. For more information, please visit http://www.health.gov.on.ca/english/providers/program/ohtac/public_engage_overview.html
Contact Information
The Medical Advisory Secretariat
Ministry of Health and Long-Term Care
20 Dundas Street West, 10th floor
Toronto, Ontario
CANADA
M5G 2N6
Email: MASinfo@moh.gov.on.ca
Telephone: 416-314-1092
ISSN 1915-7398 (Online)
ISBN 1-4249-0132-4 (PDF)
About the Medical Advisory Secretariat
The Medical Advisory Secretariat is part of the Ontario Ministry of Health and Long-Term Care. The mandate of the Medical Advisory Secretariat is to provide evidence-based policy advice on the coordinated uptake of health services and new health technologies in Ontario to the Ministry of Health and Long-Term Care and to the healthcare system. The aim is to ensure that residents of Ontario have access to the best available new health technologies that will improve patient outcomes.
The Medical Advisory Secretariat also provides a secretariat function and evidence-based health technology policy analysis for review by the Ontario Health Technology Advisory Committee (OHTAC).
The Medical Advisory Secretariat conducts systematic reviews of scientific evidence and consultations with experts in the health care services community to produce the Ontario Health Technology Assessment Series.
About the Ontario Health Technology Assessment Series
To conduct its comprehensive analyses, the Medical Advisory Secretariat systematically reviews available scientific literature, collaborates with partners across relevant government branches, and consults with clinical and other external experts and manufacturers, and solicits any necessary advice to gather information. The Medical Advisory Secretariat makes every effort to ensure that all relevant research, nationally and internationally, is included in the systematic literature reviews conducted.
The information gathered is the foundation of the evidence to determine if a technology is effective and safe for use in a particular clinical population or setting. Information is collected to understand how a new technology fits within current practice and treatment alternatives. Details of the technology’s diffusion into current practice and information from practicing medical experts and industry, adds important information to the review of the provision and delivery of the health technology in Ontario. Information concerning the health benefits; economic and human resources; and ethical, regulatory, social and legal issues relating to the technology assist policy makers to make timely and relevant decisions to maximize patient outcomes.
If you are aware of any current additional evidence to inform an existing Evidence-Based Analysis, please contact the Medical Advisory Secretariat: MASInfo@moh.gov.on.ca. The public consultation process is also available to individuals wishing to comment on an analysis prior to publication. For more information, please visit http://www.health.gov.on.ca/english/providers/program/ohtac/public_engage_overview.html
Disclaimer
This evidence-based analysis was prepared by the Medical Advisory Secretariat, Ontario Ministry of Health and Long-Term Care, for the Ontario Health Technology Advisory Committee and developed from analysis, interpretation and comparison of scientific research and/or technology assessments conducted by other organizations. It also incorporates, when available, Ontario data, and information provided by experts and applicants to the Medical Advisory Secretariat to inform the analysis. While every effort has been made to do so, this document may not fully reflect all scientific research available. Additionally, other relevant scientific findings may have been reported since completion of the review. This evidence-based analysis is current to the date of publication. This analysis may be superceded by an updated publication on the same topic. Please check the Medical Advisory Secretariat Website for a list of all evidence-based analyses: http://www.health.gov.on.ca/ohtas
Abbreviations
- AGB
Adjustable gastric banding
- BPD
Biliopancreatic diversion
- BMI
Body mass index
- CI
Confidence interval
- EWL
Excess weight loss
- LAGB
Laparoscopic adjustable gastric banding
- OR
Odds ratio
- QALY
Quality-adjusted life year
- QoL
Quality of life
- RCT
Randomized controlled trial
- RYGB
Roux-en-Y gastric bypass
- SOS (study)
Swedish obese subjects (study)
- VBG
Vertical banded gastroplasty
Footnotes
Body mass index: Body weight expressed in kilograms (kg) divided by height expressed in square metres (m2).
OHIP will pay in full for health services outside Canada if: a) the patient gets written authorization from the Ministry of Health and Long-Term Care before the treatment is given, b) the treatment is generally accepted in Ontario, and c) the treatment or an equivalent procedure is not performed in Ontario, or d) the treatment is performed in Ontario but it is necessary that the person travel outside Canada to avoid a delay that would result in death or medically significant irreversible tissue damage. To obtain consideration for full funding of treatment outside Canada, the patient’s physician must apply to the ministry for approval while the patient is in Ontario and before the patient receives out-of-country treatment.
The actual number of cases is the number of patients who actually receive the surgery. In some cases, patients may be approved for the service in one fiscal year, but actually receive it in another fiscal year. Not all patients who are approved for OOC service end up receiving the service. This is because some patients will decide not to undergo the surgery at all and others may have a change in their condition that results in them no longer being a viable candidate for the surgery.
OHIP will pay in full for health services outside Canada if: a) the patient gets written authorization from the Ministry of Health and Long-Term Care before the treatment is given, b) the treatment is generally accepted in Ontario, and c) the treatment or an equivalent procedure is not performed in Ontario, or d) the treatment is performed in Ontario but it is necessary that the person travel outside Canada to avoid a delay that would result in death or medically significant irreversible tissue damage. To obtain consideration for full funding of treatment outside Canada, the patient’s physician must apply to the ministry for approval while the patient is in Ontario and before the patient receives out-of-country treatment.
The actual number of cases is the number of patients who actually receive the surgery. In some cases, patients may be approved for the service in one fiscal year, but actually receive it in another fiscal year. Not all patients who are approved for OOC service end up receiving the service. This is because some patients will decide not to undergo the surgery at all and others may have a change in their condition that results in them no longer being a viable candidate for the surgery.
MOHLTC Claims History Database records only 230 gastric bypass procedures for FY 2003; however, discrepancies with discharge data are not uncommon owing to difficulties in assessing the exact number of procedures billed under remuneration schemes other than Fee-for-Service. There are other reasons beyond this as to why there might be a difference in numbers – for example we have found that hospitals sometimes make errors in their DAD information, just as providers might make mistakes when submitting OHIP claims. Given the relatively small number of services we are looking at here, and the small number of facilities providing bariatric surgery, it is possible that just one facility making errors on their DAD information could affect this data. Most alternative payment plan physicians are required to submit shadow claims to OHIP which still allows us reasonably accurate information on physician services.
ICD-10/CA CCI procedure code 1YS78LA
Normally, a small adjustment is made for price inflation when using cost data from a previous fiscal year; however, the wide range of costs across patients and year-to-year variations would dominate the small 1.14% annual health and personal care price inflation determined by StatsCan for the period 2002-04.
Since only about 29% of gastric bypass patients are expected to require panniculectomy, the per-case expected OHIP cost add-on is not expected to significantly add to the gastric bypass OHIP costs. Therefore, the panniculectomy OHIP costs have been left out of the calculations.
Data for FY 2004/05 may be incomplete as the ministry may not have received and processed all invoices for procedures performed in this fiscal year by the end of May 2005 when this report was finished.
This difference in costs is calculated based on the assumption that all panniculectomy procedures are performed in-province; therefore, cost savings from repatriation are calculated independent of costs associated with this procedure ($20,100 = $36,600 avg. cost for out-of-country - $16,500 in-province)
Out-of-Country costs have been decreasing substantially for bariatric surgery as the Ministry has recently negotiated discount rates with a number of U.S. providers which should continue to decrease the annual avg. cost per patient.
Ontario currently funds 508 procedures annually (283 are performed in Ontario and 225 receive out-of-country funding). It could fund 275 more procedures based on difference is costs from repatriating the 225 out-of-country cases ($3.3 M in annual savings) leaving 2,720 procedures that must be funded with additional outlays to achieve the goal of 3,500 annual procedures.
OHIP will pay in full for health services outside Canada if: a) the patient gets written authorization from the Ministry of Health and Long-Term Care before the treatment is given, b) the treatment is generally accepted in Ontario, and c) the treatment or an equivalent procedure is not performed in Ontario, or d) the treatment is performed in Ontario but it is necessary that the person travel outside Canada to avoid a delay that would result in death or medically significant irreversible tissue damage. To obtain consideration for full funding of treatment outside Canada, the patient’s physician must apply to the ministry for approval while the patient is in Ontario and before the patient receives out-of-country treatment.
The actual number of cases is the number of patients who actually receive the surgery. In some cases, patients may be approved for the service in one fiscal year, but actually receive it in another fiscal year. Not all patients who are approved for OOC service end up receiving the service. This is because some patients will decide not to undergo the surgery at all and others may have a change in their condition that results in them no longer being a viable candidate for the surgery.
References
- 1.Sjostrom L, Kindroos AK, Peltonen M, Torgerson J, Bouchard C, Carlsson B, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. New England Journal of Medicine. 2004;(351):2683–2693. doi: 10.1056/NEJMoa035622. [DOI] [PubMed] [Google Scholar]
- 2.Tsai AG, Wadden TA. Systematic review: an evaluation of major commercial weight loss programs in the United States. Annals of Internal Medicine. 2005;(142):56–66. doi: 10.7326/0003-4819-142-1-200501040-00012. [DOI] [PubMed] [Google Scholar]
- 3.Stern L, Iqbal N, Seshadri P, Chicano KL, DAily DAI, McGrory J, et al. The effects of low carbohydrate versus conventional weight loss diets in severely obese adults: one year followup of a randomized trial. Annals of Internal Medicine. 2004;(140):778–785. doi: 10.7326/0003-4819-140-10-200405180-00007. [DOI] [PubMed] [Google Scholar]
- 4.Patterson EJ, Urbach DR, Swanstrom LL. A comparison of diet and exercise therapy versus laparoscopic Roux en Y gastric bypass surgery for morbid obesity: a decision analysis model. Journal of the American College of Surgery. 2003;(196):379–384. doi: 10.1016/S1072-7515(02)01754-4. [DOI] [PubMed] [Google Scholar]
- 5.Clegg AJ, Colquitt J, Sidhu MK, Royle P, Loveman E, Walker A. The clinical effectiveness and cost-effectiveness of surgery for people with morbid obesity: a systematic review and economic evaluation. Health Technology Assessment. 2002;6(12) doi: 10.3310/hta6120. [DOI] [PubMed] [Google Scholar]
- 6.Birmingham CL, Jones PJ. Clinical nutrition: 5 How much should Canadians eat? Canadian Medical Association Journal. 2002;166(767):770. [PMC free article] [PubMed] [Google Scholar]
- 7.Douketis JD, Feightner JW, Attia J, Feldman WF. with the Canadian Task Force on Preventive Health Care. Canadian Medical Association Journal. 1999;(160):513–525. Periodic health examination, 1999 update: 1. Detection, prevention and treatment of obesity. [PMC free article] [PubMed] [Google Scholar]
- 8.Manson JE, Colditz GA, Stampfer MJ, Willet WC, Rosner B, Monson RR. A prospective study of obesity and risk of coronary heart disease and women. New England Journal of Medicine. 1990;(322):882–889. doi: 10.1056/NEJM199003293221303. [DOI] [PubMed] [Google Scholar]
- 9.Rimm EB, Stampfer MJ, Giovannucci E, Ascherio A, Spiegelman D, Colditz GA. Body size and fat distribution as predictors of coronary heart disease among middle aged and older US men. American Journal of Epidemiology. 1995;(141):1117–1127. doi: 10.1093/oxfordjournals.aje.a117385. [DOI] [PubMed] [Google Scholar]
- 10.Manson JE, Willet WC, Stampfer MJ, Colditz GA, Hunter DJ, Hankinson SE. Body weight and mortality among women. New England Journal of Medicine. 1995;(33):677–685. doi: 10.1056/NEJM199509143331101. [DOI] [PubMed] [Google Scholar]
- 11.Canadian Coordinating Office for Health Technology Assessment. Laparoscopic adjustable gastric banding for clinically severe obesity. 2003. Canadian Coordinating Office for Health Technology Assessment (CCOHTA) [Google Scholar]
- 12.Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US adults, 1999-2000. JAMA. 2002;(288):1723–1727. doi: 10.1001/jama.288.14.1723. [DOI] [PubMed] [Google Scholar]
- 13.Health Canada. Canadian Guidelines for Body Weight Classification in Adults. 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Macdonald SM, Reeder BA, Chen Y, Depres JP. Canadian Hearth Health Surveys Research Group. Obesity in Canada: a descriptive analysis. Canadian Medical Association Journal. 1997;(157(Suppl 1)):S3–S9. [PubMed] [Google Scholar]
- 15.Ontario Chief Medical Officer of Health. 2004 Chief Medical Officer of Health Report. Healthy Weights, Healthy Lives. Ontario: Ministry of Health and Long-Term Care; 2004. [Google Scholar]
- 16.Collazo-Clavell ML. Safe and effective management of the obese patient. Mayo Clinic Proceedings. 1999;(74):1255–1260. doi: 10.4065/74.12.1255. [DOI] [PubMed] [Google Scholar]
- 17.Solomon CG, Dluhy RG. Bariatric surgery - quick fix or long term solution? New England Journal of Medicine. 2004;(351):2751–2753. doi: 10.1056/NEJMe048308. [DOI] [PubMed] [Google Scholar]
- 18.Food and Drug Administration. The LAP-BAND Adjustable Gastric Banding System: summary of safety and effectiveness data. PMA Number P000008. 2001. [Google Scholar]
- 19.ECRI Bariatric Surgery for Obesity. Plymouth Meeting, PA, ECRI. 2004. Technology Assessment Report. [Google Scholar]
- 20.Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;(292):1724–1737. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- 21.Shekelle PG, Morton SC, Maglione MA, Suttopr M, Tu W, Li Z, et al. Santa Monica, CA, Prepared by the Southern California - RAND Evidence Based Practice Center. 2004. Pharmacological and surgical treatment of obesity. [PMC free article] [PubMed] [Google Scholar]
- 22.Blue Cross Blue Shield Association TEC. Special report: the relationship between weight loss and changes in morbidity following bariatric surgery for morbid obesity. Chicago IL: Blue Cross Blue Shield Association; 2003. [PubMed] [Google Scholar]
- 23.Blue Cross Blue Shield Association TEC. Newer technologies in bariatric surgery for morbid obesity. 2003. [Google Scholar]
- 24.Medical Services Advisory Committee. Laparoscopic adjustable gastric banding for morbid obesity. Canberra: Medical Services Advisory Committee (MSAC); 2003. [Google Scholar]
- 25.Chapman A, Game P, O’Brien P, Maddern G, Kiroff G, Foster B, et al. Australian Safety and Efficacy Register of New Interventional Procedures - Surgical (ASERNIP-S) 2002. A systematic review of laparoscopic adjustable gastric banding for the treatment of obesity (update and re-appraisal) [Google Scholar]
- 26.Chen J, McGregor M. The gastric banding procedure: an evaluation. Montreal: Technology Assessment Unit of the McGill University Health Centre (MUHC); 2004. [Google Scholar]
- 27.Institute for Clinical Systems Improvement. Gastric restrictive surgery for morbid obesity Technology Assessment Update. 2000. TA#14 Second Update. [Google Scholar]
- 28.Smith SC, Edwards CB, Goodman GN, Halversen RC, Simper SC. Open vs laparoscopic Roux-en-Y gastric bypass: comparison of operative morbidity and mortality. Obesity Surgery. 2004;14(1):73–76. doi: 10.1381/096089204772787329. [DOI] [PubMed] [Google Scholar]
- 29.Christou NV, Sampalis JS, Liberman M, Look D, Auger S, McLean AP, et al. Surgery decreases long-term mortality, morbidity, and health care use in morbidly obese patients. Annals of Surgery. 2004;240(3):416–423. doi: 10.1097/01.sla.0000137343.63376.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dolan K, Hatzifotis M, Newbury L, Lowe N, Fielding G. A clinical and nutritional comparison of biliopancreatic diversion with and without duodenal switch. Annals of Surgery. 2004;240(1):51–56. doi: 10.1097/01.sla.0000129280.68540.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ponce J, Haynes B, Paynter S, From R, Lindsey B, Shafer A, et al. Effect of Lap Band induced weight loss on type 2 diabetes mellitus and hyptertension. Obesity Surgery 14. 2004:1335–1342. doi: 10.1381/0960892042583932. [DOI] [PubMed] [Google Scholar]
- 32.Martikainen T, Pirinen E, Alhava E, Poikolainen E, Paakkonen M, Uusitupa M, et al. Long-term results, late complications and quality of life in a series of adjustable gastric banding. Obesity Surgery. 2004;14(5):648–654. doi: 10.1381/096089204323093435. [DOI] [PubMed] [Google Scholar]
- 33.Shen R, Dugay G, Rajaram K, Cabrera I, Siegel N, Ren CJ. Impact of patient follow-up on weight loss after bariatric surgery. Obesity Surgery. 2004;14(4):514–519. doi: 10.1381/096089204323013523. [DOI] [PubMed] [Google Scholar]
- 34.Dolan K, Hatzifotis M, Newbury L, Fielding G. A comparison of laparoscopic adjustable gastric banding and biliopancreatic diversion in superobesity [see comment] Obesity Surgery. 2004;14(2):165–169. doi: 10.1381/096089204322857500. [DOI] [PubMed] [Google Scholar]
- 35.Deveney CW, MacCabee D, Marlink K, Welker K, Davis J, McConnell DB. Roux-en-Y divided gastric bypass results in the same weight loss as duodenal switch for morbid obesity. American Journal of Surgery. 2004;187(5):655–659. doi: 10.1016/j.amjsurg.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 36.Flum DR, Dellinger EP. Impact of gastric bypass operation on survival: a population based analysis. Journal of the American College of Surgeons. 2004;(199):543–551. doi: 10.1016/j.jamcollsurg.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 37.Gallagher SF, Banasiak M, Gonzalvo JP, Paoli DP, Allwood J, Morris D, et al. The impact of bariatric surgery on the Veterans Administration healthcare system: A cost analysis. Obesity Surgery. 2003;13(2):245–248. doi: 10.1381/096089203764467144. [DOI] [PubMed] [Google Scholar]
- 38.Craig BM, Tseng DS. Cost-effectiveness of gastric bypass for severe obesity. American Journal of Medicine. 2002;113(6):491–498. doi: 10.1016/s0002-9343(02)01266-4. [DOI] [PubMed] [Google Scholar]
- 39.Cooney RN, Bryant P, Haluck R, Rodgers M, Lowery M. The impact of a clinical pathway for gastric bypass surgery on resource utilization. Journal of Surgical Research. 2001;98(2):97–101. doi: 10.1006/jsre.2001.6167. [DOI] [PubMed] [Google Scholar]
- 40.Cooney RN, Haluck RS, Ku J, Bass T, MacLeod J, Brunner H, et al. Analysis of cost outliers after gastric bypass surgery: What can we learn? Obesity Surgery. 2003;13(1):29–36. doi: 10.1381/096089203321136557. [DOI] [PubMed] [Google Scholar]
- 41.Huerta S, Heber D, Sawicki MP, Liu CD, Arthur D, Alexander P, et al. Reduced length of stay by implementation of a clinical pathway for bariatric surgery in an academic health care center. American Surgeon. 2001;67(12):1128–1135. [PubMed] [Google Scholar]
- 42.Narbro K, Agren G, Jonsson E, Naslund I, Sjostrom L, Peltonen M, et al. Pharmaceutical costs in obese individuals: comparison with a randomly selected population sample and long-term changes after conventional and surgical treatment: the SOS intervention study. Archives of Internal Medicine. 2002;162(18):2061–2069. doi: 10.1001/archinte.162.18.2061. [DOI] [PubMed] [Google Scholar]
- 43.Nguyen NT, Goldman C, Rosenquist CJ, Arango A, Cole CJ, Lee SJ, et al. Laparoscopic versus open gastric bypass: a randomized study of outcomes, quality of life, and costs. Annals of Surgery. 2001;234(3):279–289. doi: 10.1097/00000658-200109000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Monk Jr JS, Nagib ND, Stehr W. Pharmaceutical Savings after Gastric Bypass Surgery. Obesity Surgery. 2004;14(1):13–15. doi: 10.1381/096089204772787220. [DOI] [PubMed] [Google Scholar]
- 45.Potteiger CE, Paragi PR, Inverso NA, Still C, Reed MJ, Strodel W III, et al. Bariatric surgery: shedding the monetary weight of prescription costs in the managed care arena. Obesity Surgery. 2004;14(6):725–730. doi: 10.1381/0960892041590999. [DOI] [PubMed] [Google Scholar]
- 46.Sampalis JS, Liberman M, Auger S, Christou NV. The impact of weight reduction surgery on health-care costs in morbidly obese patients. Obesity Surgery. 2004;14(7):939–947. doi: 10.1381/0960892041719662. [DOI] [PubMed] [Google Scholar]
- 47.Agren G, Narbro K, Jonsson E, Naslund I, Sjostrom L, Peltonen M. Cost of in-patient care over 7 years among surgically and conventionally treated obese patients. Obesity Research. 2002;10(12):1276–1283. doi: 10.1038/oby.2002.173. [DOI] [PubMed] [Google Scholar]
- 48.DeMaria EJ, Schweitzer MA, Kellum JM, Meador J, Wolfe L, Sugerman HJ. Hand-assisted laparoscopic gastric bypass does not improve outcome and increases costs when compared to open gastric bypass for the surgical treatment of obesity. Surgical Endoscopy. 2002;16(10):1452–1455. doi: 10.1007/s00464-001-8321-5. [DOI] [PubMed] [Google Scholar]
- 49.Brechner RJ, Farris C, Harrison S, Tillman K, Salive M, Phurrough S. Centers for Medicare and Medicaid Services Summary of evidence - bariatric surgery - November 4 2004. 2004. http://www.cms.hhs.gov/mcac/id137f.pdf .
- 50.American Society for Bariatric Surgery, Society of Gastrointestinal Endoscopic Surgeons. 2000. www.asbs.org/html/lab_guidelines.html . [DOI] [PubMed]
- 51.National Heart LaBI. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults. The evidence report. 1998. NIH Publication No 98 4083. [PubMed] [Google Scholar]
- 52.Blue Cross Blue Shield Association. Blue Cross Blue Shield of Tennessee Medical Policy Manual - Bariatric Surgery for Morbid Obesity. 2004. www.bcbst.com/MPManual?Bariatric_Surgery_for_Morbid_Obesity.html .
- 53.Aetna. Clinical Policy Bulletin - Number 0157 - Obesity Surgery. 2004. www.aetna.com/cpb/data/PrtCPBA0157.html .
- 54.2004. Provider Services Branch OMoHaL-TC Gastric bypass surgeries funded by OHIP (updated August 26, 2004) [Google Scholar]
- 55.Steinbrook R. New England Journal of Medicine. 350. 2004. Surgery for severe obesity; pp. 1075–1079. [DOI] [PubMed] [Google Scholar]
- 56.ECRI Bariatric Services: Safety, Quality and Technology Guide. Plymouth Meeting, PA, ECRI 2004 [Google Scholar]


