Abstract
Interleukin-4 is a cytokine widely known for its role in CD4+ T cell polarization and its ability to alternatively activate macrophage populations. In contrast, the impact of IL-4 on the activation and function of dendritic cells (DCs) is poorly understood. We report here that DCs respond to IL-4 both in vitro and in vivo by expression of multiple alternative activation markers with a different expression pattern to that of macrophages. We further demonstrate a central role for DC IL-4Rα expression in the optimal induction of IFNγ responses in vivo in both Th1 and Th2 settings, through a feedback loop in which IL-4 promotes DC secretion of IL-12. Finally, we reveal a central role for RELMα during T-cell priming, establishing that its expression by DCs is critical for optimal IL-10 and IL-13 promotion in vitro and in vivo. Together, these data highlight the significant impact that IL-4 and RELMα can have on DC activation and function in the context of either bacterial or helminth pathogens.
Keywords: antigen presenting cells, T lymphocytes, innate immunity, adaptive immunity
Activation of dendritic cells (DCs) with bacterial or viral antigen (Ag) results in production of proinflammatory cytokines and enhanced ability to stimulate Th1/Th17 responses (1). In addition, a range of cytokines influence DC activation, with IL-12 and IL-10 playing critical roles in either promotion or regulation of DC maturation and function (2, 3). The hallmark of allergic disease and helminth infection is induction of a CD4+ Th2 response, characterized by secretion of cytokines such as IL-4 and IL-13 (4). Although DCs themselves are not thought to produce IL-4 (5), this canonical Th2 cytokine can be secreted rapidly in direct response to pathogen stimulation by a variety of other innate cells (4). As such, DCs encountering Ag or pathogen stimulation in Th2 infection or disease settings will likely be simultaneously exposed to IL-4. However, the impact of IL-4 on the functional capability of DCs remains relatively unexplored.
In comparison with DCs, the effect of IL-4 on activation of macrophage (MΦ) populations has been much more thoroughly addressed, with IL-4/IL-13 treatment resulting in “alternatively” activated macrophages (aaMΦs) (6) characterized by the expression of Arginase-1, chitinase-like molecule Ym1, resistin-like molecule α (RELMα, also known as FIZZ1), and C-type lectin receptors such as mannose receptor (MR) and Dectin-1 (6, 7). Although the individual and cumulative function of these IL-4–induced products is not clear, aaMΦs are thought to play vital roles in helminth infection and tissue remodeling, and are capable of suppressing T-cell responses (8–10). However, only a limited number of studies have addressed whether DCs express markers associated with aaMΦs (11–14), and it remains unclear how expression of IL-4–induced molecules may influence DC function in Th2 settings. Somewhat surprisingly, previous work has indicated that IL-4 can enhance production of IL-12p70 by DCs stimulated with bacterial LPS in vitro via inhibition of IL-10 (15–18). This suggests that IL-4 may facilitate DC priming of Th1 responses in ongoing, counterregulatory, Th2 settings (15, 16, 19).
We set out to delineate the role of IL-4 in influencing DC activation and function, both in vitro and in vivo. We show that treatment of DCs with IL-4 in vitro resulted in robust expression of a wide range of alternative activation markers at both the transcript and protein level, with a more selective expression of alternative activation markers RELMα and Ym1 in vivo. We further demonstrate a key role for DC-derived RELMα in promotion of optimal Th2 responses that contrasts with the previously suggested regulatory function of this alternative activation product (20–22). Together, these data reveal that the ability to respond to IL-4 is critical for optimal Th1 or Th2 priming by DCs through modulation of IL-12/IL-10 or RELMα production, respectively.
Results
IL-4 Induces DC Expression of RELMα in Vivo.
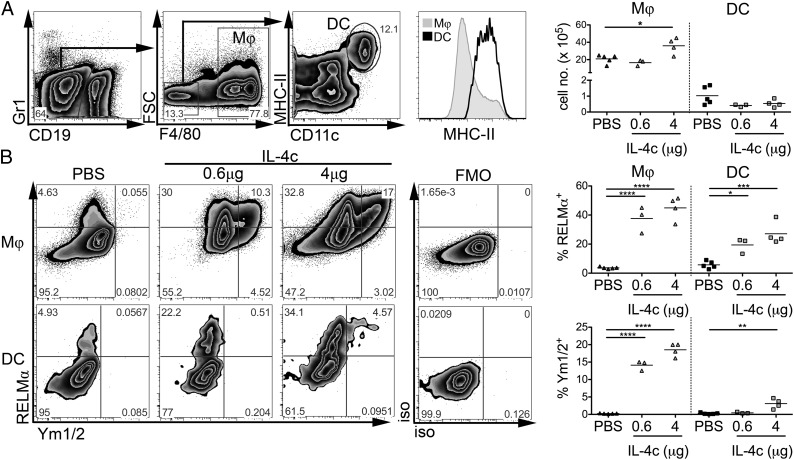
The phenotypes of aaMΦ populations, isolated from a variety of tissues in Th2 inflammatory settings, have been well characterized, with one hallmark being the abundant expression of markers RELMα and Ym1 (6). RELMα has also previously been identified in cells other than aaMΦs, including eosinophils and epithelial cells (10, 20, 22). To investigate whether DCs respond to Th2 environments in a manner similar to MΦs, mice were injected i.p. with rIL-4 complexed with anti–IL-4 mAb (IL-4c), which ensures slow release of cytokine for 2–3 d (23). Peritoneal exudate cells (PEC) were isolated 4 d after mice received a single dose of IL-4c or PBS. For flow-cytometric analysis, granulocytes, monocytes, and B cells were excluded (based on cell size, Gr1, and CD19 staining), MΦs were defined as F4/80+FSCint/hiMHC-IIlo/int and DCs as F4/80−FSCloCD11chiMHC-IIhi (Fig. 1A). IL-4c did not significantly alter the number of DCs in the PEC but, as recently reported (23), significantly increased accumulation of MΦs (P < 0.05; Fig. 1A), suggesting that, in contrast to MΦs, DCs do not proliferate in response to IL-4c injection. To assess whether IL-4 administration caused alternative activation of these populations, we performed intracellular staining for RELMα and Ym1/2. As expected, MΦs isolated from IL-4c–injected mice displayed striking levels of RELMα and Ym1/2 expression compared with MΦs isolated from PBS control animals (Fig. 1B). Furthermore, the expression of these proteins was not uniform, with RELMα+Ym1/2−, RELMα+Ym1/2+, and RELMα−Ym1/2+ MΦ populations all being identified. In contrast to the effect on MΦs, predominantly single positive RELMα−expressing DCs were observed at a low dose of IL-4c (0.6 μg) with little change in Ym1/2 evident. However, at a higher dose of IL-4c (4 μg), a significant proportion of RELMα-positive DCs expressed Ym1/2. In addition, the ability to respond to IL-4 in vivo was not restricted to peritoneal cavity DCs, as splenic MHCIIhiCD11chi DCs also significantly up-regulated RELMα and Ym1/2 expression following IL-4c injection (Fig. S1).
Fig. 1.
IL-4c induces MΦ and DC expression of RELMα in vivo. MΦs and DCs in PEC from PBS- or IL-4c–treated mice were assessed by flow cytometry (A). MΦs and DCs as defined in (A) stained for intracellular expression of RELMα and Ym1/2 (B). Data are representative of five experiments. Graphs show percent expression for individual mice, three to five per group. FMO, fluorescence minus one control.
To determine the general capacity of DCs to alternatively activate in vivo in response to stimuli other than IL-4c, we assessed RELMα and Ym1/2 production by DCs from mice infected with Litomosoides sigmodontis or Schistosoma mansoni (Fig. S2), parasitic helminths that promote strong Th2 responses. Pleural cavity (Fig. S2A) or splenic (Fig. S2B) MHCIIhiCD11chi DCs from infected mice displayed significantly increased expression of RELMα in comparison with naive control animals. Ym1/2 expression was less dramatically influenced by infection (Fig. S2). Thus, DCs from diverse tissue sites can express markers associated with aaMΦs in vivo, in response to injection with IL-4c or in the more complex setting of helminth infection.
IL-4 Up-Regulates DC Expression of Multiple Markers of Alternative Activation.
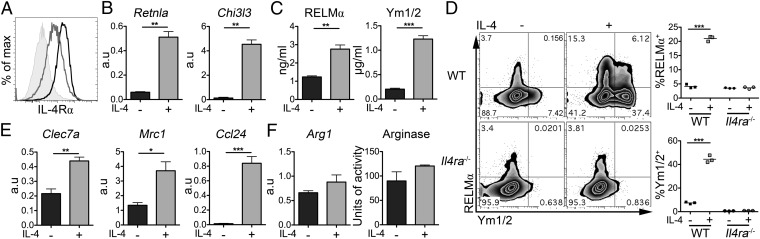
To carefully dissect the influence of IL-4 on a well-characterized population of cells, we generated DCs from murine bone marrow (BM) using GM-CSF, then exposed the DCs to recombinant IL-4 in vitro. As shown previously, DCs expressed IL-4Rα (Fig. 2A) (18), and IL-4 did not substantially alter MHC-II or the costimulatory molecules CD40, CD80 and CD86 (Fig. S3). However, IL-4 significantly increased DC mRNA levels and protein secretion of both RELMα (Retnla) and Ym1/2 (Chi3l3) (Fig. 2 B and C). Intracellular staining showed that production of these proteins was not limited to a single population, with both RELMα and Ym1/2 single and double-positive DCs clearly distinguishable (Fig. 2D: WT). Verifying that DC up-regulation of RELMα and Ym1/2 was a consequence of IL-4 responsiveness, Il4ra−/− DCs did not up-regulate RELMα and Ym1/2 in response to IL-4 (Fig. 2D: Il4ra−/−). In addition, in WT DCs, IL-4 increased transcripts for Dectin-1 (Clec7a), Mrc1 (MR) and Ccl24 (Fig. 2E), markers previously associated with aaMΦs (6, 7). However, Arginase-1, another signature molecule of aaMΦs (6), was not significantly increased in terms of either transcription or enzymatic activity in IL-4–treated DCs (Fig. 2F). Increased transcript expression of Retnla, Clec7a, and Ccl24 was also seen following in vitro exposure of FACS-purified splenic cDCs from naive mice to IL-4 (Fig. S4). Together, these data reveal that murine DCs can respond to IL-4 in a manner similar to that previously described for aaMΦs by significantly up-regulating RELMα and Ym1/2 and other aaMΦ markers, with the notable exception of Arginase-1.
Fig. 2.
IL-4 stimulates RELMα and Ym1 expression by BMDCs. IL-4Rα expression by BMDCs (A) (shaded portion = isotype control; black line = media; gray line= +IL-4). WT or Il4ra−/− BMDCs were cultured overnight with or without IL-4 and were assessed for markers of alternative activation (B–F) (black = without IL-4; gray = 20 ng/mL IL-4). Data are representative of more than three experiments. Error bars indicate SEM of triplicate wells. a.u., Arbitrary units.
Signaling to DCs via IL-4Rα Alters Their Response to Inflammatory Stimuli and Is Important for Their Ability to Induce IFNγ and IL-17.
Previous studies have shown that IL-4 can boost DC proinflammatory cytokine production upon engagement of specific pattern recognition receptors, and suggested that this may represent a mechanism to enable DC induction of adaptive Th1 responses in IL-4–rich environments (15, 19). We assessed the influence of IL-4 on DCs exposed to IL-4 concurrently with complex Ag from pathogens and individual toll like receptor (TLR) ligands. We found that DC exposure to ligands of TLR4 (LPS), TLR9 (CpG), or a heat-killed preparation of the Gram-positive bacterium Propionibacterium acnes (Pa) boosted IL-12p70 secretion, and inhibited production of IL-10, RELMα, and Ym1/2, in response to IL-4 (Fig. S5 A–D). Production of proinflammatory cytokines IL-1β and IL-6 was not significantly altered by IL-4 treatment (Fig. S5E). In contrast to Pa and TLR ligands, DCs exposed to soluble extracts from the egg stage of S. mansoni [soluble egg Ag (SEA)] in the presence or absence of IL-4 did not alter secretion of either RELMα or Ym1/2, indicating that SEA has no potential to drive or block alternative activation of DCs (Fig. S5 C and D). Similarly, unlike Pa- and TLR-pulsed DCs, IL-4 treatment did not alter production of proinflammatory or regulatory cytokines by SEA-exposed DCs. Thus, the influence of IL-4 over DC activation is context dependent: IL-4 can enhance TLR-driven IL-12p70 production, but engagement of these pattern recognition receptors by defined TLR ligands or bacteria can also dramatically inhibit alternative activation markers ordinarily induced by IL-4. In contrast, the helminth Ag SEA did not impede this process.
Having demonstrated that DCs can express molecules associated with aaMΦs in an IL-4Rα–dependent manner, and that IL-4 can boost TLR-mediated proinflammatory cytokine production, we next addressed the relevance of DC responsiveness to IL-4 for T cell priming, one major characteristic used to distinguish DCs from MΦs. We have previously shown that Pa-activated DCs generate a strong mixed Th1/Th17 response following transfer into naive recipient mice (24). In contrast, we have established that DCs pulsed with SEA are capable inducers of Th2 responses both in vitro and in vivo, despite failing to up-regulate traditional markers of activation (5). Although it has been debated recently whether DCs are needed to prime Th2 responses (4), we have definitively shown that Th2 priming to schistosomes is dependent on CD11c+ DCs (25).
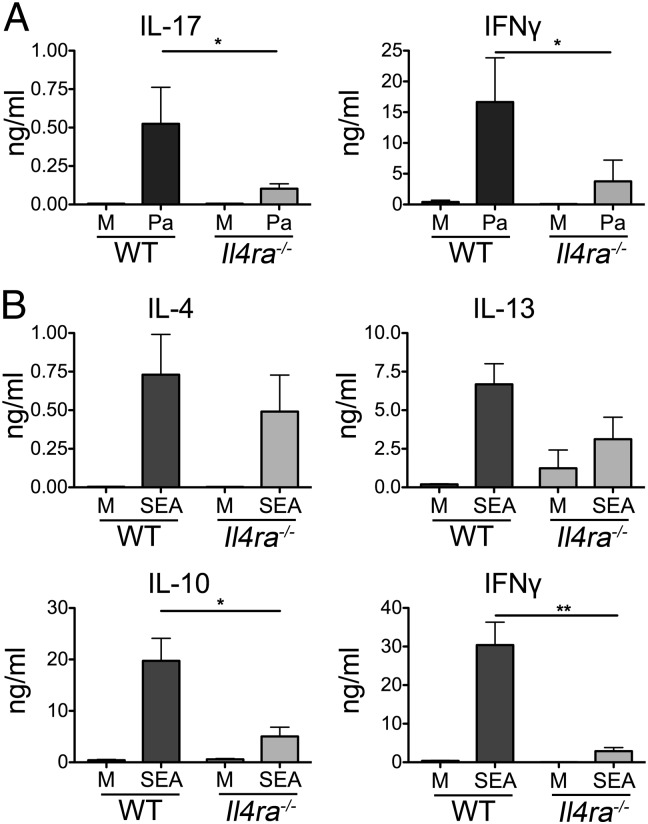
To investigate whether DC responsiveness to IL-4 alters priming of naive T cells, we generated WT or Il4ra−/− DCs, exposed them to Pa or SEA in vitro, then adoptively transferred them into WT naive recipient mice, and compared Ag-specific immune responses in the draining LN (dLN). Transfer of Pa pulsed Il4ra−/− DCs resulted in significantly lower amounts of Ag-specific IL-17 and IFNγ in recipient mice, in comparison with WT Pa DCs (Fig. 3A). This demonstrates that DC IL-4Rα signaling is required to promote optimal T-cell responses even in a Th1/Th17 polarizing environment with low levels of available Th2 cytokine. The deficiency in IL-17 priming is unlikely to be due to alteration in DC production of Th17-promoting cytokines IL-1β and IL-6, as IL-4 did not significantly influence secretion of either of these cytokines by DCs responding to Pa in vitro (Fig. S5E).
Fig. 3.
Il4ra−/− DCs are less able to induce IFNγ and IL-17 responses. WT or Il4ra−/− BMDCs were cultured overnight in medium alone (M), with Pa (A) or SEA (B), harvested, and injected s.c. into WT mice. Seven days later, the draining pLN were harvested, cells were restimulated for 72 h with Pa (A) or SEA (B), and cytokine secretion was assessed by ELISA. Data are representative of five experiments. Error bars indicate SEM of three to five mice per group.
In the context of helminth- rather than bacterial Ag–conditioned DCs, Il4ra−/− SEA DCs induced similar levels of Ag-specific IL-4 and IL-13, but significantly lower amounts of IL-10 and IFNγ in comparison with WT SEA DCs (Fig. 3B). This indicates that DC expression of IL-4Rα is not an absolute requirement for Th2 priming, but is needed for the induction of IFNγ and IL-10 responses against SEA. In keeping with previous work (26), the level of IL-17 induced by SEA-activated C57BL/6 DCs was negligible, and was not influenced by the presence or absence of IL-4Rα on the transferred DCs.
The reduced ability of Il4ra−/− DCs to promote Pa-specific IL-17 and IFNγ, and SEA-specific IL-10 and IFNγ, was not due to a fundamental defect in Ag uptake, processing, or presentation by these cells. Il4ra−/− DCs capably took up Ag in the form of FITC-labeled dextran (Fig. S6), and displayed enhanced ability to stimulate proliferation of OTII TCR Tg T cells in vitro, following exposure to ovalbumin (OVA) peptide or protein, compared with WT DCs (Fig. S7).
IL-4 Alters the Response of DCs to CD40 Ligation.
CD40 expressed by DCs binds CD154 on the surface of activated T cells, this interaction enhancing DC activation and cytokine production which is often crucial for their ability to prime Th1, Th2 and Th17 responses (24, 27). To address whether the IL-4 induced alteration in the balance of IL-12, IL-10, RELMα, and Ym1/2 production by DCs might be influenced by interaction with T cells we examined the impact of CD40 ligation on DC cytokine production following IL-4 exposure. To mimic DC–T-cell interaction in vitro, we analyzed cytokine secretion by DCs that had previously been exposed to Pa or SEA in the presence of absence of IL-4, after stimulation with agonistic αCD40 mAb. IL-4 treatment of Pa-pulsed DCs significantly enhanced production of IL-12p40, IL-6, and IL-12p70, and reduced secretion of IL-10, following addition of αCD40 mAb (Fig. S8A). However, in keeping with previously published data (28), control (medium) DCs or SEA DCs did not produce significant amounts of IL-12p70 or IL-10 following stimulation with anti-CD40, and this was not influenced by IL-4 exposure (Fig. S8A). Therefore, the influence of IL-4 on cytokine secretion by DCs responding to bacterial stimulation was not transient, and extended to secondary activation via CD40 ligation.
Previous studies have suggested that RELMα and Ym1/2 may have a role in regulating or promoting T-cell responses, respectively (14, 21, 22), and it is conceivable that during T-cell priming, CD154 expressed by T cells could engage DC CD40 and influence secretion of these molecules. However, IL-4–treated DCs stimulated with αCD40 mAb produced similar levels of RELMα and Ym1/2 compared with isotype controls (Fig. S8B), indicating that CD40 stimulation does not regulate IL-4–driven expression of these alternative activation products either in control or SEA DCs. Furthermore, in the case of Pa stimulated DCs, bacterial inhibition of RELMα and Ym1/2 was maintained throughout culture, and was not overcome by CD40 ligation (Fig. S8B). These CD40 ligation experiments also illustrate that DCs previously exposed to IL-4 still produced substantial amounts of RELMα and Ym1/2 up to 36 h after initial cytokine treatment (Fig. S8B). Thus, production of RELMα and Ym1/2 by DCs is not transient and requires neither the continued presence of IL-4 nor subsequent CD40 ligation.
DC Secretion of RELMα During Th2 Priming Regulates IFNγ and Promotes Th2 Responses.
Although our data demonstrate that DC sensitivity to IL-4 plays an important role in priming of T-cell IL-10, IL-17, and IFNγ (Fig. 3 A and B), it is not clear whether this is a consequence of alternative activation marker expression or some other facet of the diverse influence of IL-4 on DCs (Fig. 2 and Fig. S5). To specifically address the role of a defined alternative activation product in T-cell, priming we generated DCs from Retnla−/− mice (20), which were similar to WT in terms of numbers and phenotype. We adoptively transferred SEA- or Pa-pulsed WT or Retnla−/− DCs into WT-naive recipient mice and assessed the resulting Ag-specific immune response.
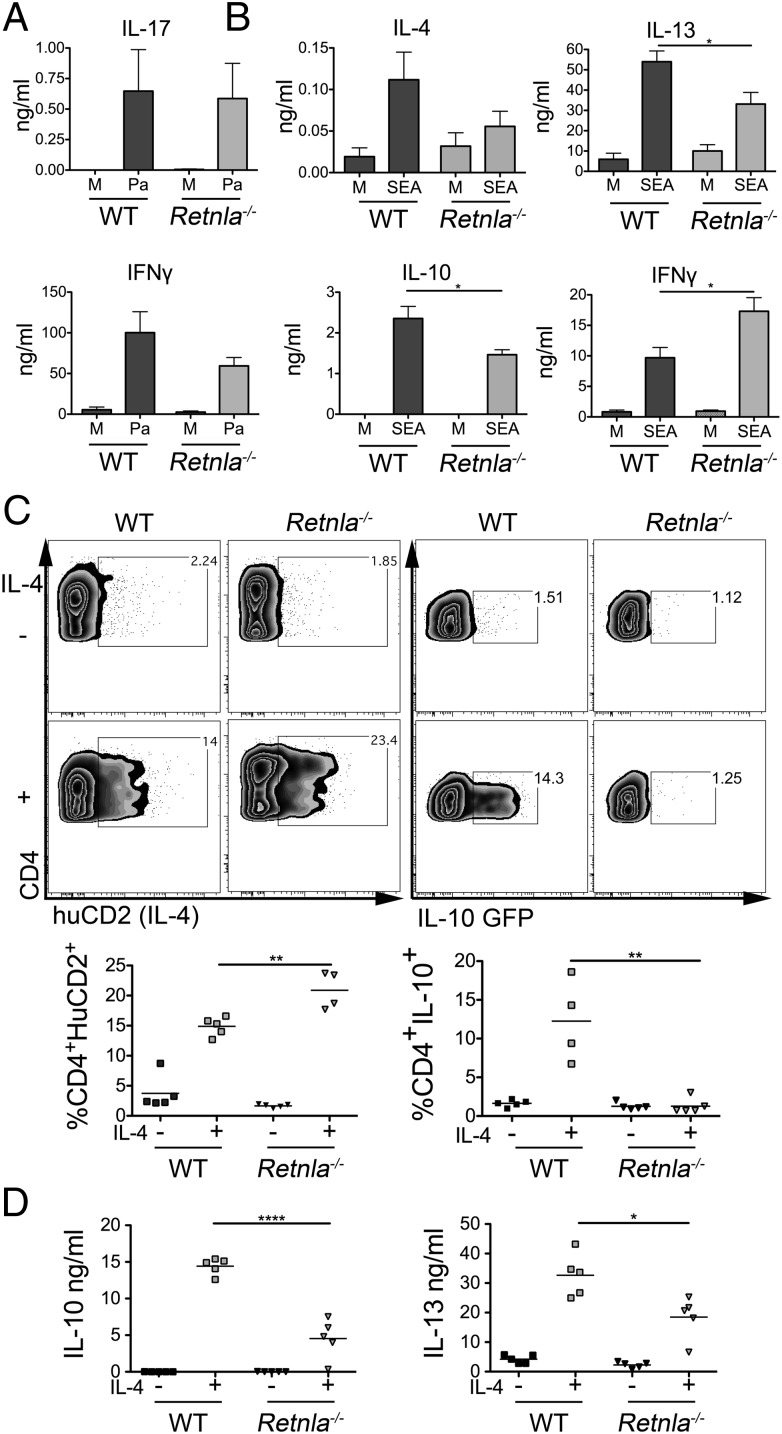
Retnla−/− Pa DCs induced similar IFNγ and IL-17 responses to WT DCs in the draining LN following transfer (Fig. 4A), demonstrating that DC-derived RELMα neither promotes nor regulates T-cell differentiation in a bacterial Th1/Th17 setting. Thus, impaired priming by Il4ra−/− DCs exposed to bacteria (Fig. 3A) is RELMα independent. The lack of a major role for RELMα in Th1/17 priming also fits with the observed inhibition of IL-4–induced RELMα expression by DCs following bacterial or TLR ligand exposure (Fig. S5).
Fig. 4.
DC expression of RELMα during Th2 priming regulates IFNγ and promotes IL-10 and IL-13 production. WT or Retnla−/− BMDCs were cultured overnight in medium alone (M), Pa (A), or SEA (B) and injected s.c. into WT mice. Next, 5–7d later, pLN were harvested, cells were restimulated for 72 h with Pa (A) or SEA (B), and cytokine secretion was assessed by ELISA. CD4+ cells sorted from KN2 IL-4 reporter mice, or IL-10eGFP−-CD4+ cells, were cultured for 4 d with WT or Retnla−/− BMDCs and anti-CD3 mAb with or without IL-4 and assessed for IL-4 protein production or IL-10 mRNA expression by flow cytometry (C) and cytokine secretion by ELISA (D). Data are representative of two (A and C) or four (B and D) experiments. Error bars indicate SEM of three to five mice (A and B) or four to five replicate wells (C and D) per group.
To directly assess the importance of DC RELMα secretion in a Th2 setting, we transferred Retnla−/− DCs pulsed with SEA into naive recipients. In repeated experiments, both WT and Retnla−/− SEA DCs stimulated similar levels of Ag-specific IL-4, but mice that received Retnla−/− DCs displayed significantly reduced levels of IL-10 and IL-13 compared with WT SEA DCs (Fig. 4B). Furthermore, Retnla−/− SEA DCs induced significantly greater amounts of IFNγ compared with WT SEA DCs (Fig. 4B). This suggests that DC production of RELMα is required for optimal Th2 cell priming by helminth-activated DCs, promoting some components of the Th2 response while regulating the level of the Th1 response.
To further dissect requirements for DC RELMα production in promoting optimal T-cell Th2 cytokine secretion, we examined the ability of WT or Retnla−/− DCs to induce CD4+ T-cell IL-4, IL-10, and IL-13 in vitro. In this assay, the addition of exogenous IL-4 to WT DCs cocultured with CD4+IL-10eGFP− T cells purified from IL-10eGFP reporter mice (29), or CD4+ T cells from KN2 mice in which IL-4–producing cells express huCD2 (30), resulted in induction of T-cell expression of eGFP or huCD2, and secretion of IL-10 and IL-13 (Fig. 4 C and D). In keeping with the lack of a significant impact of RELMα deficiency on IL-4 induction in vivo (Fig. 4B), Retnla−/− DCs capably provoked huCD2 expression on responding KN2 T cells in vitro (Fig. 4C). However, Retnla−/− DCs were incapable of promoting T-cell expression of IL-10 mRNA as detected by GFP expression (Fig. 4C). Furthermore, analysis of secreted cytokine by ELISA showed that cocultures of CD4+ T cells and WT DCs produced more IL-10 and IL-13 protein in the presence of IL-4 compared with cocultures with Retnla−/− DCs (Fig. 4D). Interestingly, T cells cultured with WT DCs secreted lower levels of IFNγ protein than their Retnla−/− counterparts, supporting observations following in vivo DC transfer of SEA activated DCs (Fig. 4B).
Mechanistically, the altered ability of Retnla−/− DCs to polarize SEA-specific cytokine responses was not due to defective Ag uptake, processing, or presentation by these cells. Similar to Il4ra−/− DCs, Retnla−/− DCs competently took up Ag (Fig. S6), and showed enhanced ability to induce proliferation of OTII TCR Tg T cells in vitro (Fig. S7), with either OVA peptide or protein. This increased proliferation is consistent with expectation, given the reduced IL-10 production evident following transfer of Retnla−/− DCs in vivo (Fig. 4B) or coculture of Retnla−/− DCs with T cells in vitro (Fig. 4 C and D).
These data strongly suggest that DC production of RELMα is an important process both for directing priming of Th2 responses and for control of Th1 responses, and provide mechanistic insight into the function of RELMα during the early stages of T-cell activation.
Discussion
A substantial body of literature documents the ability of IL-4 to generate aaMΦs in multiple settings. However, comparatively few studies have addressed how DCs respond to IL-4, whether they can express markers associated with aaMΦs (11–14), and what the functional relevance of this expression may be. The data that we present here reveal that murine DCs can respond to IL-4 both in vitro and in vivo in a manner similar to that previously described for aaMΦs, by significantly up-regulating RELMα, Ym1/2, and other aaMΦ markers, with the notable exception of Arginase-1. Furthermore, we have identified that DC expression of RELMα plays a critical role in their instruction of T cells to produce IL-10 and IL-13. This demonstrates a previously undescribed role for RELMα, and reveals that DC responsiveness to IL-4 is vital for priming optimal Th2 responses.
Exposure of DCs to IL-4 triggered expression of numerous markers associated with aaMΦ, but one notable difference was the lack of Arginase-1 up-regulation at both the mRNA and protein activity level. Arginase-1 competes with iNOS for l-arginine (6), and one consequence of increased Arginase-1 activity is l-arginine depletion from the local environment. In murine Leishmania major infection, increased arginase activity in parasite-infected MΦs depletes the skin of l-arginine, impairing proliferation of T cells in the lesion (31). Furthermore, MΦ-derived Arginase-1 is required to suppress T-cell proliferation during Th2 infection, where it limits pathology (8). The function of DCs is instead mainly to prime naive T cells in the early phase of immune response development. In this context, a high level of Arginase-1 expression by immunogenic DCs that could deplete l-arginine from the local environment might be undesirable.
In addition to RELMα and Ym1/2, we have found that IL-4–treated DCs significantly increased expression of Mrc1, Clec7a, and Ccl24, all of which have previously been associated with aaMΦs (6, 7). MR and Dectin-1 are both C-type lectin receptors that can bind carbohydrates and trigger distinct signaling pathways in DCs (32). MR has been linked mainly to internalization of Ags containing mannose motifs; however, in the absence of an additional stimulus (such as TLR ligation), engagement of the receptor is thought to be unable to mediate proinflammatory responses in DCs (32). In contrast, Dectin-1 recognition of β glucans, which are mainly found in fungal cell walls, initiates proinflammatory responses in DCs even in the absence of TLR ligation (33). Our finding that IL-4 increases DC expression of both these C-type lectin receptors suggests that they will have an enhanced ability to internalize and respond to a wide range of glycosylated pathogen motifs that could further modulate their activation and function. Increased DC expression of CCL24 could also influence this process, as this chemokine can enhance recruitment of CCR3-expressing cells such as eosinophils and basophils, as has been reported for CCL24-producing aaMΦs in the lung (34). These recruited granulocytes could, in turn, assist Th2 priming in certain situations through provision of cytokines such as IL-4.
As well as identifying that DCs can display hallmarks of alternative activation following IL-4 exposure in vitro and in vivo, we have revealed DC expression of alternative activation markers in the context of chronic helminth infection, a subject that has so far barely been addressed in the literature (11). There was some heterogeneity in the levels of specific markers expressed when comparing in vitro and in vivo settings, in particular with Ym1/2. This could reflect basic differences between BMDCs and their in vivo counterparts, or differential regulation of aspects of DC alternative activation in vivo, in line with the well-documented diversity of aaMΦs (6).
Our results also support and extend previously published work addressing how IL-4 might alter the IL-12/IL-10 balance (15, 19), but are unique in showing the impact of IL-4 on T-cell polarization by DCs in vivo. Our data highlight an important feedback loop in DC-mediated priming in vivo, where sources of IL-4/IL-13 will not only promote Th2 responses (4), but will also facilitate DC-mediated priming of Th1 responses through enhancement of IL-12p70 and inhibition of IL-10 production by DCs. Extending our understanding of the cross-talk between microbes, DCs, and their environment, we have demonstrated the ability of defined TLR ligands and heat-killed bacteria to inhibit the ability of IL-4 to prompt RELMα and Ym1/2 production by DCs. In light of our identification of a pro-Th2 role for DC-derived RELMα, inhibition of such by bacteria or their products may be important in some settings to allow optimal Th1/17 immunity to develop.
In peripheral environments, DCs migrate away from the site of Ag exposure toward draining LNs (35). In order for RELMα and Ym1/2 to play a relevant role in T-cell priming in the draining LNs, DC expression of such molecules would have to be prolonged, or responsive to subsequent interaction with T cells. We have shown that production of RELMα and Ym1/2 by DCs is not transient and requires neither the continued presence of IL-4 nor subsequent CD40 ligation. Furthermore, by adoptive transfer of Retnla−/− DCs in vivo, we have identified a previously unreported requirement for DC production of RELMα in the initiation of Th2 responses in the LN draining the site of DC introduction. A Th2-promoting role for RELMα contrasts with the down-regulatory role that has previously been described using global Retnla−/− mice, which identified RELMα as a negative regulator of ongoing Th2 inflammation against helminth Ag, with Retnla−/− mice displaying elevated Th2 responses and greater pathology (21, 22). These reports would suggest that the ultimate role for RELMα produced by multiple cellular sources in vivo (including eosinophils, epithelial cells, and MΦs) is to regulate chronic Th2 pathology by influencing T-cell differentiation and cellular recruitment while regulating wound repair. In the present study, we focused on the early events in Th2 priming by DCs in vitro and in vivo. By restricting RELMα deficiency to DCs alone, our results extend our fundamental understanding of the cellular, temporal, and spatial diversity of RELMα function, demonstrating an alternative function of DC expression of this molecule early in immune response development. Our identification of a critical role for DC-derived RELMα in promotion of T-cell production of IL-10 reveals a mechanism that may, in part, explain the dysregulated immune pathology previously observed in Th2 infection models using global Retnla−/− mice (21, 22).
It is clear from our results that RELMα production does not solely account for Th2 induction by DCs, but helps promote specific facets of this response (IL-10 and IL-13). Because we have shown that IL-4 stimulates production or expression of a wide range of alternative activation-related molecules in DCs, and that Il4ra−/− and Retnla−/− DCs do not display identical functional ability, it is likely that a combination of IL-4–induced products will work together and in balance to ultimately dictate the character of the resultant Th2 response. For example, as Ym1/2 has previously been suggested to promote some Th2 cytokines, at least in vitro, its production by DCs responding to IL-4 may act in concert with RELMα and other alternative activation products to generate optimal Th2 immunity.
In summary, we have shown that the canonical Th2 cytokine IL-4 can have a profound impact on DC activation and function, triggering both in vivo and in vitro expression of a range of molecules ordinarily associated with aaMΦs, altering responsiveness to challenge with bacteria and TLR ligands, and dramatically influencing T-cell response induction and polarization. Although the cumulative impact of IL-4 on DC activation and function is complex, in the context of pathogenic stimulation, the over-riding effect of IL-4 appears to be to enable IFNγ induction through enhanced DC IL-12p70 production. Furthermore, DC production of the IL-4 induced protein, RELMα, is important in priming of T-cell IL-10 and IL-13. Together these findings indicate that IL-4 has a more influential and diverse role in directly regulating DC-mediated T-cell priming than has been previously appreciated.
Materials and Methods
Mice and IL-4c Injections.
C57BL/6, BALB/c, Retnla−/−, IL-10eGFPxDOG, KN2 (C57BL/6), Il4ra−/−, and OTII mice (20, 23, 25, 29, 30) were maintained under specific pathogen-free conditions at the University of Edinburgh. Experiments were conducted under a Project License granted by the Home Office (United Kingdom) in accordance with local guidelines. IL-4/anti-IL-4 mAb complexes (IL-4c) were prepared as described previously (23). Mice were injected i.p. with 50 μL PBS or 0.625–10μg IL-4 complexed to 11B11, and peritoneal exudate cells (PEC) and spleens harvested 4 d later.
Cell Culture and DC Transfer.
BM-derived DCs were generated with GM-CSF as previously described (36). Following 10 d of culture, BMDCs were harvested and replated at 2 × 106/mL for 18 h in the presence or absence of rIL-4 (20 ng/mL; Peprotech). In coculture experiments, CD4+GFP− T cells were sorted from IL-10eGFPxDOG or KN2B6xB6 mice using BD FACs Aria-II and cultured with WT or Retnla−/− DCs. For DC transfer, WT, Il4ra−/−, or Retnla−/− BMDCs were cultured as above, with SEA, Pa, or medium alone. BMDCs were injected s.c. into recipient WT mice (2.5 × 105 per foot) and 5–7d later the draining popliteal LNs and restimulated as previously described (24, 36). Supernatants were harvested after 72 h, and cytokine production was assessed by ELISA. Further details are provided in SI Materials and Methods.
Flow Cytometry and ELISA.
Details of mAb used are given in SI Materials and Methods. Samples were acquired using FACS LSR II or FACS Canto II using BD FACSDiva software and analyzed with FlowJo v.9 software (Tree Star). ELISAs were performed on culture supernatants using paired mAb, and recombinant cytokine standards, or DuoSets (eBioscience, BD Pharmingen, R&D Systems, and Peprotech). Arginase assay details are provided in SI Materials and Methods.
RNA Isolation and Quantitative PCR.
RNA was recovered from cells using TRIzol (Invitrogen), cDNA was generated using SuperScript-III (Invitrogen). Relative quantification of the gene of interest was performed by quantitative PCR. Primer details are given in SI Materials and Methods.
Statistical Analysis.
Statistical analyses were carried out using GraphPad Prism 5. The Student's t test or one-way analysis of variance was used to determine significant differences between sample groups (in figures, *P < 0.05 **P < 0.01 ***P < 0.001 ****P < 0.0001).
Supplementary Material
Acknowledgments
We thank Rob Thompson for preparation of bone marrow samples, Martin Waterfall for cell sorting and assistance with flow cytometry, and David Gray and Markus Mohrs for provision of IL-10eGFP and KN2 mice. We also thank Rachel Lundie, Alex Phythian-Adams, Lauren Webb, Dominik Rückerl, and Elia Tait for support with experiments and critique of the manuscript. Schistosomes used to generate soluble egg Ag for this research were supplied by the National Institute of Allergy and Infectious Diseases (NIAID) Schistosomiasis Resource Center at the Biomedical Research Institute (Rockville, MD) through Contract N01-AI-30026. This work was supported by Medical Research Council UK G0701437 (to A.S.M.) and G0600818 (to J.E.A.) and by the Wellcome Trust (L.H.J.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1121231109/-/DCSupplemental.
References
- 1.Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations (*) Annu Rev Immunol. 2010;28:445–489. doi: 10.1146/annurev-immunol-030409-101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Langrish CL, et al. IL-12 and IL-23: Master regulators of innate and adaptive immunity. Immunol Rev. 2004;202:96–105. doi: 10.1111/j.0105-2896.2004.00214.x. [DOI] [PubMed] [Google Scholar]
- 3.Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 4.Paul WE, Zhu J. How are T(H)2-type immune responses initiated and amplified? Nat Rev Immunol. 2010;10:225–235. doi: 10.1038/nri2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.MacDonald AS, Maizels RM. Alarming dendritic cells for Th2 induction. J Exp Med. 2008;205:13–17. doi: 10.1084/jem.20072665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 7.Mantovani A, et al. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 8.Pesce JT, et al. Arginase-1-expressing macrophages suppress Th2 cytokine-driven inflammation and fibrosis. PLoS Pathog. 2009;5:e1000371. doi: 10.1371/journal.ppat.1000371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Allen JE, Wynn TA. Evolution of Th2 immunity: A rapid repair response to tissue destructive pathogens. PLoS Pathog. 2011;7:e1002003. doi: 10.1371/journal.ppat.1002003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cook PC, et al. Multiple helminth infection of the skin causes lymphocyte hypo-responsiveness mediated by Th2 conditioning of dermal myeloid cells. PLoS Pathog. 2011;7:e1001323. doi: 10.1371/journal.ppat.1001323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nair MG, et al. Chitinase and Fizz family members are a generalized feature of nematode infection with selective upregulation of Ym1 and Fizz1 by antigen-presenting cells. Infect Immun. 2005;73:385–394. doi: 10.1128/IAI.73.1.385-394.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Munder M, et al. Th1/Th2-regulated expression of arginase isoforms in murine macrophages and dendritic cells. J Immunol. 1999;163:3771–3777. [PubMed] [Google Scholar]
- 13.Arora M, et al. Simvastatin promotes Th2-type responses through the induction of the chitinase family member Ym1 in dendritic cells. Proc Natl Acad Sci USA. 2006;103:7777–7782. doi: 10.1073/pnas.0508492103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cai Y, Kumar RK, Zhou J, Foster PS, Webb DC. Ym1/2 promotes Th2 cytokine expression by inhibiting 12/15(S)-lipoxygenase: Identification of a novel pathway for regulating allergic inflammation. J Immunol. 2009;182:5393–5399. doi: 10.4049/jimmunol.0803874. [DOI] [PubMed] [Google Scholar]
- 15.Hochrein H, et al. Interleukin (IL)-4 is a major regulatory cytokine governing bioactive IL-12 production by mouse and human dendritic cells. J Exp Med. 2000;192:823–833. doi: 10.1084/jem.192.6.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guenova E, et al. IL-4-mediated fine tuning of IL-12p70 production by human DC. Eur J Immunol. 2008;38:3138–3149. doi: 10.1002/eji.200838463. [DOI] [PubMed] [Google Scholar]
- 17.Yao Y, Li W, Kaplan MH, Chang CH. Interleukin (IL)-4 inhibits IL-10 to promote IL-12 production by dendritic cells. J Exp Med. 2005;201:1899–1903. doi: 10.1084/jem.20050324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lutz MB, et al. Differential functions of IL-4 receptor types I and II for dendritic cell maturation and IL-12 production and their dependency on GM-CSF. J Immunol. 2002;169:3574–3580. doi: 10.4049/jimmunol.169.7.3574. [DOI] [PubMed] [Google Scholar]
- 19.Biedermann T, et al. IL-4 instructs TH1 responses and resistance to Leishmania major in susceptible BALB/c mice. Nat Immunol. 2001;2:1054–1060. doi: 10.1038/ni725. [DOI] [PubMed] [Google Scholar]
- 20.Munitz A, et al. Resistin-like molecule alpha enhances myeloid cell activation and promotes colitis. J Allergy Clin Immunol. 2008;122:1200–1207. doi: 10.1016/j.jaci.2008.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nair MG, et al. Alternatively activated macrophage-derived RELM-alpha is a negative regulator of type 2 inflammation in the lung. J Exp Med. 2009;206:937–952. doi: 10.1084/jem.20082048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pesce JT, et al. Retnla (relmalpha/fizz1) suppresses helminth-induced Th2-type immunity. PLoS Pathog. 2009;5:e1000393. doi: 10.1371/journal.ppat.1000393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jenkins SJ, et al. Local macrophage proliferation, rather than recruitment from the blood, is a signature of TH2 inflammation. Science. 2011;332:1284–1288. doi: 10.1126/science.1204351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perona-Wright G, et al. A pivotal role for CD40-mediated IL-6 production by dendritic cells during IL-17 induction in vivo. J Immunol. 2009;182:2808–2815. doi: 10.4049/jimmunol.0803553. [DOI] [PubMed] [Google Scholar]
- 25.Phythian-Adams AT, et al. CD11c depletion severely disrupts Th2 induction and development in vivo. J Exp Med. 2010;207:2089–2096. doi: 10.1084/jem.20100734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shainheit MG, et al. Dendritic cell IL-23 and IL-1 production in response to schistosome eggs induces Th17 cells in a mouse strain prone to severe immunopathology. J Immunol. 2008;181:8559–8567. doi: 10.4049/jimmunol.181.12.8559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elgueta R, et al. Molecular mechanism and function of CD40/CD40L engagement in the immune system. Immunol Rev. 2009;229:152–172. doi: 10.1111/j.1600-065X.2009.00782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.MacDonald AS, Straw AD, Dalton NM, Pearce EJ. Cutting edge: Th2 response induction by dendritic cells: A role for CD40. J Immunol. 2002;168:537–540. doi: 10.4049/jimmunol.168.2.537. [DOI] [PubMed] [Google Scholar]
- 29.Kamanaka M, et al. Expression of interleukin-10 in intestinal lymphocytes detected by an interleukin-10 reporter knockin tiger mouse. Immunity. 2006;25:941–952. doi: 10.1016/j.immuni.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 30.Mohrs K, Wakil AE, Killeen N, Locksley RM, Mohrs M. A two-step process for cytokine production revealed by IL-4 dual-reporter mice. Immunity. 2005;23:419–429. doi: 10.1016/j.immuni.2005.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Modolell M, et al. Local suppression of T cell responses by arginase-induced L-arginine depletion in nonhealing leishmaniasis. PLoS Negl Trop Dis. 2009;3:e480. doi: 10.1371/journal.pntd.0000480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Geijtenbeek TB, Gringhuis SI. Signalling through C-type lectin receptors: Shaping immune responses. Nat Rev Immunol. 2009;9:465–479. doi: 10.1038/nri2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kerrigan AM, Brown GD. Syk-coupled C-type lectin receptors that mediate cellular activation via single tyrosine based activation motifs. Immunol Rev. 2010;234:335–352. doi: 10.1111/j.0105-2896.2009.00882.x. [DOI] [PubMed] [Google Scholar]
- 34.Kurowska-Stolarska M, et al. IL-33 amplifies the polarization of alternatively activated macrophages that contribute to airway inflammation. J Immunol. 2009;183:6469–6477. doi: 10.4049/jimmunol.0901575. [DOI] [PubMed] [Google Scholar]
- 35.Banchereau J, et al. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 36.MacDonald AS, Straw AD, Bauman B, Pearce EJ. CD8- dendritic cell activation status plays an integral role in influencing Th2 response development. J Immunol. 2001;167:1982–1988. doi: 10.4049/jimmunol.167.4.1982. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.