Abstract
GABAρ1 receptors are highly expressed in bipolar neurons of the retina and to a lesser extent in several areas of the central nervous system (CNS), and dopamine and serotonin are also involved in the modulation of retinal neural transmission. Whether these biogenic amines have a direct effect on ionotropic GABA receptors was not known. Here, we report that GABAρ1 receptors, expressed in X. laevis oocytes, were negatively modulated by dopamine and serotonin and less so by octopamine and tyramine. Interestingly, these molecules did not have effects on GABAA receptors. 5-Carboxamido-tryptamine and apomorphine did not exert evident effects on any of the receptors. Schild plot analyses of the inhibitory actions of dopamine and serotonin on currents elicited by GABA showed slopes of 2.7 ± 0.3 and 6.1 ± 1.8, respectively, indicating a noncompetitive mechanism of inhibition. The inhibition of GABAρ1 currents was independent of the membrane potential and was insensitive to picrotoxin, a GABA receptor channel blocker and to the GABAρ-specific antagonist (1,2,5,6-tetrahydropyridine-4-yl)methyl phosphinic acid (TPMPA). Dopamine and serotonin changed the sensitivity of GABAρ1 receptors to the inhibitory actions of Zn2+. In contrast, La3+ potentiated the amplitude of the GABA currents generated during negative modulation by dopamine (EC50 146 μM) and serotonin (EC50 196 μM). The functional role of the direct modulation of GABAρ receptors by dopamine and serotonin remains to be elucidated; however, it may represent an important modulatory pathway in the retina, where GABAρ receptors are highly expressed and where these biogenic amines are abundant.
Keywords: GABAρ1, GABAA, dopamine, receptor modulation, 5-HT, Xenopus oocyte
γ-Aminobutyric acid (GABA) is a major inhibitory neurotransmitter in the adult mammalian brain and retina. The ionotropic receptors for GABA are ligand-gated chloride channels that are targets for a variety of clinically prescribed therapeutic compounds.1 Among the different isoforms of GABA receptors, those made up by ρ1–3 subunits (GABAρ) display a pharmacological profile that is different from the more ubiquitous GABAA receptors composed of α, β, and γ subunits. GABAρ receptors are blocked by picrotoxin (Ptx) and by (1,2,5,6-tetrahydropyridine-4-yl)methylphosphinic acid (TPMPA), and they are insensitive to bicuculline, barbiturates, and benzodiazepines.2−7
GABAρ subunits have been found in several regions of the central nervous system, including cerebellar Purkinje neurons8,9 and the amygdala.10 In Purkinje neurons, GABAρ mediate a component of phasic inhibitory transmission,11 whereas in the amygdala, pharmacological evidence suggests that GABAρ-mediated activity participates in the modulation of fear and anxiety.10 In the retina, GABAρ receptors are highly expressed at the axon terminal of bipolar neurons where they are involved in regulating visual signaling.12,13
Several studies have shown indirect modulation of GABAρ by dopamine (DA) in horizontal cells of the catfish and in bipolar cells of the tiger salamander.14,15 Moreover, it has been suggested that G protein-coupled receptors, such as those for glutamate or serotonin (5-HT), indirectly reduce GABAρ-mediated responses in bipolar cells.16 Because cross-talk between dopamine and serotonin G protein-coupled receptors has been reported,4,16,17 and because GABAρ subunits are modulated by several molecules besides GABA,19−22 it is possible that DA or 5-HT directly interacts with GABAρ receptors. However, in spite of their pharmacological importance, such interactions have not been explored.
In previous studies, we have demonstrated a negative serotonergic modulation of nicotinic acetylcholine receptors in isolated muscle fibers and in cloned receptors.23,24 Here, we extend these observations to cloned GABA receptors, in which we assessed the effect of biogenic amines. Our results suggest a direct inhibition of GABAρ1 by DA, 5-HT, tyramine, and octopamine. In contrast, this modulation was not observed for GABAA receptors composed of α1β2γ2 subunits. Thus, we found yet another peculiar characteristic of the GABAρ subunits that sets them apart from the classic GABAA receptors and that may help in the design of new subunit-specific antagonists.
Results and Discussion
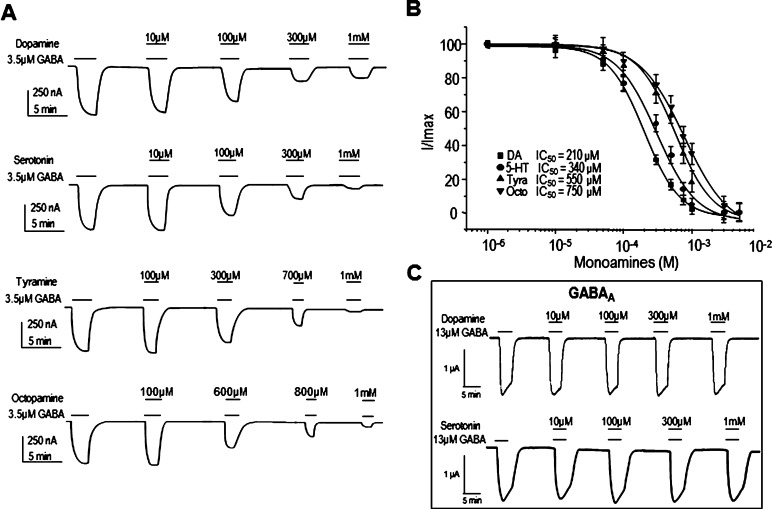
Xenopus oocytes expressing GABAρ1 receptors generated typical nondesensitizing ionic currents of 1.4 ± 0.5 μA (n = 19) upon perfusion of 1 mM GABA. The EC50 for GABA was 3.5 ± 0.4 μM (n = 19). The same oocytes did not generate detectable responses during perfusion of 300 μM DA or 5-HT. Similarly, perfusion of tyramine (Tyra) or octopamine (Octo), molecules related in structure to DA, did not elicit responses when tested at concentrations up to 300 μM, indicating that none of these biogenic amines directly activate GABAρ1 (data not shown). Interestingly, when each of these compounds was coapplied with 3.5 μM GABA, a concentration near the EC50 for GABA, we observed a reversible decrease of GABA currents that was dependent on the concentration of the amine tested (Figure 1A). DA was the most potent inhibitor of the GABAρ1 current (IC50 of 210 ± 11.2 μM; n = 9) followed by 5-HT (IC50 of 340 ± 22.3 μM; n = 9), Tyra (550 ± 10.3 μM; n = 9), and Octo (750 ± 11.7 μM; n = 9) (Figure 1B). Apomorphine, a nonselective agonist of DA receptors and 5-carboxamido-tryptamine, a nonselective full agonist of 5-HT receptors, did not inhibit GABAρ1 responses. The reduction of GABAρ1 responses by DA and 5-HT was not correlated with changes of the activation (τact) or deactivation times (τdeac) of the GABA current. The τact and τdeac for GABA alone were 1.08 ± 0.5 s and 2.22 ± 0.9 s, respectively. The τ’s were not modified when we coapplied either 210 μM DA (1.18 ± 0.3 s and 2.17 ± 0.7 s for τact and τdeac; P > 0.05) or 310 μM 5-HT (1.91 ± 0.5 s and 2.31 ± 0.7 s; P > 0.05). In contrast, GABAA receptors (α1β2γ2) activated by 13 μM GABA (equal to the EC50) were not affected by any of the biogenic amines at the range of concentrations tested (10 μM to 1 mM). In this case, GABA was applied every 10–15 min to avoid the desensitization of the receptor (Figure 1C).
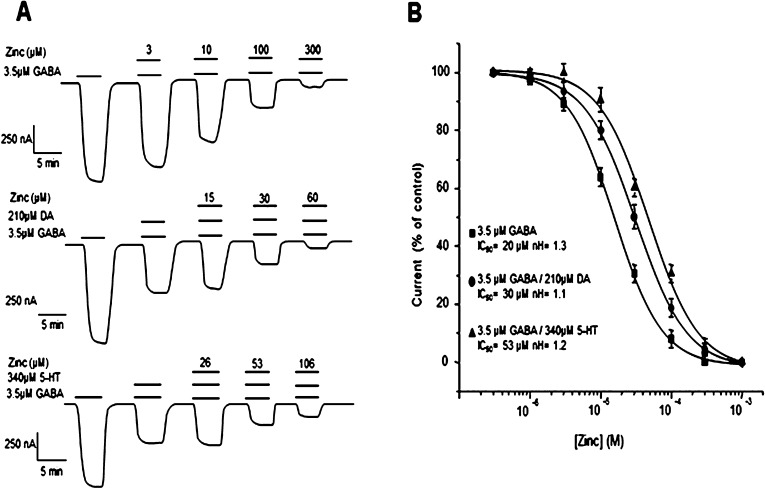
Figure 1.
Effects of the biogenic amines on GABA-currents in oocytes expressing GABAρ1 receptors. (A) Effect of biogenic amines at several concentrations upon currents activated by 3.5 μM GABA. (B) Monoamine concentration–response relationships showing the IC50 for each one. Data were normalized to the maximal GABA response (3.5 μM) of each oocyte (C) DA and 5-HT did not modulate GABA-A receptors. DA (■), 5-HT (●), Tyra (▲), and Octo (▼). Data points are the means ± SEM obtained from at least 9 oocytes (n = 9) from 5 different frogs (N = 5).
Even though DA, Tyra, and Octo share similar core structures, DA was the most potent inhibitor of GABAρ1, indicating that the two adjacent hydroxyl groups in the phenolic ring of DA form an important moiety that enhances the affinity of DA for GABAρ1. The absence of inhibitory modulation by apomorphine and 5-carboxamido-tryptamine suggests that the binding site for DA and 5-HT on the GABAρ1 receptor may not be structurally similar to the agonist binding site on the DA and 5-HT receptors.
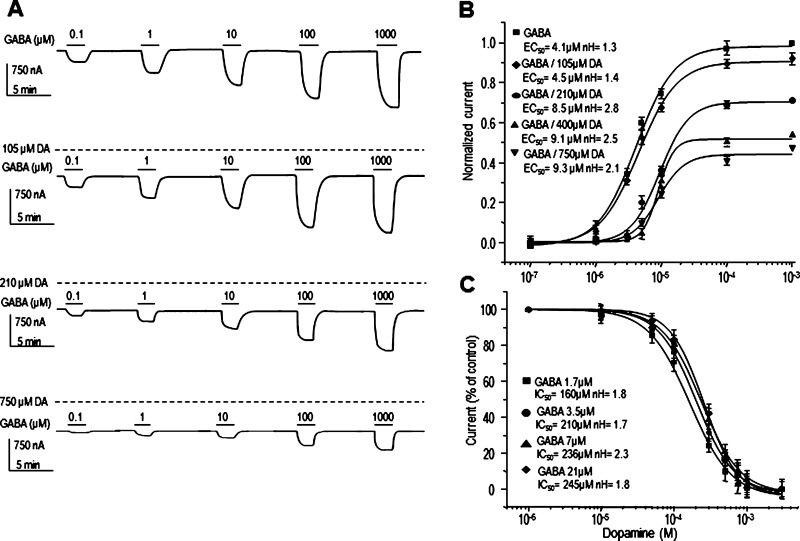
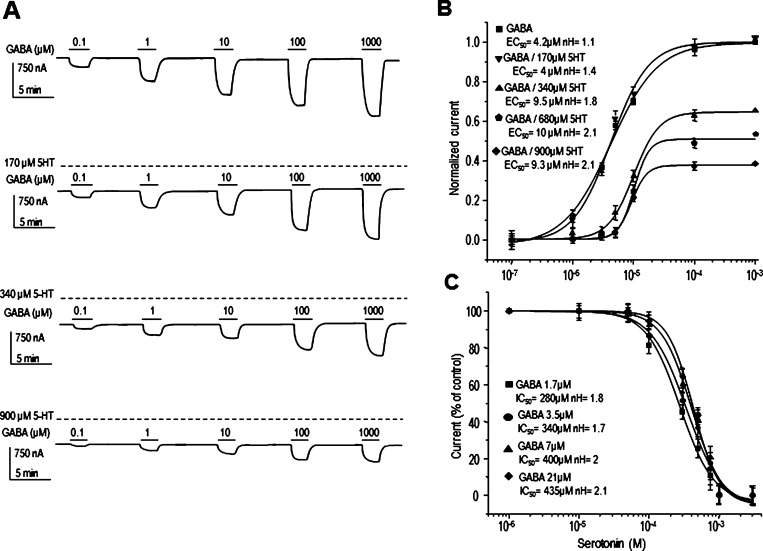
To determine whether DA and 5-HT are competing with GABA for the same binding site, we constructed concentration–response curves for GABA in the presence of different concentrations of DA or 5-HT. Figures 2 and 3 show that DA and 5-HT shifted the GABA curves to the right in a nonparallel manner (n = 9 each). Moreover, DA and 5-HT caused a nonsurmountable antagonism of the response to GABA, indicating a noncompetitive antagonism. Linear regressions of Schild plots for DA and 5-HT yielded straight lines with mean slopes different from 1. The slopes of the Schild plots were 2.7 ± 0.3 for DA and 6.1 ± 1.8 for 5-HT; both values confirmed that DA and 5-HT do not compete with GABA for the same binding site on the receptor. The intercepts of the line with the abscissa (pA2 value) were 3.7 ± 0.9 for DA and 4.7 ± 0.3 for 5-HT, corresponding to apparent equilibrium dissociation constants (KB) of 4.8 ± 0.5 μM and 5.2 ± 0.9 μM, respectively. Because the inhibition was noncompetitive, we used the method of Gaddum et al.25 to calculate the KB, and the values obtained were 3.04 and 4.9 μM for DA and 5-HT, respectively; both values were similar to those estimated by Schild plots. The inhibitory effects of DA or 5-HT on the GABA currents were independent of the concentrations of GABA used to activate the channel (range of EC25 to EC75) (Figures 2C and 3C; n = 9 each; P > 0.05).
Figure 2.
Competition assays. (A) Sample currents and (B) concentration–response relationships of GABA-current modulation by DA (105, 210, 400, and 750 μM) in oocytes expressing GABAρ1. DA concentration–response curves of currents elicited by 1.7, 3.5, 7, and 21 μM GABA. Notice that DA did not shift the GABA dose–responses curve (P > 0.05). Data points are the means ± SEM obtained from at least 9–11 oocytes from 5 frogs.
Figure 3.
Competition assays. (A) Sample currents and (B) concentration–response relation of GABA-current modulation by 5-HT (140, 340, 680, and 900 μM) in oocytes expressing GABAρl (C) 5-HT concentration–response curves of currents elicited by 1.7, 3.5, 7, and 21 μM GABA Notice that 5-HT did not affect the GABA dose–responses curve (P > 0.05). Data points are the means ± SEM obtained from at least 5 oocytes (n = 4–7) from 5 frogs (N = 5).
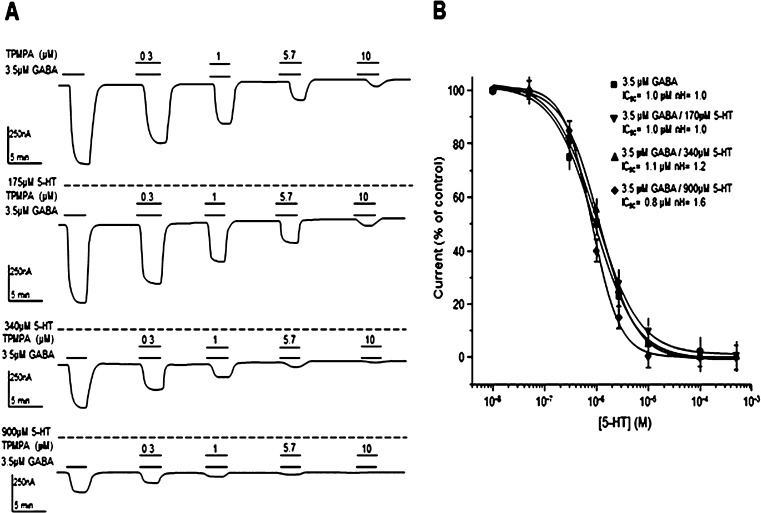
We also analyzed if the negative modulation of DA or 5-HT on GABA currents modified the inhibitory effects of TPMPA, a highly specific competitive antagonist of GABAρ1.6,7 As observed in Figure 4, at a concentration of 105 μM, DA did not affect the inhibitory activity of TPMPA; however, at higher concentrations, DA gradually increased the slope of the TPMPA inhibitory curve (nH = 0.8 ± 0.08, 0.9 ± 0.08, 1.3 ± 0.03, and 2.0 ± 0.02 for GABA alone and with 105, 210, and 750 μM DA, respectively), thus indicating a cooperative effect between TPMPA and DA. The IC50 for TPMPA did not change with the different concentrations of DA (IC50 = 1.1 ± 0.07, 1.1 ± 0.09, 1.0 ± 0.09, and 0.7 ± 0.1 μM for GABA alone and with 105, 210, and 750 μM DA, respectively; P > 0.05).The additive antagonism of TPMPA and DA indicates that these two compounds bind to different sites within GABAρ1. 5-HT also gradually increased the Hill coefficient for the TPMPA antagonist effect; however, its effects were not as strong as those of DA (nH =1 ± 0.05, 1 ± 0.05, 1.2 ± 0.07, and 1.6 ± 0.09 for GABA alone and with 170, 340, and 900 μM 5-HT, respectively) (Figure 5). 5-HT did not modify the IC50 for TPMPA at any of the concentrations tested (IC50 = 1 ± 0.09, 1 ± 0.04, 1.1 ± 0.1, and 0.8 ± 0.1 μM for GABA alone and with 179, 340, and 900 μM 5-HT, respectively; P > 0.05).
Figure 4.
Effect of the GABAρ receptor antagonist TPMPA on the GABA-elicited currents modulated by DA. (A) Sample records showing that TPMPA effectively and reversibly blocked the currents generated by 3.5 μM GABA and those modulated by 105, 210, and 750 μM DA. Note that the coapplication of DA concentration, higher than 105 μM, and TPMPA enhanced the inhibitory effect. (B) TPMPA antagonism on currents elicited by coapplication of GABA and either DA at 105, 210, and 750 μM. Data points are the means ± SEM from 6 oocytes (n = 6) from 5 frogs (N = 3).
Figure 5.
Effect of the GABAρ receptor antagonist TPMPA on the GABA-elicited currents modulated by 5-HT. (A) Sample records showing that TPMPA effectively and reversibly blocked the currents generated by 3.5 μM GABA and modulated by 175, 340, and 900 μM 5-HT. (B) TPMPA antagonism on currents elicited by coapplication of GABA and either 5-HT at 175, 340, and 900 μM. The currents were adjusted to the amplitude the maximum current for each curve. Data points are the means ± SEM from 6 oocytes (n = 6) from 5 frogs (N = 5).
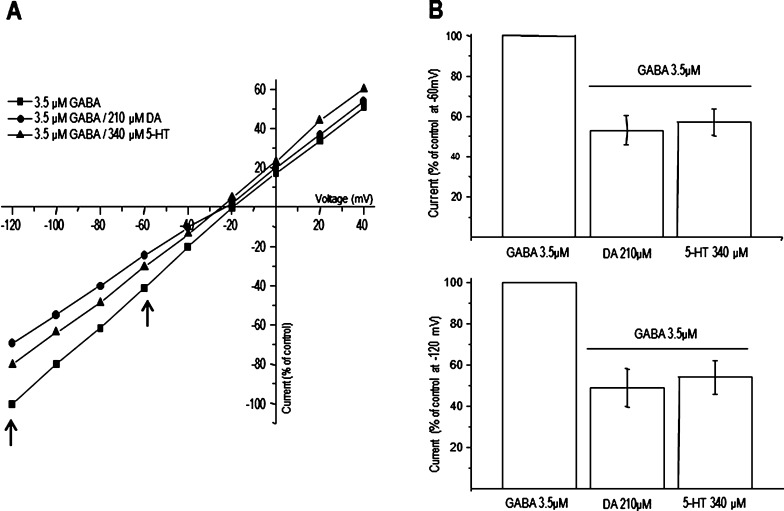
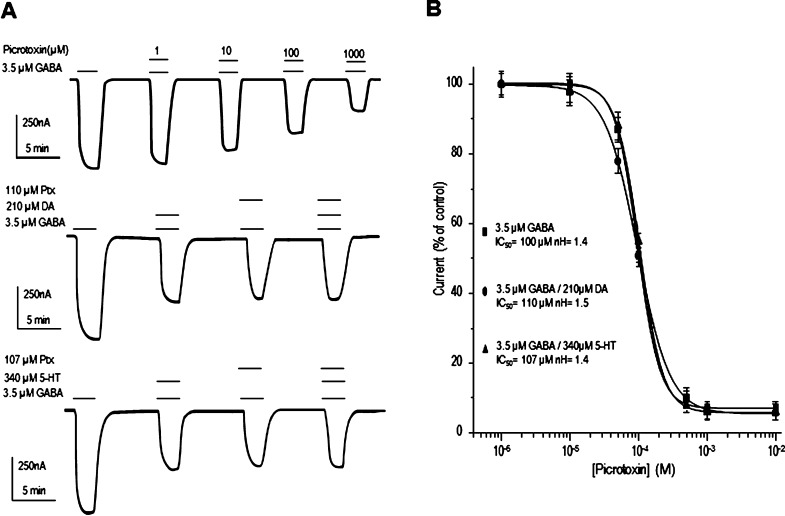
Available evidence indicates that DA may interact with other ligand-gated ion channels by blocking the ion pathway (e.g., glutamate NMDA receptors expressed in oocytes or present in neurons grown in culture26,27). Since the voltage dependence of the antagonism is a shared characteristic among several compounds that bind inside the pore,27 we explored whether the effects of DA and 5-HT were voltage dependent. Current–voltage relationships were constructed for the activation of the receptors with 3.5 μM GABA alone and during coapplication of GABA with DA or 5-HT IC50 values (210 and 340 μM, respectively). As shown in Figure 6A, in all cases, the current–voltage relationships were linear within the range explored (−120 to +40 mV), indicating that the effects of DA and 5-HT were voltage independent and inhibited equally at −60 and −120 mV (Figure 6B). None of these biogenic amines changed the ion selectivity of the channel, as suggested by their inversion potential which was −23 ± 1.9 mV (n = 9) for GABA and −25 ± 0.8 mV (n = 9) or −28 ± 0.6 mV (n = 9) in the presence of DA or 5-HT, indicating that chloride remains as the main ion flowing through the GABA-gated channel. To further explore a possible ion pore blockade mechanism, we analyzed if DA and 5-HT affect the blocking of GABA-currents by picrotoxin (Ptx), a noncompetitive antagonist of GABA receptors whose binding site is in the ion pathway.28 As shown in Figure 7, the inhibitory actions of Ptx were not affected by DA or 5-HT. We did not observe any synergic inhibitory effect when Ptx was coapplied with DA or 5-HT. One possible explanation for this is that Ptx binding to GABAρ1 alters the conformation such that the binding site for the biogenic amines is not exposed. The IC50’s and Hill coefficients for Ptx were 100 ± 1.0 μM and 1.4 ± 0.9 for GABA (n = 5), 100 ± 1.5 μM and 1.5 ± 0.8 for GABA+DA (n = 6), and 107 ± 0.9 μM and 1.4 ± 0.7 for GABA+5-HT (n = 5–6 each). These results contrast with those observed in NMDA receptors, in which DA, 5-HT, and even Tyra clearly interact with the narrowest region of the channel pore of the receptor.26−28 Thus, according to the competition assays, competition with Ptx, and current–voltage relationships, the binding site for DA and 5-HT in GABAρ1 is different from the GABA binding site, and it is probably not located inside the channel pore. Future point-mutation studies will precisely determine if DA and 5-HT bind inside the pore of the channel.
Figure 6.
Current–voltage relationships for GABA-elicited currents modulated by DA and 5-HT at indicated concentrations. Note that in all cases the current–voltage relationship is linear and not affected by DA or 5-HT (A). The extent of inhibition is the same at −120 and −60 mV (B). Data points are the means ± SEM obtained from at least 9 oocytes (n = 9) from 5 frogs (N = 5).
Figure 7.
Effect of Picrotoxin on the GABA-elicited currents modulated by DA and 5-HT. (A) Sample records showing that Ptx reversibly blocked the currents generated by 3.5 μM GABA and modulated by 210 μM DA and 340 μM 5-HT. Note that the coapplication of the biogenic amines and Ptx did not enhance the inhibitory effect. (B) Picrotoxin antagonism of the currents elicited by coapplication of GABA and either DA or 5-HT. The currents were normalized to the maximum amplitude elicited by GABA+DA or GABA+5HT in absence of Ptx. Data points are the means ± SEM from at least 8 oocytes (n = 8) from 5 frogs (N = 5).
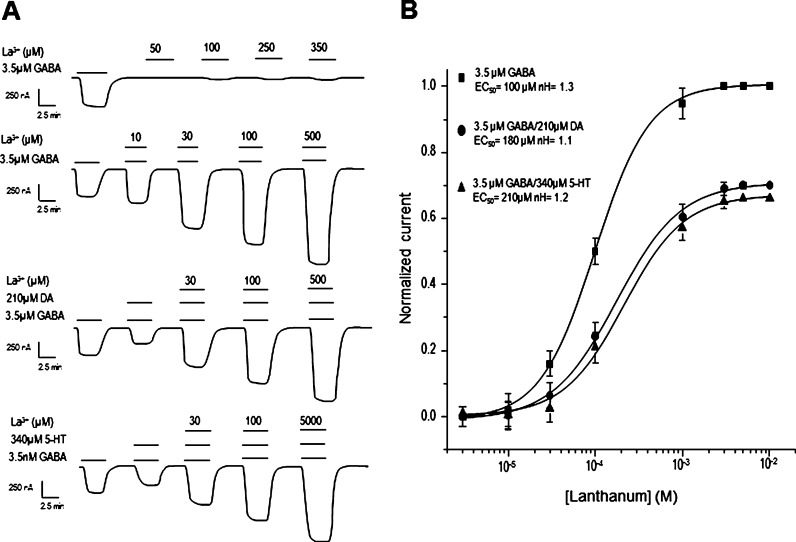
La3+ acts on GABAA receptors at a site different from those used by barbiturates, benzodiazepines, Ptx, or Zn2+, and in some biological preparations La3+ induces a Cl– current by directly activating GABAA receptors.31−36 It was previously shown that La3+ and other lanthanides positively modulate GABAρ1 receptors,37 suggesting the existence of a La3+ allosteric site in these receptors.38 In our experiments, the application of 100 μM La3+ alone induced small inward currents in oocytes that expressed GABAρ1 (Figure 8A). This current was not observed in noninjected oocytes, suggesting a direct activation of the GABAρ1 receptors by La3+. Lanthanum also increased, in a concentration-dependent manner, the amplitude of the currents elicited by GABA alone. The potentiation of GABA-currents by La3+ was not prevented by the negative modulation of DA or 5-HT (Figure 8B); nevertheless, the magnitude of the GABA-induced currents modulated by DA or 5-HT in presence of La3+ was smaller than the control. The presence of the amines reduced the potency of La3+, as observed by the right-shift of the dose response from 100 ± 1.8 μM for GABA alone (n = 5) to 186 ± 1.1 μM for GABA + DA (n = 5; P > 0.05, different than the control) and 210 ± 1.2 μM for GABA + 5-HT modulated currents (n = 5; P > 0.05, different than the control). Furthermore, the negative modulation of DA and 5-HT was not overcome by larger concentrations of La3+ (Figure 8B). No changes in the Hill coefficients were observed (1.3 ± 0.1, 1.1 ± 0.07, and 1.2 ± 0.08, for GABA alone, GABA+DA, and GABA+5HT respectively). Since these data suggest a noncompetitive interaction between the amines and La3+, we investigated whether DA and 5-HT affected the inhibition of the receptor by Zn2+, which apparently binds to a different allosteric site than La3+.39
Figure 8.
Lanthanum modulation. (A) Sample records illustrating the activation of GABAρ1 receptors and the potentiation of GABA-currents by La3+ and their modulation by DA and 5-HT. (B) La3+ concentration–response relations of the currents generated by 3.5 μM GABA and modulated by 210 μM DA and 340 μM 5-HT. Observe the blocking effect of DA and 5-HT on the GABA-elicited current in presence of La3+. The currents were normalized to the amplitude of that elicited by 3.5 μM GABA alone. Data points are the means ± SEM from at least 8 oocytes (n = 8) from 5 frogs (N = 5).
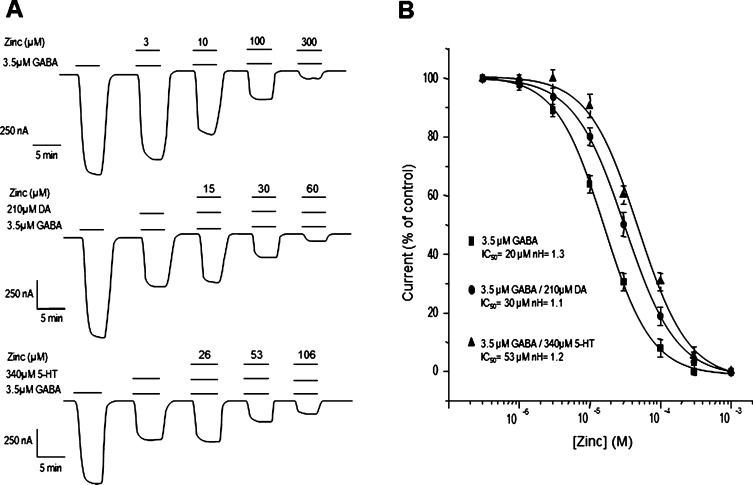
The GABA-currents negatively modulated by DA and 5-HT were further inhibited by Zn2+. The additive inhibitory effects of Zn2+ and either DA or 5-HT reached about 80% total inhibition in both cases (Figure 9A). Interestingly, DA and 5-HT had different effects on the sensitivity of GABA-currents to Zn2+. In Figure 9B, it is shown that DA increased the sensitivity of GABA-currents to inhibition by Zn2+. In contrast, 5-HT right-shifted the concentration–response curve of Zn2+. The concentration–response relationship for Zn2+ gave IC50 values of 30 ± 0.7 μM (n = 7) for GABA alone, 20 ± 0.5 μM (n = 7) for GABA+DA, and 53 ± 0.8 μM for GABA+5-HT (n = 7) (P > 0.05, different to the control). No significant differences among the Hill coefficients were observed: 1.3 ± 0.08, 1.1 ± 0.1, and 1.2 ± 0.09, for GABA, GABA+DA, and GABA+5-HT, respectively (Figure 9B). One possible explanation for the opposite effects of DA and 5-HT on GABAρ1 sensitivity to Zn2+ is that the allosteric modulation could occur via different structural rearrangements. Interestingly, competition between Zn2+ and DA for the same binding site has been described in the DA type 1 and 2 receptors expressed in HEK and CHO cells.40−42
Figure 9.
Zinc inhibition. (A) Inhibition of the currents elicited by GABA alone and modulated by DA and 5-HT. The coapplication of either DA or 5-HT with Zn2+ enhances the inhibitory effect. (B) Concentration–response relationships of the currents elicited by 3.5 μM GABA and modulated by 210 μM DA and 340 μM 5-HT. Note the displacement of the curves in the presence of DA or 5-HT. The currents were normalized to the amplitude of that elicited by GABA+DA or GABA+5HT in absence of Zn2+. Data points are the means ± SEM from at least 7 oocytes (n = 7) from 5 frogs (N = 5).
In conclusion, we found that DA and 5-HT directly modulate homomeric GABAρ1 receptors but not GABAA receptors. Since the concentrations of DA and 5-HT in the retina vary between 0.9 and 470 μM and 0.1 and 590 μM,32,43−45 respectively, both of which are within the range of modulation we found for GABAρ1, it is plausible that these interactions occur in the retina. Such possibilities will be explored in future studies.
Methods
Expression of Human GABAA-Receptors
All the animals were handled in accordance with the guidelines of the National Institute of Health Guide for Care and Use of Laboratory Animals, and with the approval of the Institutional Animal Care and Use Committee of the National University of Mexico. X. laevis frogs were anesthetized with 0.17% 3-aminobenzoic acid methyl ester (MS-222) for 20–30 min. Follicles were manually removed, enzymatically defolliculated (with 0.3 μg/μL collagenase type I at room temperature for 45 min), and then kept at 16 °C in Barth’s medium: 88 mM NaCl, 1 mM KCl, 0.33 mM Ca2(NO)3, 0.41 mM CaCl2, 0.82 mM MgSO4, 2.4 mM NaHCO3, 5 mM HEPES, pH 7.4, containing 0.1 mg/mL gentamicin sulfate. The next day, 50 nL (0.5 μg/μL) of human GABAρ1 mRNA or 18 nL (0.5 μg/μL) of GABAA (α1β1γ2) cDNA were injected in the equator or in the nucleus of the oocyte, respectively. The electrophysiological recordings were obtained 3–5 days after injection.
Voltage-Clamp Recordings
The membrane currents elicited by the agonists were recorded using the two-microelectrode voltage-clamp technique.46 Oocytes were placed in a 500 μL chamber, impaled with two glass microelectrodes filled with 3 M KCl (0.5–2.5 MΩ) and clamped at −60 mV. To obtain the equilibrium membrane potential of the agonist transmitter action, current–voltage relationships were constructed by stepping the oocyte’s membrane potential from −60 to −120 mV for 1 s and then from −120 to +40 mV (in 20 mV steps) in the absence or presence of GABA, DA, 5-HT, Tyra, Octo, the dopaminergic agonist apomorphine, or the serotonergic agonist 3-carboxamido-tryptamine. All recordings were done at room temperature (20–23 °C) in a chamber continually perfused (5–10 mL/min) with frog Ringer solution: 115 mM NaCl, 2 mM KCl, 1.8 mM CaCl2, 5 mM HEPES, pH 7.4. All drugs were purchased from SIGMA. The stock solution of GABA (1 M) was stored frozen, and fresh dilutions were used for the experiments. All monoamines were prepared the day of the experiment by dissolving them directly in frog Ringer solution. The pH of all solutions was adjusted to 7.4.
Data Analysis
Results are reported as mean ± SEM of the values obtained from several cells. Data from each experiment were collected from at least seven oocytes. Agonist concentration–response curves were constructed by measuring the maximum response evoked by each agonist concentration. The half-maximal concentration (EC50) and Hill coefficient (nH) were estimated for each curve by fitting the data to the logistic type equation (Origin 6.0, Northampton, MA): A = Amax/(1 + 10[logEC50–[agonist]nH]). The half-inhibitory concentration (IC50) of DA, 5-HT, TPMPA, or Zn2+ was estimated by fitting the following equation: A = Amax/(1 + 10[agonist]–logIC50). To determine the time constants for the activation (τact) and deactivation (τdeac) of GABA-current responses, a decay function of the form I(τ) = exp(−t/τd) + C, where I is the current and t is time, was fit to the experimental data (Origin 6.0 software; Northampton, MA). Differences between groups were statistically analyzed by ANOVA and a Tukey-Kramer post-test. Differences were considered significant at the level P > 0.05. The pA2 values (−log of the molar concentration of agonist that reduces the agonist EC50 by a factor of 2) for DA and 5-HT were determined from Schild plots using GABA as agonist (GraphPad Prism 4). The concentration ratio (the ratio between the EC50 values for GABA in the presence and absence of an agonist) at different antagonist concentrations for the different GABA/antagonist pairs were plotted in a Schild diagram using regression analysis, and the pA2 value was obtained from the intercept of the regression line with the abscissa. Since DA and 5-HT showed a noncompetitive antagonistic effect on GABAρ1 receptors, the affinity was calculated by the method of Gaddum.25 Logistic equations of the form I(x) = Imin + (Imax – Imin)/[1 + (x/EC50)k] were fitted to agonist curves, and the equiactive concentrations at EC30, EC40, EC50, and EC60 in the absence and in the presence of different concentrations of DA or 5-HT were calculated from these curves. The reciprocals of the equiactive concentrations are correlated according to the equation 1/[GABA] = (1/[GABA′])(1 + [DA]/KB) + (α[DA])/(KAKB), where [GABA] is the concentration of agonist (M) in absence of DA, [GABA′] is the equiactive concentration in the presence of a specific concentration of DA (M), α is a modifying term that denotes the change in affinity of one ligand produced by binding of the other, and KA is the equilibrium dissociation constant for the agonist. Since the reciprocals 1/[GABA] and 1/[GABA′] are linearly correlated, they were plotted, and the linear regression to these data was used to calculate the KB, using the equation KB = [DA]/(slope – 1). Control responses to GABA were obtained before and after each drug application to account for possible shifts in the amplitude of the control current.
Acknowledgments
We thank E. Ruiz Alcibar and E. Espino Saldaña for technical support and Dr. D. Pless for editing the manuscript.
Glossary
Abbreviations
- GABA
γ-aminobutyric acid
- DA
dopamine
- 5-HT
5-hydroxytryptamine
- Tyra
tyramine
- Octo
octopamine
- Ptx
picrotoxin
- TPMPA
(1,2,5,6-tetrahydropyridine-4-yl) methylphosphinic acid
- MS-222
ethyl 3-aminobenzoate methanesulfonate
- HEPES
N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid
- LGIC
ligand-gated ion channels
- NMDA
N-methyl-d-aspartate
- SCH23390
R-(+)-7-chloro-8-hydroxy-3-methyl-1-phenyl-2,3,4,5-tetrahydro-1H-3benzazepine hydrochloride
- SKF81297
R-(+)-6-chloro-7,8-dihydroxy-1-phenyl-2,3,4,5-tetrahydro-(1H)-3-benzazepine-7,8-diol hydrochloride
Author Contributions
A.E.-M. and A.M.-T. purified the plasmids carrying the GABA receptors and synthesized the cRNA, R.M. and L.D.O.P. did the electrophysiology and pharmacology in oocytes, and A.L. and L.D.O.P. did the statistic tests. All the authors participated in the experimental design and writing of the paper.
L.D.O.P. was supported by IACOD-UNAM (IA202411-22) at UNAM. A.E.-M. was supported by the CONACYT Fellowship Program. A.L. was supported by the King Abdul Aziz City for Science and Technology, Riyadh (Grant KACST-46749). This work was supported by grants from CONACYT (101851) and PAPIIT-UNAM (IN202609 and IN205308) to A.M.-T. The authors acknowledge the support of The Shedid Fund.
The authors declare no competing financial interest.
References
- Olsen R. W.; Sieghart W. (2008) International Union of Pharmacology. LXX. Subtypes of γ-aminobutyric acid (A) receptors: classification on the basis of subunit composition, pharmacology, and function. Update. Pharmacol. Rev. 60, 243–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polenzani L.; Woodward R. M.; Miledi R. (1991) Expression of mammalian γ-amino butyric acid receptors with distinct pharmacology in Xenopus oocytes. Proc. Natl. Acad. Sci. U.S.A. 88, 4318–4322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutting G. R.; Lu L.; O’Hara B. F.; Kash L. M.; Montrose-Refizadeh C.; Donovan D. M.; Shimada S.; Antonaraski S. E.; Guggino W. B.; Uhl G. R. (1991) Cloning of the γ-aminobutyric acid (GABA) ρ1 cDNA: a GABA receptor subunit highly expressed in the retina. Proc. Natl. Acad. Sci. U.S.A. 88, 2673–2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward R. M.; Polenzani L.; Miledi R. (1992) Characterization of bicuculline/ baclofen-isensitive γ-aminobutyric acid expressed in Xenopus oocytes. Part I. Effect of Cl– channels inhibitors. Mol. Pharmacol. 42, 165–173. [PubMed] [Google Scholar]
- Woodward R. M.; Polenzani L.; Miledi R. (1993) Characterization of bicuculline/ baclofen-insensitive (ρ-like) γ-aminobutyric acid receptors expressed in Xenopus oocytes. Part II. Pharmacology of γ-aminobutyric acid A and γ-aminobutyric acid B receptor agonists and antagonists. Mol. Pharmacol. 43, 609–625. [PubMed] [Google Scholar]
- Murata Y.; Woodward R. M.; Miledi R.; Overman L. E. (1996) The first selective antagonist selective for a GABAc receptor. Bioorg. Med. Chem. Lett. 6, 2073–2076. [Google Scholar]
- Ragozzino D.; Woodward R. M.; Murata Y.; Eusebi F.; Overman L. E.; Miledi R. (1996) Design and in vitro pharmacology of a selective γ-aminobutyric acid C receptor antagonist. Mol. Pharmacol. 50, 1024–1030. [PubMed] [Google Scholar]
- Harvey V. L.; Duguid I. C.; Krasel C; Stephens G. J. (2006) Evidence that GABAρ subunits contribute to functional ionotropic GABA receptors in mouse cerebellar Purkinje cells. J. Physiol. 577, 127–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mejía C; García-Alcocer G; Berumen L. C.; Rosas-Arellano A; Miledi R; Martínez-Torres A. (2008) Expression of GABAρ subunits during rat cerebellum development. Neurosci. Lett. 432, 1–6. [DOI] [PubMed] [Google Scholar]
- Flores-Gracia C; Nuche-Bricaire A; Crespo-Ramírez M; Miledi R; Fuxe K; Pérez de la Mora M. (2010) GABA(A) ρ receptor mechanisms in the rat amygdala and its role in the modulation of fear and anxiety. Psychopharmacology 212, 475–484. [DOI] [PubMed] [Google Scholar]
- Harvey V. L.; Duguid I. C.; Krasel C.; Stephens G. J. (2006) Evidence that GABAρ subunits contribute to functional ionotropic GABA receptors in mouse cerebellar Purkinje cells. J. Comp. Neurol. 4, 693–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chávez A. E.; Grimes W. N.; Diamond J. S. (2010) Mechanisms underlying lateral GABAergic feedback onto rod bipolar cells in rat retina. J. Neurosci. 30, 2330–2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S. M.; Palmer M. J. (2009) Activation of the ionic GABAC receptor current in retinal bipolar cell terminals by nonvesicular GABA release. J. Neurophysiol. 2, 691–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong C. J.; Werblin F. S. (1994) Dopamine modulation of GABAc receptor function in an isolated retinal neuron. J. Neurophysiol. 71, 1258–1260. [DOI] [PubMed] [Google Scholar]
- Wellis D. P.; Werblin F. S. (1995) Dopamine modulates GABAc receptors mediating inhibition of calcium entry into and transmitter release from bipolar cell terminals in tiger salamander retina. J. Neurosci. 15, 4748–4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feigenspan A.; Bormann J. (1994) Modulation of GABAC receptors in rat retinal bipolar cells by protein kinase C. J. Physiol. 481, 325–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya S.; Raote I.; Bhattacharya A.; Miledi R; Panicker M. M. (2006) Activation, internalization, and recycling of the serotonin 2A receptor by dopamine. Proc. Natl. Acad. Sci. U.S.A. 41, 15248–15253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F.; Wan Q.; Pristupa Z. B.; Yu X. M.; Wang Y. T.; Niznik H. B. (2000) Direct protein-protein coupling enables cross-talk between dopamine D5 and γ-aminobutyric acid a receptors. Nature 403, 274–280. [DOI] [PubMed] [Google Scholar]
- Calvo D. J.; Miledi R. (1995) Activation of GABAρ1 receptors by glycine and β-alanine. NeuroReport 8, 1118–1120. [DOI] [PubMed] [Google Scholar]
- Demuro A.; Martinez-Torres A.; Miledi R. (2000) Functional and pharmacological properties of GABAρ1 Δ51 receptors. Neurosci. Res. 36, 141–146. [DOI] [PubMed] [Google Scholar]
- Horikoshi T.; Asanuma A.; Yanagisawa K.; Anzai K.; Goto S. (1988) Taurine and β-alanine act on both GABA and glycine receptors in Xenopus oocyte injected with Mouse brain Messenger RNA. Brain Res. 2, 97–105. [DOI] [PubMed] [Google Scholar]
- Ochoa-de la Paz L. D.; Martinez-Davila I. A.; Miledi R.; Martinez-Torres A. (2008) Modulation of human GABAρ1 receptors by taurine. Neurosci. Res. 61, 302–308. [DOI] [PubMed] [Google Scholar]
- Garcia-Colunga J; Miledi R. (1995) Effects of serotonergic agents on neuronal nicotinic acetylcholine receptors. Proc. Natl. Acad. Sci. U.S.A. 92, 2919–2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Colunga J; Miledi R. (1996) Serotonergic modulation of muscle acetylcholine receptors by different subunit composition. Proc. Natl. Acad. Sci. U.S.A. 93, 3990–3994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaddum J. H.; Hameed K, A.; Hathway D. E.; Stephens F. F. (1955) Quantitative studies of antagonists for 5-hydroxitryptamine. Q. J. Exp. Physiol. 40, 49–74. [DOI] [PubMed] [Google Scholar]
- Masuko T.; Suzuki I.; Kizawa Y.; Kusama-Eguchi K.; Watanabe K.; Kashiwagi K.; Igarashi K.; Kusama T. (2004) Monoamines directly inhibit N-methyl-D-aspartate receptors expressed in Xenopus oocytes in a voltage-dependent manner. Neurosci. Lett. 371, 30–33. [DOI] [PubMed] [Google Scholar]
- Castro N. G.; de Mello M. C. F.; de Mello F. G.; Aracava Y. (1999) Direct inhibition of the N-methyl-d-aspartate receptor channel by dopamine and (+)-SKF38393. Br. J. Pharmacol. 126, 1847–1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D.; Pan Z. H.; Zhang X.; Brideau A. D.; Lipton S. A. (1995) Cloning of a γ-aminobutyric acid type C receptor subunit in rat retina with a methionine residue critical for picrotoxin channel block. Proc. Natl. Acad. Sci. U.S.A. 92, 11756–11760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quian H.; Pan Y.; Khalili P. (2005) Picrotoxin accelerates relaxation of GABAc receptors. Mol. Pharmacol. 67, 470–479. [DOI] [PubMed] [Google Scholar]
- Kashiwagi K.; Masuko T.; Nguyen C. D.; Kuno T.; Tanaka I.; Igarashi K.; Williams K. (2002) Channel blockers acting at N-methyl-d-aspartate receptors: differential effects of mutations in the vestibule and ion channel pore. Mol. Pharmacol. 61, 533–545. [DOI] [PubMed] [Google Scholar]
- Zhu W. J.; Wang J. F.; Corsi L.; Vicini S. (1998) Lanthanum-mediated modification of GABAA receptor deactivation, desensitization and inhibitory synaptic currents in rat cerebellar neurons. J. Physiol. 511, 647–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im M. S.; Hamilton B. J.; Carter D. B.; Im W. B. (1992) Selective potentiation of GABA-mediated Cl-current by lanthanum ion in subtypes of cloned GABAA receptors. Neurosci. Lett. 144, 165–168. [DOI] [PubMed] [Google Scholar]
- Im W. B.; Pregenzer J. F. (1993) Interaction of La3+ with GABAA receptors in rat cerebrocortical membranes as detected with [35S]t-butylbicyclophosphorothionate binding. Eur. J. Pharmacol. 245, 111–117. [DOI] [PubMed] [Google Scholar]
- Ma J. Y.; Narahashi T. (1993a) Enhancement of γ-aminobutyric acid-activated chloride channel currents by lanthanides in rat dorsal root ganglion neurons. J. Neurosci. 11, 4872–4879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J. Y.; Narahashi T. (1993b) Differential modulation of GABAA receptor channel complex by polyvalent cations in rat dorsal root ganglion neurons. Brain Res. 607, 222–232. [DOI] [PubMed] [Google Scholar]
- Reichling D. B.; MacDermott A. B. (1991) Lanthanum actions on excitatory amino acid-gated currents and voltage-gated calcium currents in rat dorsal horn neurons. J. Physiol. 441, 199–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo D. J.; Vazquez A. E.; Miledi R. (1994) Cationic modulation of r1-type γ-aminobutyrate receptors expressed in Xenopus oocytes. Proc. Natl. Acad. Sci. U.S.A. 91, 12725–12729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goutman J. D.; Escobar A. L.; Calvo D. J. (2005) Analysis of macroscopic ionic currents mediated by GABAρ1 receptors during lanthanide modulation predicts novel states controlling channel gating. Br. J. Pharmacol. 146, 1000–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wand T. L.; Hackman A.; Guggino W. B.; Cutting G. R. (1995) A single histidine residue is essential for a zinc inhibition of a GABA rho1 receptors. J. Neurosci. 15, 7684–7691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schetz J. A.; Sibley D. R. (1997) Zinc allosterically modulates antagonist binding to cloned D1 and D2 dopamine receptors. J. Neurochem. 68, 1990–1997. [DOI] [PubMed] [Google Scholar]
- Schetz J. A.; Chu A.; Sibley D. R. (1999) Zinc modulates antagonist interactions with D2-like dopamine receptors through distinct molecular mechanisms. J. Pharmacol. Exp. Ther. 289, 956–964. [PubMed] [Google Scholar]
- Liu Y.; Teeter M. M.; DuRand C. J.; Neve K. A. (2006) Identification of a Zn2+-binding site on the dopamine D2 receptor. Biochem. Biophys. Res. Commun. 339, 873–879. [DOI] [PubMed] [Google Scholar]
- Di Paolo T.; Harnois C.; Daigle M. (1987) Assay of dopamine and its metabolites in human and rat retina. Neurosci. Lett. 74, 250–254. [DOI] [PubMed] [Google Scholar]
- Osborne N. N. (1982) Uptake, localization and release of serotonin in the chick retina. J. Physiol. 331, 469–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima L.; Urbina M. (1994) Dopamine and Serotonin turnover rate in the retina of rabbit, rat, goldfish and Eugerres Plumieri: light effects in goldfish and rat. J. Neurosc. Res. 39, 595–603. [DOI] [PubMed] [Google Scholar]
- Miledi R. (1982) A calcium-dependent transient outward current in Xenopus laevis oocytes. Proc. R. Soc. London, Ser. B 215, 491–497. [DOI] [PubMed] [Google Scholar]