Abstract
We show that anandamide (AEA) externally added to model membrane vesicles containing trapped fatty acid amide hydrolyase (FAAH) can be readily hydrolyzed, demonstrating facile, rapid anandamide movement across the lipid bilayer. The rate of hydrolysis is significantly facilitated by cholesterol and coprostanol, but not by cholesterol sulfate. The effects of sterol upon hydrolysis by FAAH bound to the outer surface of the bilayer were much smaller, although they followed the same pattern. We propose the facilitation of hydrolysis is a combination of the effects of sterol on accessibility of membrane-inserted endocannabinoids to surface protein, and on the rate of endocannabinod transport across the membrane bilayer.
Keywords: Anandamide, liposome, LUV, transporter, fatty acid amide hydrolase, FAAH, cholesterol
Metabolic enzymes and plasma membrane protein transporters are classical drug targets for most neurotransmitter systems. FAAH falls into this category as discussed in the accompanying papers. However, the situation for the anandamide transporter is unique. Owing to its water insolubility, after traversing the plasma membrane, it must be transported through the cytoplasm to intracellular FAAH for inactivation. Although there is literature in support of an anandamide transmembrane transporter, recent direct evidence has shifted the focus of the transporter away from the cell membrane to the cytoplasm.1 Four intracellular transporters (carriers, chaperones) have been proposed and in two cases they have been shown to be the targets for “transport inhibitors”, endocannabinoid analogues whose function were originally ascribed to binding a plasma membrane protein.2−5
Here, we employed synthetic lipid vesicles with FAAH restricted to the vesicle interior, to explore whether anandamide can cross the cell membrane in a protein-independent manner. This model system also allows us to address observations in the literature regarding passive diffusion of anandamide, the effects of cholesterol upon its uptake and the involvement of lipid rafts.6−11 Our results suggest that a cell membrane protein is an unlikely drug target for endocannabinoid transport.
Results and Discussion
Efficient transmembrane and intracellular transport are indispensable for AEA inactivation.2−5 In contrast to intracellular trafficking, the mechanisms mediating AEA membrane transport are poorly characterized and controversial.7,12−14 Although originally characterized as a process of facilitated diffusion,15 recent evidence implicates simple diffusion in AEA transport.16,17
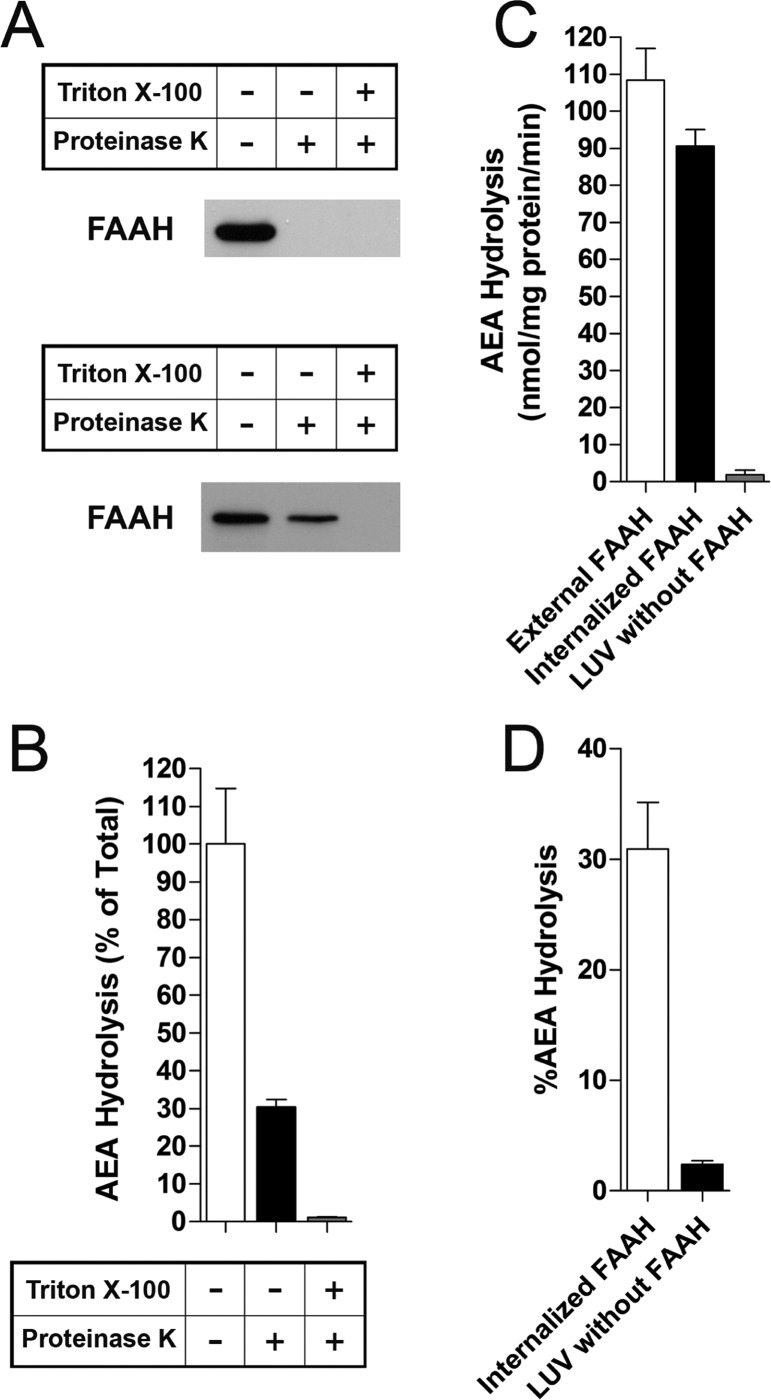
To explore AEA membrane transport, we prepared large unilamellar vesicles (LUVs) with internalized/trapped FAAH bound to the inner leaflet of the LUV membrane (Figure 1). The LUVs contained a physiological phospholipid mixture that exists in a liquid disordered (nonlipid raft) state.18 We employed a FAAH variant that lacks its N-terminal transmembrane domain yet retains the activity of wild-type FAAH.19 AEA hydrolysis by FAAH serves as an indicator of AEA membrane transport as nontransported AEA cannot be used by trapped FAAH as a substrate. To ensure that AEA hydrolysis originated exclusively from trapped FAAH, we treated the LUVs with proteinase K to cleave FAAH that was bound to the liposome exterior. As expected, proteinase K completely cleaved externally bound FAAH while internalized FAAH was inactivated only in the presence of a membrane-permeabilizing detergent (Figure 2A). FAAH internalization occurred with a ∼30% efficiency (Figure 2B), indicating adequate FAAH incorporation into the LUVs.
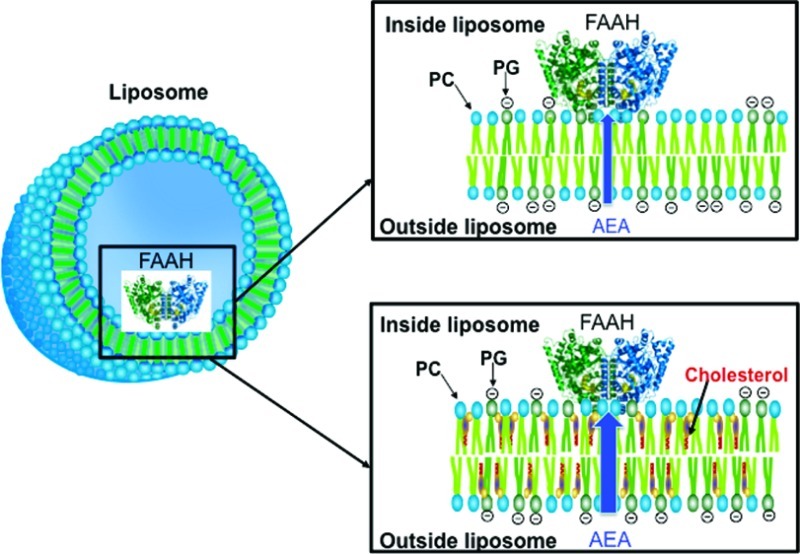
Figure 1.
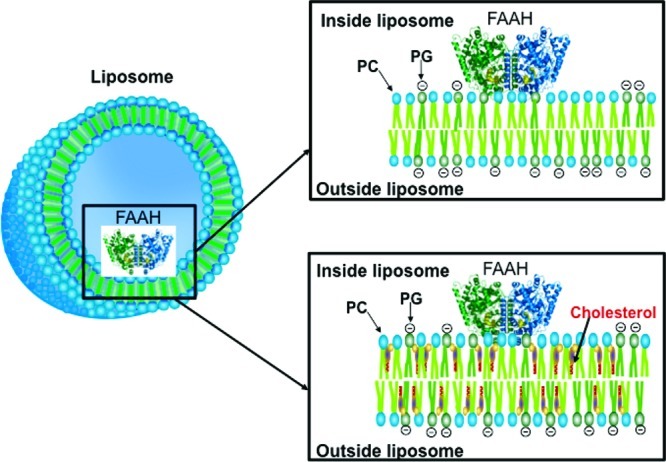
Schematic of LUVs with internalized FAAH. PC/PG-containing LUVs were prepared in the absence (top panel) or presence (bottom panel) of sterols (coprostanol or cholesterol).
Figure 2.
Characterization of LUVs with internalized FAAH. (A) Treatment of LUVs with 30 μg/mL proteinase K completely cleaves externally facing FAAH (top panel). When added to the lipid mixtures before vesicle formation to permit internalization, only noninternalized FAAH is susceptible to proteinase K cleavage (bottom panel). Addition of 0.5% Triton X-100 to permeabilize membranes results in cleavage of trapped FAAH. (B) Hydrolysis of [14C]AEA by internalized FAAH is revealed following addition of proteinase K to cleave externally facing FAAH (n = 3). (C) Similar rates of [14C]AEA hydrolysis by externally or internally bound FAAH (n = 4). (D) Percentage of [14C]AEA hydrolysis of total AEA added to the reaction tubes by internalized FAAH (n = 5).
We analyzed AEA membrane transport by examining AEA hydrolysis in LUVs containing externally bound or internalized FAAH. Externally and internally restricted FAAH hydrolyzed AEA with similar rates (Figure 2C), indicating that AEA readily diffused through the membrane. Interestingly, ∼30% of the added AEA was hydrolyzed by internalized FAAH over the 5 min time course employed in these experiments (Figure 2D). These results indicate that AEA readily diffuses through a membrane in the absence of a putative membrane transporter.
Previous reports indicate that membrane cholesterol potentiates, while its absence reduces, AEA membrane transport.9,20,21 These effects were attributed to cholesterol-enriched lipid rafts, liquid ordered domains within the plasma membrane.22 However, to date, there is no direct experimental evidence for the involvement of lipid rafts in AEA membrane transport. Therefore, we sought to determine whether the ability of cholesterol to potentiate AEA uptake is related to its ability to increase membrane order and promote lipid raft formation or to its biophysical properties unrelated to lipid rafts. To uncouple these two properties of cholesterol, we prepared LUVs containing cholesterol or coprostanol, a non-raft-forming sterol.23 Additionally, to explore whether the ability of cholesterol to rapidly flip-flop across membranes might aid AEA movement across the membrane,9 we prepared LUVs containing cholesterol sulfate, a charged sterol that should not be able to rapidly flip across membranes.
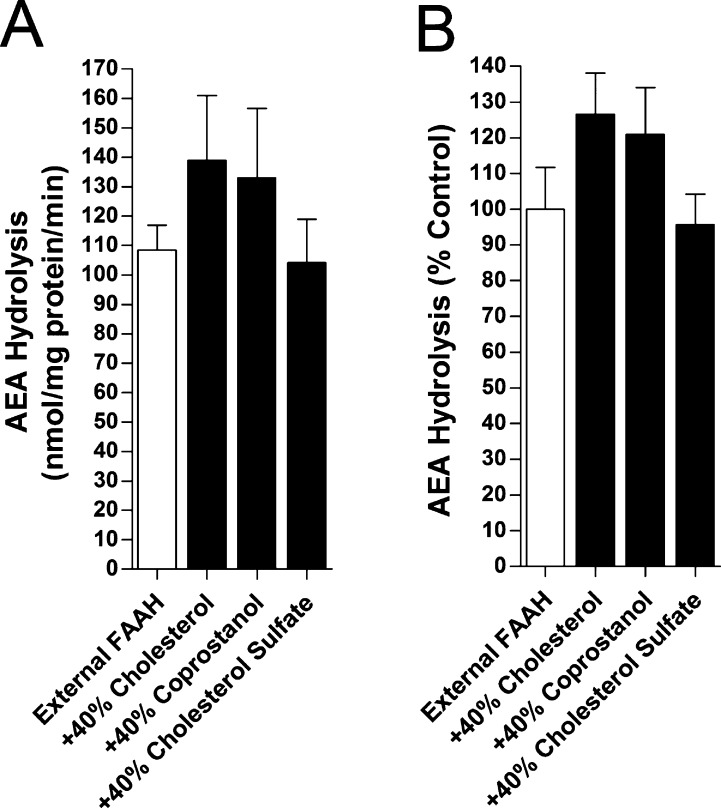
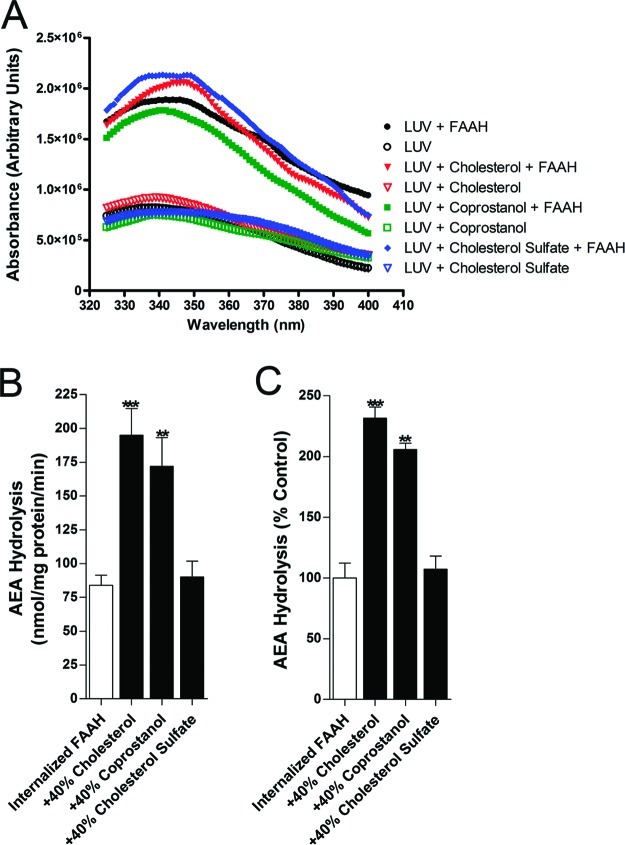
The activity of externally bound FAAH (Figure 3A and B) was weakly sterol dependent, with at most a slight increase in hydrolysis in the presence of cholesterol and coprostanol, but not statistically significant. FAAH trapping (Figure 4A) was unaffected by membrane sterols, but AEA hydrolysis by vesicle-trapped FAAH was significantly enhanced in LUVs enriched in cholesterol or coprostanol, while cholesterol sulfate was without effect (Figure 4B and C). The effect of cholesterol and coprostanol was about 5-fold greater for trapped FAAH than for external FAAH. The observation that the enhancement of AEA hydrolysis was similar in magnitude in the presence of cholesterol or coprostanol suggests a common mechanism underlying these effects.
Figure 3.
Effect of sterols upon AEA hydrolysis by FAAH bound to the exterior face of LUVs. The specific activities (A) and normalized activities (B) of [14C]AEA hydrolysis by FAAH in LUVs containing or lacking cholesterol, coprostanol, or cholesterol sulfate. The activities were normalized to FAAH activity on LUVs lacking sterols (n = 3).
Figure 4.
Cholesterol and coprostanol potentiate [14C]AEA hydrolysis by internalized FAAH. (A) Representative tryptophan fluorescence intensities of LUVs with internalized FAAH reveal similar levels of FAAH incorporation in the presence or absence of sterols. (B) [14C]AEA hydrolysis by FAAH in the presence or absence of cholesterol, coprostanol, or cholesterol sulfate. (C) Normalized [14C]AEA hydrolysis by FAAH in the presence or absence of sterols. **p < 0.01; ***p < 0.001 (n = 5).
The present study employed a defined system to mechanistically define AEA membrane transport and its modulation by sterols. Our data demonstrate that the transmembrane transport of AEA is robust and occurs in the absence of a membrane transporter, corroborating similar observations in cultured cell models.16,17 Although we cannot rule out the existence of an endocannabinoid membrane transporter, such a protein is unnecessary for efficient endocannabinoid transport.2,9,24
Membrane cholesterol modulates AEA internalization through unknown mechanisms. Although originally attributed to lipid rafts,9,20,21 our findings show that the lipid raft-forming cholesterol and the lipid raft-excluded coprostanol almost equipotently enhanced AEA membrane transport. Therefore, the potentiating effects of these molecules are independent of their ability to promote lipid raft formation and must stem from other biophysical properties common to both sterols. We propose several possible models that may explain these effects. One possibility is that cholesterol and coprostanol physically interact with AEA as previously postulated,9 and enhance its diffusion across the membrane. However, this model does not by itself explain why the absolute rate of hydrolysis in the presence of cholesterol is higher for trapped FAAH than external FAAH. If AEA transport were rate limiting, then one would expect a slower rate of hydrolysis for trapped FAAH that would increase to a value close to that of external FAAH when cholesterol was added.
An alternative is that the effect of sterols involves their influence on the accessibility of AEA to FAAH. It has been shown in many studies that the exposure of membrane bound hydrophobic molecules with small polar groups (e.g., cholesterol) to aqueous solution, and thus to proteins in solution, is controlled by the “umbrella effect”.25 The umbrella effect refers to the (limited) ability of large phospholipid headgroups to shield/hide lipids with small headgroups from unfavorable contact with water. When two molecules with small polar headgroups are present in a membrane, they compete for this shielding and exhibit increased exposure to the aqueous solution. This results in an increase in their reactivity with surface bound or aqueous proteins with which the small headgroup lipids interact.26−29 As AEA is a small polar headgroup lipid, the presence of cholesterol is expected to increase its exposure to FAAH, and thus its rate of hydrolysis. Consistent with the experimental results, this effect should also be observed with coprostanol, but not with cholesterol sulfate, which has a large hydrophilic sulfate group, and thus will not compete with AEA for sites that shield AEA from contact with water. The effect of sterols may be more pronounced in the inner leaflet due to differential distribution of sterols between the inner and outer leaflets or due to an effect of membrane curvature upon AEA exposure. It is likewise possible that the potentiation of AEA uptake and hydrolysis observed with trapped FAAH reflects a combination of enhanced reactivity of AEA with FAAH due to umbrella effect competition and increased AEA transport across the bilayer.
Methods
Chemicals
1,2-Dioleoyl-sn-glycero-3-phospho-(1′-rac-glycerol) (PG), 1,2-di-(9,10-dibromo)stearoyl-sn-glycero-3-phosphocholine (BrPC), 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (PC), cholesterol, and cholesterol 3-sulfate were purchased from Avanti Polar Lipids (Alabaster, AL). Coprostanol was from Steraloids Inc. (Newport, RI). [14C]AEA was kindly provided by the drug supply program of the National Institute on Drug Abuse. Proteinase K was from Sigma (St. Louis, MO). FAAH was prepared as the truncated form (residues 29–579) with an N-terminal 6XHIs tag30
LUVs with Externally Bound FAAH
LUVs (2 mM total lipid) were prepared in PBS (pH 7.4) and contained 30% PG, 15% BrPC, 15% or 55% POPC ± 40% sterols (mol:mol). The lipids were mixed in chloroform and subsequently dried under a stream of nitrogen. The samples were further dried under high vacuum for 1 h. After vacuum, the samples were rehydrated with 1 mL PBS at 70 °C and underwent five freeze–thaw cycles in dry ice/acetone mixtures. The resulting LUVs were incubated with 7 μg of purified FAAH as described19 and then incubated on ice for 1 h. These LUVs were subsequently pelleted by centrifugation at 85 000g for 35 min at 4 °C with approximately 50–70% of FAAH bound to the vesicles. Once bound to the liposome surface, 95% of the enzyme activity remained associated with the pellet throughout the experimental procedure. The LUVs were resuspended in PBS and the tryptophan fluorescence emission intensity was measured at room temperature on a SPEX Fluorolog 3 spectrofluoremeter with excitation and emission wavelengths of 280 nm and 325–400 nm, respectively. Fluorescence intensities of LUVs lacking FAAH were also measured. The intensities at 340 nm were used to normalize activity for variations in FAAH levels between preparations.
LUVs Containing Internalized FAAH
LUVs were prepared as described above with the exception that FAAH was added to the lipid mixture preceding the freeze–thaw procedure. The resulting LUVs possessing internalized and externally associated FAAH were treated with 30 μg/mL proteinase K for 15 min at room temperature. The LUVs were subsequently pelleted by centrifugation, and their tryptophan fluorescence measured as described above.
Western Blotting
Western blotting was performed as described.31 Identical volumes of FAAH-bound LUVs (∼30 ng FAAH) were treated with 30 μg/mL proteinase K for 15 min at room temperature in the presence or absence of 0.5% Triton X-100 and subsequently subjected to SDS-PAGE. The blots were probed with anti-6x His antibodies (1:2000) (Abcam, Cambridge, MA) followed by goat anti-mouse HRP antibodies (Molecular Probes, Eugene, OR).
AEA Uptake and Hydrolysis
LUVs containing bound FAAH (0.5 μg) in PBS were incubated with 1 μM [14C]AEA for 5 min at 37 °C with shaking. LUVs lacking FAAH were used as controls. For experiments with internalized FAAH, the LUVs were preincubated with 30 μg/mL proteinase K for 10 min at room temperature to ensure that only intact vesicles with internalized FAAH contributed to AEA hydrolysis. The reactions were stopped by the addition of 2 volumes of 1:1 chloroform/methanol followed by centrifugation to separate the phases. The methanol phase containing [14C]ethanolamine was sampled and quantified using a Beckman LS6500 scintillation counter.
Statistics
Results are expressed as means ± SEM of at least three independent experiments performed in triplicate. Significance was determined using two tailed unpaired student t tests.
Author Contributions
Participated in research design: M.K., E.L., B.F.C., and D.G.D. Conducted experiments: M.K., Q.L., L.D.N., and M.K.M. Performed data analysis: M.K. and E.L. Wrote or contributed to the writing of the manuscript: M.K., L.D.N., E.L., and D.G.D.
This work was supported by NIH DA032232 (M.K.) NIH DA02710301 (D.G.D.), NIH DA 02695301 (D.G.D.), NSF MCB1019986 (E.L.), and NIH DA017259 (B.F.C).
The authors declare no competing financial interest.
Author Contributions
§ Authors contributed equally to the manuscript.
Funding Statement
National Institutes of Health, United States
References
- Howlett A. C.; Reggio P. H.; Childers S. R.; Hampson R. E.; Ulloa N. M.; Deutsch D. G. (2011) Endocannabinoid tone versus constitutive activity of cannabinoid receptors. Br. J. Pharmacol. 163, 1329–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczocha M.; Glaser S. T.; Deutsch D. G. (2009) Identification of intracellular carriers for the endocannabinoid anandamide. Proc. Natl. Acad. Sci. U.S.A. 106, 6375–6380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczocha M.; Vivieca S.; Sun J.; Glaser S. T.; Deutsch D. G. (2012) Fatty acid binding proteins transport N-acylethanolamines to nuclear receptors and are targets of endocannabinoid transport inhibitors. J. Biol. Chem. 287, 3415–3424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oddi S.; Fezza F.; Pasquariello N.; D’Agostino A.; Catanzaro G.; De Simone C.; Rapino C.; Finazzi-Agro A.; Maccarrone M. (2009) Molecular identification of albumin and Hsp70 as cytosolic anandamide-binding proteins. Chem. Biol. 16, 624–632. [DOI] [PubMed] [Google Scholar]
- Fu J.; Bottegoni G.; Sasso O.; Bertorelli R.; Rocchia W.; Masetti M.; Guijarro A.; Lodola A.; Armirotti A.; Garau G.; Bandiera T.; Reggiani A.; Mor M.; Cavalli A.; Piomelli D. (2011) A catalytically silent FAAH-1 variant drives anandamide transport in neurons. Nat. Neurosci. 15, 64–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczocha M.; Hermann A.; Glaser S. T.; Bojesen I. N.; Deutsch D. G. (2006) Anandamide uptake is consistent with rate-limited diffusion and is regulated by the degree of its hydrolysis by fatty acid amide hydrolase. J. Biol. Chem. 281, 9066–9075. [DOI] [PubMed] [Google Scholar]
- Glaser S. T.; Kaczocha M.; Deutsch D. G. (2005) Anandamide transport: a critical review. Life Sci. 77, 1584–1604. [DOI] [PubMed] [Google Scholar]
- Yates M. L.; Barker E. L. (2009) Organized trafficking of anandamide and related lipids. Vitam. Horm. 81, 25–53. [DOI] [PubMed] [Google Scholar]
- Di Pasquale E.; Chahinian H.; Sanchez P.; Fantini J. (2009) The insertion and transport of anandamide in synthetic lipid membranes are both cholesterol-dependent. PLoS One 4, e4989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillard C. J.; Jarrahian A. (2005) Accumulation of anandamide: evidence for cellular diversity. Neuropharmacology 48, 1072–1078. [DOI] [PubMed] [Google Scholar]
- Ortega-Gutierrez S.; Hawkins E. G.; Viso A.; Lopez-Rodriguez M. L.; Cravatt B. F. (2004) Comparison of anandamide transport in FAAH wild-type and knockout neurons: evidence for contributions by both FAAH and the CB1 receptor to anandamide uptake. Biochemistry 43, 8184–8190. [DOI] [PubMed] [Google Scholar]
- McFarland M. J.; Barker E. L. (2004) Anandamide transport. Pharmacol. Ther. 104, 117–135. [DOI] [PubMed] [Google Scholar]
- Hillard C. J.; Jarrahian A. (2003) Cellular accumulation of anandamide: consensus and controversy. Br. J. Pharmacol. 140, 802–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler C. J.; Tiger G.; Ligresti A.; Lopez-Rodriguez M. L.; Di Marzo V. (2004) Selective inhibition of anandamide cellular uptake versus enzymatic hydrolysis--a difficult issue to handle. Eur. J. Pharmacol. 492, 1–11. [DOI] [PubMed] [Google Scholar]
- Beltramo M.; Stella N.; Calignano A.; Lin S. Y.; Makriyannis A.; Piomelli D. (1997) Functional role of high-affinity anandamide transport, as revealed by selective inhibition. Science 277, 1094–1097. [DOI] [PubMed] [Google Scholar]
- Glaser S. T.; Abumrad N. A.; Fatade F.; Kaczocha M.; Studholme K. M.; Deutsch D. G. (2003) Evidence against the presence of an anandamide transporter. Proc. Natl. Acad. Sci. U.S.A. 100, 4269–4274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasia L.; Karava V.; Siafaka-Kapadai A. (2003) Uptake and metabolism of [3H]anandamide by rabbit platelets. Lack of transporter?. Eur. J. Biochem. 270, 3498–3506. [DOI] [PubMed] [Google Scholar]
- Bakht O.; Pathak P.; London E. (2007) Effect of the structure of lipids favoring disordered domain formation on the stability of cholesterol-containing ordered domains (lipid rafts): identification of multiple raft-stabilization mechanisms. Biophys. J. 93, 4307–4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patricelli M. P.; Lashuel H. A.; Giang D. K.; Kelly J. W.; Cravatt B. F. (1998) Comparative characterization of a wild type and transmembrane domain-deleted fatty acid amide hydrolase: identification of the transmembrane domain as a site for oligomerization. Biochemistry 37, 15177–15187. [DOI] [PubMed] [Google Scholar]
- Bari M.; Battista N.; Fezza F.; Finazzi-Agro A.; Maccarrone M. (2005) Lipid rafts control signaling of type-1 cannabinoid receptors in neuronal cells. Implications for anandamide-induced apoptosis. J. Biol. Chem. 280, 12212–12220. [DOI] [PubMed] [Google Scholar]
- McFarland M. J.; Porter A. C.; Rakhshan F. R.; Rawat D. S.; Gibbs R. A.; Barker E. L. (2004) A role for caveolae/lipid rafts in the uptake and recycling of the endogenous cannabinoid anandamide. J. Biol. Chem. 279, 41991–41997. [DOI] [PubMed] [Google Scholar]
- Dainese E.; Oddi S.; Bari M.; Maccarrone M. (2007) Modulation of the endocannabinoid system by lipid rafts. Curr. Med. Chem. 14, 2702–2715. [DOI] [PubMed] [Google Scholar]
- Xu X.; London E. (2000) The effect of sterol structure on membrane lipid domains reveals how cholesterol can induce lipid domain formation. Biochemistry 39, 843–849. [DOI] [PubMed] [Google Scholar]
- Bojesen I. N.; Hansen H. S. (2005) Membrane transport of anandamide through resealed human red blood cell membranes. J. Lipid Res. 46, 1652–1659. [DOI] [PubMed] [Google Scholar]
- Huang J.; Feigenson G. W. (1999) A microscopic interaction model of maximum solubility of cholesterol in lipid bilayers. Biophys. J. 76, 2142–2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zitzer A.; Bittman R.; Verbicky C. A.; Erukulla R. K.; Bhakdi S.; Weis S.; Valeva A.; Palmer M. (2001) Coupling of cholesterol and cone-shaped lipids in bilayers augments membrane permeabilization by the cholesterol-specific toxins streptolysin O and Vibrio cholerae cytolysin. J. Biol. Chem. 276, 14628–14633. [DOI] [PubMed] [Google Scholar]
- Nelson L. D.; Johnson A. E.; London E. (2008) How interaction of perfringolysin O with membranes is controlled by sterol structure, lipid structure, and physiological low pH: insights into the origin of perfringolysin O-lipid raft interaction. J. Biol. Chem. 283, 4632–4642. [DOI] [PubMed] [Google Scholar]
- Sokolov A.; Radhakrishnan A. (2010) Accessibility of cholesterol in endoplasmic reticulum membranes and activation of SREBP-2 switch abruptly at a common cholesterol threshold. J. Biol. Chem. 285, 29480–29490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Megha; London E. (2004) Ceramide selectively displaces cholesterol from ordered lipid domains (rafts): implications for lipid raft structure and function. J. Biol. Chem. 279, 9997–10004. [DOI] [PubMed] [Google Scholar]
- McKinney M. K.; Cravatt B. F. (2006) Structure-based design of a FAAH variant that discriminates between the N-acyl ethanolamine and taurine families of signaling lipids. Biochemistry 45, 9016–9022. [DOI] [PubMed] [Google Scholar]
- Kaczocha M.; Glaser S. T.; Chae J.; Brown D. A.; Deutsch D. G. (2010) Lipid droplets are novel sites of N-acylethanolamine inactivation by fatty acid amide hydrolase-2. J. Biol. Chem. 285, 2796–2806. [DOI] [PMC free article] [PubMed] [Google Scholar]