Abstract
Articular cartilage repair remains a significant and growing clinical challenge with the aging population. The native extracellular matrix (ECM) of articular cartilage is a 3D structure composed of proteinaceous fibers and a hydrogel ground substance that together provide the physical and biological cues to instruct cell behavior. Here we present fibrous scaffolds composed of poly(vinyl alcohol) and the biological cue chondroitin sulfate with fiber dimensions on the nanoscale for application to articular cartilage repair. The unique, low-density nature of the described nanofiber scaffolds allows for immediate cell infiltration for optimal tissue repair. The capacity for the scaffolds to facilitate cartilage-like tissue formation was evaluated in vitro. Compared with pellet cultures, the nanofiber scaffolds enhance chondrogenic differentiation of mesenchymal stems cells as indicated by increased ECM production and cartilage specific gene expression while also permitting cell proliferation. When implanted into rat osteochondral defects, acellular nanofiber scaffolds supported enhanced chondrogenesis marked by proteoglycan production minimally apparent in defects left empty. Furthermore, inclusion of chondroitin sulfate into the fibers enhanced cartilage-specific type II collagen synthesis in vitro and in vivo. By mimicking physical and biological cues of native ECM, the nanofiber scaffolds enhanced cartilaginous tissue formation, suggesting their potential utility for articular cartilage repair.
Keywords: biomaterials, electrospin, tissue engineering, regenerative medicine
Cartilage damage and associated clinical joint dysfunction affects a growing number of individuals. Articular cartilage has a limited ability to self-repair after traumatic injury, excessive wear, or age-related degeneration as a result of the tissue avascularity along with the low mitotic activity of the resident cells, chondrocytes (1). Surgical strategies to repair cartilage defects have been used to restore joint function and eliminate associated pain, including the microfracture technique and autologous chondrocyte transplantation (2). Although surgical strategies reduce patient pain and increase joint mobility, the repair tissue is a mechanically inferior fibrocartilage with short clinical efficacy. Additional surgery is often required to regain complete function, resulting in the progression to partial or total knee replacement. Therefore, there is a tremendous need for new regenerative medicine approaches to augment the repair process to facilitate adequate tissue regeneration and longevity.
Polymer-based scaffolds have been used to augment tissue regeneration by recapitulating a variety of physical cues of native tissue (3). To recapitulate the fibrous collagen network of native ECM, numerous methods of producing fibrous scaffolds have been developed, including fiber self-assembly, phase separation, and electrospinning (4). Electrospun fiber scaffolds have been investigated for musculoskeletal, neural, cardiac, and other regenerative medicine applications (5). Recent work has demonstrated the potential of electrospun polyester-based fibers for cartilage tissue engineering (6, 7). However, the standard electrospinning procedure results in dense fibrous mats that are collected over time, giving rise to extremely compact fibrous scaffolds that are not conducive to cell infiltration (8, 9). These materials present fibrous topographical cues more suitable for tissues such as skin, eye, blood vessels, and nerve guidance conduits, but lack a 3D presentation to cells analogous to bulk tissue ECM such as cartilage that allows for cell infiltration. Therefore, a significant need exists for a low-density, nanofibrous scaffold that can efficiently entrap cells through the entire scaffold and provide the necessary cues to stimulate new tissue development (10).
In addition to the physical cues of native matrix, cells are exposed to an array of biological cues throughout the ECM that direct cellular behavior. Cells are constantly interacting with the surrounding ECM, which gives rise to a dynamic transfer of information between the extracellular and intracellular space. To better recapitulate the ECM environment for cartilage tissue engineering, researchers have introduced several biological signals, including chondroitin sulfate (CS), hyaluronic acid, and collagen, into tissue-engineered scaffolds to encourage tissue specificity (11–14). CS, hyaluronic acid, and type II collagen have been shown to promote or enhance chondrogenesis of mesenchymal stem cells (MSCs) in hydrogel-based culture systems.
Here, we used a low-density, 3D nanofiber network composed of poly(vinyl alcohol) (PVA)-methacrylate (PVA-MA) and a composite of PVA-MA/CS-methacrylate (CS-MA) that supports cell infiltration at the time of cell seeding, denoted as PVA fibers and CS fibers, respectively. The nanofibers were evaluated based on their morphology, diameter, and CS content. In vitro studies were performed to characterize chondrogenesis of bone marrow-derived MSCs. Furthermore, we used a rat osteochondral defect model to investigate the effect of acellular fiber scaffolds on articular tissue repair in a physiologically relevant environment. In this work, we demonstrated that the nanofiber scaffolds can support and enhance in vitro chondrogenesis, along with support early cell infiltration and augment tissue repair in an in vivo osteochondral defect model. The inclusion of the CS biological signal native to cartilage ECM further directs tissue specificity in vitro and in vivo. Collectively, these results present a nanofibrous scaffold for augmentation of surgical strategies to promote endogenous tissue repair processes for articular cartilage repair via a nanofiber network.
Results
Fabrication and Characterization of Nanofibers.
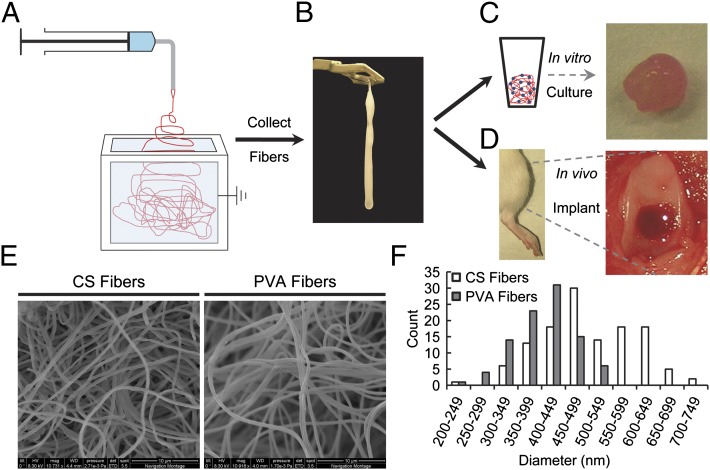
Low-density, 3D fiber scaffolds were synthesized to create networks that resembled the native physical structure of the ECM (Fig. 1). The base polymer network was created from PVA-MA, a bioinert polymer. Polymer solutions containing PVA-MA were electrospun into an ethanol bath to achieve low-density, fibrous scaffolds. Spinning into the aqueous solution eliminated fiber compaction and fusion that occurs in standard electrospinning when fibers are collected on a solid substrate. The scaffolds did not have a defined overall morphology (Fig. 1B), but could be manipulated and conformed to a mold or tissue defect space. After formation, the fibers were subsequently rendered insoluble by UV light crosslinking of the methacrylate groups. Maintenance of the fiber morphology was confirmed after crosslinking via SEM imaging (Fig. 1E). The PVA fibers had a diameter of 500 ± 100 nm (Fig. 1F).
Fig. 1.
Schematic of experimental design. (A) Electrospinning was carried out by using a system in which the nanofibers were collected into an ethanol bath and (B) removed at predefined time intervals. (C) In vitro characterization of chondrogenesis of bone marrow derived-MSCs was performed over 42 d. (D) Nanofibers were implanted into osteochondral defects in the trochlear groove of rat hind limbs and evaluated after 6 wk. (E) SEM imaging of water-insoluble CS and PVA fibers depicted the fibrous morphology. (F) Size distribution of CS and PVA fibers.
A biological cue in the form of CS was incorporated into the PVA nanofiber networks. CS-MA was combined with the PVA-MA and processed in a similar manner as the PVA fibers. The CS fibers had a diameter of 410 ± 60 nm (Fig. 1F). The precrosslinked concentration of CS in the fibers was 34 ± 1% (wt/wt; 1.09 ± 0.07 mg CS per scaffold). After crosslinking and extensive washing to remove unreacted components, the resulting CS concentration was 43 ± 8% (0.57 ± 0.08 mg CS per scaffold).
In Vitro Chondrogenesis in Nanofiber Network.
Next, we sought to quantitatively evaluate the ability of the low-density nanofiber scaffolds to support and promote chondrogenesis of goat MSCs as well as compare the two nanofiber formulations. Goat MSCs were used for these experiments, as they have been extensively used for evaluating tissue-engineering strategies for cartilage and bone (12, 15–18). Cells were distributed throughout the fibrous scaffolds, and the constructs were cultured under chondrogenic induction conditions for as long as 42 d. After 42 d of culture, the fiber scaffolds appeared as discrete cartilage-like constructs with a shiny appearance similar to hyaline cartilage (see Fig. 3 A and B). Mechanically, the constructs transitioned from a loose fibrous material as depicted in Fig. 1B to an elastic-like, discrete mass of tissue.
Fig. 3.
Representative gross images and histological evaluation of scaffolds after 42 d of culture. Gross image of CS fibers (A) and PVA fibers (B) and pellets (C). (D–I) Histological staining for proteoglycans by using safranin-O and immunohistochemical staining for type II collagen (J–L) and type I collagen (M–O) of the CS fibers (D, G, J, and M), PVA fibers (E, H, K, and N), and PVA pellet culture (F, I, L, and O). (G) Inset: Background staining of CS fibers in region void of cells. (Scale bars: A–F, 1 mm; G–I, 50 μm; J–O, 200 μm.)
To understand the impact of the nanofiber network physical structure and incorporation of a biopolymer on tissue formation, new ECM production was investigated. First, cell proliferation was monitored by quantifying the DNA content of the scaffolds (Table S1) over the culture period at day 21 and day 42. Both fiber groups facilitated cell proliferation as indicated by the increased DNA content at day 42 compared with day 21 (Fig. 2A). Specifically, DNA increased by 2.6 ± 0.7-fold and 2.03 ± 0.9-fold in the CS fibers and PVA fibers, respectively. In contrast, the pellet culture exhibited cell death as indicated by the 0.66 ± 0.1-fold decrease in DNA content (Fig. 2A).
Fig. 2.
Biochemical and gene expression analysis of in vitro chondrogenesis. (A) Total DNA content as quantified by Hoechst 33342 dye (n = 5; **P < 0.01 and ***P < 0.001). Statistical evaluation was not performed between groups as the two nanofiber scaffolds and pellets had different initial cell numbers. (B) Total collagen accumulation relative to DNA content of the scaffolds quantified via hydroxyproline content. (C) sGAG accumulation relative to DNA content of the scaffolds quantified by using dimethylmethylene blue dye (n = 5; *P < 0.05 and ***P < 0.001). Real-time PCR analysis of markers for cartilage (D–F), bone formation (G), and type I collagen (H), along with type II collagen/type I collagen ratio (I), a measure of cartilage quality. Data are presented as average ± SD (n = 3); *P < 0.05, D–H, PVA and CS fiber scaffold vs. pellet culture at specified time point; I, CS scaffold vs. pellet culture, P = 0.084 for CS scaffold vs. PVA scaffold.
To determine whether the nanofiber scaffolds enhanced chondrogenesis, sulfated glycosaminoglycan (sGAG) and total collagen content of each group was examined (Table S1). At day 21, cells within the CS fibers produced significantly more total collagen compared with cells within the PVA fibers or pellets. However, by day 42, cells in all three groups produced similar amounts of total collagen (Fig. 2B). As a result of the incorporation of CS into the nanofibers and the fact that the cells naturally degrade this molecule, accurate quantification of sGAG production by using dimethylmethylene blue assay was not possible with the CS fiber group. Accumulation of sGAG was significantly higher within the PVA fibers compared with pellet (Fig. 2C). Histological staining for proteoglycans by using safranin-O demonstrated that cells within both fiber scaffolds had significant pericellular sGAG accumulation and lacunae formation, a unique characteristic of hyaline cartilage (Fig. 3 D–I).
To further investigate the effects of CS in the fibers, we characterized the type of collagen produced by cells via immunohistochemical staining for type II collagen, specific to hyaline cartilage, and type I collagen, a ubiquitously synthesized collagen in numerous tissue types and associated with fibrocartilage. CS fibers facilitated an increase in type II collagen deposition compared with PVA fibers (Fig. 3 J and K). However, both fiber groups contained similar amounts of type I collagen (Fig. 3 M and N). Pellet cultures stained positive for both types of collagen (Fig. 3 L and O). The pellet stained more intensely for type I collagen compared with type II collagen, with the fibrous type I collagen tissue located at the periphery.
We next investigated changes in tissue-specific gene expression to evaluate lineage specificity of the differentiating stem cells in the various scaffolds. Gene expression was evaluated through the first 21 d of in vitro culture. The cartilage specific markers aggrecan, type II collagen, and Sox9 were up-regulated in both fiber scaffolds compared with pellet culture after 3 d of culture (Fig. 2 D–F). Runx2, an early bone transcription factor, and type I collagen had similar expression levels across all three groups after 3 d (Fig. 2 G and H). An indicator of the quality of hyaline-like cartilage being produced is the ratio of type II collagen to type I collagen. The presence of CS in the fibers resulted in a higher type II/type I collagen gene expression ratio by day 21 compared with the other two groups (Fig. 2I). This increase in the ratio of type II/type I collagen gene expression along with the increase in type II collagen immunohistochemical staining is indicative of enhanced chondrogenesis.
Tissue Repair in Rat Osteochondral Defects.
To determine the in vivo tissue forming capabilities of the nanofiber scaffolds under physiological conditions, cell-free scaffolds were implanted into rat osteochondral defects. Early assessment of the defects 3 d after implantation confirmed that the scaffolds were maintained in the defects. Opaque areas were present in defects containing nanofibrous scaffolds, whereas the empty defects appeared clear (Fig. 4 A–C). Histologically, fiber implants had higher cellularity throughout the material and defect space, presumably because of increased cell retention or scaffold-facilitated cell migration (Fig. 4 D–F).
Fig. 4.
Evaluation of rat osteochondral defects 3 d after implantation. Gross observation of defects containing CS fibers (A), PVA fibers (B), or left empty (C). Safranin-O staining of histological sections of defects containing CS fibers (D and G), PVA fibers (E and H), or left empty (F and I). Safranin-O–positive nanofibers can be seen in the CS fiber containing defects (D and G) along with increased cellularity in nanofiber containing defects (G and H) compared with (I) empty defects. (Scale bars: A–F, 500 μm; G–I, 50 μm.)
To examine the ability of the nanofiber scaffolds to increase tissue repair, cartilage repair within the defects was examined 6 wk after implantation. Gross observation of the fiber-containing defects revealed a less homogenous appearance compared with the empty defect controls (Fig. 5 A–C). All defects had clearly discernible boundaries between the new repair tissue and the surrounding cartilage. Although clear differences between the mature (adjacent) and immature (repair) cartilage are apparent histologically, no gaps between the two can be observed, indicating the early development of tissue integration (Fig. 5 E–G). Histological staining for proteoglycans indicated that both fiber groups elicited proteoglycan-containing repair tissue (Fig. 5 E and F), whereas the empty defect group exhibited minimum proteoglycan deposition (Fig. 5G and see Fig. S2C). The O’Driscoll histological grading scale was used to evaluate the repair tissue quality (Table S2). This semiquantitative grading indicated that both nanofiber-containing groups produced repair tissue with incompletely differentiated mesenchyme containing areas of both hyaline and fibrocartilage and slight to moderate safranin-O staining that was significantly greater than the empty defects. To further characterize the repair tissue, we quantified the percent of proteoglycan positive tissue within the cartilage region of the repair tissue by using histomorphometric analysis (Fig. 5J). No differences in the tissue area stained positive for proteoglycans were found between the two fiber groups (Fig. 5I). However, nanofiber-containing defects had statistically more proteoglycan deposition than empty defects (Fig. 5I), although significantly less compared with native articular cartilage (Fig. 5 H and I). We further investigated the quality of cartilage tissue repair by evaluating type I collagen (Fig. S1 A–C) and type II collagen (Fig. 5 K–M) deposition. Type II collagen is an indicator of hyaline cartilage repair tissue, whereas type I collagen indicates inferior fibrocartilage repair. All defects contained type I and type II collagen. However, CS fibers induced statistically higher type II collagen production compared with the PVA fibers and empty defects (Fig. 5L), whereas all groups contained similar amounts of type I collagen (Fig. S1E). Native articular cartilage contained significantly more type II collagen (Fig. 5 N and L) and significantly less type I collagen (Fig. S1 D and E) compared with all the treated defect areas. Although the nanofiber implants resulted in significant improvements in tissue architecture compared with empty defects, the repair tissue did not replicate that of native articular cartilage at the relatively early time point studied.
Fig. 5.
Cartilage repair 6 wk after implantation. Gross images of defects containing CS fibers (A), PVA fibers (B), and left empty (C). Gross image of healthy cartilage (D). Safranin-O staining of the CS fiber implants (E) and PVA fiber implants (F) showed significant proteoglycan deposition compared with empty defects (G). All defects had significantly less proteoglycan deposition than native cartilage (H). (I) Quantitative analysis of proteoglycan positive area of the regenerating tissue within the cartilage region of the defect. (J) Inlaid image depicting the region chosen for quantification. (K–N) Immunohistochemical staining for type II collagen and (O) quantitative analysis of type II collagen-positive area of the regenerating cartilage region of the defects (n = 6 or 7; *P < 0.05 and ***P < 0.001). (Scale bars: 500 μm.)
Numerous other histological observations supported the conclusion that fiber-containing defects augmented endogenous articular cartilage regeneration. These structural findings in the nanofiber-treated defects include columnar cell orientation radiating from cell-rich cavities (Fig. S2 A–C) with subchondral bone containing numerous pockets of cellular cavities (Fig. S2 D and E). Additionally, the early establishment of the multilayer architecture of articular cartilage (Fig. S2 G and H) can be observed, including a gradient of proteoglycan deposition highest near the subchondral bone and cells oriented perpendicular and parallel to the cartilage surface in the deep and superficial zone, respectively.
Discussion
Therapies to regenerate articular cartilage after injury significantly lack in the quality of repaired tissue. Routine surgical techniques to repair cartilage often result in significant fibrocartilage tissue formation marked with substantial type I collagen synthesis. To date, there has been minimal progress in the way of augmenting surgical strategies to promote hyaline cartilage-like repair tissue. In this study, we used tissue-engineering concepts and ECM mimicry to produce scaffolds composed of physical and biological cues to enhance the quality of surgically induced endogenous articular cartilage repair.
Recent approaches to mimic the fibrous protein structure of ECM have used electrospinning to obtain polymer-based fiber scaffolds. Li et al. showed that nanofibers composed of polycaprolactone support chondrogenesis of human MSCs (6), and that maintenance of the chondrocytic phenotype is enhanced on nanofibers that have dimensions comparable to native ECM compared with microfibers (7, 19). However, cell infiltration and nutrient diffusion are severely limited in standard electrospun scaffolds. Methods have been developed to increase cell infiltration into electrospun fiber scaffolds collected onto solid substrates, however, with clear limitations in the effectiveness of immediate cell infiltration (8, 9). Engineering a low-density, nanofiber network was critical in the present study so the scaffold could support homogenous cell distribution in vitro and immediate cell infiltration in vivo, a significant advantage over previous electrospun scaffolds. Additionally, these nanofibrous scaffolds have fiber dimensions similar to that of native ECM (20), with nonrigid fiber spacing that supports the ability of cells to manipulate this microenvironment.
Toward the use of a nanofibrous scaffold that mimics native ECM and enables cartilage repair, we used the base PVA nanofibers as a material to present a biological cue in the form of CS. Previously, Varghese et al. reported enhanced chondrogenesis when CS was covalently incorporated into poly(ethelene glycol)-diacrylate hydrogels (12). Nguyen et al. found that stem cell differentiation could be spatially regulated based on the inclusion of various glycosaminoglycans into poly(ethelene glycol)-diacrylate hydrogels (21). During embryogenesis, a variety of CS sulfation patterns are distributed in specific spatiotemporal patterning (22–24). Additionally, aggrecan, a proteoglycan with a high CS-containing domain, is found in abundance in mature articular cartilage. Finally, another study found that treating chondrogenic cells with chondroitinase ABC to remove CS from versican before aggrecan expression reduced the chondrogenic potential of the cells, possibly through altering cell-matrix interactions (25), evidence that CS presentation is important for chondrogenesis.
Physical and biological cues likely played a role in supporting cartilage development. PVA fibers enhance proteoglycan accumulation based on sGAG quantification. As PVA is bioinert and essentially a blank slate for cells, the enhanced sGAG production is likely caused by the low-density, fibrous architecture of the scaffolds. Additionally, the nonadhesive nature of PVA may reduce fibroblast invasion in vivo giving rise to a more hyaline-like repair tissue. The presence of CS in the fibers had a positive impact on increasing type II collagen synthesis. This finding was supported by an increase in the type II/type I collagen gene expression ratio, similar to previous results with CS hydrogels (12, 21).
Although cell-based in vitro characterization of materials is necessary to assess the biological response before in vivo testing, it does not accurately reflect the complex biological and mechanical environment of the joint. Clinical translation of cell-based therapies is challenging because of issues associated with consistent cell manufacturing, high cost, and complex delivery to the patient. Therefore, we evaluated acellular nanofibrous scaffolds in rat osteochondral defects for their ability to facilitate endogenous articular cartilage repair. After 3 d of implantation, the nanofiber scaffolds contained a large number of cells entrapped throughout the scaffold compared with empty defects. Cells trapped in the nanofiber matrix may perform a number functions, including release of trophic factors that influence tissue regeneration (26), stimulation of stem cells to migrate to the defect, and tissue production. Indeed, homing of MSCs to the site of an injury has been shown in multiple disease models (27–29).
The nanofiber scaffolds had an impact on bone remodeling, potentially contributing to altered cartilage repair. Nanofiber implanted defects did not have dense subchondral bone surrounding the cartilaginous tissue compared with the empty defects. In fact, trabecular bone in nanofiber implanted defects contained numerous cell-rich spaces resembling bone marrow cavities that were immediately adjacent to the proteoglycan-containing areas. These cell-rich areas may play a role in providing the cells and nutrients for tissue repair. Hoemann et al. found that hyaline-like repair tissue was associated with porous subchondral bone in a rabbit microfracture model implanted with chitosan/glycerol phosphate implants (30).
Hyaline cartilage has unique mechanical properties that are relevant to its physiological function of withstanding joint loading and providing low-friction movement. The significant compressive strength of articular cartilage results from highly charged proteoglycans trapped within the collagen fiber matrix forming a highly hydrated fiber-reinforced composite (1). The repair tissue after surgical intervention is predominantly composed of fibrocartilage, with limited proteoglycan synthesis, which generally results in mechanically inferior tissue compared with native tissue (31). In the present study, the nanofiber implant groups produced repair tissue with portions of proteoglycan-positive tissue, beginning to resemble hyaline cartilage, which would likely give rise to tissue with higher compressive stiffness compared with the fibrocartilage in the empty defects. Similar to the in vitro studies, the presence of CS increased the type II collagen deposition critical for an established chondrocytic phenotype. Bone overgrowth into the cartilage region of the repair tissue is observed in all the defect groups. Rehabilitation, including splinting and postsplinting motion control, although not possible in many animal models, may reduce bone overgrowth resulting from abnormal mechanical loading of the joint during the initial stages of repair. Further maturation of the repair tissue is likely to occur over longer implantation times, which would enhance the delineation of the cartilage and bone interface. Two recent publications that used scaffolds combined with biological materials implanted into osteochondral defects showed mixed hyaline and fibrous repair cartilage below the osteochondral junction at comparable time points to those in the present study (32, 33). However, both research groups evaluated the repair tissue at 12 wk, which showed segregation of the cartilage and bone layers, whereby the cartilage layer was similar in thickness to, and better resembled, the surrounding hyaline cartilage.
In summary, we demonstrated the ability of low-density, nanofiber scaffolds to mediate cartilage repair in an articular joint environment. These nanofiber scaffolds serve to enhance the endogenous repair process without exogenous cells. Furthermore, this study indicates the importance of designing tissue engineered scaffolds that mimic the physical and biological components of ECM to produce optimal tissue repair in vivo.
Materials and Methods
Detailed methods on polymer methacrylation, biochemical analysis, real-time gene expression list of real-time primers (Table S3), histology and immunohistochemistry, histological grading, histomorphometry, and SEM imaging are provided in SI Materials and Methods.
Fiber Formation and Preparation.
Polymer solutions of 9.1% (wt/wt) PVA-MA in ultrapure water or 50 mg CS-MA dissolved in 1 mL of the PVA-MA solution were loaded into a syringed fitted with a 20-gauge 90° bent needle. A voltage of 13 to 17 kV was applied to the needle. The solution flow rate was 0.62 mL/h or 0.41 mL/h for the PVA-MA or CS-MA/PVA-MA solutions, respectively. The resulting fibers were collected into a grounded, 100% (vol/vol) ethanol bath and removed every 4 min. To crosslink the fibers, 50 μL of 10% (wt/vol) Irgacure 2959 (BASF Corporation) in 100% ethanol was mixed with the collected fibers, followed by UV exposure for 10 min. This crosslinked the fiber stands without any noticeable differences in the gross properties of the nanofiber network. Fibers were washed once with 70% (vol/vol) ethanol followed by five washes with PBS solution under sterile conditions. For cell culturing, the final wash was performed by using DMEM and the volume was brought to 40 μL.
Chondrogenic Differentiation of Goat MSCs.
Goat bone marrow-derived MSCs were isolated following a previously published protocol (18). After four passages, MSCs were trypsinized, centrifuged, and resuspended in expansion medium [high-glucose DMEM containing 10% defined FBS (HyClone), 100 U/mL penicillin, 100 μg/mL streptomycin, and 2 mM L-glutamine] at a concentration of 200 million cells per milliliter. Ten microliters of the cell suspension was added to the nanofibers, thoroughly mixed, and incubated for 20 min, followed by the addition of 100 μL of expansion medium. After 2 h, the cell/nanofiber matrices were moved to 24-well plates precoated with agarose and maintained in chondrogenic induction medium composed of high-glucose DMEM, supplemented with 100 nM dexamethasone, 50 μg/mL ascorbic acid-2-phosphate, 40 μg/mL L-proline, 100 μg/mL sodium pyruvate, 1% ITS-premix (6.25 μg/mL insulin, 6.25 μg/mL transferrin, 6.25 ng/mL selenous acid, 1.25 mg/mL BSA, 5.35 μg/mL linoleic acid; Collaborative Biomedical; BD Bioscience), and 10 ng/mL TGF-β1 (Fitzgerald). Scaffolds were cultured for as long as 6 wk. For pellet culturing, 300,000 cells were centrifuged at 1,000 rpm (CL2 Centrifuge, 236 Aerocarrier Rotor) for 5 min and cultured in chondrogenic induction medium. Samples were evaluated based on biochemical content, gene expression, and histology (SI Materials and Methods).
In Vivo Rat Osteochondral Defect Model.
Osteochondral defects were created in 25 male Sprague–Dawley rats (6 wk old) following previously published methods (34–37). Rats were anesthetized with 3% isoflurane for ∼5 min and maintained under anesthesia with 2% isoflurane during the surgical procedure. Under aseptic conditions, a midline skin incision was made in the left leg with the knee bent, followed by an incision through the muscle to expose the fibrous capsule. A capsulotomy was performed to expose the patella and the patella translated laterally to expose the trochlear groove. A 1.5-mm round defect ∼1.4 to 1.5 mm deep was made in the trochlear groove. Defects were implanted with CS fibers or PVA fibers or left empty. The nanofiber scaffolds were packed into the defects and trimmed to prevent fibers from protruding out of the defects. The patella was relocated to the trochlear groove and the muscle and skin sutured closed independently. For the 3-d time point, bilateral defects were made (three rats, n = 2 per group). At 3 d and 6 wk, rats were euthanized and the femoral condyles were removed and processed for histologic assessment. Two knees were excluded from further analysis as a result of patella destabilization. One knee had no histological evidence of fibers and was removed from histomorphometric quantification. The final number of repeats was as follows: six for PVA fibers and empty defects and seven for CS fibers.
Statistical Analysis.
Data are expressed as mean ± SD. Statistical significance was determined by two-tailed unpaired t test, one-way ANOVA followed by Tukey honestly significant differences test, or Kruskal–Wallis test in SPSS software (version 18.0; SPSS) as applicable. Significance was determined at P < 0.05.
Supplementary Material
Acknowledgments
The authors thank J. M. McCaffery of the Integrated Imaging Center (Department of Biology, The Johns Hopkins University) for assistance with the SEM imaging, Jacob Simson for histological scoring, and Dr. Lorenzo Moroni for his scientific discussion concerning development of fibrous scaffolds. This work was supported by National Institutes of Health Grants R01 EB 05517 (to J.H.E.), F31 AG 033999 (to J.M.C.), and F30 AG 034807 (to M.G.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1121605109/-/DCSupplemental.
References
- 1.Mow VC, Ratcliffe A, Poole AR. Cartilage and diarthrodial joints as paradigms for hierarchical materials and structures. Biomaterials. 1992;13:67–97. doi: 10.1016/0142-9612(92)90001-5. [DOI] [PubMed] [Google Scholar]
- 2.Kalson NS, Gikas PD, Briggs TWR. Current strategies for knee cartilage repair. Int J Clin Pract. 2010;64:1444–1452. doi: 10.1111/j.1742-1241.2010.02420.x. [DOI] [PubMed] [Google Scholar]
- 3.Deans TL, Elisseeff JH. Stem cells in musculoskeletal engineered tissue. Curr Opin Biotechnol. 2009;20:537–544. doi: 10.1016/j.copbio.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 4.Vasita R, Katti DS. Nanofibers and their applications in tissue engineering. Int J Nanomedicine. 2006;1:15–30. doi: 10.2147/nano.2006.1.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dahlin RL, Kasper FK, Mikos AG. Polymeric nanofibers in tissue engineering. Tissue Eng Part B Rev. 2011;17:349–364. doi: 10.1089/ten.teb.2011.0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li W-J, et al. A three-dimensional nanofibrous scaffold for cartilage tissue engineering using human mesenchymal stem cells. Biomaterials. 2005;26:599–609. doi: 10.1016/j.biomaterials.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 7.Li W-J, Danielson KG, Alexander PG, Tuan RS. Biological response of chondrocytes cultured in three-dimensional nanofibrous poly(ε-caprolactone) scaffolds. J Biomed Mater Res A. 2003;67:1105–1114. doi: 10.1002/jbm.a.10101. [DOI] [PubMed] [Google Scholar]
- 8.Skotak M, Ragusa J, Gonzalez D, Subramanian A. Improved cellular infiltration into nanofibrous electrospun cross-linked gelatin scaffolds templated with micrometer-sized polyethylene glycol fibers. Biomed Mater. 2011;6:055012. doi: 10.1088/1748-6041/6/5/055012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nam J, Huang Y, Agarwal S, Lannutti J. Improved cellular infiltration in electrospun fiber via engineered porosity. Tissue Eng. 2007;13:2249–2257. doi: 10.1089/ten.2006.0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coburn J, et al. Biomimetics of the extracellular matrix: An integrated three-dimensional fiber-hydrogel composite for cartilage tissue engineering. Smart Struct Syst. 2011;7:213–222. doi: 10.12989/sss.2011.7.3.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Erickson IE, et al. Macromer density influences mesenchymal stem cell chondrogenesis and maturation in photocrosslinked hyaluronic acid hydrogels. Osteoarthritis Cartilage. 2009;17:1639–1648. doi: 10.1016/j.joca.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Varghese S, et al. Chondroitin sulfate based niches for chondrogenic differentiation of mesenchymal stem cells. Matrix Biol. 2008;27:12–21. doi: 10.1016/j.matbio.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 13.Bryant SJ, Arthur JA, Anseth KS. Incorporation of tissue-specific molecules alters chondrocyte metabolism and gene expression in photocrosslinked hydrogels. Acta Biomater. 2005;1:243–252. doi: 10.1016/j.actbio.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 14.Bosnakovski D, et al. Chondrogenic differentiation of bovine bone marrow mesenchymal stem cells (MSCs) in different hydrogels: influence of collagen type II extracellular matrix on MSC chondrogenesis. Biotechnol Bioeng. 2006;93:1152–1163. doi: 10.1002/bit.20828. [DOI] [PubMed] [Google Scholar]
- 15.Hwang NS, Varghese S, Li H, Elisseeff J. Regulation of osteogenic and chondrogenic differentiation of mesenchymal stem cells in PEG-ECM hydrogels. Cell Tissue Res. 2011;344:499–509. doi: 10.1007/s00441-011-1153-2. [DOI] [PubMed] [Google Scholar]
- 16.Hwang NS, Varghese S, Puleo C, Zhang Z, Elisseeff J. Morphogenetic signals from chondrocytes promote chondrogenic and osteogenic differentiation of mesenchymal stem cells. J Cell Physiol. 2007;212:281–284. doi: 10.1002/jcp.21052. [DOI] [PubMed] [Google Scholar]
- 17.Wang DA, et al. Bioresponsive phosphoester hydrogels for bone tissue engineering. Tissue Eng. 2005;11:201–213. doi: 10.1089/ten.2005.11.201. [DOI] [PubMed] [Google Scholar]
- 18.Williams CG, et al. In vitro chondrogenesis of bone marrow-derived mesenchymal stem cells in a photopolymerizing hydrogel. Tissue Eng. 2003;9:679–688. doi: 10.1089/107632703768247377. [DOI] [PubMed] [Google Scholar]
- 19.Li W-J, Jiang YJ, Tuan RS. Chondrocyte phenotype in engineered fibrous matrix is regulated by fiber size. Tissue Eng. 2006;12:1775–1785. doi: 10.1089/ten.2006.12.1775. [DOI] [PubMed] [Google Scholar]
- 20.Baer E, Cassidy James J, Hiltner A. Viscoelasticity of Biomaterials. ACS Symposium Series. Vol 489. Washington, DC: American Chemical Society; 1992. Hierarchical structure of collagen composite systems; pp. 2–23. [Google Scholar]
- 21.Nguyen LH, Kudva AK, Saxena NS, Roy K. Engineering articular cartilage with spatially-varying matrix composition and mechanical properties from a single stem cell population using a multi-layered hydrogel. Biomaterials. 2011;32:6946–6952. doi: 10.1016/j.biomaterials.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 22.Choocheep K, et al. Versican facilitates chondrocyte differentiation and regulates joint morphogenesis. J Biol Chem. 2010;285:21114–21125. doi: 10.1074/jbc.M109.096479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shepard JB, Krug HA, LaFoon BA, Hoffman S, Capehart AA. Versican expression during synovial joint morphogenesis. Int J Biol Sci. 2007;3:380–384. doi: 10.7150/ijbs.3.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fernandez-Teran M, Bayliss M, Archer CW. Molecular heterogeneity of chondroitin sulphate in the early developing chick wing bud. Anat Embryol (Berl) 1993;188:189–199. doi: 10.1007/BF00186252. [DOI] [PubMed] [Google Scholar]
- 25.Kamiya N, et al. Versican/PG-M regulates chondrogenesis as an extracellular matrix molecule crucial for mesenchymal condensation. J Biol Chem. 2006;281:2390–2400. doi: 10.1074/jbc.M509341200. [DOI] [PubMed] [Google Scholar]
- 26.Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98:1076–1084. doi: 10.1002/jcb.20886. [DOI] [PubMed] [Google Scholar]
- 27.Mahmood A, Lu D, Lu M, Chopp M. Treatment of traumatic brain injury in adult rats with intravenous administration of human bone marrow stromal cells. Neurosurgery. 2003;53:697–702. doi: 10.1227/01.neu.0000079333.61863.aa. [DOI] [PubMed] [Google Scholar]
- 28.Barbash IM, et al. Systemic delivery of bone marrow-derived mesenchymal stem cells to the infarcted myocardium: feasibility, cell migration, and body distribution. Circulation. 2003;108:863–868. doi: 10.1161/01.CIR.0000084828.50310.6A. [DOI] [PubMed] [Google Scholar]
- 29.Horwitz EM, et al. Isolated allogeneic bone marrow-derived mesenchymal cells engraft and stimulate growth in children with osteogenesis imperfecta: Implications for cell therapy of bone. Proc Natl Acad Sci USA. 2002;99:8932–8937. doi: 10.1073/pnas.132252399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoemann CD, et al. Chitosan-glycerol phosphate/blood implants elicit hyaline cartilage repair integrated with porous subchondral bone in microdrilled rabbit defects. Osteoarthritis Cartilage. 2007;15:78–89. doi: 10.1016/j.joca.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 31.Simon TM, Jackson DW. Articular cartilage: Injury pathways and treatment options. Sports Med Arthrosc. 2006;14:146–154. doi: 10.1097/00132585-200609000-00006. [DOI] [PubMed] [Google Scholar]
- 32.Jin CZ, et al. The maturity of tissue-engineered cartilage in vitro affects the repairability for osteochondral defect. Tissue Eng Part A. 2011;17:3057–3065. doi: 10.1089/ten.tea.2010.0605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun Y, Feng Y, Zhang CQ, Chen SB, Cheng XG. The regenerative effect of platelet-rich plasma on healing in large osteochondral defects. Int Orthop. 2010;34:589–597. doi: 10.1007/s00264-009-0793-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Toh WS, et al. Cartilage repair using hyaluronan hydrogel-encapsulated human embryonic stem cell-derived chondrogenic cells. Biomaterials. 2010;31:6968–6980. doi: 10.1016/j.biomaterials.2010.05.064. [DOI] [PubMed] [Google Scholar]
- 35.Ahern BJ, Parvizi J, Boston R, Schaer TP. Preclinical animal models in single site cartilage defect testing: A systematic review. Osteoarthritis Cartilage. 2009;17:705–713. doi: 10.1016/j.joca.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 36.Hwang NS, et al. In vivo commitment and functional tissue regeneration using human embryonic stem cell-derived mesenchymal cells. Proc Natl Acad Sci USA. 2008;105:20641–20646. doi: 10.1073/pnas.0809680106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dausse Y, et al. Cartilage repair using new polysaccharidic biomaterials: Macroscopic, histological and biochemical approaches in a rat model of cartilage defect. Osteoarthritis Cartilage. 2003;11:16–28. doi: 10.1053/joca.2002.0859. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.