Abstract
The sacred texts of five world religions (Buddhism, Christianity, Hinduism, Islam, and Judaism) use similar belief systems to set limits on sexual behavior. We propose that this similarity is a shared cultural solution to a biological problem: namely male uncertainty over the paternity of offspring. Furthermore, we propose the hypothesis that religious practices that more strongly regulate female sexuality should be more successful at promoting paternity certainty. Using genetic data on 1,706 father–son pairs, we tested this hypothesis in a traditional African population in which multiple religions (Islam, Christianity, and indigenous) coexist in the same families and villages. We show that the indigenous religion enables males to achieve a significantly (P = 0.019) lower probability of cuckoldry (1.3% versus 2.9%) by enforcing the honest signaling of menstruation, but that all three religions share tenets aimed at the avoidance of extrapair copulation. Our findings provide evidence for high paternity certainty in a traditional African population, and they shed light on the reproductive agendas that underlie religious patriarchy.
Keywords: evolution, extrapair paternity, mating, nonpaternity, Y DNA
The major world religions sprang from patriarchal societies in which the resources critical to reproduction, whether in the form of land or livestock, were inherited from father to son down the male line (1–3). Consistent with patrilineal inheritance, the sacred texts set forth harsh penalties for adultery and other behaviors that lower the husband’s probability of paternity (4–8) (SI Discussion). The scriptures also place greater emphasis on female than on male chastity, including the requirement of modest attire for women and the idealization of virginity for unmarried females (6, 8). Previous studies have considered the evolutionary biology of patriarchy, but have focused on primate antecedents or cultural factors rather than religion (2, 9–11). Here we test the hypothesis that religions that more strongly regulate female sexuality are more successful at limiting the incidence of cuckoldry, defined as offspring sired by extrapair copulations (EPCs).
Genetic data on paternity have altered previous assumptions about the mating systems of many species (12–14), but comparable data for humans that link behavior and paternity have been lacking. Our data set on paternity, in contrast, includes religion, year of birth, and Y-chromosome short tandem repeats (STRs) for 1,706 father–son pairs in 29 patrilineages, as well as genealogical pedigrees with a depth of up to 11 generations (15,000 individuals). We have autosomal STRs for a random sample, and data on wealth and polygyny for recent generations. Analyses of the genetic samples exploit the accompanying fine-scale data from a longitudinal study of behavioral ecology (15, 16).
The study population is the Dogon of Mali, West Africa, who practice four religions: Evangelical Protestantism introduced by conservative American missionaries; Catholicism introduced by French Jesuits who focused on humanitarian projects; Islam; and the indigenous Dogon religion, which is monotheist. Our quantitative analysis supports the hypothesis that religious ideology serves the purpose of defense against EPCs. Discovery of the reproductive consequences of religious practices is helpful for understanding the interface between the sacred and the secular, a neglected terrain despite growing interest in the evolutionary biology of religion (17–22).
We show that paternity certainty was higher in the indigenous religion than in Christianity, which we attribute to the abandonment of menstrual taboos by the Christians. Women in the traditional religion are exiled for five nights to uncomfortable places called menstrual huts; during the day menstruating women work in the fields (23, 24). The indigenous religion uses the ideology of menstrual pollution as the supernatural enforcement mechanism to coerce women to disclose their menses by going to the menstrual hut. Hormonal data showed that fear of breaking these religious taboos enforced honest signaling to the men of the husband’s family, who situate the menstrual huts in close proximity to the toguna, which is a shade shelter specific to the males of a given patrilineage (23). The Dogon do not practice contraception, and 83% of women have high fertility (7–13 live births) (25). The median duration of lactational amenorrhea is 20 mo, and menstruation is a rare event quickly followed by pregnancy (23, 26, 27). When a woman resumes going to the menstrual hut following her last birth, the husband’s patrilineage is informed of the immanency of conception and cuckoldry risk. Precautions include postmenstrual copulation initiated by the husband and enhanced vigilance by his family (23, 24).
Results and Discussion
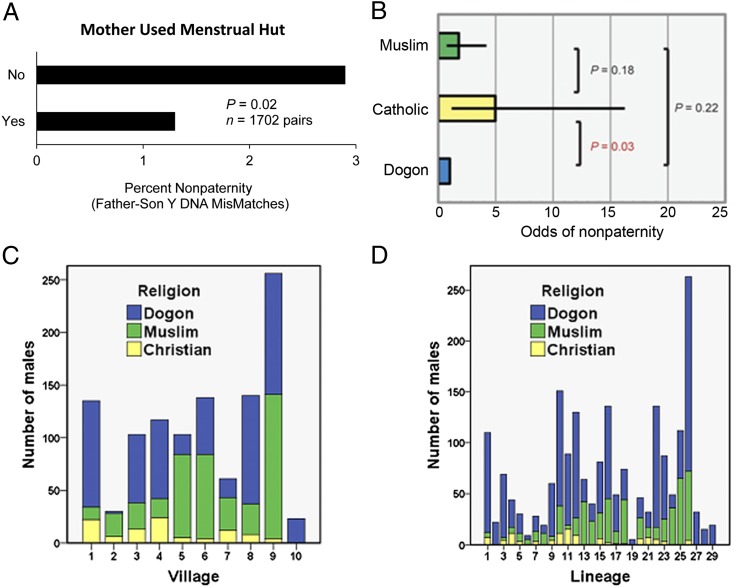
Across all religions, we detected father–son Y DNA mismatches in only 1.8% of father–son pairs (Fig. 1), a finding that contradicts the prevailing view that traditional populations have high rates of cuckoldry (28, 29). Although a similar rate has been found in several modern populations (29, 30), a key difference is that the Dogon do not use contraception (16, 25). The prevalence of mismatches was 1.3% when the mother used the menstrual hut and 2.9% when she did not, a difference that was statistically significant (P = 0.019, one-sided Fisher’s exact test; n = 1702 father–son pairs) (Fig. 2A). These results support the hypothesis (23, 24) that Dogon menstrual huts promote cuckoldry defense.
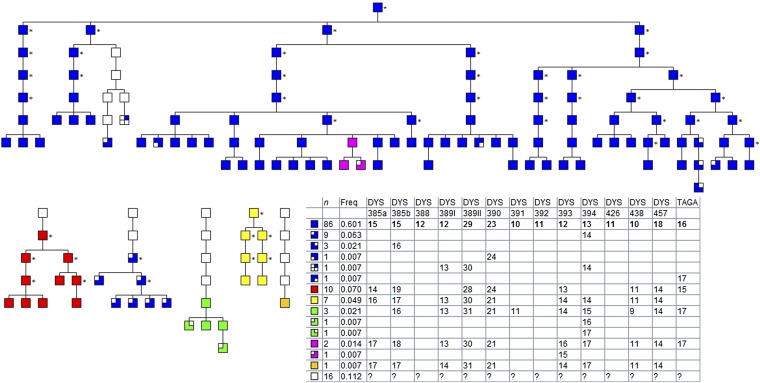
Fig. 1.
An anonymized pedigree for a Dogon patrilineage based on oral genealogies with Y DNA haplotypes mapped onto it (this is one of 29 similar pedigrees). A cuckoldry event is shown where the Y DNA haplotype changes from blue to pink. The table gives the Y DNA short tandem repeats at each locus. Asterisks indicate inferred haplotypes (SI Materials and Methods).
Fig. 2.
(A) The prevalence of father–son Y DNA mismatches by menstrual hut use. (B) Exact odds ratio (± 95% confidence limits) for cuckoldry by religion (the Dogon religion is the reference and by definition has an odds ratio of exactly 1.00). Protestants are omitted on account of small sample size (indigenous religion: n = 1,136, Islam: n = 470, Catholicism: n = 64, Protestantism: n = 34). (C) Religion by village. (D) Religion by patrilineage.
In defining a Y DNA mismatch, it was important to distinguish cuckoldry from mutation on the Y chromosome. The Y STR mutation rate was 0.0043 (exact 95% confidence limits of 0.0027–0.0064; n = 5,376 meioses), and the probability of a mutation occurring at exactly 3 of 14 Y STR loci was only 2.7 × 10−5. The probability of mutations arising at exactly 2 loci was much higher: 1.6 × 10−2. Accordingly, we defined nonpaternity as a mismatch between fathers and sons at three or more Y STR loci, regardless of the number of steps per locus. This definition is consistent with previous studies (31). The mean (±SD) number of loci that differed between fathers and sons in the nonpaternity pairs was 8.29 ± 2.00 and in the matched pairs was 0.05 ± 0.24. The mean (±SD) number of discrepant steps for the nonpaternity pairs was 16.03 ± 5.82 and for the matched pairs was 0.06 ± 0.30. Nonpaternity events were scattered thinly across the lineages and did not cluster in particular families. No man was cuckolded twice (Table S1).
Figs. S1–S3 show the distribution of Y STR haplotypes among the 29 patrilineages and 10 villages. Eighty three (75%) of 111 haplotypes were unique to a given patrilineage. We further differentiated the shared haplotypes by genotyping 11 additional Y STR loci for the 4 haplotypes whose prevalence was >4% in the total population. Analysis of molecular variance showed that 59% of the genetic variation was within patrilineages and 41% was between patrilineages; 79% was within villages and 21% was between villages (Table S2). The strong structuring of Y DNA haplotypes by patrilineage, combined with the ethnographic evidence that men do not seek EPCs within their own patrilineage, made it possible to use Y STRs to detect cuckoldry in this study.
EPCs within the patrilineage are severely punished because they disrupt the cooperation of male patrilineage members over mate guarding each other’s wives. Such a violation is considered incest. In a rare case of intralineage cuckoldry, the patrilineage elders took away 85% of the land of the accused and eventually punished the offending male and his sons with expulsion from the patrilineage. By contrast, men of different patrilineages submit to the authority of their own sets of elders, with each set exclusively controlling the resources of only one lineage. Thus, when cuckoldry happens between lineages, the elders cannot mete out punishments (23). Men from the same patrilineage also do not steal each other’s wives for the purpose of marriage, but they do steal wives from other lineages (23). The cultural prohibition against wife theft within the patrilineage stipulates that after divorce a woman must never again return to her former husband’s patrilineage even for a social visit.
As Y STRs do not permit one to test for cuckoldry by paternally related males, we also analyzed 15 autosomal loci for 61 randomly chosen father–son pairs that shared the same Y DNA haplotype. This analysis uncovered no instances of cuckoldry. On the basis of the binomial distribution, we are 84% confident that the true proportion of cuckoldry within the haplotype is less than 3%, and 92% confident that the true proportion is less than 4%. These results are consistent with the ethnographic evidence that cuckoldry within the patrilineage is rare; however, we would need a larger sample size to determine whether it is less frequent than cuckoldry between lineages.
To investigate whether the indigenous religion (with its menstrual taboos) is more effective at defending against cuckoldry in the Dogon than Catholicism, Evangelical Protestantism, or Islam, we directly compared the probability of nonpaternity by religion for 1,704 father–son pairs (Dogon religion: n = 1,136; Catholicism: n = 64; Protestantism: n = 34; Islam: n = 470). The four different religions coexist within the same villages (Fig. 2C) and even within the same patrilineages (Fig. 2D), providing a natural control for many factors other than religion that might influence paternity certainty in the Dogon. We used exact logistic regression with nonpaternity as the dependent variable and Catholic or Muslim as the predictor variables and the Dogon religion as the reference category. As shown in Fig. 2B, the odds of nonpaternity were 5.0 times higher for Catholic males versus males who followed the Dogon religion (exact odds ratio = 4.97, 95% confidence limits = 1.17–16.25, P = 0.031).
Dogon women in Catholic families are not required to notify their husbands when they are menstruating and they may attend church. Under Catholicism it is easier for women to conceal their menses and hence the onset of pregnancy. We suggest that this is the most likely reason why nonpaternity was highest among the Dogon Catholics. This finding cannot be generalized to other societies where Catholicism has deeper roots and where Catholicism’s own strictures against cuckoldry may be better enforced (SI Discussion). In the Dogon, Catholicism is a new religion that is strongly influenced by former customs—for example, polygyny is accepted. It is in transition—having lost the menstrual huts without having put something else in place. Nonpaternity was higher in the Protestant males than in those who practiced the Dogon religion, but the statistical power for this test was very low, and the results were nonsignificant (exact odds ratio = 2.26, 95% confidence limits = 0.05–15.58, P = 0.76). When the Catholics and Protestants are grouped together, the odds of nonpaternity were 4.0 times higher for Christians versus males who followed the Dogon religion (exact odds ratio = 4.01, 95% confidence limits = 1.12–11.94, P = 0.034).
We also conducted a further analysis restricted to men born from 1930 onward, effectively eliminating the period before the advent of religious change (n = 1,317 father–son pairs, including 24 nonpaternity events). In this analysis, the odds of nonpaternity in the Christians was five times higher than in the Dogon religion (exact odds ratio = 4.96, 95% confidence limits = 1.25–17.63, P = 0.023) (SI Results).
Menstruating Dogon women in Muslim families do not visit separate huts, but unlike the Christians, they must notify their husbands and are not allowed to pray. Together with the Qur’an’s emphasis on female sexual purity (8), these practices help to compensate for the absence of menstrual huts (SI Discussion). Consistent with this argument, the difference in nonpaternity between Islam and the Dogon religion was not statistically significant (exact odds ratio = 1.78, 95% confidence limits = 0.74–4.19, P = 0.22, n = 470) (Fig. 2B).
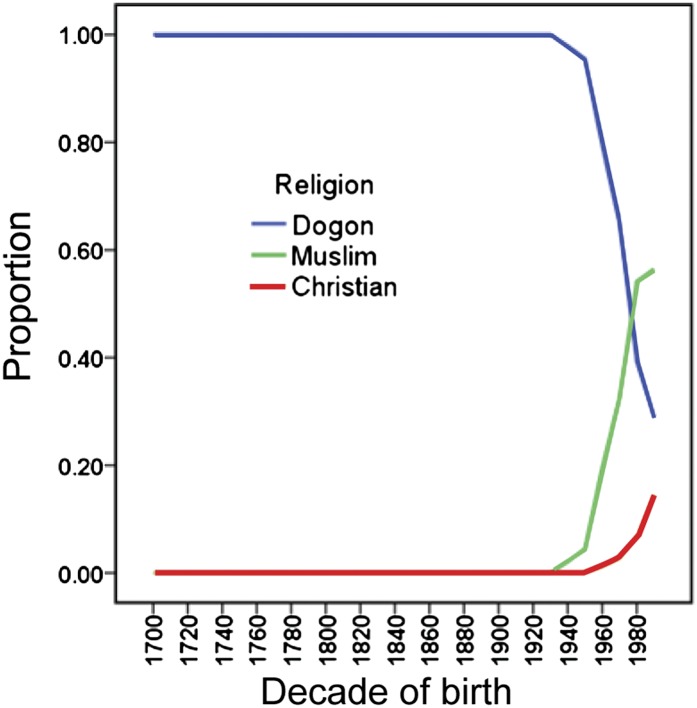
Increased susceptibility to cuckoldry due to greater poverty is an alternative hypothesis to explain the relatively high nonpaternity in the Christians. To test this hypothesis, we identified the set of fathers who practiced the traditional religion and then asked: What religions do their sons practice? We found that, in wealthier families, sons were more likely to convert from the indigenous religion to Islam (Fig. 3). Specifically, as family wealth increased by 1 SD (Fig. S4), the odds that a son converted to Islam, instead of Christianity, increased by 68% (exact odds ratio = 1.68, 95% confidence limits = 1.15–2.46, P = 0.007). In the Dogon, Christianity requires the fewest costly expenditures for funerals and holidays, and this may be one of the reasons why it has greater appeal for the poor.
Fig. 3.
Religious change in the study population over 300 y.
However, the mean wealth of the families of the men who were conceived through nonpaternity was not statistically different from that of the other men (t test for equality of means: t = −0.665, df = 1236, P = 0.506, mean difference = 0.118, standard error difference = 0.178, 95% confidence interval of the difference = −0.467–0.230). In a model with wealth and Catholic as predictors of nonpaternity, Catholic was significant (P = 0.007) with an odds ratio of 4.96 (95% confidence interval = 1.55–15.82), and wealth was not significant (P = 0.259, odds ratio = 1.34, 95% confidence interval = 0.81–2.23). Because controlling for wealth did not eliminate the significant effect for Catholic or diminish its effect size, poverty cannot account for the higher rate of nonpaternity in the Christians.
We also tested the hypothesis that nonpaternity was more prevalent under polygyny. For the matched father–son pairs, the median number of wives was 2.0 (mean ± SEM = 1.74 ± 0.169), and, for the nonpaternity cases, the median number of fathers’ wives was also 2.0 (mean ± SEM = 1.73 ± 0.024). We were unable to reject the null hypothesis that the distributions for the number of wives were the same (n = 911, mean rank(matched cases) = 456.12, mean rank(nonpaternity cases) = 451.33, Mann–Whitney U test, P(2-sided) = 0.925). The median number of fathers’ wives was two in each of the four religions (Catholicism, Protestantism, Islam, and the indigenous Dogon religion); thus religion was not confounded with polygyny in the Dogon (n = 911 father–son pairs; median test, df = 3; P2-sided = 0.391). In a model with polygyny and Catholic as the predictors of nonpaternity, Catholic was borderline significant with a right-skewed confidence interval (P = 0.059, odds ratio = 2.91, 95% confidence interval = 0.96–8.82), and polygyny was not significant (P = 0.935, odds ratio = 1.02, 95% confidence interval = 0.58–1.81). Thus, controlling for polygyny did not alter our conclusions about the variable Catholic.
There were no indications that nonpaternity was higher in the Christians due to confounding with an unmeasured variable. Christians did not spend more time in the city than the men who practiced the Dogon religion, and they spent significantly less time away than the Muslims (23). Whereas the menstrual taboos are embedded in the indigenous religion, genital cutting is a ubiquitous cultural practice that is not tied to religion (23, 24). Aside from the ongoing process of religious conversion, the study site is still very traditional: electricity, television, and contraception are totally absent (SI Discussion).
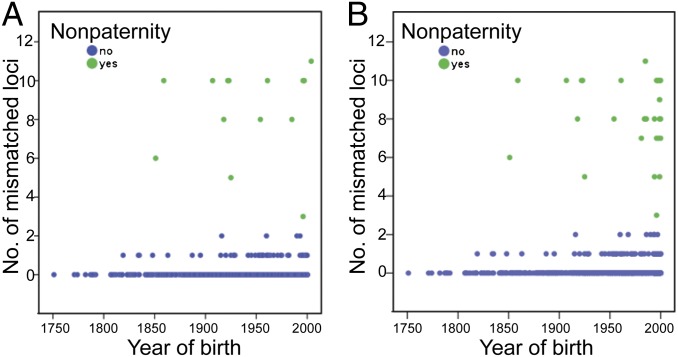
As menstrual hut use was required by the Dogon religion, but not by Islam and Christianity, which arrived at the study site much later (Fig. 3), we hypothesized that the prevalence of nonpaternity increased over time. In support of this hypothesis, we found that the median year of birth for the nonpaternity cases was significantly later than that for the males who were a genetic match to their fathers (n = 1,703, mean rank for the 31 nonpaternity cases = 1,096.50, mean rank for the 1,672 matched cases = 847.47, standardized test statistic = 2.795, P value(2-sided) = 0.005). We attribute this result to the strong correlation between year of birth and whether or not a male’s mother used the menstrual hut (Spearman’s Ρ = −0.619, P < 0.0001). Restricting the analysis to the males (n = 1,136) whose fathers practiced the Dogon religion, year of birth (1750–2000) did not predict nonpaternity (exact odds ratio = 1.003, 95% confidence limits = 0.991–1.016, P = 0.67) (Fig. 4). Similarly, within the Muslim father and son pairs (n = 470), year of birth did not predict nonpaternity (exact odds ratio = 1.027, 95% confidence limits = 0.970–1.108, P = 0.45). Both of these religions had a large enough sample size to pick up a cohort effect if it had existed. We conclude that religious change, rather than temporal change unrelated to religion, was critical for any increase in nonpaternity over time.
Fig. 4.
Matched (blue) and mismatched (green) father–son pairs by year of birth for son. We used exact logistic regression to calculate the odds of nonpaternity (yes, no) with year of birth (years) as the predictor variable. (A) Dogon religion only (exact odds ratio = 1.00, 95% confidence limits = 0.99–1.02, P = 0.677). (B) All religions (exact odds ratio = 1.01, 95% confidence limits = 1.00–1.02, P = 0.064).
Finally, we considered multivariable logistic regression models using the method of best subsets, which identifies the top models for a given number of predictor variables. For all models, the dependent variable was nonpaternity. In models with one predictor variable, the χ2 was higher for Catholic (χ2 = 7.46, P = 0.01) and menstrual hut use (χ2 = 5.32, P = 0.02) than for other variables such as year of birth (χ2 = 3.09, P = 0.08) (Table S3). The best two-variable model (χ2 = 10.27) included Catholic (odds ratio = 2.78, P = 0.08) and menstrual hut use (odds ratio = 0.52, P = 0.08), both of which lost statistical significance due to collinearity. In all 16 models generated (Table S3), there was never more than one significant term, and adding variables beyond two did not substantially improve the χ2. It is noteworthy that inclusion of the variable, year of birth, did not improve the fit in any of the multivariable models nor did it eliminate the significance of the variable Catholic or substantially affect its odds ratio.
In summary, we were able to exclude the possibility that higher nonpaternity in the Catholics was due to confounding with wealth, polygyny, and year of birth. It is more plausible that the higher nonpaternity in the Catholics was due to changes in their religious observances, especially their abandonment of the menstrual taboos.
Conclusion
Taking advantage of the natural experiment afforded by the ongoing religious transition in the Dogon, we found genetic support for the hypothesis that menstrual huts help to assure paternity. Although the world religions do not have menstrual huts, they do share common tenets that may foster cuckoldry avoidance (SI Discussion). For example, in Judaism, menstrual purity laws increase coital frequency around the time of ovulation (32). In Islam, paternity confusion is prevented by the Qur’an’s rule that, after divorce, a woman must wait for three menstrual periods before remarrying (8, 23). The Hindu text, The Laws of Manu, admonishes against cuckoldry or “sowing in another man’s field” (7). Strong statements against adultery and extramarital children are found in the Bible (6), and, in Buddhism, adultery is a form of sexual misconduct (5). In preventing cuckoldry, religions use the dual strategy of social control in the public sphere (attendance at a place of worship or at a menstrual hut) and the fear of divine or supernatural punishment. In the United States, frequent church attendance and belief that the Bible is the word of God were the two most robust predictors of lower rates of self-reported EPCs (33). We posit that the ideological and tactical similarities between the world religions and the Dogon religion have arisen in response to the same biological pressures.
Materials and Methods
Field Methods.
Informed consent was obtained from each participant, and approval for this study was obtained from village elders, the Malian government, and the University of Michigan Health and Behavioral Sciences Institutional Review Board (H03-00001208-R2). The 29 Dogon patrilineages requested and were given poster-size copies of their patrilineal pedigrees with the genetic data omitted.
Each male (n = 1,218) provided a DNA sample in the form of a cheek swab or a whole saliva sample and gave genealogical information for his paternal and maternal ancestry as far back as he could go. From these oral histories, we made patrilineal pedigrees in the genetic program Progeny (versions 6 and 7) (34) that have a depth of up to 11 generations from the youngest generation to the common ancestor [mean ± SD = 5.7 (1.9) generations]. We mapped each man’s Y DNA haplotype onto the 29 pedigrees (Fig. 1). Discrepant samples were regenotyped in the laboratory up to four times and recollected in the field to minimize the possibility of sampling or laboratory error. Field trips for DNA sampling took place in 2001, 2004–2005, 2005–2006, and 2007.
At the time the cheek swabs were collected, we also asked each male the following questions: (i) his father’s religion, (ii) whether or not his mother used the menstrual hut, and (iii) the number of wives his father had (Table S4). The time point of reference for these interviews was “the time the man was born,” which was a proxy for when he was conceived. If the man deferred our question to a relative of the previous generation, then we asked that person instead. As the Dogon religion was the sole religion until the 1940s, we assumed that the fathers of males born before 1940 practiced the Dogon religion and that their mothers used the menstrual huts. B.I.S. is fluent in Dogon and conducted these interviews in the local language.
Sampling.
The males belong to 29 patrilineages in 10 villages that are located within a 5-km radius of their shared market place. Only 2.2% had emigrated from other villages, and the remaining 97.8% shared the same last name and belonged to the same clan. It is extremely unlikely that males who were conceived through extrapair copulation selectively avoided participation in our study as, in the absence of DNA testing, such males would not be able to identify themselves. Informants stated that, if a male is believed to have been conceived out of wedlock, then he is not accepted into the patrilineage and will emigrate, but known examples are extremely rare. Our analysis does not include males who emigrated and instead gives a detailed assessment of the predictors of nonpaternity among males living with their natal patrilineages (Fig. S5).
Genotyping.
We genotyped 14 Y chromosome STRs (DYS385a, DYS385b, DYS388, DYS389I, DYS389II, DYS390, DYS391, DYS392, DYS393, DYS394, DYS426, DYS438, DYS457, TAGA = DYS439 + 4 repeats) in two multiplex reactions. A subset of samples was further genotyped for 11 additional Y chromosome STRs (CDYa, CDYb, DYS442, DYS456, DYS460, DYS570, DYS576, DYS607, YCAIIa, YCAIIb, and H4) in two multiplex reactions according to Redd et al. (35, 36) with slight modifications. To genotype autosomal markers, we used the AmpFLSTR Identifiler Plus ID PCR amplification kit according to manufacturer’s conditions (CSF1P0, D7S820, D8S1179, D21S11, D2S1338, D3S1358, D13S317, D16S539, TH01, D18S51, D19S433, TPOX, vWA, D5S818, and FGA). We also developed two multiplex protocols to genotype the following autosomal loci: D12S1301, D18S535, D8S1048, D12S373, D9S922, D4S2417, D2S434, D1S3669, D2S2944, D6S2436, D5S2849, D8S2324, D10S2470, D3S1763, and D8S1128. All protocols and primer information are available upon request.
Supplementary Material
Acknowledgments
We thank the Malian government for research permission; the Dogon people for their generous participation; C. Vincenz, D. C. Queller, and J. E. Strassmann for helpful comments on the manuscript; M. E. Kaplan, E. R. Landeen, and B. B. Fransway for genotyping; K. B. Welch and Y. V. Rodgers for statistical advice; and M. Soc and H. G. Freeman for assistance with data management. This work was supported by National Science Foundation Grant BCS 0509019.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. R.S. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1110442109/-/DCSupplemental.
References
- 1.Friedman RE. Who Wrote the Bible? New York: HarperCollins; 1989. [Google Scholar]
- 2.Dickemann M. Paternal confidence and dowry competition: A biocultural analysis of purdah. In: Alexander RD, Tinkle D, editors. Natural Selection and Social Behavior. New York: Chiron; 1981. pp. 417–438. [Google Scholar]
- 3.Michaels A. Hinduism Past and Present. Princeton, NJ: Princeton Univ Press; 2004. [Google Scholar]
- 4.Gotama Buddha. Anguttara-nikāya: The Numerical Discourses of the Buddha. 2009 IV-66 (Theravada Tipitaka Press, Par-la-Ville, MD) [Google Scholar]
- 5.Carter JR, Palihawadana M, editors. The Dhammapada. New York: Oxford Univ Press; 1987. pp. 309–310. [Google Scholar]
- 6.Coogan MD, Brettler MZ, Newsom CA, Perkins P, editors. The New Oxford Annotated Bible. New York: Oxford Univ Press; 2001. Deuteronomy 2.13–21 (virginity); Leviticus 20.10; Exodus 20.14; Deuteronomy 5.18, 22.22–23; Matthew 5.27–32, 19.9, 18 (adultery); 1 Corinthians 6.9, 7.2 (adultery, sexual immorality); 1 Peter 3:3–5 (women’s adornment) [Google Scholar]
- 7.Doniger W, Smith BK, editors. The Laws of Manu. New York: Penguin Group; 1991. Chaps 8:226, 359, 374–378; 9:10–17, 30, 33–41, 149. [Google Scholar]
- 8.Arberry AJ, editor. The Koran Interpreted. New York: Touchstone; 1996. Light 24:2–3, 30–31; Women 4:3, 19, 38; Cow 2:228. [Google Scholar]
- 9.Smuts B. The evolutionary origins of patriarchy. Hum Nat. 1995;6:1–32. doi: 10.1007/BF02734133. [DOI] [PubMed] [Google Scholar]
- 10.Hrdy SB. Raising Darwin’s consciousness. Hum Nat. 1997;8:1–49. doi: 10.1007/s12110-997-1003-9. [DOI] [PubMed] [Google Scholar]
- 11.Hartung J. On natural selection and the inheritance of wealth. Curr Anthropol. 1976;17:607–622. [Google Scholar]
- 12.Reichard UH. Monogamy: Past and present. In: Reichard UH, Boesch C, editors. Monogamy: Mating Strategies and Partnerships in Birds, Humans and Other Mammals. Cambridge, UK: Cambridge Univ Press; 2003. pp. 3–25. [Google Scholar]
- 13.Petrie M, Kempenaers B. Extra-pair paternity in birds: Explaining variation between species and populations. Trends Ecol Evol. 1998;13:52–58. doi: 10.1016/s0169-5347(97)01232-9. [DOI] [PubMed] [Google Scholar]
- 14.Hughes C. Integrating molecular techniques with field methods in studies of social behaviour: A revolution results. Ecology. 1998;79:383–399. [Google Scholar]
- 15.Strassmann BI. Social monogamy in a human society: Marriage and reproductive success among the Dogon. In: Reichard UH, Boesch C, editors. Monogamy: Mating Strategies and Partnerships in Birds, Humans and Other Mammals. Cambridge, UK: Cambridge Univ Press; 2003. pp. 177–189. [Google Scholar]
- 16.Strassmann BI. Cooperation and competition in a cliff-dwelling people. Proc Natl Acad Sci USA. 2011;108(Suppl 2):10894–10901. doi: 10.1073/pnas.1100306108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boyer P. Religion Explained: The Evolutionary Origins of Religious Thought. New York: Basic Books; 2001. [Google Scholar]
- 18.Sosis R, Alcorta C. Signaling, solidarity, and the sacred: The evolution of religious behaviour. Evol Anthropol. 2003;12:264–274. [Google Scholar]
- 19.Atran S, Norenzayan A. Religion’s evolutionary landscape: Counterintuition, commitment, compassion, communion. Behav Brain Sci. 2004;27:713–730, discussion 730–770. doi: 10.1017/s0140525x04000172. [DOI] [PubMed] [Google Scholar]
- 20.Alcorta CS, Sosis R. Ritual, emotion, and sacred symbols: The evolution of religion as an adaptive complex. Hum Nat. 2005;16:323–359. doi: 10.1007/s12110-005-1014-3. [DOI] [PubMed] [Google Scholar]
- 21.Norenzayan A, Shariff AF. The origin and evolution of religious prosociality. Science. 2008;322:58–62. doi: 10.1126/science.1158757. [DOI] [PubMed] [Google Scholar]
- 22.Henrich J. The evolution of costly displays, cooperation and religion: Credibility enhancing displays and their implications for cultural evolution. Evol Hum Behav. 2009;30:244–260. [Google Scholar]
- 23.Strassmann BI. The function of menstrual taboos among the Dogon. Hum Nat. 1992;3:89–131. doi: 10.1007/BF02692249. [DOI] [PubMed] [Google Scholar]
- 24.Strassmann BI. Menstrual hut visits by Dogon women: A hormonal test distinguishes deceit from honest signaling. Behav Ecol. 1996;7:304–315. [Google Scholar]
- 25.Strassmann BI, Gillespie B. Life history theory, female fertility, and reproductive success in humans. Proc R Soc Lond B Biol Sci. 2002;269:553–562. doi: 10.1098/rspb.2001.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Strassmann BI. The biology of menstruation in Homo sapiens: Total lifetime menses, fecundity, and nonsynchrony in a natural fertility population. Curr Anthropol. 1997;38:123–129. [Google Scholar]
- 27.Strassmann BI, Warner JH. Predictors of fecundability and conception waits among the Dogon of Mali. Am J Phys Anthropol. 1998;105:167–184. doi: 10.1002/(SICI)1096-8644(199802)105:2<167::AID-AJPA5>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 28.Diamond JM. Why Is Sex Fun? The Evolution of Human Sexuality. New York: Basic Books; 1997. [Google Scholar]
- 29.Simmons LW, Firman RC, Rhodes G, Peters M. Human sperm competition: Testis size, sperm production and rates of extrapair copulations. Anim Behav. 2004;68:297–302. [Google Scholar]
- 30.Anderson KG. How well does paternity confidence match actual paternity? Evidence from worldwide nonpaternity rates. Curr Anthropol. 2006;47:513–520. [Google Scholar]
- 31.Kayser M, Sajantila A. Mutations at Y-STR loci: Implications for paternity testing and forensic analysis. Forensic Sci Int. 2001;118:116–121. doi: 10.1016/s0379-0738(00)00480-1. [DOI] [PubMed] [Google Scholar]
- 32.Boster JS, Hudson RR, Gaulin SJC. High paternity certainties of Jewish priests. Am Anthropol. 1998;100:967–971. [Google Scholar]
- 33.Burdette AM, Ellison CG, Sherkat DE, Gore KA. Are there religious variations in marital infidelity? J Fam Issues. 2007;28:1553–1581. [Google Scholar]
- 34.Progeny Software LLC 2007 Progeny 7.6.03 (Delray Beach, FL) [Google Scholar]
- 35.Redd AJ, et al. Forensic value of 14 novel STRs on the human Y chromosome. Forensic Sci Int. 2002;130:97–111. doi: 10.1016/s0379-0738(02)00347-x. [DOI] [PubMed] [Google Scholar]
- 36.Redd AJ, et al. Genetic structure among 38 populations from the United States based on 11 U.S. core Y chromosome STRs. J Forensic Sci. 2006;51:580–585. doi: 10.1111/j.1556-4029.2006.00113.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.