Abstract
In anaerobic biota, reducing equivalents (electrons) are transferred between different species of microbes [interspecies electron transfer (IET)], establishing the basis of cooperative behaviors and community functions. IET mechanisms described so far are based on diffusion of redox chemical species and/or direct contact in cell aggregates. Here, we show another possibility that IET also occurs via electric currents through natural conductive minerals. Our investigation revealed that electrically conductive magnetite nanoparticles facilitated IET from Geobacter sulfurreducens to Thiobacillus denitrificans, accomplishing acetate oxidation coupled to nitrate reduction. This two-species cooperative catabolism also occurred, albeit one order of magnitude slower, in the presence of Fe ions that worked as diffusive redox species. Semiconductive and insulating iron-oxide nanoparticles did not accelerate the cooperative catabolism. Our results suggest that microbes use conductive mineral particles as conduits of electrons, resulting in efficient IET and cooperative catabolism. Furthermore, such natural mineral conduits are considered to provide ecological advantages for users, because their investments in IET can be reduced. Given that conductive minerals are ubiquitously and abundantly present in nature, electric interactions between microbes and conductive minerals may contribute greatly to the coupling of biogeochemical reactions.
Keywords: bioenergy, biogeochemistry, electrode respiration, microbial ecology
Biological energy conservation generally depends on the coupling of oxidative and reductive chemical reactions. The most prominent is organics oxidation coupled to oxygen reduction; most of organisms, including animals and plants, exploit this type of catabolism for energy conservation (1). Recent studies, however, have revealed that some bacteria perform anodic and cathodic half reactions using electrodes (mostly graphite carbons) as electron donors and acceptors, respectively. For example, dissimilatory iron-reducing bacteria (DMRB) (such as Shewanella and Geobacter) oxidize organics and transfer electrons to anodes, resulting in the current generation in microbial fuel cells (2, 3). On the other hand, G. metallireducens accepts electrons from graphite cathodes and use them for nitrate reduction (4).
Although microbe/electrode interactions promise to be applied to bioelectrochemical processes, such as microbial fuel/electrolysis cells (5–7) and bioremediation (8, 9), their ecological and evolutionary origins remain enigmatic. Although some scientists deduce that microbe/electrode interactions stem from the abilities of bacteria to reduce extracellular solid metals as terminal electron acceptors (5), recent studies have disclosed that electron transfer to electrodes does not necessarily share identical cellular components to those for solid-metal reduction (10, 11). It is, therefore, likely that electrodes (that do not accept electrons by themselves but transfer electrons to electron acceptors) may also exist in the natural environment, allowing microbes to evolve abilities to interact with electrodes (either natural or artificial).
Concerning this idea, a recent study has suggested that spatially separated biogeochemical redox processes in marine sediment (i.e., sulfide oxidation at depths of >10 mm and oxygen reduction at the surface) are connected by electric currents, but not by diffusion of redox molecules (12). Although that study did not identify electron conduits and electrodes that could work in marine sediments, possible mechanisms for electron transfer have been discussed, including those via microbial nanowires, direct contact of outer-membrane cytochromes, and natural minerals, such as pyrite (12). Among them, we consider that natural minerals are likely candidates for natural electron conduits for the following reasons. First, (semi)conductive minerals [e.g., pyrite (semiconductive), magnetite (conductive), and hematite (semiconductive)] are abundant in natural soil and sediments (13, 14); for instance, marine sediments in the Aarhus bay contain 30 μmol of pyrite per gram (12). Second, it has recently been shown that some bacteria use iron-oxide mineral nanoparticles (magnetite and hematite) as conduits of electrons toward working electrodes in electrochemical chambers (15, 16).
The present study was conducted to evaluate a possibility that conductive minerals can serve as electric conduits that connect redox reactions catalyzed by different species of microbes. In anaerobic biota, electrons are transferred between different species of microbes [interspecies electron transfer (IET)], establishing a basis of cooperative behaviors and community functions (17–19). IET mechanisms described so far are based on diffusion of redox chemical species (20) and/or direct contact in cell aggregates (21). To address the involvement of conductive minerals in IET, we constructed mixed cultures of two representative soil bacteria, G. sulfurreducens (2, 5) and Thiobacillus denitrificans (22), in the presence and absence of iron-oxide minerals, and their IET-dependent cooperative catabolic reactions were investigated.
Results
Electrochemical Characteristics of Catabolic Reactions in Monocultures.
We attempted to construct IET-dependent mixed cultures consisting of two soil bacteria, G. sulfurreducens and T. denitrificans. G. sulfurreducens is a widely studied DMRB (5) and has abilities to respire a metal-oxide electrode [a tin-doped In2O3 (ITO) glass electrode] coupled to acetate oxidation (Fig. 1A). The anodic current [at +0.4 V vs. standard hydrogen electrode (SHE)] was initially stable at ∼7 μA cm−2, although it increased afterward, probably owing to the formation of conductive biofilms (23). In contrast, T. denitrificans is a chemolithoautotrophic bacterium and known to use solid iron minerals (e.g., pyrite) as electron donors (22). We confirmed that the metal-oxide electrode (at −0.4 V vs. SHE) served as an electron donor for T. denitrificans in the presence of nitrate (Fig. 1B), generating a constant cathodic current at 4–5 μA cm−2. The stable current density could be attributed to limited electron transfer by bacterial cells that directly attached to the electrode. In the absence of nitrate, a current density was negligible; e.g., below 0.2 μA cm−2 at hour 20. Electrochemical characteristics of G. sulfurreducens and T. denitrificans were further analyzed by linear sweep voltammetry (Fig. 1C). It is shown that G. sulfurreducens generated anodic current in a potential range of over −0.3 V (vs. SHE), whereas T. denitrificans generated cathodic current in a potential range of below 0 V (vs. SHE). These results suggest that electric currents from G. sulfurreducens to T. denitrificans can be generated in a potential range between −0.3 and 0 V (vs. SHE), if there are appropriate electron conduits between them.
Fig. 1.
Electrochemical characteristics of G. sulfurreducens (blue lines) and T. denitrificans (black lines) on ITO electrodes. Anodic currents were measured using an electrode poised at +0.4 V vs. SHE after supplemented with 20 mM acetate (A), whereas cathodic currents were measured at −0.4 V vs. SHE with 30 mM nitrate (B). Linear sweep voltammograms (C) were measured at a scan rate of 1 mV s−1 30 h after initiating the incubation.
Cooperative Catabolism in the Presence of Conductive Iron Minerals.
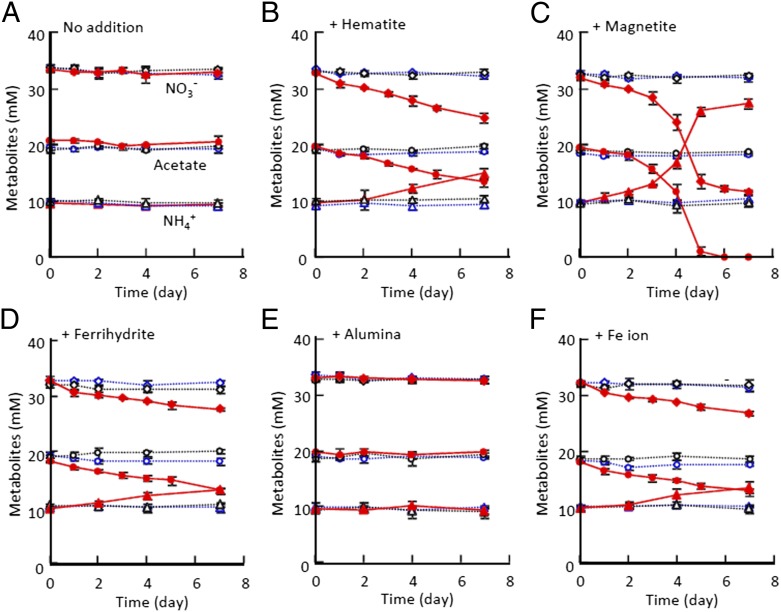
To examine IET between G. sulfurreducens and T. denitrificans, anaerobic culture media were inoculated with these bacteria and supplemented with acetate (20 mM) and nitrate (30 mM) as potential electron donor and acceptor, respectively. In a single culture of either of these bacteria, neither acetate oxidation nor nitrate reduction was observed (Fig. 2A), because G. sulfurreducens is able to oxidize acetate but unable to reduce nitrate and vice versa for T. denitrificans. Under this condition, IET between the two bacterial species is required for accomplishing the cooperative catabolism (acetate oxidation coupled to nitrate reduction). The reaction, however, did not occur even in their coculture (Fig. 2A). IETs mediated by H2 and formate do not occur in their coculture, because T. denitrificans cannot use these compounds as electron donors (22). This result also ruled out possibilities for IETs via direct cell contact and conductive biological appendages [e.g., extracellular cytochromes (21) and nanowires (24)], because large portions of the inoculated Geobacter and Thiobacillus cells were precipitated at the bottom of the culture bottle within several days after the initiation of incubation (without agitation), where they could exploit cell contacts.
Fig. 2.
Changes in substrates and metabolites in single cultures of G. sulfurreducens (blue dashed lines), single cultures of T. denitrificans (black dashed lines), and their cocultures (red solid lines) in the absence (A) and presence (B–E) of different mineral species (indicated above the graphs) or in controls supplemented with Fe ion (F). Changes in concentrations of nitrate (diamonds), acetate (circles), and ammonium (triangles) are shown. Data points represent means of three independent cultures; error bars indicate SDs.
Iron oxides show distinct electrochemical properties depending on their crystal structures; for example, magnetite (Fe3O4) is conductive, whereas hematite (α-Fe2O3) is semiconductive (13). We added these crystalline iron-oxide particles (10–20 nm in diameter; 10 mM as Fe atom) to examine whether they could mediate IET between G. sulfurreducens and T. denitrificans. For comparison, insulating ferrihydrite (FeOOH) (2.5 mM) and alumina (Al2O3) particles were also examined. It was found that, when supplemented with nanoparticles of hematite or magnetite, acetate and nitrate were decreased in their coculture concomitant with an increase in an ammonium concentration at an approximate stoichiometry of 1:1:1 (Fig. 2 B and C). Nitrite was also produced during the incubations, albeit small amounts (<0.2 mM). These changes were not observed in their single cultures. This result suggests that electrons were transferred from G. sulfurreducens to T. denitrificans, facilitating the coupling of acetate oxidation by G. sulfurreducens (CH3COO− + 4H2O → 2HCO3− + 9H+ + 8e−) to nitrate reduction by T. denitrificans (NO3− + 10H+ + 8e− → NH4+ + 3H2O). The fact that the catabolic reactions occurred only in the presence of these two organisms also indicates that abiotic oxidation and reduction of these compounds were negligible.
It is important to note that, in the magnetite-supplemented culture, the increase in the ammonia concentration and the decrease in the acetate and nitrate concentrations were exponential (Fig. 2C), whereas they were liner in the hematite-supplemented culture (Fig. 2B). Linear changes were also observed in the ferrihydrite-supplemented culture (Fig. 2D), whereas there were no changes in the alumina-supplemented culture (Fig. 2E). The different trends in substrate concentrations suggest that there existed different IET mechanisms between the magnetite- and hematite-supplemented cultures.
IET Mechanisms.
The redox cycling of Fe electron shuttles that were produced from iron-oxide minerals could have contributed to IET between G. sulfurreducens to T. denitrificans. To examine this, concentrations of 0.5 N HCl-extractable iron were measured throughout the incubations (Fig. S1). This analysis revealed that 2–2.5 mM acid-extractable iron was present in the hematite- and magnetite-supplemented cocultures and in the G. sulfurreducens single cultures. In the cocultures, most of acid-extractable iron existed as Fe(III), whereas they were Fe(II) in the G. sulfurreducens single cultures. The reduction of 2 mM iron in the single G. sulfurreducens cultures may have been coupled with the oxidation of 0.25 mM acetate, and a slight decrease in acetate concentration was detected (Fig. 2). It is suggested that G. sulfurreducens reduced iron-oxide crystals and produced acid-extractable Fe(II), whereas T. denitrificans oxidized them to form Fe(III), resulting in diffusive IET.
To evaluate whether or not the redox cycle of Fe(II)/Fe(III) is sufficient for the IET observed between G. sulfurreducens and T. denitrificans, we examined IET in cocultures of these bacteria after supplemented with soluble Fe ions (2.5 mM ferric citrate). We found that the acetate oxidation, nitrate reduction, and ammonium production occurred in the Fe ion-supplemented coculture (but not in single cultures of these bacteria) (Fig. 2F). Acid-extractable Fe(II) concentrations in the coculture were less than those in the G. sulfurreducens pure culture (Fig. S1), indicating that the redox cycle of Fe(II)/Fe(III) mediated IET in these systems. In these cultures, similar to the hematite-supplemented culture, the substrates and metabolite were linearly changed (Fig. 2).
Amounts of electrons transferred from G. sulfurreducens to T. denitrificans were estimated from the nitrate-depletion curves in Fig. 2, and they were compared among the cocultures supplemented with different iron species (Fig. 3). From a slope of the curve, an IET rate in the presence of hematite was estimated to be 7.6 ± 0.8 mM equivalent (mMeq) electrons d−1. This rate was comparable to those for the ferrihydrite- and Fe ion-supplemented cocultures (5.8 ± 0.9 and 5.4 ± 0.6 mMeq electrons d−1, respectively), indicating that IET in the hematite-supplemented culture was mostly mediated by the Fe redox cycles. Contrary to the other cases, IET in the magnetite-supplemented culture did not show a linear increase; it was exponential, and the IET rate reached at 83.8 ± 6.4 mMeq electrons d−1 (more than 10-fold higher than those for the other cultures). The nonlinear (exponential) curve for the magnetite-supplemented culture (Fig. 3) suggests that microbial growth limited the electron transfer only in this culture. During the incubation, a precipitate was subjected to a powder X-ray diffraction (XRD) analysis to check whether the magnetite structure was maintained even in the presence of microbes (Fig. S2). It is shown that the spectra were unchanged during the incubation, indicating that magnetite was stably present.
Fig. 3.
Cumulative curves showing amounts of electrons (mM equivalent) transferred from G. sulfurreducens to T. denitrificans. Data points represent means of three independent cultures; error bars indicate SDs.
Scanning Electron Micrography.
In the magnetite-supplemented culture, black precipitates were formed at the bottom of culture bottles. Precipitates were also observed in hematite- and ferrihydrite-supplemented cultures, whereas, owing to poor bacterial growth, amounts of precipitates were less than those observed in the magnetite-supplemented culture. Without iron oxides, G. sulfurreducens and T. denitrificans cells were also precipitated and formed a thin orange layer at the bottom of the culture bottle.
We observed precipitates formed in the nonmetal (Fig. 4A) and magnetite-supplemented (Fig. 4B) cultures by scanning electron micrography (SEM). In the electron micrographs, T. denitrificans cells were slightly larger and thicker than G. sulfurreducens cells. In preparing SEM samples, loosely precipitated magnetite nanoparticles were washed away, and those attaching tightly to bacterial cells could be seen in the SEM images. In Fig. 4B, nanoparticles were seen at the surface of bacterial cells, and, in some parts, these two bacteria were connected via these particles. Extracellular appendages (24) and polysaccharide networks (25, 26) were not observed, even though our sample-preparation procedure was the same as that used in a previous study (25), in which polysaccharide networks were observed. In the hematite-supplemented culture, nanoparticles also adhered to surfaces of bacterial cells (Fig. S3). Cell-surface nanoparticles in the magnetite-supplemented culture were examined by an energy dispersive X-ray (EDX) analysis (Fig. 4 C and D). A comparison in EDX spectra of a Geobacter cell surface (immediately left of the lower blue box in Fig. 4C) and a cell-attaching nanoparticle (immediately left of the upper blue box) indicates that the nanoparticle is abundant in iron and oxygen (Fig. 4D), supporting the idea that cell-surface nanoparticles are magnetite.
Fig. 4.
SEM images for precipitates formed in cocultures of G. sulfurreducens and T. denitrificans 6 d after initiating the cultivation in the absence (A) and presence (B and C) of magnetite. Points at which EDX spectra were obtained are indicated in C (immediately left of the blue boxes), whereas EDX spectra are shown in D (a yellow area, spectrum 1 for the upper point in C; a red line, spectrum 2 for the lower point). (Scale bars: 1 μm.)
Discussion
Iron works as an electron shuttle in microbial ecosystems (20, 27). In the coculture of G. sulfurreducens and T. denitrificans, the data (Fig. S1) indicate that IET between them occurred via Fe electron shuttles that were either artificially supplemented or dissolved from iron-oxide minerals. Because acid-extractable Fe concentrations in all of the iron-supplemented cocultures were similar, the resultant IET-dependent cooperative catabolism should have occurred at similar speeds. Another mechanism was, therefore, necessary for explaining the high IET rate observed in the magnetite-supplemented culture. For considering this point, it is noteworthy that, although magnetite and hematite exhibit similar surface chemistry (13), their electrochemical properties are different; magnetite is conductive, whereas hematite is semiconductive (it is conductive at a potential range of over 0 V vs. SHE) (13). As shown in Fig. 1, however, the electric connection of G. sulfurreducens and T. denitrificans is possible in a potential range between −0.3 and 0 V (vs. SHE). This indicates that only magnetite can serve as an electron conduit between these two bacteria. Although magnetite nanoparticles are known to be formed by G. sulfurreducens from other iron species (e.g., ferrihydrite) (28), partial formation of magnetite in the presence of nonconductive species may have been insufficient to promote IET between them. In the ferrihydrite-supplemented culture, total acid-extractable Fe was not substantially decreased (Fig. S1D). This indicates that the formation of magnetite was minor, because biogenic magnetite is known to be hardly dissolved in 0.5 N HCl (29). The idea of electric connection between them is also supported by our finding that conductive graphite particles also facilitated their cooperative catabolism (Fig. S4). Based on these results, we conclude that IET between G. sulfurreducens and T. denitrificans occurs via electric currents through conductive magnetite particles.
We consider that the present study provides experimental evidences that help explain mechanisms underlying biogeochemical long-distance electron transfer observed in natural environments, such as marine sediments (12). In that report, they found that sulfide oxidation at the depth of over 12 mm in marine sediments was rapidly initiated after the sediment surface was exposed to oxygen-containing seawater, resulting in a pH elevation at the sediment surface. This observation neglects a possibility for the involvement of diffusive electron-shuttle compounds in the electron transfer, and they have argued that bacterial nanowires may have served as conductors of electrons in the sediment. They have also discussed a possibility that pyrite grains may have assisted the electron transfer. This idea is based on implications provided by geochemical studies (14, 30); for instance, the existence of large-scale natural electric currents was proposed in 1960 for biogeochemical processes in conductive pyrite ores (31). Before the present study, however, there had been no experimental evidence regarding the involvement of electric currents through conductive minerals in connecting biogeochemical redox reactions. In the marine-sediment study (12), oxygen-reduction rates at the surface were reported to be 9.7–46 mmol m−2 d−1, which are equivalent to current densities of 4–20 μA cm−2. These current densities are comparable to those seen in Fig. 1 (using the metal-oxide electrodes) and lower than those reported previously for microbial electron transfer in the presence of iron-oxide nanoparticles (∼100 μA cm−2 or higher) (15, 16). This estimation also suggests that conductive minerals have sufficient potentials to serve as electron conduits in marine sediments.
Conductive mineral particles are abundant and ubiquitous in nature; for instance, the Aarhus bay sediment contains 30 μmol of pyrite per gram (12). It has also been reported that conductive minerals, such as magnetite, abundantly exist on the present and ancient Earth (14). Furthermore, reports have documented that dissimilatory iron-oxidizing and iron-reducing microbes generate crystalline iron-oxide nanoparticles as a result of respiration (28, 32). We, therefore, consider that microbes have evolved biomolecular machineries for discharging electrons to and accepting them from mineral particles around their cells, resulting in IETs via electric currents. Because conductivities of metallic minerals are, in general, much higher than those of organic and biological materials (33), microbes should preferentially use mineral particles for IETs, in particular for long-distance electron transfer. Another merit of using conductive minerals is that they do not have intrinsic limitation in potential ranges for transferring electrons between microbes. Furthermore, because the synthesis of proteins needs a large energy investment, it must be difficult for microbes that produce large amounts of extracellular electron conduits (e.g., conductive nanowires) to gain ecological advantages. It is, therefore, likely that conductive mineral particles contribute a lot to biogeochemical long-distance electron transfers observed in natural environments, e.g., marine sediments (12).
Materials and Methods
Bacterial Strains and Culture Conditions.
G. sulfurreducens DSM12127 (German Collection of Microorganisms) and T. denitrificans ATCC25259 (American Type Culture Collection) were routinely cultivated in PS medium (16) under a N2/CO2 [80:20 (vol/vol)] atmosphere at 30 °C without agitation. Vitamin solution (16) (1 mL L−1) and appropriate electron donor and acceptor (10 mM sodium acetate and 40 mM sodium fumarate for G. sulfurreducens, or 20 mM sodium thiosulfate and 20 mM sodium nitrate for T. denitrificans) were supplemented from sterilized oxygen-free stocks. The pH of the medium was kept constant at 7.0 with 30 mM 4-(2-hydroxyethyl)-1-piperazineethane sulfonic acid (Hepes).
Electrochemical Analyses.
A single-chamber three-electrode electrochemical cell [14 mL in capacity (13)] was equipped with a working electrode (ITO; 6.2 cm2 in surface area) at the bottom, an Ag/AgCl (KCl sat., +0.2 V vs. SHE) reference electrode (HX-5; Hokuto Denko), and a platinum-wire counter electrode. The working electrode was poised at a certain potential using a HA-1510 potentiostat (Hokuto Denko). After sterilized, a cell was filled with 12 mL of PS medium and inoculated with G. sulfurreducens or T. denitrificans cells at an optical density at 600 nm (OD600) of 0.8. Oxygen-free stock solutions of sodium acetate and sodium nitrate were injected into a cell to give final concentrations of 20 and 30 mM, respectively. The system was incubated at 30 °C without shaking, and current was measured at 10-min intervals. After incubation for 30 h, a linear sweep voltammogram was taken using a HSV-100 potentiostat (Hokuto Denko).
IET Assay.
G. sulfurreducens and T. denitrificans were precultivated to early stationary phases as described above. Cells were collected by centrifugation (8,000 × g; 5 min), washed twice, and suspended in fresh PS medium. The cell suspension was inoculated into a bottle containing PS medium supplemented with 20 mM acetate and 30 mM nitrate as electron donor and acceptor, respectively, at a final OD600 of 0.8. Hematite nanoparticles were synthesized by slowly adding 1 M FeCl3 solution into vigorously mixed boiling water and purified by dialysis against water (34). Magnetite nanoparticles were synthesized by slowly adding Fe(II)/Fe(III) acidic solution (0.8 M FeCl3 and 0.4 M FeCl2 in 0.4 N HCl) into vigorously mixed 1.5 N NaOH solution, purified by centrifugation, and suspended in deoxygenated water (35). Ferrihydrite nanoparticles were synthesized by neutralizing 0.1 M FeCl3 solution with 5 N NaOH, purified by centrifugation, and suspended in water (36). Average diameters of these synthesized nanoparticles were 10–20 nm (15). Graphite (Vulcan XC-72; Showa Cabot) and Al2O3 (Sigma Aldrich) nanoparticles were purchased and were ∼50 nm in average diameters.
A cell suspension was incubated at 30 °C under a N2/CO2 [80:20 (vol/vol)] atmosphere without agitation. Nitrate and nitrite concentrations were determined using HPLC (CLASS-VP5; Shimadzu) equipped with Shim-pack IC-A3 column (Shimadzu). Acetate concentration was determined by HPLC (1100 series; Agilent) with a Zorbax column SB-Aq (Agilent) (37). Ammonium was measured using F-kit (Roche Diagnostics). Concentrations of 0.5 N HCl-extractable Fe(II) was determined by a ferrozine method as described elsewhere (38). To determine Fe(II) + Fe(III) (total HCl-extractable iron), a sample was reduced with 0.25 M hydroxylamine before the acid extraction. An XRD spectrum was obtained using a diffractometer [Rigaku RINT 2100; Cu Kα (λ = 1.5418 Å)] by a conventional 2θ/θ method.
SEM Analyses.
Cells were incubated in the presence of a glass plate for 6 d, and cells on the plate were fixed with 2.5% (wt/vol) glutaraldehyde. The fixed cells were dehydrated using a graded series of ethanol solutions, and dried with t-butanol. The dried samples were mounted on aluminum stubs, coated with platinum, and imaged using FE-SEM SU4800 (Hitachi High-Technologies). An elementary analysis of an SEM sample at a certain point was conducted by an EDX spectroscopy using an X-max80 machine (Horiba).
Supplementary Material
Acknowledgments
We thank Ken Nealson for helpful advice and encouragement and Reiko Hirano and Ayako Matsuzawa for technical assistance. This work was supported by the Exploratory Research for Advanced Technology (ERATO) program of the Japanese Science and Technology Agency (JST).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1117592109/-/DCSupplemental.
References
- 1.Lehninger AL. Bioenergetics. NY: WA Benjamin Inc.; 1965. [Google Scholar]
- 2.Bond DR, Lovley DR. Electricity production by Geobacter sulfurreducens attached to electrodes. Appl Environ Microbiol. 2003;69:1548–1555. doi: 10.1128/AEM.69.3.1548-1555.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim HJ, et al. A mediator-less microbial fuel cell using a metal reducing bacterium, Shewanella putrefaciens. Enzyme Microb Technol. 2002;30:145–152. [Google Scholar]
- 4.Gregory KB, Bond DR, Lovley DR. Graphite electrodes as electron donors for anaerobic respiration. Environ Microbiol. 2004;6:596–604. doi: 10.1111/j.1462-2920.2004.00593.x. [DOI] [PubMed] [Google Scholar]
- 5.Lovley DR. Bug juice: Harvesting electricity with microorganisms. Nat Rev Microbiol. 2006;4:497–508. doi: 10.1038/nrmicro1442. [DOI] [PubMed] [Google Scholar]
- 6.Rozendal RA, Jeremiasse AW, Hamelers HV, Buisman CJ. Hydrogen production with a microbial biocathode. Environ Sci Technol. 2008;42:629–634. doi: 10.1021/es071720+. [DOI] [PubMed] [Google Scholar]
- 7.Cheng S, Xing D, Call DF, Logan BE. Direct biological conversion of electrical current into methane by electromethanogenesis. Environ Sci Technol. 2009;43:3953–3958. doi: 10.1021/es803531g. [DOI] [PubMed] [Google Scholar]
- 8.Thrash JC, et al. Electrochemical stimulation of microbial perchlorate reduction. Environ Sci Technol. 2007;41:1740–1746. doi: 10.1021/es062772m. [DOI] [PubMed] [Google Scholar]
- 9.Zhang T, Gannon SM, Nevin KP, Franks AE, Lovley DR. Stimulating the anaerobic degradation of aromatic hydrocarbons in contaminated sediments by providing an electrode as the electron acceptor. Environ Microbiol. 2010;12:1011–1020. doi: 10.1111/j.1462-2920.2009.02145.x. [DOI] [PubMed] [Google Scholar]
- 10.Holmes DE, et al. Microarray and genetic analysis of electron transfer to electrodes in Geobacter sulfurreducens. Environ Microbiol. 2006;8:1805–1815. doi: 10.1111/j.1462-2920.2006.01065.x. [DOI] [PubMed] [Google Scholar]
- 11.Richter H, Lanthier M, Nevin KP, Lovley DR. Lack of electricity production by Pelobacter carbinolicus indicates that the capacity for Fe(III) oxide reduction does not necessarily confer electron transfer ability to fuel cell anodes. Appl Environ Microbiol. 2007;73:5347–5353. doi: 10.1128/AEM.00804-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nielsen LP, Risgaard-Petersen N, Fossing H, Christensen PB, Sayama M. Electric currents couple spatially separated biogeochemical processes in marine sediment. Nature. 2010;463:1071–1074. doi: 10.1038/nature08790. [DOI] [PubMed] [Google Scholar]
- 13.Cornell RM, Schwertmann U. The Iron Oxides: Structure, Properties, Reactions, Occurrences and Uses. Weinheim, Germany: Wiley-VCH; 2003. [Google Scholar]
- 14.Hochella MF, Jr, et al. Nanominerals, mineral nanoparticles, and Earth systems. Science. 2008;319:1631–1635. doi: 10.1126/science.1141134. [DOI] [PubMed] [Google Scholar]
- 15.Nakamura R, Kai F, Okamoto A, Newton GJ, Hashimoto K. Self-constructed electrically conductive bacterial networks. Angew Chem Int Ed Engl. 2009;48:508–511. doi: 10.1002/anie.200804750. [DOI] [PubMed] [Google Scholar]
- 16.Kato S, Nakamura R, Kai F, Watanabe K, Hashimoto K. Respiratory interactions of soil bacteria with (semi)conductive iron-oxide minerals. Environ Microbiol. 2010;12:3114–3123. doi: 10.1111/j.1462-2920.2010.02284.x. [DOI] [PubMed] [Google Scholar]
- 17.Schink B. Energetics of syntrophic cooperation in methanogenic degradation. Microbiol Mol Biol Rev. 1997;61:262–280. doi: 10.1128/mmbr.61.2.262-280.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keller L, Surette MG. Communication in bacteria: An ecological and evolutionary perspective. Nat Rev Microbiol. 2006;4:249–258. doi: 10.1038/nrmicro1383. [DOI] [PubMed] [Google Scholar]
- 19.Kato S, Watanabe K. Ecological and evolutionary interactions in syntrophic methanogenic consortia. Microbes Environ. 2010;25:145–151. doi: 10.1264/jsme2.me10122. [DOI] [PubMed] [Google Scholar]
- 20.Watanabe K, Manefield M, Lee M, Kouzuma A. Electron shuttles in biotechnology. Curr Opin Biotechnol. 2009;20:633–641. doi: 10.1016/j.copbio.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 21.Summers ZM, et al. Direct exchange of electrons within aggregates of an evolved syntrophic coculture of anaerobic bacteria. Science. 2010;330:1413–1415. doi: 10.1126/science.1196526. [DOI] [PubMed] [Google Scholar]
- 22.Beller HR, et al. The genome sequence of the obligately chemolithoautotrophic, facultatively anaerobic bacterium Thiobacillus denitrificans. J Bacteriol. 2006;188:1473–1488. doi: 10.1128/JB.188.4.1473-1488.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lovley DR. The microbe electric: Conversion of organic matter to electricity. Curr Opin Biotechnol. 2008;19:564–571. doi: 10.1016/j.copbio.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 24.Reguera G, et al. Extracellular electron transfer via microbial nanowires. Nature. 2005;435:1098–1101. doi: 10.1038/nature03661. [DOI] [PubMed] [Google Scholar]
- 25.Ishii S, Shimoyama T, Hotta Y, Watanabe K. Characterization of a filamentous biofilm community established in a cellulose-fed microbial fuel cell. BMC Microbiol. 2008;8:6. doi: 10.1186/1471-2180-8-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rollefson JB, Stephen CS, Tien M, Bond DR. Identification of an extracellular polysaccharide network essential for cytochrome anchoring and biofilm formation in Geobacter sulfurreducens. J Bacteriol. 2011;193:1023–1033. doi: 10.1128/JB.01092-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wielinga B, Mizuba MM, Hansel CM, Fendorf S. Iron promoted reduction of chromate by dissimilatory iron-reducing bacteria. Environ Sci Technol. 2001;35:522–527. doi: 10.1021/es001457b. [DOI] [PubMed] [Google Scholar]
- 28.Lovley DR, Stolz JF, Nord GL, Jr, Phillips EJP. Anaerobic production of magnetite by a dissimilatory iron-reducing microorganism. Nature. 1987;330:252–254. [Google Scholar]
- 29.Fredrickson JK, et al. Biogenic iron mineralization accompanying the dissimilatory reduction of hydrous ferric oxide by a groundwater bacterium. Geochim Cosmochim Acta. 1998;62:3239–3257. [Google Scholar]
- 30.Bigalke J, Grabner EW. The geobattery model: A contribution to large scale electrochemistry. Electrochim Acta. 1997;42:3443–3452. [Google Scholar]
- 31.Sato M, Mooney HM. The electrochemical mechanism of sulphide selfpotentials. Geophysics. 1960;25:226–249. [Google Scholar]
- 32.Jiao Y, Kappler A, Croal LR, Newman DK. Isolation and characterization of a genetically tractable photoautotrophic Fe(II)-oxidizing bacterium, Rhodopseudomonas palustris strain TIE-1. Appl Environ Microbiol. 2005;71:4487–4496. doi: 10.1128/AEM.71.8.4487-4496.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.El-Naggar MY, et al. Electrical transport along bacterial nanowires from Shewanella oneidensis MR-1. Proc Natl Acad Sci USA. 2010;107:18127–18131. doi: 10.1073/pnas.1004880107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mulvaney P, Cooper R, Grieser F, Meisel D. Charge trapping in the reductive dissolution of colloidal suspensions of iron(III) oxides. Langmuir. 1988;4:1206–1211. [Google Scholar]
- 35.Kang YS, Risbud S, Rabolt JF, Stroeve P. Synthesis and characterization of nanometer-size Fe3O4 and γ-Fe2O3 particles. Chem Mater. 1996;8:2209–2211. [Google Scholar]
- 36.Lovley DR, Phillips EJ. Organic matter mineralization with reduction of ferric iron in anaerobic sediments. Appl Environ Microbiol. 1986;51:683–689. doi: 10.1128/aem.51.4.683-689.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Newton GJ, Mori S, Nakamura R, Hashimoto K, Watanabe K. Analyses of current-generation mechanisms of Shewanella loihica PV-4 in microbial fuel cells in comparison with Shewanella oneidensis MR-1. Appl Environ Microbiol. 2009;75:7674–7681. doi: 10.1128/AEM.01142-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lovley DR, Phillips EJ. Rapid assay for microbially reducible ferric iron in aquatic sediments. Appl Environ Microbiol. 1987;53:1536–1540. doi: 10.1128/aem.53.7.1536-1540.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.