Abstract
Osteoclasts are generated from monocyte/macrophage-lineage precursors in response to colony-stimulating factor 1 (CSF-1) and receptor activator of nuclear factor-κB ligand (RANKL). CSF-1–mutated CSF-1op/op mice as well as RANKL−/− mice exhibit osteopetrosis (OP) caused by osteoclast deficiency. We previously identified RANKL receptor (RANK)/CSF-1 receptor (CSF-1R) double-positive cells as osteoclast precursors (OCPs), which existed in bone in RANKL−/− mice. Here we show that OCPs do not exist in bone but in spleen in CSF-1op/op mice, and spleen acts as their reservoir. IL-34, a newly discovered CSF-1R ligand, was highly expressed in vascular endothelial cells in spleen in CSF-1op/op mice. Vascular endothelial cells in bone also expressed IL-34, but its expression level was much lower than in spleen, suggesting a role of IL-34 in the splenic generation of OCPs. Splenectomy (SPX) blocked CSF-1–induced osteoclastogenesis in CSF-1op/op mice. Osteoclasts appeared in aged CSF-1op/op mice with up-regulation of IL-34 expression in spleen and bone. Splenectomy blocked the age-associated appearance of osteoclasts. The injection of 2-methylene-19-nor-(20S)-1α,25(OH)2D3 (2MD), a potent analog of 1α,25-dihidroxyvitamin D3, into CSF-1op/op mice induced both hypercalcemia and osteoclastogenesis. Administration of 2MD enhanced IL-34 expression not only in spleen but also in bone through a vitamin D receptor-mediated mechanism. Either splenectomy or siRNA-mediated knockdown of IL-34 suppressed 2MD-induced osteoclastogenesis. These results suggest that IL-34 plays a pivotal role in maintaining the splenic reservoir of OCPs, which are transferred to bone in response to diverse stimuli, in CSF-1op/op mice. The present study also suggests that the IL-34 gene in vascular endothelial cells is a unique target of vitamin D.
Keywords: platelet endothelial cell adhesion molecule 1-positive cells, osteoblasts, blood stream
Osteoclasts are bone-resorbing cells generated from monocyte/macrophage-lineage precursors. The differentiation of osteoclast precursors (OCPs) into osteoclasts is regulated by bone-forming osteoblasts. Osteoblastic cells express two cytokines responsible for osteoclastogenesis: one is colony-stimulating factor 1 [CSF-1, also called macrophage colony-stimulating factor (M-CSF)] and the other is receptor activator of nuclear factor-κB ligand (RANKL). OCPs express CSF-1 receptor (CSF-1R, also called c-Fms) and RANK (receptor for RANKL) and differentiate into osteoclasts in response to CSF-1 and RANKL. The expression of RANKL is up-regulated by osteoclast-inducing factors such as parathyroid hormone (PTH) and 1α,25-dihydroxyvitamin D3 [1α,25(OH)2D3] (1, 2).
CSF-1 is the most potent growth factor for monocytes/macrophages (3), but its synthesis by osteoblasts occurs independently of PTH and 1α,25(OH)2D3 (2). CSF-1op/op mice cannot produce a functionally active CSF-1 (4), and therefore, exhibit monocytopenia and osteopetrosis (OP) (5, 6). However, several curious phenomena have been observed in CSF-1op/op mice. First, osteoclasts are totally absent in young CSF-1op/op mice, but appear in aged CSF-1op/op mice (7). Second, osteopetrotic characteristics of CSF-1R−/− mice are more severe than those of CSF-1op/op mice (8). Third, F4/80+ [F4/80(+)] macrophages exist in the splenic red pulp in CSF-1op/op mice as well as in WT mice, and their number is regulated by a mechanism independently of CSF-1 (9, 10). Fourth, the administration of vascular endothelial growth factor (VEGF) rescues osteopetrosis in CSF-1op/op mice (11, 12), but VEGF cannot substitute for CSF-1 to induce osteoclast formation in vitro (13).
Recently, Lin et al. (14) discovered IL-34, as a new ligand for CSF-1R. The amino acid sequence of IL-34 was quite different from that of CSF-1, but IL-34 promoted macrophage colony formation like CSF-1 did. IL-34 was specifically expressed in splenic tissues, predominantly in the red pulp region. When IL-34 was expressed under the control of the CSF-1 promotor in CSF-1op/op mice, the osteopetrotic phenotype was rescued (15). IL-34 in combination with RANKL induced osteoclastic differentiation of progenitor cells in mouse (16, 17) and human (17) cell culture systems. However, it remains unclear why IL-34 cannot substitute for CSF-1 in CSF-1op/op mice in vivo.
Using RANKL−/− mice and CSF-1op/op mice, we identified cell-cycle–arrested RANK/CSF-1R double-positive [RANK(+)/CSF-1R(+)] cells as the direct OCPs in vivo (18). When RANKL was administered to RANKL−/− mice and CSF-1 to CSF-1op/op mice, OCPs similarly differentiated into osteoclasts in bone tissue without cell cycle progression. OCPs were detected in the vicinity of osteoblastic cells in RANKL−/− mice, suggesting the existence of OCPs in bone in WT mice. However, our preliminary experiments showed that OCPs were not present in bone in CSF-1op/op mice.
The active form of vitamin D3 [1α,25(OH)2D3] regulates calcium homeostasis by acting on various types of cells such as intestinal endothelial cells, renal tubular cells, and osteoblastic cells (19). Shevde et al. (20) reported that 2-methylene-19-nor-(20S)-1α,25(OH)2D3 (2MD), a highly potent analog of 1α,25(OH)2D3, strongly enhanced osteoblastic cell-mediated osteoclast formation and also induced bone formation in vitro and in vivo. We have synthesized a derivative of 2MD at carbon 2 (2α-methyl-19-nor-(20S)-1α,2β,25(OH)3D3, 2-methyl-2MD), and showed that osteoclastic bone resorption is indispensable for the hypercalcemic action of the 2MD analog in vivo (21). Recently, increasing evidence has been accumulating in showing that 1α,25(OH)2D3 directly regulates activities of vascular endothelial cells through vitamin D receptors (22–24). These results suggest that 1α,25(OH)2D3 plays a unique role in the vascular system beyond the classical role in calcium homeostasis.
In the present study, we examined how osteoclasts were formed in CSF-1op/op mice in response to various stimuli. We found that OCPs existed in spleen but not in bone in CSF-1op/op mice. OCPs in CSF-1op/op mice were transferred from spleen to bone and differentiated into osteoclasts in response to CSF-1, VEGF, and 2MD administrations, and also to aging. IL-34 appeared to play a pivotal role in the generation and storage of OCPs in spleen and osteoclastogenesis in CSF-1op/op mice. In addition, we have shown that the IL-34 gene in the vascular endothelial cells is a unique target of vitamin D.
Results
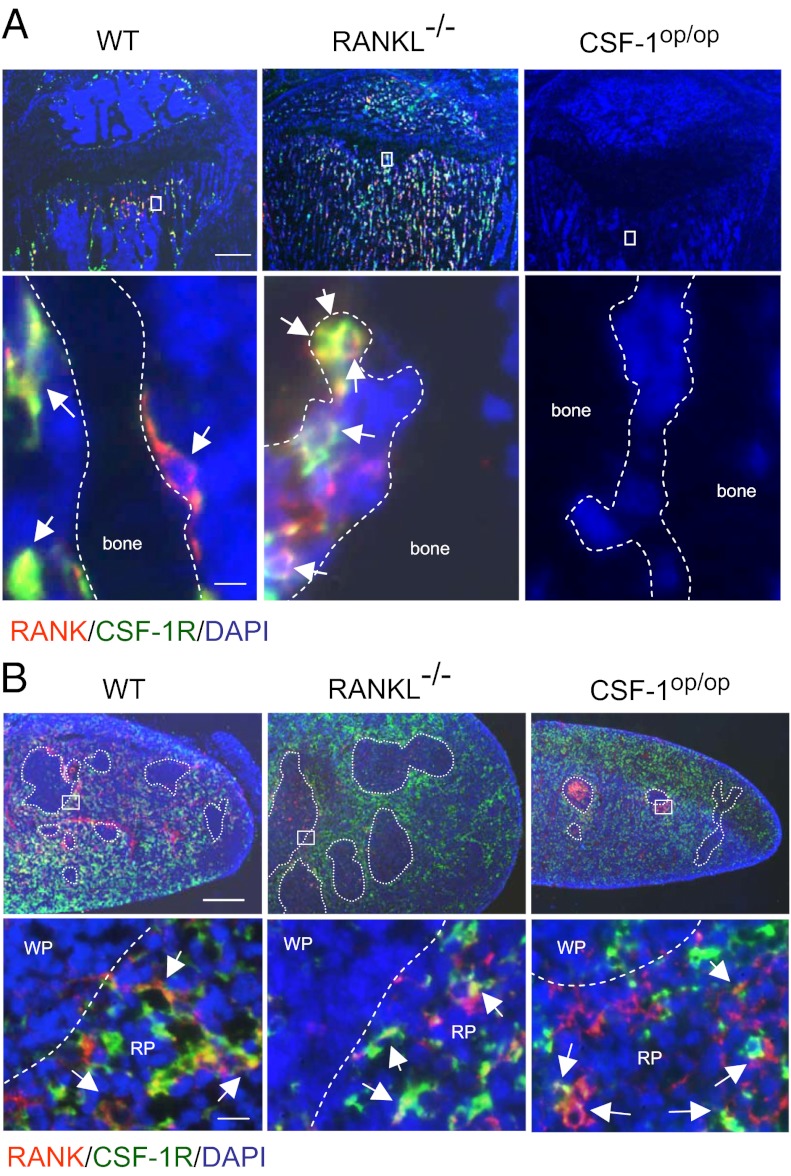
Immunohistochemical analysis showed that RANK(+) cells and CSF-1R(+) cells were present in the proximal region of tibiae obtained from RANKL−/− mice as well as from WT mice (Fig. 1A). Most of the RANK(+) cells expressed CSF-1R in WT mice. Cells double positive for RANK and CSF-1R [RANK(+)/CSF-1R(+) cells] were also detected in RANKL−/− mice and identified as the direct OCPs in vivo (18). Conversely, neither RANK(+) cells nor CSF-1R(+) cells were detected in bone tissue in CSF-1op/op mice (Fig. 1A, Right). In contrast, RANK(+) cells, CSF-1R(+) cells, and RANK(+)/CSF-1R(+) cells (arrows) were detected in spleen in CSF-1op/op mice as well as in WT mice and RANKL−/− mice (Fig. 1B, Right). RANK(+)/CSF-1R(+) cells were mainly observed in the red pulp region and marginal zones. These results suggest that OCPs do not exist in bone but do exist in spleen in CSF-1op/op mice.
Fig. 1.
Distribution of OCPs in bone and spleen in WT, RANKL−/−, and CSF-1op/op mice. (A) Localization of RANK (red) and CSF-1R (green) in proximal tibiae in WT, RANKL−/−, and CSF-1op/op mice. Nuclei were stained with DAPI (blue). Lower panels show magnified views of the boxed areas in the Upper panels. Dashed lines represent bone surface. Arrows indicate RANK(+)/CSF-1R(+) cells. [Scale bar, 400 μm (Upper), 5 μm (Lower).] (B) Localization of RANK (red) and CSF-1R (green) in spleen in WT, RANKL−/−, and CSF-1op/op mice. Dashed circles represent the white pulp. Lower panels show magnified views of the boxed areas in the Upper panels. WP; white pulp, RP; red pulp. [Scale bar, 400 μm (Upper), 10 μm (Lower).]
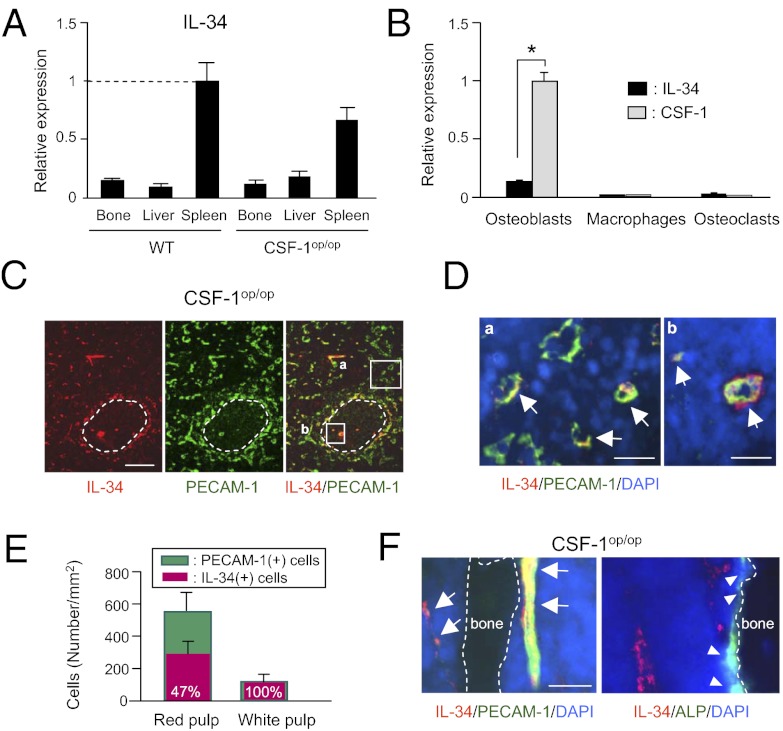
We then examined the tissue distribution of IL-34 mRNA (Fig. 2A). Consistent with the previous report (14), IL-34 was expressed predominantly in spleen but slightly in bone (Fig. 2A). The osteoblast level of IL-34 mRNA was much lower compared with that of CSF-1 mRNA (Fig. 2B). Little IL-34 mRNA or CSF-1 mRNA was expressed in bone marrow (BM) macrophages and osteoclastic cells (Fig. 2B). IL-34(+) cells were mainly distributed in the splenic red pulp region and marginal zones (Fig. 2C), where RANK(+)/CSF-1R(+) cells were detected (Fig. 1B). IL-34(+) cells were similarly distributed in the spleen in WT mice (Fig. S1 A and B). Most IL-34(+) cells expressed platelet endothelial cell adhesion molecule 1 (PECAM-1), a marker of vascular endothelial cells (Fig. 2 C–E and Fig. S1 A and B). Cells double positive for IL-34 and PECAM-1 [IL-34(+)/PECAM-1(+) cells] were detected as endothelial cells in blood vessels. Most of the endothelial cells found in white pulp also expressed IL-34 (Fig. 2 C–E). Notably, endothelial cells in central arterioles robustly expressed IL-34 (Fig. 2D). Next, we examined the distribution of IL-34 expression in bone. PECAM-1(+) cells expressed IL-34 but alkaline phosphatase (ALP)(+) osteoblastic cells did not (Fig. 2F and Fig. S1C). The endothelial cell population was much lower in bone than in spleen, consistent with the result of real-time RT-PCR (Fig. 2A). These results suggest that IL-34 is involved in the generation of OCPs in spleen in CSF-1op/op mice.
Fig. 2.
Distribution of IL-34 expression in WT and CSF-1op/op mice. (A) Real-time RT-PCR measurements of IL-34 mRNA expression in bone, liver, and spleen in 3-wk-old WT and CSF-1op/op mice. Data obtained from triplicate PCRs using RNA from different mice are expressed as the mean ± SD (n = 3). (B) Real-time RT-PCR measurements of IL-34 and CSF-1 mRNA expression in osteoblastic cells, BM macrophages, and osteoclasts. Data obtained from triplicate PCRs are expressed as the mean ± SD *P < 0.01. (C) Localization of IL-34 (red) and PECAM-1 (green) in spleen in CSF-1op/op mice. Dashed circles represent the white pulp. The outsides of the circles show the marginal zones and red pulp. (Scale bar, 100 μm.) (D) Magnified views of the boxed areas [(a) red pulp and (b) white pulp] in C. Nuclei were stained with DAPI (blue). Arrows indicate IL-34(+)/PECAM-1(+) cells. (Scale bar, 20 μm.) (E) Number of IL-34(+) cells and PECAM-1(+) cells in the red and white pulp. Values in red bars represent percentages of IL-34(+) cells among PECAM-1(+) cells. Data are expressed as the mean ± SD for four optical fields. (F) Localization of IL-34 (red) and PECAM-1 (green) (Left) and that of IL-34 (red) and ALP activity (green) (Right) in the proximal tibiae in CSF-1op/op mice. Dashed lines represent bone surface. Arrows indicate IL-34(+)/PECAM-1(+) cells. Arrowheads indicate ALP(+) osteoblastic cells. (Scale bar, 20 μm.)
Then, we examined the biological activities of IL-34 in several assays and found them to be similar to those of CSF-1 (Fig. S2). Consistent with the previous reports (14–17), IL-34 promoted not only the proliferation of BM macrophages (Fig. S2A), but also the formation of osteoclasts (Fig. S2B), both of which were similarly inhibited by adding αCSF-1R Ab. IL-34 as well as CSF-1 supported the survival of osteoclasts, which was similarly inhibited by adding αCSF-1R Ab (Fig. S2C). Thus, IL-34 is concluded to stimulate osteoclastogenesis through CSF-1R. These results suggest that splenic OCPs are transferred from spleen to bone in response to CSF-1/IL-34 administration in CSF-1op/op mice.
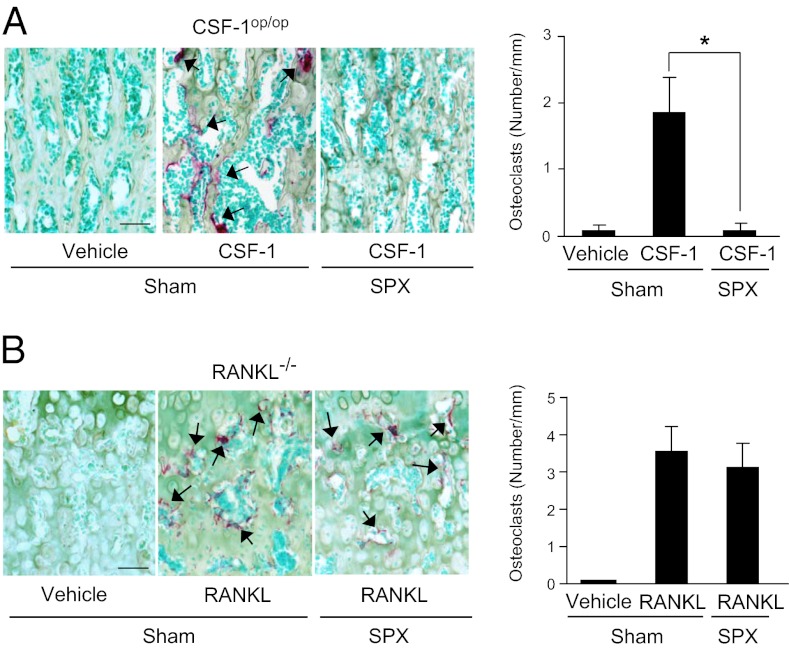
We then examined the effect of splenectomy (SPX) on osteoclast formation in CSF-1op/op mice (Fig. 3A). Three-week-old CSF-1op/op mice were subjected to SPX or a sham operation (Sham) and injected with CSF-1 4 d after the surgery. The CSF-1 injection produced tartrate-resistant acid phosphatase (TRAP, an osteoclast marker)(+) osteoclasts in bone in Sham CSF-1op/op mice, but not in SPX CSF-1op/op mice. RANKL−/− mice were also subjected to SPX or Sham and examined for osteoclastogenesis in response to RANKL (Fig. 3B). The RANKL injection produced osteoclasts in bone both in Sham and SPX RANKL−/− mice. RANKL appeared to induce osteoclasts to form from RANK(+)/CSF-1R(+) cells preexisting in bone in RANKL−/− mice.
Fig. 3.
Effects of SPX on osteoclastogenesis in CSF-1op/op mice and RANKL−/− mice. Three-week-old CSF-1op/op mice (A) and 8-wk-old RANKL−/− mice (B) were subjected to SPX or Sham. Four days later, CSF-1op/op mice and RANKL−/− mice were administered CSF-1 (107 units/kg) and RANKL (2.5 mg/kg), respectively, daily for 4 d and killed 24 h after the last injection. Sections of tibiae were double stained for TRAP and methyl green (Left), and the number of TRAP(+) osteoclasts was counted (Right). Arrows indicate TRAP(+) osteoclasts. Data are expressed as the mean ± SD for four optical fields from four mice. *P < 0.01. (Scale bar, 50 μm.)
The administration of VEGF improved the phenotype of osteopetrosis in CSF-1op/op mice (11, 12), and a deficiency of VEGFR1 worsened it (12). VEGF may stimulate the growth and IL-34 synthesis of vascular endothelial cells. Consistent with the previous reports (11, 12), the injection of VEGF-A120 (VEGF120) into CSF-1op/op mice increased the appearance of TRAP(+) osteoclasts (Fig. S3A). SPX prevented VEGF-A120–induced osteoclastogenesis in CSF-1op/op mice. However, the VEGF120 injection did not increase the expression of IL-34 mRNA in bone, liver, or spleen in CSF-1op/op mice (Fig. S3B). These results suggest that VEGF induces loosening of the endothelial cell contacts (25), and the subsequent entry of OCPs into the blood stream.
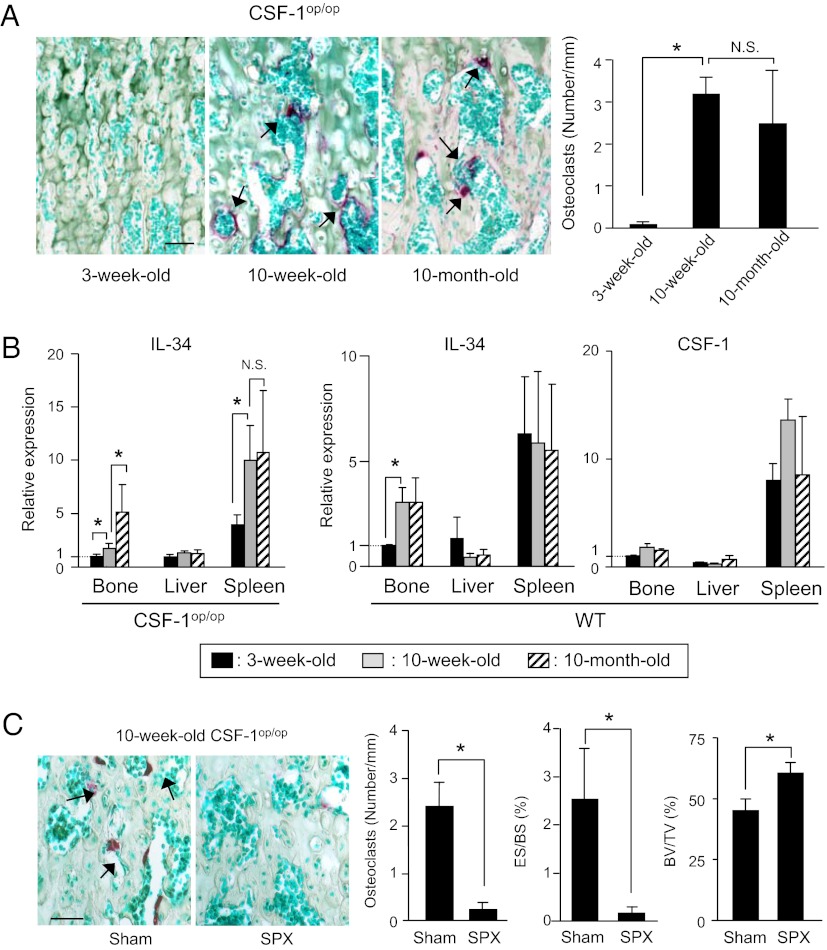
We next examined the possibilities of whether the spleen acts as a reservoir of OCPs in aged CSF-1op/op mice and whether IL-34 is involved in this process as well (Fig. 4). TRAP(+) osteoclasts were detected in bone in 10-wk-old and 10-mo-old CSF-1op/op mice (Fig. 4A). The expression of IL-34 mRNA in bone and spleen but not in the liver increased with aging in CSF-1op/op mice (Fig. 4B). WT mice also exhibited an age-associated increase in IL-34 expression in bone. CSF-1 mRNA expression showed no correlation with aging in WT mice (Fig. 4B). Then, 5-wk-old CSF-1op/op mice were subjected to SPX or Sham. Five weeks later, tibiae were recovered and examined for osteoclastogenesis (Fig. 4C). Histomorphometric analysis of tibiae showed that SPX suppressed the age-associated appearance of osteoclasts, erosion surface/bone surface (ES/BS), and increased bone volume/tissue volume (BV/TV) in aged CSF-1op/op mice (Fig. 4C). These results suggest that spleen acts as a reservoir of OCPs in the age-associated appearance of osteoclasts in CSF-1op/op mice.
Fig. 4.
Role of spleen for age-associated osteoclastogenesis in CSF-1op/op mice. (A) Appearance of osteoclasts in CSF-1op/op mice in an age-dependent manner. Sections of tibiae obtained from CSF-1op/op mice at different ages were double stained for TRAP and methyl green (Left), and the number of osteoclasts was counted (Right). Arrows indicate TRAP(+) osteoclasts. Data are expressed as the mean ± SD for four optical fields from four mice. (B) Expression of IL-34 and CSF-1 mRNAs in bone, liver, and spleen in CSF-1op/op mice and WT mice at various ages. Data were obtained from triplicate PCRs using RNA from three mice and expressed as the mean ± SD. (C) Effect of splenectomy on age-associated osteoclastogenesis. Five-week-old CSF-1op/op mice were subjected to SPX or Sham. Five weeks later, they were killed. Sections of tibiae were double stained for TRAP and methyl green (Left). Osteoclast number (number/mm), erosion surface/bone surface (ES/BS, %), and bone volume/tissue volume, (BV/TV, %) were measured (Right). Data are expressed as the mean ± SD for four optical fields from four mice. (Scale bar, 50 μm.) *P < 0.01. NS, not significant.
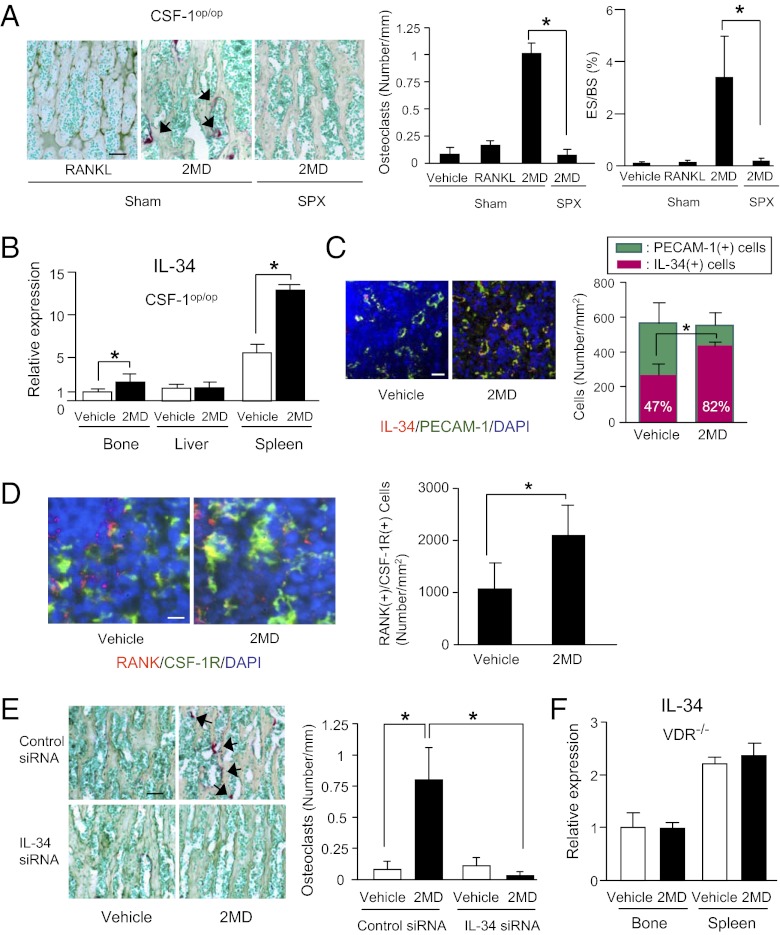
We previously reported that a large amount of a 2MD analog, 2-methyl-2MD, induced hypercalcemia in WT mice, but not in c-Fos−/− mice (21). The potency of 2-methyl-2MD as well as 2MD in inducing osteoclastogenesis was 100 times higher than that of 1α,25(OH)2D3 (21). In the course of investigating further, we found that administration of 2-methyl-2MD to CSF-1op/op mice induced hypercalcemia (Fig. S4). We, then, examined whether administration of the original 2MD induces osteoclastogenesis in CSF-1op/op mice. CSF-1op/op mice subjected to SPX or Sham were injected with a large amount of 2MD (Fig. 5A). The 2MD injection induced the appearance of TRAP(+) osteoclasts in bone in Sham but not in SPX CSF-1op/op mice. 2MD increased erosion surface in parallel with the increase of osteoclast number in Sham but not in SPX CSF-1op/op mice (Fig. 5A). 2MD is known to induce RANKL expression in osteoblastic cells (20, 21). Then, RANKL was injected into CSF-1op/op mice, but neither the osteoclast formation nor the increase in erosion surface (ES/BS) was observed (Fig. 5A). The administration of 2MD stimulated the expression of IL-34 mRNA in spleen and bone in CSF-1op/op mice (Fig. 5B). The number of IL-34(+)/PECAM-1(+) cells and that of RANK(+)/CSF-1R(+) cells were also increased in response to 2MD administration (Fig. 5 C and D). We then performed siRNA-mediated knockdown of IL-34. Using fluorescence-labeled control siRNA, we confirmed that the siRNA was successfully delivered to spleen and bone (Fig. S5A). Then IL-34 siRNA or control siRNA was injected into CSF-1op/op mice 24 h before the administration of 2MD. The expression of IL-34 mRNA in spleen was reduced by up to 80% by adding IL-34 siRNA (Fig. S5B). IL-34 siRNA but not control siRNA suppressed the 2MD-induced osteoclastogenesis in CSF-1op/op mice (Fig. 5E). These results indicate that IL-34 is involved in the 2MD-induced mobilization of OCPs from spleen to bone in CSF-1op/op mice. We finally examined whether 2MD-induced up-regulation of IL-34 expression is mediated by the vitamin D receptor (VDR) using VDR−/− mice. Although comparable levels of IL-34 mRNA expression were detected in bone and spleen in VDR−/− mice, the IL-34 expression was not enhanced by 2MD administration (Fig. 5F).
Fig. 5.
Effects of SPX and IL-34 siRNA on 2MD-induced osteoclastogenesis in CSF-1op/op mice. (A) Effects of 2MD and RANKL on osteoclastogenesis. Three-week-old CSF-1op/op mice were subjected to SPX or Sham. Four days later, mice were injected with 2MD (2 nmol/kg) twice 2 d apart, and killed 48 h after the second injection. Sham mice were administered daily RANKL (2.5 mg/kg) daily for 4 d, and killed 24 h after the last injection. Sections of tibiae were double stained for TRAP and methyl green (Left), and osteoclast number (Center) and erosion surface (ES/BS) (Right) were measured. Arrows indicate TRAP(+) osteoclasts. Data are expressed as the mean ± SD for four optical fields from four mice. (Scale bar, 50 μm.) (B–D) Effects of 2MD on IL-34 expression (B and C) and RANK(+)/CSF-1R(+) cells (D). Three-week-old CSF-1op/op mice were injected with vehicle or 2MD (2 nmol/kg) and killed 24 h after the injection. (B) Effects of 2MD on IL-34 mRNA expression. Expression of IL-34 mRNA in bone, liver, and spleen was examined by real-time RT-PCR. Data were obtained from triplicate PCRs using RNA from two mice and expressed as the mean ± SD. (C) Effects of 2MD on IL-34 protein expression. Spleen sections were stained for IL-34 (red) and PECAM-1 (green) (Left). Number of IL-34(+)/PECAM-1(+) cells in the red pulp was counted (Right). Values in red bars represent percentages of IL-34(+) cells among PECAM-1(+) cells. Data are expressed as the mean ± SD for four optical fields. (D) Effects of 2MD on RANK(+)/CSF-1R(+) cells in spleen. Spleen sections were stained for RANK (red) and CSF-1R (green) (Left). Number of RANK(+)/CSF-1R(+) cells in the red pulp was counted (Right). Data are expressed as the mean ± SD for four optical fields. (Scale bar, 10 μm.) (E) Effects of IL-34 siRNA injection on osteoclastogenesis. Three-week-old CSF-1op/op mice were i.v. injected with control siRNA or IL-34 siRNA (10 mg/kg) or its control. One day later, they were injected with 2MD (2 nmol/kg) twice 2 d apart and killed 48 h after the second injection. Sections of tibiae were double stained for TRAP and methyl green (Left), and the number of osteoclasts was counted (Right). Arrows indicate TRAP(+) osteoclasts. Data are expressed as the mean ± SD for four optical fields from four mice. *P < 0.01. (Scale bars, 50 μm.) (F) Effects of 2MD on IL-34 mRNA expression in VDR−/− mice. Eight-week-old VDR−/− mice were injected with vehicle or 2MD (2 nmol/kg) and killed 24 h after the injection. Data obtained from triplicate PCRs using RNA from three mice are expressed as the mean ± SD (n = 3).
Discussion
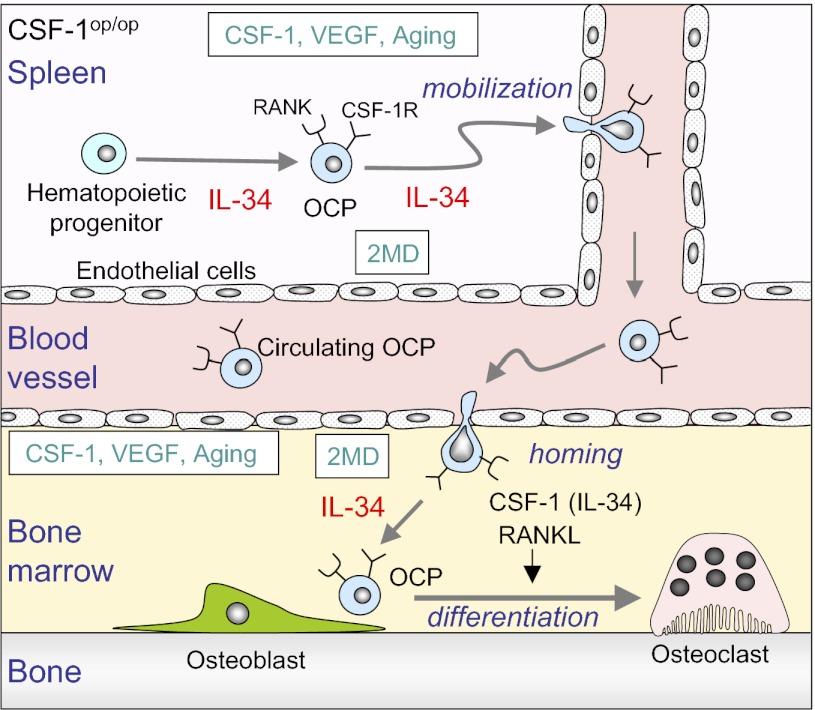
We have shown that spleen in CSF-1op/op mice acts as a reservoir of OCPs, which are transferred to bone and differentiate into osteoclasts in response to diverse stimuli (Fig. 6). The existence of OCPs in spleen seems to be supported by IL-34 expressed in vascular endothelial cells. The mysterious phenomena observed in CSF-1op/op mice (7–12) may be explained by the transfer of OCPs from spleen to bone.
Fig. 6.
A hypothetical model for osteoclastogenesis in CSF-1op/op mice. OCPs exist as RANK(+)/CSF-1R(+) cells in spleen but not in bone in CSF-1op/op mice. IL-34 expressed by endothelial cells appears to be involved in the differentiation of OCPs from hematopoietic progenitor cells. Administration of CSF-1, 2MD, or VEGF into CSF-1op/op mice produces osteoclasts in bone. Osteoclasts also develop in bone in aged CSF-1op/op mice. OCPs in spleen are mobilized into the circulation in response to diverse stimuli. Circulating OCPs settle down in bone and differentiate into osteoclasts in response to RANKL and CSF-1 or IL-34. IL-34 expression is increased in spleen and bone with aging or 2MD administration. IL-34 appears to play pivotal roles in the development in spleen and transfer of OCPs from spleen to bone in CSF-1op/op mice.
The exclusive localization of OCPs in spleen was observed in CSF-1op/op mice but not in RANKL−/− mice. SPX prevented osteoclastogenesis in 2MD-induced osteoclastogenesis in CSF-1op/op mice. However, 2MD-induced osteoclastogenesis in normal mice was not impaired by SPX (Fig. S6). Therefore, it is unlikely that spleen acts as a reservoir of OCPs in osteoclastogenesis under the physiological condition in normal animals. However, 2MD administration increased the number of OCPs as well as the expression of IL-34 in spleen in CSF-1op/op mice. SPX suppressed the age-associated appearance of osteoclasts in CSF-1op/op mice. These results suggest that OCPs are generated and maintained in spleen through IL-34 during the lifetime of CSF-1op/op mice.
OCPs were released from spleen into the blood stream in CSF-1op/op mice. Using an in vivo system of bone morphogenetic protein 2 (BMP-2)–induced ectopic bone formation in RANKL−/− mice, we demonstrated that OCPs existed in the peripheral blood as well as in bone marrow (26). Circulating OCPs were cell-cycle–arrested cells committed to the osteoclast lineage. When CSF-1 was injected into CSF-1op/op mice, osteoclasts detected in bone were generated from cell-cycle–arrested OCPs (18). Our findings also support the notion that the lineage-committed OCPs circulate in the blood stream and fix to the correct site for osteoclastogenesis. Ishii et al. (27) reported that an agonist of sphingosine 1 phosphate (S1P) increased the migration of OCPs between blood and bone. CSF-1 administration increased the mobilization of OCPs from spleen to bone, suggesting that CSF-1 as well as IL-34 plays important roles not only in the osteoclastic differentiation of OCPs in bone but also in the mobilization of OCPs from spleen to bone in CSF-1op/op mice. Future studies will further clarify the mechanism by which OCPs are transferred from spleen into blood and home to bone.
The expression level of IL-34 was much lower in bone than in spleen. This may explain why OCPs and osteoclasts are absent in bone in young CSF-1op/op mice. Osteoclasts appeared in aged CSF-1op/op mice with concomitant up-regulation of IL-34 expression in bone. 2MD administration also enhanced IL-34 expression in bone. These results suggest that IL-34 generated by vascular endothelial cells contributes to osteoclastogenesis induced by aging and 2MD administration in CSF-1op/op mice. VEGF also induced osteoclastogenesis in CSF-1op/op mice. However, the ability of VEGF to induce osteoclastogenesis was much weaker than that of CSF-1 and 2MD, and VEGF failed to up-regulate IL-34 expression. These results suggest that IL-34 expressed in vascular endothelial cells in bone is essentially involved in osteoclastogenesis in CSF-1op/op mice.
Administration of 2MD induced osteoclastogenesis with up-regulation of IL-34 expression in CSF-1op/op mice. The RNA interference experiment further supported the notion that IL-34 is involved in the 2MD-induced osteoclastogenesis in CSF-1op/op mice. When 2MD was administered to WT mice, the expression of IL-34 mRNA was significantly increased in WT mice (Fig. S7). The stimulatory effect of 2MD on IL-34 expression was not observed in VDR−/− mice. Administration of a large amount of 1α,25(OH)2D3 into CSF-1op/op mice also induced osteoclastogenesis (Fig. S8). These findings suggest that the IL-34 gene is a unique target of 1α,25(OH)2D3. Using the program Pattern Search for Transcription Factor Binding Sites (PATCH 1.0), we found five putative binding sites of VDR within the 2 kb upstream of the transcription start site of the mouse IL-34 gene. The expression level of IL-34 mRNA in bone and spleen in VDR−/− mice was comparable to that in wild-type mice. These results suggest that VDR-mediated signals are not essential for IL-34 expression, but are involved in the up-regulation of IL-34 expression in endothelial cells.
We have not succeeded in showing the stimulation of IL-34 expression by 2MD in mouse splenic endothelial cell cultures. Vascular microenvironment or blood vascular networks may be required for 2MD-induced up-regulation of IL-34 expression. Vascular endothelial cells are shown to regulate the recruitment of monocyte/macrophage lineage cells, which play a role in angiogenesis (28–31). At the amino acid sequence level, IL-34 gene is more conserved than the CSF-1 gene during evolution (32). These results suggest that the vitamin D system and IL-34 may fundamentally work in normal angiogenesis.
Recently, it was reported that IL-34 was expressed in synovial tissues obtained from rheumatoid arthritis patients, and tumor necrosis factor α (TNFα) stimulated IL-34 expression in those synovial fibroblasts in culture (33, 34). Giant cell tumors of bone have been shown to express IL-34 (17). Therefore, we examined the effects of osteotropic factors such as 1α,25(OH)2D3, 2MD, TNFα, interleukin 1β (IL-1β) and prostaglandin E2 (PGE2) on IL-34 expression in mouse osteoblastic cells in culture (Fig. S9). Real-time RT-PCR analysis showed that TNFα, IL-1β, and PGE2, respectively, failed to increase IL-34 mRNA expression in osteoblastic cells in our cell culture conditions. 2MD and 1α,25(OH)2D3 significantly increased IL-34 mRNA expression in osteoblastic cells. However, it is unlikely that IL-34 expressed by osteoblastic cells in response to 1α,25(OH)2D3 can substitute for CSF-1 for osteoclastogenesis, because 1α,25(OH)2D3 failed to support osteoclast formation in cocultures of hematopoietic osteoclast precursors and CSF-1op/op mouse-derived osteoblastic cells (35). At present, the cause of the difference between our result and the results reported previously is not known. Further studies will elucidate the regulatory mechanism of IL-34 expression in fibroblastic cells as well as endothelial cells.
Both CSF-1op/op and RANKL−/− osteopetrotic mice develop splenic extramedullary hematopoiesis due to the impaired bone microenvironment. This suggests that spleen acts as the reservoir of hematopoietic precursors under pathological conditions such as osteopetrosis. Recently, Miyamoto et al. (36) reported that the mobilization of hematopoietic stem and progenitor cells (HSPCs) occurring after granulocyte colony-stimulating factor (G-CSF) injection was comparable or even increased in osteopetrotic CSF-1op/op, RANKL−/− and c-Fos−/− mice, compared with that in WT mice. Contrary to OCPs, the mobilization of HSPCs was not suppressed by SPX in CSF-1op/op mice in the G-CSF treatment (36). These results suggest that spleen cannot act as the reservoir of HSPCs in CSF-1op/op mice, although HSPCs exist in the enlarged spleen. Swirski et al. (37) identified unique monocytes in spleen, which exited the spleen en masse, accumulated in the injured tissue, and participated in wound healing, in response to ischemic myocardial injury. CSF-1 has been shown to be involved in the coordinated dynamics of the tissue distribution of macrophages and dendritic cells (38). Thus, spleen plays important roles in the maintenance and tissue distribution of monocyte–macrophage lineage cells through IL-34 expression.
In conclusion, IL-34 plays pivotal roles in the maintenance and mobilization of splenic OCPs in CSF-1op/op mice. IL-34 and CSF-1 play dominant roles in determining the distribution of OCPs. The IL-34 gene in vascular endothelial cells is a unique target of vitamin D. Clarifying how splenic OCPs enter the blood stream and reach bone may provide a unique strategy to control bone resorption.
Materials and Methods
Detailed protocols are given in SI Materials and Methods.
Animals.
Breeding pairs of CSF-1op /+ mice (B6C3Fe genetic background) were purchased from The Jackson Laboratory and F2 mice were raised in our laboratory. Homozygous CSF-1op/op mice, identified by a lack of incisors at postnatal day 10, and RANKL−/− mice (39) (C57BL/6 background) were fed a softened rodent chow (Oriental Yeast) with water after weaning. VDR−/− mice (C57BL/6 background) were generated by cross-breeding of VDR-floxed mice with CMV-Cre mice (40). VDR−/− mice were fed a high calcium diet (CE-2 supplemented with 2% (wt/wt) calcium, 1.25% (wt/wt) phosphate, and 20% (wt/wt) lactose; CLEA Japan) to normalize serum calcium level. Eight-week-old male ddY mice and newborn ddY mice (Japan SLC) were used as WT mice for preparation of BM cells and osteoblastic cells, respectively. All experiments were conducted in accordance with the guidelines for studies with laboratory animals of the Matsumoto Dental University Experimental Animal Committee.
Splenectomy.
Mice were anesthetized with isoflurane (Isoflu; Dainippon Sumitomo Pharma) using a vaporizer (DS Pharma Biomedical). The spleen was identified after a transverse laparotomy incision just to the left of the spinal cord and removed after appropriate blood vessel ligation. Sham-operated animals underwent the laparotomy without a splenectomy.
Statistics.
Statistical analyses were performed using the one-tailed Student t test and Fisher’s exact probability test, as appropriate. P < 0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
This work was supported in part by Ministry of Education, Culture, Sports, Science, and Technology of Japan Grants-in-Aid 21791817 (to Y.N.), 22791804 (to T.M.), 21390551 (to Y.K.), and 22390351 (to N.T.) and by a grant from the Naito Foundation for Natural Science (to T.M.).
Footnotes
Conflict of interest statement: H.Y. is an employee of the Oriental Yeast Co.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1207361109/-/DCSupplemental.
References
- 1.Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423:337–342. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- 2.Suda T, et al. Modulation of osteoclast differentiation and function by the new members of the tumor necrosis factor receptor and ligand families. Endocr Rev. 1999;20:345–357. doi: 10.1210/edrv.20.3.0367. [DOI] [PubMed] [Google Scholar]
- 3.Stanley ER, Berg KL, Einstein DB, Lee PS, Yeung YG. The biology and action of colony stimulating factor-1. Stem Cells. 1994;12(Suppl 1):15–24, discussion 25. [PubMed] [Google Scholar]
- 4.Yoshida H, et al. The murine mutation osteopetrosis is in the coding region of the macrophage colony stimulating factor gene. Nature. 1990;345:442–444. doi: 10.1038/345442a0. [DOI] [PubMed] [Google Scholar]
- 5.Wiktor-Jedrzejczak WW, Ahmed A, Szczylik C, Skelly RR. Hematological characterization of congenital osteopetrosis in op/op mouse. Possible mechanism for abnormal macrophage differentiation. J Exp Med. 1982;156:1516–1527. doi: 10.1084/jem.156.5.1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kodama H, et al. Congenital osteoclast deficiency in osteopetrotic (op/op) mice is cured by injections of macrophage colony-stimulating factor. J Exp Med. 1991;173:269–272. doi: 10.1084/jem.173.1.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Begg SK, et al. Delayed hematopoietic development in osteopetrotic (op/op) mice. J Exp Med. 1993;177:237–242. doi: 10.1084/jem.177.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dai XM, et al. Targeted disruption of the mouse colony-stimulating factor 1 receptor gene results in osteopetrosis, mononuclear phagocyte deficiency, increased primitive progenitor cell frequencies, and reproductive defects. Blood. 2002;99:111–120. doi: 10.1182/blood.v99.1.111. [DOI] [PubMed] [Google Scholar]
- 9.Cecchini MG, et al. Role of colony stimulating factor-1 in the establishment and regulation of tissue macrophages during postnatal development of the mouse. Development. 1994;120:1357–1372. doi: 10.1242/dev.120.6.1357. [DOI] [PubMed] [Google Scholar]
- 10.Yamamoto T, et al. Macrophage colony-stimulating factor is indispensable for repopulation and differentiation of Kupffer cells but not for splenic red pulp macrophages in osteopetrotic (op/op) mice after macrophage depletion. Cell Tissue Res. 2008;332:245–256. doi: 10.1007/s00441-008-0586-8. [DOI] [PubMed] [Google Scholar]
- 11.Niida S, et al. Vascular endothelial growth factor can substitute for macrophage colony-stimulating factor in the support of osteoclastic bone resorption. J Exp Med. 1999;190:293–298. doi: 10.1084/jem.190.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Niida S, et al. VEGF receptor 1 signaling is essential for osteoclast development and bone marrow formation in colony-stimulating factor 1-deficient mice. Proc Natl Acad Sci USA. 2005;102:14016–14021. doi: 10.1073/pnas.0503544102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lean JM, Fuller K, Chambers TJ. FLT3 ligand can substitute for macrophage colony-stimulating factor in support of osteoclast differentiation and function. Blood. 2001;98:2707–2713. doi: 10.1182/blood.v98.9.2707. [DOI] [PubMed] [Google Scholar]
- 14.Lin H, et al. Discovery of a cytokine and its receptor by functional screening of the extracellular proteome. Science. 2008;320:807–811. doi: 10.1126/science.1154370. [DOI] [PubMed] [Google Scholar]
- 15.Wei S, et al. Functional overlap but differential expression of CSF-1 and IL-34 in their CSF-1 receptor-mediated regulation of myeloid cells. J Leukoc Biol. 2010;88:495–505. doi: 10.1189/jlb.1209822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen Z, Buki K, Vääräniemi J, Gu G, Väänänen HK. The critical role of IL-34 in osteoclastogenesis. PLoS ONE. 2011;6:e18689. doi: 10.1371/journal.pone.0018689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baud’huin M, et al. Interleukin-34 is expressed by giant cell tumours of bone and plays a key role in RANKL-induced osteoclastogenesis. J Pathol. 2010;221:77–86. doi: 10.1002/path.2684. [DOI] [PubMed] [Google Scholar]
- 18.Mizoguchi T, et al. Identification of cell cycle-arrested quiescent osteoclast precursors in vivo. J Cell Biol. 2009;184:541–554. doi: 10.1083/jcb.200806139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DeLuca HF. Overview of general physiologic features and functions of vitamin D. Am J Clin Nutr. 2004;80(6) Suppl:1689S–1696S. doi: 10.1093/ajcn/80.6.1689S. [DOI] [PubMed] [Google Scholar]
- 20.Shevde NK, et al. A potent analog of 1alpha,25-dihydroxyvitamin D3 selectively induces bone formation. Proc Natl Acad Sci USA. 2002;99:13487–13491. doi: 10.1073/pnas.202471299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sato M, et al. New 19-nor-(20S)-1alpha,25-dihydroxyvitamin D3 analogs strongly stimulate osteoclast formation both in vivo and in vitro. Bone. 2007;40:293–304. doi: 10.1016/j.bone.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 22.Wong MS, Delansorne R, Man RY, Vanhoutte PM. Vitamin D derivatives acutely reduce endothelium-dependent contractions in the aorta of the spontaneously hypertensive rat. Am J Physiol Heart Circ Physiol. 2008;295:H289–H296. doi: 10.1152/ajpheart.00116.2008. [DOI] [PubMed] [Google Scholar]
- 23.Mantell DJ, Owens PE, Bundred NJ, Mawer EB, Canfield AE. 1 α,25-dihydroxyvitamin D(3) inhibits angiogenesis in vitro and in vivo. Circ Res. 2000;87:214–220. doi: 10.1161/01.res.87.3.214. [DOI] [PubMed] [Google Scholar]
- 24.Hisa T, et al. Vitamin D inhibits endothelial cell migration. Arch Dermatol Res. 1996;288:262–263. doi: 10.1007/BF02530097. [DOI] [PubMed] [Google Scholar]
- 25.Broermann A, et al. Dissociation of VE-PTP from VE-cadherin is required for leukocyte extravasation and for VEGF-induced vascular permeability in vivo. J Exp Med. 2011;208:2393–2401. doi: 10.1084/jem.20110525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muto A, et al. Lineage-committed osteoclast precursors circulate in blood and settle down into bone. J Bone Miner Res. 2011;26:2978–2990. doi: 10.1002/jbmr.490. [DOI] [PubMed] [Google Scholar]
- 27.Ishii M, et al. Sphingosine-1-phosphate mobilizes osteoclast precursors and regulates bone homeostasis. Nature. 2009;458:524–528. doi: 10.1038/nature07713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grunewald M, et al. VEGF-induced adult neovascularization: Recruitment, retention, and role of accessory cells. Cell. 2006;124:175–189. doi: 10.1016/j.cell.2005.10.036. [DOI] [PubMed] [Google Scholar]
- 29.Bergmann CE, et al. Arteriogenesis depends on circulating monocytes and macrophage accumulation and is severely depressed in op/op mice. J Leukoc Biol. 2006;80:59–65. doi: 10.1189/jlb.0206087. [DOI] [PubMed] [Google Scholar]
- 30.Lobov IB, et al. WNT7b mediates macrophage-induced programmed cell death in patterning of the vasculature. Nature. 2005;437:417–421. doi: 10.1038/nature03928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kubota Y, et al. M-CSF inhibition selectively targets pathological angiogenesis and lymphangiogenesis. J Exp Med. 2009;206:1089–1102. doi: 10.1084/jem.20081605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garceau V, et al. Pivotal Advance: Avian colony-stimulating factor 1 (CSF-1), interleukin-34 (IL-34), and CSF-1 receptor genes and gene products. J Leukoc Biol. 2010;87:753–764. doi: 10.1189/jlb.0909624. [DOI] [PubMed] [Google Scholar]
- 33.Chemel M, et al. Interleukin 34 expression is associated with synovitis severity in rheumatoid arthritis patients. Ann Rheum Dis. 2012;71:150–154. doi: 10.1136/annrheumdis-2011-200096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hwang SJ, et al. Interleukin-34 produced by human fibroblast-like synovial cells in rheumatoid arthritis supports osteoclastogenesis. Arthritis Res Ther. 2012;14:R14. doi: 10.1186/ar3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tanaka S, et al. Macrophage colony-stimulating factor is indispensable for both proliferation and differentiation of osteoclast progenitors. J Clin Invest. 1993;91:257–263. doi: 10.1172/JCI116179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miyamoto K, et al. Osteoclasts are dispensable for hematopoietic stem cell maintenance and mobilization. J Exp Med. 2011;208:2175–2181. doi: 10.1084/jem.20101890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Swirski FK, et al. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science. 2009;325:612–616. doi: 10.1126/science.1175202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tagliani E, et al. Coordinate regulation of tissue macrophage and dendritic cell population dynamics by CSF-1. J Exp Med. 2011;208:1901–1916. doi: 10.1084/jem.20110866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kong YY, et al. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature. 1999;397:315–323. doi: 10.1038/16852. [DOI] [PubMed] [Google Scholar]
- 40.Li M, et al. Topical vitamin D3 and low-calcemic analogs induce thymic stromal lymphopoietin in mouse keratinocytes and trigger an atopic dermatitis. Proc Natl Acad Sci USA. 2006;103:11736–11741. doi: 10.1073/pnas.0604575103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.