Abstract
In the last few years, the insula has been the focus of many brain-imaging studies, mostly devoted to clarify its role in emotions and social communication. Physiological data, however, on which one may ground these correlative findings are almost totally lacking. Here, we investigated the functional properties of the insular cortex in behaving monkeys using intracortical microstimulation. Behavioral responses and heart rate changes were recorded. The results showed that the insula is functionally formed by two main subdivisions: (i) a sensorimotor field occupying the caudal–dorsal portion of the insula and appearing as an extension of the parietal lobe; and (ii) a mosaic of orofacial motor programs located in the anterior and centroventral insula sector. These programs show a progressive shift from dorsally located nonemotional motor programs (ingestive activity) to ventral ones laden with emotional and communicative content. The relationship between ingestive and other behaviors is discussed in an evolutionary perspective.
The insula is one of the parts of the cerebral cortex that is less understood. Its location, buried in the depth of the Sylvian fissure, its opercularization, and its high vascularization are some of the anatomical features that render it difficult to study.
In recent years, brain-imaging studies provided a series of correlative data on its functions. The results of these studies were, however, not crystal clear. According to them, the insula is involved in many diverse functions. The work by Craig (1), for example, claims that the insula is involved in maternal love, bowel distention, orgasm, sudden insight, and decision making as well as awareness and consciousness (2). The interpretation of brain-imaging studies would be much more convincing if they could be grounded on a physiological basis. Unfortunately, there are only few physiological data, mostly obtained with electrical stimulation in epileptic patients (3–6).
As far as monkeys are concerned, besides a series of single-neuron studies focused on the sensory properties of the insula (7–13), the only systematic data on the insula organization derive from electrical stimulation studies carried out in the middle of the last century (14–16). These studies, however, are of rather limited use. First, they were carried out in general anesthesia. Second, they were often performed after surgical removal of large portions of the surrounding opercula. Third, the stimulation was carried out with surface macroelectrodes, which allow only an approximate localization of the observed responses.
In contrast to the paucity of physiological data, there is an excellent and exhaustive picture of the anatomical organization of the insula of both humans and nonhuman primates (17–19). One of the main conclusions of these studies is that the “plan of anatomical organization is virtually identical [in humans] to that of the macaque monkey” (17). This similarity renders, therefore, a particularly valuable picture of the functional organization of the insula in nonhuman primates.
In the present study, we investigated the functional properties of the monkey insular cortex using behavioral timescale intracortical microstimulation (ICMS). This technique employs prolonged electrical trains to reveal the behaviors that are controlled by the stimulated region. Prolonged electrical stimulation of the hypothalamus and other subcortical structures was used in the past to investigate complex behaviors such as feeding or mating (20, 21). More recently, it was used in the works by Graziano (22) and Graziano et al. (23) to map the global organization of the motor/premotor complex in the monkey and by neurosurgeons in epileptic patients (6, 7) (detailed information on stimulation parameters, reasons that justify prolonged stimulation, and data indicating that stimulation parameters similar to our parameters activate selective anatomical pathways is discussed in Methods, SI Methods, and Fig. S1).
The results of our study can be summarized as follows. The insula is functionally constituted of two major subdivisions: (i) a sensorimotor sector occupying its caudal–dorsal portion and (ii) an anterior/centroventral sector consisting of a mosaic of orofacial motor programs. These programs show a progressive dorsal to ventral shift from motor programs related to ingestion to motor programs with emotional and communicative content. A preliminary report on the social and emotional aspects of the insula has been published elsewhere (24).
Results
We stimulated 4,771 sites in the insula and surrounding perisylvian region in two monkeys (M1 = 2,999 sites; M2 = 1,772 sites). Responses to ICMS were elicited from 74.9% of the sites (74.4% in M1; 75.4% in M2). The elicited behavior was classified into five main categories: sensorimotor responses, feeding behavior, disgust-related behavior, affiliative behavior, and movement inhibition.
Sensorimotor Responses.
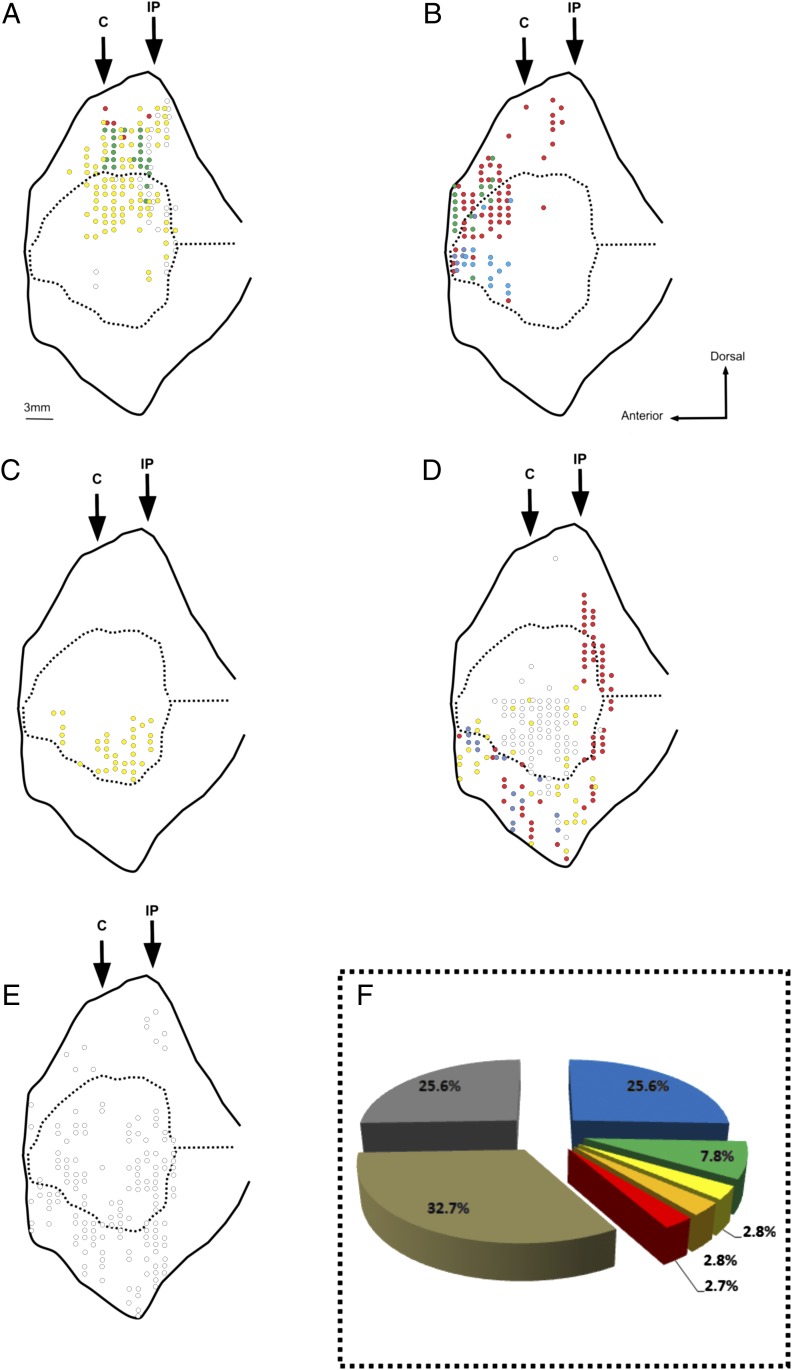
These responses consisted of elementary movements involving mouth, face, hand, or upper or lower limbs. They were evoked by stimulation of the upper bank of the Sylvian fissure and dorsal insula (Fig. 1A and Fig. S2A). Sensorimotor responses were elicited from 28.4% of the stimulated sites (25.6% in M1; 31.2% in M2).
Fig. 1.
Unfolded view of the lateral sulcus of the left hemisphere of M1 depicting its upper and lower banks and the insula. Each dot indicates the entrance point of the electrode. Black arrows indicate the antero-posterior (AP) position of the central sulcus (C) and the intraparietal sulcus (IP). In all penetrations, several sites were stimulated every 500 mm below the entrance point and above the exit point. (A) Posterior dorsal field. Red dots, mouth movements; yellow dots, hand movements; green dots, face movements; gray dots, lower and upper limbs. (B) Anterior field. Red dots, ingestive behavior; blue dots, disgust behavior; green dots, movement inhibition. (C) Ventral field. Yellow dots, affiliative behavior. (D) Miscellaneous responses. Gray dots, discomfort reactions; purple dots, gaze–trunk contralateral displacement; yellow dots, twitch of the chest; red dots, tremors. (E) Unresponsive sites. (F) Percentage of sites per each category. Blue, sensorimotor sites; green, ingestive sites; yellow, disgust sites; orange, affiliative sites; red, movement inhibition sites; tan, miscellaneous responses sites; gray, unresponsive sites.
Mouth movements were elicited from the outer part of the Sylvian upper bank (Fig. 1A and Fig. S2A, red dots) and constituted 6.2% of the sensorimotor responses (3.9% in M1; 8.5% in M2). The movements consisted of mouth opening or closing. They lasted the whole duration of the stimulation.
Face movements were elicited from the middle part of the Sylvian upper bank (Fig. 1A and Fig. S2A, green dots). They constituted 9.5% of the sensorimotor responses (10.3% in M1; 8.7% in M2). The observed movements were eye blinking or contraction of the facial musculature. They lasted the whole duration of the stimulation time. Occasionally, face movements were accompanied by upper limb movements.
Hand movements were elicited from the mediocaudal sector of the Sylvian upper bank and dorsal insula (Fig. 1A and Fig. S2A, yellow dots). They constituted 66.0% of the sensorimotor responses (63.2% in M1; 68.8% in M2). The observed movements were flexion or extension of the contralateral fingers, movements of the contralateral wrist, joint finger closure, and wrist rotation (grasping movements). Loss of muscular tone was also observed. Occasionally, hand movements were accompanied by the adduction or abduction of the arm. Goal-related movements of the upper limb were also sometimes elicited from the same region, including hand movements directed to the mouth, hand movements directed to body parts, compulsive attempts to withdraw something from the mouth, and visual inspections of the hand triggered by the stimulation onset (Movie S1).
Upper and lower limbs movements were elicited mostly from the most caudal part of the upper bank and dorsal insula (Fig. 1A and Fig. S2A, gray dots). They constituted 18.3% of the sensorimotor responses (22.6% in M1; 13.9% in M2). ICMS of these sites elicited nonspecific movements of abduction or adduction of the contralateral hindlimbs and forelimbs.
The analysis of the heart instantaneous frequency (IF) (Fig. S3) showed that stimulation of this region did not evoke any significant effect to the heart rate frequency but a slight bradycardic effect (peak deviation per behavioral outcome = −3.5%).
Ingestive Behavior.
Ingestive behavior was evoked from the rostral sector of the Sylvian upper bank and dorsal insula. It consisted of chewing and mouthing, and it was occasionally followed by swallowing (Fig. 1B, Fig. S2B, red dots, and Movie S2). The evoked movements were very similar to the movements observed during spontaneous oroalimentary behavior. It was elicited from 12.7% of all stimulated sites (7.8% in M1; 17.6% in M2).
Stimulations applied during spontaneous chewing changed the masticatory rhythm into the rhythm triggered by ICMS. Furthermore, ICMS could evoke licking movements that, in some cases, were context-dependent. Thus, when ICMS was applied while the monkey was still, the stimulation evoked a repetitive protrusion of the tongue constantly directed to the same direction. However, when the left or right part of the monkey lips was wetted with juice, the evoked tongue protrusion was directed to that part (Movie S3).
The analysis of the IF (Fig. S3) showed that ICMS applied to the ingestive field evoked a slight bradycardic effect (peak deviation per behavioral outcome = −7.7%).
Disgust and Retching.
ICMS of the region ventral to the ingestive field evoked disgust behavior (Fig. 1B and Fig. S2B, blue dots). It was elicited from 2.3% of the stimulated sites (2.8% in M1; 1.9% in M2).
The disgust behavior most commonly observed was a typical facial expression characterized by the curling of the upper lip and the wrinkling of the nose. ICMS of the same sites could also determine food refusal. In particular, when stimulation was applied while the monkey was bringing food to the mouth, the monkey immediately threw it away; when stimulation was applied with the monkey chewing the food already in its mouth, the monkey spat it (Movie S4) (24). Occasionally, the stimulation of the same sites could evoke retching (Movie S5).
The analysis of the IF (Fig. S3) showed that ICMS applied to the disgust field evoked a strong bradycardic effect (peak deviation per behavioral outcome = −17.9%).
Affiliative Behavior.
Affiliative behavior was evoked by stimulating the middle part of the ventral insula (Fig. 1C and Fig. S2C, yellow dots). It was elicited from 3.4% of the stimulated sites (2.8% in M1; 4.1% in M2). Its typical response was lip smacking (that is, a repetitive up–down movement of the jaw with the lips also repetitively opening and closing) (Movie S6). The affiliative behavior occurred only when ICMS was applied in the presence of eye contact between the monkey and the experimenter. It was lacking in the absence of it. When stimulation was applied with the monkey exhibiting another emotional expressions (i.e., threat), ICMS interrupted this spontaneous behavior and replaced it with affiliative behavior. The original behavior was resumed again after the end of the stimulation (24).
The analysis of the IF (Fig. S3) showed that ICMS applied to affiliative sites evokes a slight bradycardic effect (peak deviation per behavioral outcome = −5.1%) starting during the stimulation and lasting a few seconds after its offset.
Inhibition of Ongoing Movements.
Movement inhibition was observed after stimulation of the rostralmost part of the Sylvian upper bank (Fig. 1B and Fig. S2B, green dots). It was elicited from 3.7% of the stimulated sites (2.7% in M1; 4.8% in M2). ICMS of these sites produced an immediate inhibition of any arm movement performed by the monkey, including food grasping movements and movements bringing food to the mouth. During the whole stimulation time, the arm remained still where it was located at the stimulation onset.
The analysis of the IF (Fig. S3) showed that ICMS applied to the movement inhibition sites evokes a clear bradycardic effect (peak deviation per behavioral outcome = −8.5%).
Miscellaneous Responses.
In addition to the responses already described, other types of reactions were evoked from the ventral insula and the lower bank of the Sylvian fissure (Fig. 1D and Fig. S2D). They included (i) signs of discomfort reactions, (ii) contralateral orienting of the gaze or the trunk, (iii) twitches of chest muscles, and (iv) tremors. Globally, these reactions were elicited from 24.4% of the stimulated sites (32.7% in M1; 16% in M2).
Reactions interpreted as caused by discomfort were elicited from the ventral insula. They were evoked from 9.3% of the stimulated sites (12.4% in M1; 6.1% in M2) (Fig. 1D and Fig. S2D, gray dots). ICMS of these sites produced compulsive repetitive movements of the hands or feet and postural adjustments. The intensity of the discomfort reactions ranged from small postural adjustments to a clear psychomotor agitation. In some cases, ICMS elicited a facial grimace of distress; the grimace was also often accompanied by psychomotor agitation.
Contralateral orienting of gaze and trunk was elicited from the lower bank of the Sylvian fissure (Fig. 1D and Fig. S2D, purple dots). It consisted of a rotation of the trunk to the contralateral space and simultaneously, a shift of gaze in the same direction. This response was elicited from 2.3% of the stimulated sites (1.2% in M1; 3.3% in M2).
Twitches of chest muscles were also elicited from the ventral insula and Sylvian lower bank (Fig. 1D and Fig. S2D, yellow dots). They were elicited from 3.8% of the stimulated sites (5.7% in M1; 1.8% in M2). Often, these movements were followed by general psychomotor agitation.
Tremors were mostly evoked from the caudal insula (Fig. 1D and Fig. S2D, red dots). They were elicited from 9.1% of the stimulated sites (13.3% in M1; 4.8% in M2). Tremors were diffused to the limbs or even the whole body. There was no evidence of a somatotopic arrangement. The effect did not last beyond stimulation time. Interestingly, during ICMS-evoked tremors, the monkey did not show any sign of discomfort or other emotional facial expressions.
The analysis of the IF (Fig. S3) showed different types of heart rate modulation in different behaviors. Discomfort reactions and twitch of the chest sites showed a biphasic pattern (i.e., a strong bradycardic phase quickly replaced by a tachycardic phase; −9.4%). The contralateral displacement of gaze and trunk sites evoked a bradycardic effect (−8.6%). Twitches sites evoked a very strong bradycardic effect (−18.7%). Tremors-evoking sites elicited a tachycardic effect (8.4%).
Unresponsive Sites.
The stimulation of 25.1% (25.6% in M1; 24.5% in M2) of the sites did not elicit any detectable behavioral response. Although unresponsive sites were found in the entire stimulated region, the vast majority of them were concentrated in the lower Sylvian bank and the ventral insula (Fig. 1E and Fig. S2E). The analysis of the IF showed that ICMS applied to the unresponsive sites often evoked a very slight bradycardic effect (peak deviation per behavioral outcome = −5.4%).
Discussion
The aim of the present study was to assess the functional organization of the insula and the surrounding opercular regions. The main results of our study can be summarized as follows. (i) There is a clear functional separation between a large sensorimotor field located in the dorsocaudal portion of the insula and the rest of it. (ii) The anterior and centroventral insula consist of a mosaic of different motor programs, all of them related to specific orofacial motor behaviors. (iii) In the anterior and centroventral insula, there is a progressive dorsoventral shift from motor programs without emotional content to motor programs with such a content.
Sensorimotor Dorsal Field.
The dorsocaudal part of insula and the adjacent Sylvian upper bank are somatotopically organized. Mouth movements are represented dorsally, face movements are in an intermediate position, and hand movements are represented ventrally. The hand representation occupies the dorsal part of the insula for most of its extension. Caudally, there is a small representation of upper and lower limbs.
Our data are based on electrical stimulation, which obviously stresses the motor aspect of an area. There is no doubt, however, that the sensorimotor field is essentially a sensory field. This finding is shown (besides the simplicity of the observed movements elicited by ICMS) by its cytoarchitectonic granular structure and single neuron studies showing the presence of somatosensory responses in this field (12, 13).
Considering the lack of agreement on the extension and parcellation of area SII, it is difficult to match the part of our sensorimotor field located in the Sylvian upper bank with SII or some parts of it. One possibility is that the map that we just described is independent of SII. In favor of this interpretation is the cytoarchitectonic data in the work by Roberts and Akert (25), which localized SII in a more caudal position with respect to our sensorimotor field (ref. 26, figure 10). Furthermore, the somatotopic organization of the sensorimotor field described here is distributed along a dorsoventral axis rather than along a rostrocaudal one as in SII.
According to the work by Schneider et al. (12), sensory information coming from a region approximately corresponding to our sensorimotor field and information coming from SII (27) link primary somatosensory centers to the ventromedial limbic regions, thus playing a crucial role in somatosensory learning. In agreement with this interpretation are the connectivity data showing that the posterior part of the insula is connected with SII complex and the inferior parietal lobule but not the rostral insula (18). Eberstaller, cited in the work by Cunningham (28), wrote that “the anterior insula is connected entirely with the frontal lobe, whilst the posterior insula is exclusively connected with the parietal and temporal lobes” (28).
Finally, the absence of clear modification of the heart rate after stimulation of this field is consistent with a sensorimotor interpretation of its functional role.
Ingestive Behavior.
Ingestive behavior was evoked from the anterior sector of the dorsal insula and the adjacent part of upper Sylvian bank. The elicited motor acts included chewing, mouthing, and swallowing. Anatomically, this region occupies the orbitofrontal cortex of the Sylvian upper bank and the dorsal disgranular insula shown in the work by Roberts and Akert (25).
This region, from which we evoked ingestive motor acts, is classically considered a gustatory cortex. Indeed, taste (7, 8) and some specific aspects of somatosensory modalities (9, 10) are represented. However, the percentage of neurons responsive to such stimuli is rather low. In fact, less than 10% of the recorded neurons are responsive to taste stimuli, whereas about 20% of neurons respond to specific somatosensory stimuli such as texture, viscosity, etc. It is plausible that these last neurons, providing information on food consistency and texture, may control and drive chewing, swallowing, and more generally, ingestive behaviors. In conclusion, our data suggest that the main role of this part of anterior insula sector is integrating somatosensory and gustatory information to achieve an effective ingestive behavior. Note that this sector of the insula is disgranular, a structure consistent with a motor function. Finally, our proposal is in accord with clinical cases where oroalimentary movements have been described during epileptic seizures originating in this part of the insula (4).
Disgust-Related Field.
Ventral to the ingestive field, electrical stimulation elicited disgust-related responses (24). These responses were characterized by the grimace typical of disgust and occasionally followed by retching. More complex behaviors, such as the refusal of food intake or food spitting from the mouth, were also observed.
Unlike the stimulation of the ingestive field, which determined only slight modification of the heart rate, stimulation of this field showed potent bradycardic effects accompanying the motor expression of disgust. These data are in agreement with previous findings showing that disgust, unlike other negative emotions, is typically associated with a decrease in heart rate (29). In line with this finding is also the clinical data in the work by Catenoix et al. (30), which found that the occurrence of ictal vomiting correlated in time with a discharge affecting exclusively the anterior part of the insula.
Note the continuity between two ingestive-related behaviors: a positive one, located dorsally, and a negative one, located ventrally. It is worth stressing that IF data show that the ventrally located ICMS-evoked motor behavior is associated with a strong emotional component.
Affiliative Field.
The stimulation of the most ventral part of the midposterior insula evoked communicative responses. These responses mostly consisted of lip smacking (an affiliative gesture). They were evoked only if there was eye contact between the experimenter and the monkey. The stimulation of the same site, in the absence of eye contact, did not produce any overt behavior. When ICMS was applied during a spontaneous grimace expressing threat, the monkey’s behavior changed, switching from an aggressive to an affiliative behavior, and it resumed its previous behavior at the end of the stimulation.
Macaques are social animals. A very important element in social interactions among them is eye contact. Direct eye contact starts the interaction between two individuals and is considered an aggressive message during conflict situations. The necessity of eye contact to evoke lip smacking suggests that this sector of the insula plays an important role in activating the motor programs necessary to establish the social hierarchic positions and ranks within a given social group. Note that, unlike the other behaviors elicited by ICMS, affiliative behavior required, in addition to electrical stimulation, a specific social element involving another individual. This finding suggests that the electrical stimulation, in addition to triggering a specific motor behavior, also modified the mood of the stimulated monkey, rendering it clear to the observer.
As far as the heart rate is concerned, our data showed a slight bradycardic effect. This finding is in line with the observation that, in humans, affiliative behavior is accompanied with an increase of the parasympathetic tone.
Miscellaneous Responses.
A series of repetitive and stereotyped responses, rather difficult to decipher in terms of their behavioral meaning, were also observed in the ventral insula and adjacent lower bank of the Sylvian sulcus. As far as the ventral insula is concerned, these responses consisted of repetitive movements of the hands or feet, possibly caused by unpleasant sensations. The intensity of these putative discomfort reactions ranged from small postural adjustments to a clear psychomotor agitation. In some cases, ICMS elicited a facial grimace of distress. It is important to stress that these results are not in contrast with the finding that affiliative behavior was elicited in a partially overlapping region (SI Methods shows statistical evidence). In fact, the affiliative behavior also includes a submission component and therefore, a stressful and anxiety-related feeling.
In cases in which the discomfort was more evident, the motor responses were accompanied by biphasic cardiac responses, suggesting a dual activation of the sympathetic–parasympathetic system. The work by Paton et al. (31) reported that, unlike the classic inverse relation between sympathetic and parasympathetic activity, coactivation of the two vegetative systems occurs during the nociception. Thus, the heart pattern produced by ICMS supports our interpretation of the presence of nociceptive responses in the ventral insula.
Stimulation of the lower bank of the Sylvian sulcus evoked body rotation to the contralateral side, which was generally accompanied by gaze shifting to that same side. It is interesting to note that the work by Ferrier (32) already showed that the electrical stimulation of the outer portion of the temporal operculum elicits deviation of eyes and neck contralaterally. Clinical observations report that, during epileptic seizures involving the posterior inferior quadrant of the insula and extending to the superior temporal gyrus, head rotation is frequently observed (4). Taken together, these data suggest that the contralateral orienting behavior could be related to the excitation of auditory fields.
Functional Organization of the Insula.
The conventional wisdom is that insula is a center related to emotions. More specifically, it would constitute an intermediate station between the neocortical association areas and the core structures mediating emotions, such as the hypothalamus, the periacqueductal gray, and the centromedial nuclei of the amygdala (33). The present data offer a more complex and articulated picture of the insula functional organization.
Insula is formed by two separate sectors: a dorsal–caudal sector and the anterior one that ventrally extends into caudal direction. The dorsal–caudal sector is functionally similar to the somatosensory areas located in the parietal lobe. Its connections are almost exclusively with the posterior part of this lobe. In contrast, the anterior and central insula consists of a mosaic of motor programs mostly related to mouth and facial movements. Some of these programs are related with the emotional core structures and therefore, are endowed with an emotional valence. Thus, the anterior insula contains mouth programs related to ingestive behavior, negative (aversive) food responses including disgust and retching, affiliative gestures mostly expressed by mouth and face movements, and intermixed, aversive responses triggered by unpleasant and painful sensations.
As far as the organization of the motor mosaic is concerned, there is a clear dorsal-to-ventral trend from nonemotionally related motor programs (ingestive field) to motor programs with emotional valence (disgust and affiliative fields). In agreement with these functional data are the connections of the ingestive insula. These connections are with the frontal lobe (18) and include the masticatory field (34) located in the ventral premotor cortex and the part of F5 that contains ingestive neurons (35).
According to the work by Mesulam and Mufson (17), the human insula has “a plan of anatomical organization virtually identical to that of the macaque monkey” (17). Do the functional data support this statement? A recent metaanalysis by Kurth et al. (36), based on a large number of functional MRI studies, provided a comprehensive correlative functional picture of human data to which the present findings can be compared. According to the work by Kurth et al. (36), there are four distinct functional fields in the human insula: the sensorimotor, the socioemotional, the olfactory–gustatory, and finally, the cognitive fields. A conjunction analysis across these domains revealed that, aside from the sensorimotor field, all of the others share activations in a sector of the anterior dorsal insula.
The sensorimotor field described in the work by Kurth et al. (36) is located in the dorsal–posterior part of the insula. This field corresponds, for its functional properties and location, to our sensorimotor field. As discussed above, this field is related to the elaboration of sensory information somehow similar to the information carried out in SII and adjacent areas, and it is not related to emotions.
We already discussed the sensory properties of insular ingestive field. Our conclusion was that the sensory responses of the neurons in this field represent only one, albeit a very important, functional aspect of it. However, its fundamental function is of using this sensory information for organizing ingestive actions. The similar anatomical location of the olfactory–gustatory field in humans and monkeys suggests similar ingestive functions in both species.
In strict agreement with the present data, the part of the insula related to emotional behaviors is located in humans in the ventral part of the insula. As stated in the work by Kurth et al. (36), “after testing for specific effects evoked by emotional processing, only the anterior–ventral insula and a small cluster on the central region remained significant” (36). These locations are in full accord with our data, which also showed two ventral fields related to emotion: a rostral one related to aversive food behavior (disgust) and a central one related to affiliative behavior.
A field described in humans that we were unable to find in the monkey is the so-called cognitive field. This field is functionally very heterogeneous, being activated by language processing (e.g., lexical decision or semantic judgment), overt speech, working memory, and finally, episodic and short-term memory retrieval. It is hard to believe that all these many and diverse cognitive functions are clustered in a small insular sector. It is much more plausible that these activations occurred as a consequence of a common factor. This factor is, most likely, internal verbalization, obviously necessary for some verbal tasks and underpinning the other cognitive functions mentioned above.
Note that the notion that a sector of the anterior insula is a language center is supported by a vast amount of clinical data. The work by Ojemann and Whitaker (37), for example, by using electrical stimulation of the human insula concluded that the rostral insula is an extension of the language center situated in the frontal lobe. Consistent with this proposal is the data reported in the work by Vignolo et al. (38), which described cases of global aphasia with lesions centered on the insula. Finally, there is evidence coming from single cases showing aphasia after left anterior insular infarction (39).
Some Evolutionary Considerations.
On the basis of the data of the present study, it is interesting to discuss some ethological hypotheses concerning the evolution of communication in primates. According to the work by Van Hoff (40), many of the most common communicative gestures in monkeys, such as lip smacking or lips protruding from faces, are ritualizations of ingestive actions that monkeys use for affiliative purposes (41). Similarly, the work by Redican (42) stressed the similarity between lip smacking and other facial communicative gestures and ingestive cyclicities. The presence of an ingestive center in the insula, shown in the present study, and its proximity with the affiliative region in the insula provide support to these ethological proposals.
Another consequence of the notion that the more advanced functions of the insula derived from mouth ingestive motor programs is the possible link between ingestive behavior and speech. As suggested in the work by MacNeilage (43), a species-specific organization property of speech is a continual mouth open–close alternation, the two phases of which are subject to continual articulatory modulation. The cycle constitutes the syllable, and the open–closed phases are segments, vowels, and consonants, respectively. It is plausible that this communication related frames evolved when the ingestive-related cyclicities of mandibular oscillations (associated to mastication, sucking ,and licking) assumed communicative significance (see lip smacking and other communicative gestures of nonhuman primates). Finally, the proximity of the insular primitive speech center with the premotor areas controlling fine mouth and orolaringeal movements (area 44 and ventral premotor cortex) might have allowed the transformation of simple open–close mouth movements into the motorically complex activity necessary for speech.
Methods
The experiments were carried out on two behaving macaque monkeys (Macaca mulatta). Before the experiments, the monkeys were operated under general anesthesia, and a head-holder and two recording chambers were implanted (SI Methods).
ICMS were performed through low-impedance (<200 KΩ) tungsten microelectrodes with epoxylite insulation (FHC). Penetrations were made perpendicularly to the lateral sulcus, and they were spaced at 1- (M1) and 2-mm (M2) intervals in the rostrocaudal axis and 0.5- (M1) and 1-mm (M2) intervals in the mediolateral axis. A microdrive was attached to a stereotaxic arm and fixed to the monkey head-holder. The electrodes were inserted through the dura that was left intact and moved by a hydraulic micromanipulator. Neuronal activity was amplified (Bak Electronics) and monitored on an oscilloscope. The position of the microelectrodes in the deep cortical regions was monitored by means of ultrasound technique (Logiq 400CL ProSeries; General Electric Medical System). When the target region was reached, ICMS was applied every 500 μm from the upper bank to the lower bank of the lateral sulcus. ICMS was applied by means of a Biphasic Pulse Generator (BAK) connected to an isolation unit (Stimulus Isolator; WPI). Stimulation was triggered by a hand-held button and consisted of a train of 200-μs biphasic pulses with cathodal pulse leading. Trains were delivered at 50 Hz with an intensity of 4 mA for 3 s. The behavioral responses were included in the dataset only when two observers recognized the elicited behavior, and this behavior could be evoked in more than 50% of ICMS trials. For each site, the first ICMS was delivered after a period of 60 s, during which time the monkey was quiet and its heart rate was around 120 bpm. Electrocardiogram (EKG) traces (SI Methods) were recorded in each site during the first stimulation and the next 10 s after stimulation. After the first stimulation, additional ICMS was applied at least five times. All experiments were videotaped.
Supplementary Material
Acknowledgments
We thank Giuseppe Luppino, Stefano Rozzi, Marzio Gerbella, and Elena Borra for technical support and histological analysis. This study was supported by the Italian Ministero Istruzione Università Ricerca (G.R.), European Union Contract 027017 (Neuroprobes), and Rete Tecnologica Multidisciplinare-Italian Instituite of Technology.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1200143109/-/DCSupplemental.
References
- 1.Craig AD. How do you feel—now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- 2.Lamm C, Singer T. The role of anterior insular cortex in social emotions. Brain Struct Funct. 2010;214:579–591. doi: 10.1007/s00429-010-0251-3. [DOI] [PubMed] [Google Scholar]
- 3.Krolak-Salmon P, et al. An attention modulated response to disgust in human ventral anterior insula. Ann Neurol. 2003;53:446–453. doi: 10.1002/ana.10502. [DOI] [PubMed] [Google Scholar]
- 4.Isnard J, Guénot M, Sindou M, Mauguière F. Clinical manifestations of insular lobe seizures: A stereo-electroencephalographic study. Epilepsia. 2004;45:1079–1090. doi: 10.1111/j.0013-9580.2004.68903.x. [DOI] [PubMed] [Google Scholar]
- 5.Ostrowsky K, et al. Representation of pain and somatic sensation in the human insula: A study of responses to direct electrical cortical stimulation. Cereb Cortex. 2002;12:376–385. doi: 10.1093/cercor/12.4.376. [DOI] [PubMed] [Google Scholar]
- 6.Stephani C, Fernandez-Baca Vaca G, Maciunas R, Koubeissi M, Lüders HO. Functional neuroanatomy of the insular lobe. Brain Struct Funct. 2011;216:137–149. doi: 10.1007/s00429-010-0296-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sudakov K, MacLean PD, Reeves A, Marino R. Unit study of exteroceptive inputs to claustrocortex in awake, sitting, squirrel monkey. Brain Res. 1971;28:19–34. doi: 10.1016/0006-8993(71)90521-x. [DOI] [PubMed] [Google Scholar]
- 8.Yaxley S, Rolls ET, Sienkiewicz ZJ. Gustatory responses of single neurons in the insula of the macaque monkey. J Neurophysiol. 1990;63:689–700. doi: 10.1152/jn.1990.63.4.689. [DOI] [PubMed] [Google Scholar]
- 9.Ogawa H, Ito S, Nomura T. Oral cavity representation at the frontal operculum of macaque monkeys. Neurosci Res. 1989;6:283–298. doi: 10.1016/0168-0102(89)90021-7. [DOI] [PubMed] [Google Scholar]
- 10.Verhagen JV, Kadohisa M, Rolls ET. Primate insular/opercular taste cortex: Neuronal representations of the viscosity, fat texture, grittiness, temperature, and taste of foods. J Neurophysiol. 2004;92:1685–1699. doi: 10.1152/jn.00321.2004. [DOI] [PubMed] [Google Scholar]
- 11.Smith-Swintosky VL, Plata-Salaman CR, Scott TR. Gustatory neural coding in the monkey cortex: Stimulus quality. J Neurophysiol. 1991;66:1156–1165. doi: 10.1152/jn.1991.66.4.1156. [DOI] [PubMed] [Google Scholar]
- 12.Schneider RJ, Friedman DP, Mishkin M. A modality-specific somatosensory area within the insula of the rhesus monkey. Brain Res. 1993;621:116–120. doi: 10.1016/0006-8993(93)90305-7. [DOI] [PubMed] [Google Scholar]
- 13.Robinson CJ, Burton H. Somatotopic organization in the second somatosensory area of M. fascicularis. J Comp Neurol. 1980;192:43–67. doi: 10.1002/cne.901920104. [DOI] [PubMed] [Google Scholar]
- 14.Kaada BR, Pribram KH, Epstein JA. Respiratory and vascular responses in monkeys from temporal pole, insula, orbital surface and cingulate gyrus; a preliminary report. J Neurophysiol. 1949;12:347–356. doi: 10.1152/jn.1949.12.5.347. [DOI] [PubMed] [Google Scholar]
- 15.Frontera JG. Some results obtained by electrical stimulation of the cortex of the island of Reil in the brain of the monkey (Macaca mulatta) J Comp Neurol. 1956;105:365–394. doi: 10.1002/cne.901050303. [DOI] [PubMed] [Google Scholar]
- 16.Showers MJC, Lauer EW. Somatovisceral motor patterns in the insula. J Comp Neurol. 1961;117:107–115. doi: 10.1002/cne.901170109. [DOI] [PubMed] [Google Scholar]
- 17.Mesulam MM, Mufson EJ. Insula of the old world monkey. I. Architectonics in the insulo-orbito-temporal component of the paralimbic brain. J Comp Neurol. 1982a;212:1–22. doi: 10.1002/cne.902120102. [DOI] [PubMed] [Google Scholar]
- 18.Mesulam MM, Mufson EJ. Insula of the old world monkey. III: Efferent cortical output and comments on function. J Comp Neurol. 1982c;212:38–52. doi: 10.1002/cne.902120104. [DOI] [PubMed] [Google Scholar]
- 19.Gallay DS, Gallay MN, Jeanmonod D, Rouiller EM, Morel A. The insula of Reil revisited: Multiarchitectonic organization in macaque monkeys. Cereb Cortex. 2012;22:175–190. doi: 10.1093/cercor/bhr104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caggiula AR, Hoebel BG. “Copulation-reward site” in the posterior hypothalamus. Science. 1966;153:1284–1285. doi: 10.1126/science.153.3741.1284. [DOI] [PubMed] [Google Scholar]
- 21.Hoebel BG. Feeding and self-stimulation. Ann N Y Acad Sci. 1969;157:758–778. doi: 10.1111/j.1749-6632.1969.tb12919.x. [DOI] [PubMed] [Google Scholar]
- 22.Graziano MSA. The organization of behavioral repertoire in motor cortex. Annu Rev Neurosci. 2006;29:105–134. doi: 10.1146/annurev.neuro.29.051605.112924. [DOI] [PubMed] [Google Scholar]
- 23.Graziano MSA, Aflalo TN, Cooke DF. Arm movements evoked by electrical stimulation in the motor cortex of monkeys. J Neurophysiol. 2005;94:4209–4223. doi: 10.1152/jn.01303.2004. [DOI] [PubMed] [Google Scholar]
- 24.Caruana F, Jezzini A, Sbriscia-Fioretti B, Rizzolatti G, Gallese V. Emotional and social behaviors elicited by electrical stimulation of the insula in the macaque monkey. Curr Biol. 2011;21:195–199. doi: 10.1016/j.cub.2010.12.042. [DOI] [PubMed] [Google Scholar]
- 25.Roberts TS, Akert K. Insular and opercular cortex and its thalamic projection in Macaca mulatta. Schweiz Arch Neurol Neurochir Psychiatr. 1963;92:1–43. [PubMed] [Google Scholar]
- 26.Gerbella M, Belmalih A, Borra E, Rozzi S, Luppino G. Cortical connections of the anterior (F5a) subdivision of the macaque ventral premotor area F5. Brain Struct Funct. 2011;216:43–65. doi: 10.1007/s00429-010-0293-6. [DOI] [PubMed] [Google Scholar]
- 27.Mishkin M. Analogous neural models for tactual and visual learning. Neuropsychologia. 1979;17:139–151. doi: 10.1016/0028-3932(79)90005-8. [DOI] [PubMed] [Google Scholar]
- 28.Cunningham DJ. Development of the gyri and sulci on the surface of the island of reil of the human brain. J Anat Physiol. 1891;25:338–348. [PMC free article] [PubMed] [Google Scholar]
- 29.Rozin P, Haidt J, McCauley CR. Disgust. In: Lewis M, Haviland-Jones JM, editors. Handbook of Emotions. 2nd Ed. New York: Guilford; 2000. [Google Scholar]
- 30.Catenoix H, et al. The role of the anterior insular cortex in ictal vomiting: A stereotactic electroencephalography study. Epilepsy Behav. 2008;13:560–563. doi: 10.1016/j.yebeh.2008.06.019. [DOI] [PubMed] [Google Scholar]
- 31.Paton JFR, Boscan P, Pickering AE, Nalivaiko E. The yin and yang of cardiac autonomic control: Vago-sympathetic interactions revisited. Brain Res Brain Res Rev. 2005;49:555–565. doi: 10.1016/j.brainresrev.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 32.Ferrier D. Experiments on the brain of the monkeys, N.1. Proc R Soc London. 23:409–430. [Google Scholar]
- 33.Gothard K, Hoffman K. Circuits of emotion in the primate brain. In: Platt M, Ghazanfar A, editors. Primate Neuroethology. New York: Oxford University Press; 2010. pp. 292–315. [Google Scholar]
- 34.Luschei ES, Goldberg LJ. Neural mechanisms of mandibular control: Mastication and voluntary biting. In: Brookhart JM, Mountcastle VB, Brooks VB, editors. Handbook of Physiology, Section I. The Nervous System, Vol. II. Motor Control. Washington, DC: American Physiological Society; 1981. [Google Scholar]
- 35.Ferrari PF, Gallese V, Rizzolatti G, Fogassi L. Mirror neurons responding to the observation of ingestive and communicative mouth actions in the monkey ventral premotor cortex. Eur J Neurosci. 2003;17:1703–1714. doi: 10.1046/j.1460-9568.2003.02601.x. [DOI] [PubMed] [Google Scholar]
- 36.Kurth F, Zilles K, Fox PT, Laird AR, Eickhoff SB. A link between the systems: Functional differentiation and integration within the human insula revealed by meta-analysis. Brain Struct Funct. 2010;214:519–534. doi: 10.1007/s00429-010-0255-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ojemann GA, Whitaker HA. Language localization and variability. Brain Lang. 1978;6:239–260. doi: 10.1016/0093-934x(78)90061-5. [DOI] [PubMed] [Google Scholar]
- 38.Vignolo LA, Boccardi E, Caverni L. Unexpected CT-scan findings in global aphasia. Cortex. 1986;22:55–69. doi: 10.1016/s0010-9452(86)80032-6. [DOI] [PubMed] [Google Scholar]
- 39.Shuren J. Insula and aphasia. J Neurol. 1993;240:216–218. doi: 10.1007/BF00818707. [DOI] [PubMed] [Google Scholar]
- 40.Van Hoff JARAM. The facial display of the Catarrhine monkeys and apes. In: Morris D, editor. Primate Ethology. Chicago: Aldine Transaction; 1967. pp. 7–65. [Google Scholar]
- 41.Darwin C. The Expressions of the Emotions in Men and Animals. London: John Murray; 1872. [Google Scholar]
- 42.Redican W. Facial expression in nonhuman primates. In: Rosenblum LA, editor. Primate Behavior. New York: Academic; 1975. [Google Scholar]
- 43.MacNeilage PF. The frame/content theory of evolution of speech production. Behav Brain Sci. 1998;21:499–511. doi: 10.1017/s0140525x98001265. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.