Abstract
Cyclin-dependent kinase 1 (Cdk1) kinase dephosphorylation and activation by Cdc25 phosphatase are essential for mitotic entry. Activated Cdk1 phosphorylates Cdc25 and other substrates, further activating Cdc25 to form a positive feedback loop that drives the abrupt G2/mitosis switch. Conversely, mitotic exit requires Cdk1 inactivation and reversal of Cdk1 substrate phosphorylation. This dephosphorylation is mediated, in part, by Clp1/Cdc14, a Cdk1-antagonizing phosphatase, which reverses Cdk1 phosphorylation of itself, Cdc25, and other Cdk1 substrates. Thus, Cdc25 phosphoregulation is essential for proper G2–M transition, and its contributions to cell cycle control have been modeled based on studies using Xenopus and human cell extracts. Because cell extract systems only approximate in vivo conditions where proteins interact within dynamic cellular environments, here, we use Schizosaccharomyces pombe to characterize, both experimentally and mathematically, the in vivo contributions of Cdk1-mediated phosphorylation of Cdc25 to the mitotic transition. Through comprehensive mapping of Cdk1 phosphosites on Cdc25 and characterization of phosphomutants, we show that Cdc25 hyperphosphorylation by Cdk1 governs Cdc25 catalytic activation, the precision of mitotic entry, and unvarying cell length but not Cdc25 localization or abundance. We propose a mathematical model that explains Cdc25 regulation by Cdk1 through a distributive and disordered phosphorylation mechanism that ultrasensitively activates Cdc25. We also show that Clp1/Cdc14 dephosphorylation of Cdk1 sites on Cdc25 controls the proper timing of cell division, a mechanism that is likely due to the double negative feedback loop between Clp1/Cdc14 and Cdc25 that controls the abruptness of the mitotic exit switch.
Keywords: mitotic bistability, multisite phosphorylation
Cyclin-dependent kinases (CDKs) are key regulators of the eukaryotic cell cycle. At mitotic entry, the Cdc25 family phosphatases activate Cdk1-CyclinB complexes by removing inhibitory phosphorylations on Cdk1 catalyzed by Wee1 family kinases. Activated Cdk1-CyclinB phosphorylates its substrates and drives mitotic entry (1, 2). Cell cycle modeling showed that a bistable trigger facilitates the switch-like transition between interphase and mitosis (3–5). Bistability ensures that there can only be two stable steady states for the system (interphase or mitosis); it predicts a Cdk1 activity threshold for mitotic entry and a lower activity threshold for mitotic exit, thus giving rise to hysteresis in the system.
Xenopus laevis and human cell extract studies found that the bistable mitotic switch is modulated by at least two feedback loops: the Cdk1-Wee1 double negative feedback loop, in which Cdk1 and Wee1 inactivate one another by phosphorylation, and the Cdk1-Cdc25 positive feedback loop, where Cdk1 phosphorylates and activates Cdc25, while Cdc25 dephosphorylates and further activates Cdk1 (3–7). In addition to a positive or double negative feedback loop, bistability requires an ultrasensitive response of at least one component of a feedback loop (8, 9). It has been proposed that Cdc25 activation and Wee1 inactivation by Cdk1 are ultrasensitive in nature; their activity follows sigmoid signal response curves (rising and decreasing, respectively) as a function of Cdk1 activity (10, 11). In Xenopus egg extracts, ultrasensitivity in Wee1 inactivation is attributed to competition between essential and nonessential Cdk1 phosphosites on Wee1 and between Wee1 and other Cdk1 substrates (6). In addition, Cdk1 multisite phosphorylation of XCdc25C contributes to its ultrasensitive activation (7). Although ex vivo and mathematical models suggest that both Cdk1-Wee1 and Cdk1-Cdc25 feedback loops contribute to the robustness of the mitotic entry switch, perturbation of the feedback loops in vivo in cycling cells has yet to be analyzed.
Mitotic exit and the spindle assembly checkpoint may also be modulated by a bistable switch (12–14). In Saccharomyces cerevisiae, Cdc14, a phosphatase that dephosphorylates Cdk1 substrates (15, 16), adds abruptness to the metaphase–anaphase switch by interacting with Securin, a protein that protects sister chromatid separation until anaphase onset in an ultrasensitive positive feedback loop. Cdc14 dephosphorylates Securin to target it for ubiquitylation and degradation. Degradation of Securin activates Separase, which also activates Cdc14 (13). Our laboratory and other groups found that Clp1, the Schizosaccharomyces pombe Cdc14 ortholog, dephosphorylates Cdc25 on Cdk1 phosphosites, and this dephosphorylation correlates with Cdc25 inactivation and degradation (17, 18). Because Cdc25 activates Cdk1, the activity of which inhibits Clp1 activity (19), the interaction between Clp1 and Cdc25 may form a feedback loop that contributes to the mitotic exit switch in S. pombe.
Here, we use S. pombe to further understand how Cdc25 phosphorylation by Cdk1 contributes to the mitotic entry and exit switches in cycling cells. Using this in vivo model, we suggest a mechanism of direct Cdk1 activation and Clp1 inactivation of Cdc25. Also, we find that the Cdk1-Cdc25 positive feedback loop is important for the precision of mitotic entry and maintenance of uniform cell length. Finally, we suggest that the interactions of Clp1, Cdk1, and Cdc25 create a double negative feedback loop that significantly contributes to the robustness of mitotic exit, specifically controlling the timing of cell division.
Results
Characterization of Cdk1- and Clp1-Specific Phosphosites on Cdc25.
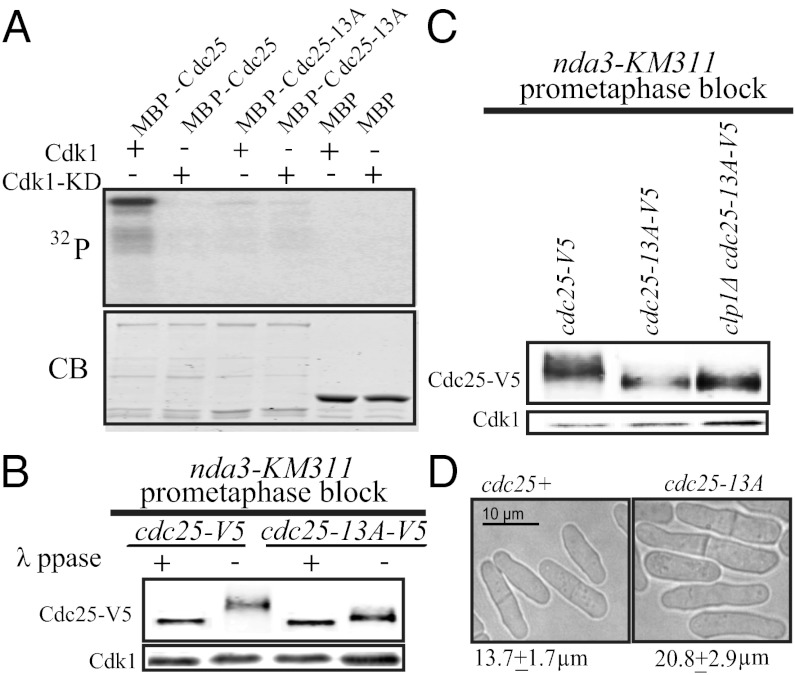
To examine how Cdk1 phosphorylation of Cdc25 affects the mitotic entry and exit switches, we eliminated Cdk1 phosphosites on Cdc25 by mutating all 13 Ser and Thr in the minimal Cdk1 consensus sites (Ser/Thr-Pro) outside of the Cdc25 catalytic domain to nonphosphorylable alanines (Cdc25-13A). In vitro, active recombinant Cdk1-CycB phosphorylated recombinant maltose binding protein tagged Cdc25 (MBP-Cdc25) but not MBP-Cdc25-13A (Fig. 1A), indicating that all major in vitro Cdk1 sites were abolished in Cdc25-13A. Through tandem affinity purification of Cdc25 from prometaphase-arrested cells, when Cdc25 is maximally phosphorylated (17, 18, 20) followed by liquid chromatography-tandem mass spectrometry, we identified phosphorylation on all but two of these Cdk1 consensus sites (Fig. S1 and Table S1).
Fig. 1.
Characterization of Cdc25 phosphomutant. (A) Recombinant MBP-Cdc25, MBP-Cdc25-13A, or MBP was incubated with active Cdk1-CyclinB (Cdk1) or kinase-dead Cdk1-CyclinB (Cdk1-KD) in an in vitro kinase assay. Proteins were separated by SDS-PAGE and visualized by Coomassie blue (CB; Lower) and autoradiography (Upper). (B and C) Cdc25-V5 was immunoprecipitated from the indicated strains arrested in prometaphase, and immunoprecipitates were treated or not with λ-phosphatase before immunoblotting with anti-Cdc25 antibody (Upper). Cdk1 levels from lysates used for immunoprecipitates are visualized with PSTAIRE antibody (Lower). (D) Light microscopy images of cdc25+ and cdc25-13A cells. Cell lengths at septation were measured, and SEM is presented.
We next looked at Cdc25-13A phosphostatus in vivo. Endogenously tagged Cdc25-13A-V5 or Cdc25-V5 phosphorylation was assessed by SDS-PAGE mobility in cells arrested in prometaphase. Most of the gel shift caused by phosphorylation was eliminated in Cdc25-13A-V5, consistent with Cdk1 being the major kinase for Cdc25 in mitosis; however, some remained, suggesting that another kinase(s) contributes to mitotic Cdc25 phosphorylation (Fig. 1B).
Clp1 reverses Cdk1-dependent phosphorylation on Cdc25 (17, 18). We, therefore, expected that the mobility shift of Cdc25-13A would not be affected by clp1Δ. Indeed, Cdc25-13A-V5 displayed the same SDS-PAGE mobility with or without Clp1 (Fig. 1C and Fig. S2C), indicating that Cdc25-13A is not a Cdk1 substrate in vivo and that Clp1 is unable to affect the phosphorylation of Cdc25 caused by another protein kinase(s).
Abolishing Cdk1 Phosphosites on Cdc25 Delays Mitotic Entry.
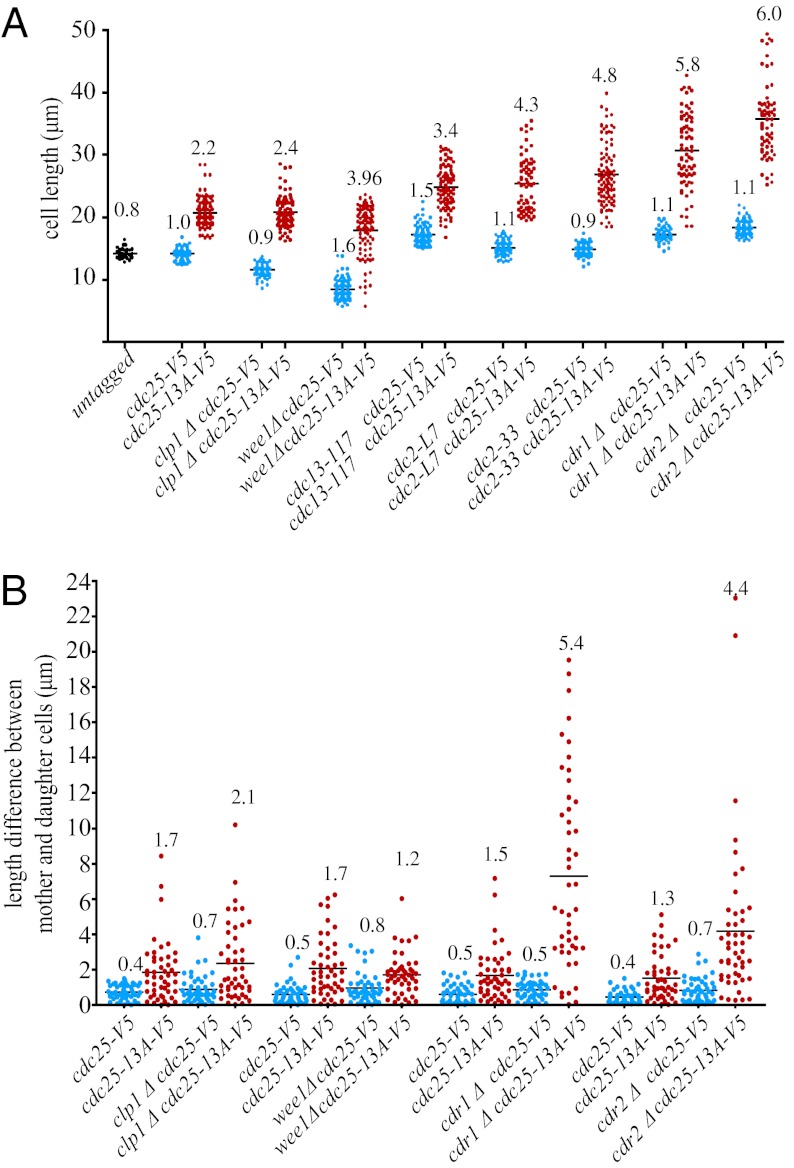
We next asked if eliminating Cdk1 phosphosites on Cdc25 altered mitotic entry. S. pombe grows lengthwise during interphase and stops growing at mitotic entrance, and therefore, cell length at septation equates to cell length at mitotic entrance (21). The increased septation length of cdc25-13A cells relative to wildtype (WT) (Fig. 1D) indicated a mitotic entrance delay. In mitotic entrance in mutants that are already longer than WT (cdc2-L7, cdc13-117, cdc2-33, cdr1Δ, and cdr2Δ), cdc25-13A-V5 exacerbated their defects (Fig. 2A). In strains that enter mitosis prematurely (clp1Δ and wee1Δ), cdc25-13A-V5 delayed their mitotic entry (Fig. 2A). Thus, direct Cdk1 phosphorylation of Cdc25 is important for promoting mitotic entry.
Fig. 2.
Disruption of Cdk1 phosphorylation on Cdc25 delays mitotic entrance. (A) Scatter plot of the septation lengths of the indicated strains; >100 cells of each strain were measured. Mean lengths are indicated by the horizontal bars. (B) Scatter plot of length differences between mother and daughter cells of the indicated strains. For each strain, n = 50. (A and B) The SD is displayed above each scatter plot.
If the Cdk1-Cdc25 positive feedback loop affects the precision of mitotic entrance, then disrupting this loop would be expected to increase the variation of mitotic entry timing and thus, septation length within the population. Indeed, cdc25-13A-V5 strains exhibited a two- to sixfold increase in variation of cell length, determined by increased standard deviation, compared with cdc25-V5 cells (Fig. 2A and Fig. S2A). To examine the regulation of mitotic timing on a single-cell level, we followed individual cells for two to five divisions by time-lapse microscopy and calculated the difference in septation lengths between mother and daughter cells. Compared with cdc25+ cells, cdc25-13A cells in every genetic background had significantly more varied lengths between generations (Fig. 2B and Fig. S3, representative examples). The increased spectrum of cdc25-13A cell lengths within a population and between generations shows that the precision of mitotic entrance is disrupted when Cdk1 cannot phosphorylate Cdc25.
Cdk1 Phosphorylation Does Not Affect Cdc25 Concentration.
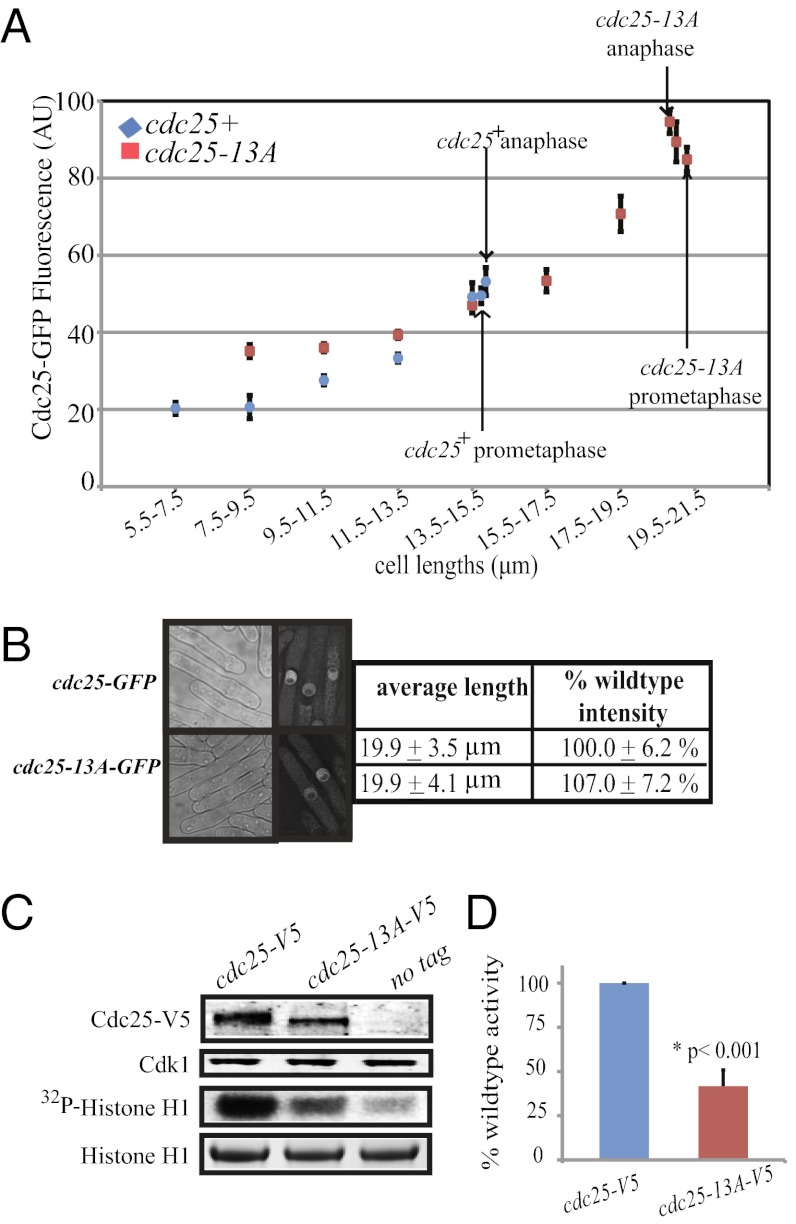
Cdk1 phosphorylation of Cdc25 could influence Cdc25 activity, Cdc25 abundance, or both. We asked if Cdk1 phosphorylation modulates Cdc25 abundance by measuring GFP fluorescence of Cdc25-GFP or Cdc25-13A-GFP cells. Cells in interphase were grouped according to cell lengths in 2-μm intervals, and mitotic cells, as judged by spindle pole body (SPB) separation, were separated into prometaphase and anaphase cells (Fig. 3A).
Fig. 3.
Cdk1 phosphorylation regulates Cdc25 activity at mitotic entrance. (A) Cdc25-GFP or Cdc25-13A-GFP fluorescence was quantified in cells at various lengths (25–50 cells per group). Cells in different phases of mitosis, determined by Sid4-RFP–marked SPB positions, are indicated with arrows, interphase cells are unmarked, and average length and fluorescence intensity of these cell groups were measured separately. SEM is presented as error bars, and SD is listed. (B) The indicated strains were blocked in S phase with HU, and cells were visualized by DIC and fluorescence microscopy. Tip to tip lengths were measured. Cdc25-GFP or Cdc25-13A-GFP fluorescence was averaged and calculated as a percentage of average WT Cdc25-GFP intensity. SEM is presented. (C) nda3-KM311, nda3-KM311 cdc25-V5, and nda3-KM311 cdc25-13A-V5 were blocked at prometaphase, and protein lysates were prepared. Cdk1 levels in each lysate were visualized with PSTAIRE antibody (row 2). The remaining lysates were subjected to immunoprecipitation with anti-V5 antibody, and the immunoprecipitated proteins were incubated in protein lysates from cdc25-22–arrested cells. Immunoprecipitated Cdc25 was resolved by SDS-PAGE and detected by immunoblotting (row 1). Next, Cdk1-Cdc13 was immunoprecipitated from each lysate and incubated with histone H1 in an in vitro kinase assay. Histone H1 was resolved by SDS-PAGE and visualized by CB staining (row 4) or autoradiography (row 3). (D) The specific activities of Cdc25 and Cdc25-13A in five separate experiments performed as in C were averaged, and the SEM is presented. P value was determined using the Student's t test.
During interphase, Cdc25-GFP and Cdc25-13A-GFP fluorescence increased as a function of cell length. cdc25-GFP cells reached mitosis at 13.5–15.5 μm with Cdc25-GFP fluorescence at 49.2 ± 4.0 arbitrary units (AUs). At the same length, cdc25-13A-GFP cells compared with cdc25-GFP had no significant difference in GFP fluorescence (47.0 ± 2.0 AU). However, cdc25-13A cells did not enter mitosis until they were significantly longer (with 89.4 ± 5.2 AU) (Fig. 3A). These results suggest that more Cdc25-13A-GFP than Cdc25-GFP is needed to induce mitotic entry.
To pursue this idea further, we used hydroxyurea (HU) to block cdc25-GFP and cdc25-13A-GFP cells in S phase and monitored Cdc25 abundance and cell length as cells were released back into the cell cycle. During the S-phase arrest, cdc25-GFP and cdc25-13A-GFP cells were not significantly different in Cdc25 protein levels, which was assessed by GFP fluorescence, or length (Fig. 3B). Since HU blocks cells in S phase by activating the DNA replication checkpoint and cdc25-13A cells arrested upon HU treatment (Fig. 3B and Fig. S2B), we conclude that Cdc25-13A remains responsive to checkpoint kinases that phosphorylate Cdc25 upon DNA damage (22). After release from the HU block, cdc25-13A-GFP cells septated after cdc25-GFP cells (Fig. S2B). These results suggest that the delay in mitotic entry in cdc25-13A cells results from a change in Cdc25-specific activity.
We also measured Cdc25 abundance in late interphase cells compared with cells in mitosis. There was no significant change in GFP fluorescence levels between late interphase and mitotic cdc25-GFP or cdc25-13A-GFP cells (Fig. 3A), suggesting again that the increased Cdk1 activity at mitotic entry does not affect Cdc25 abundance. Interestingly, neither cdc25-GFP nor cdc25-13A-GFP cells had significant changes in GFP fluorescence levels between metaphase and anaphase (Fig. 3A), suggesting that Clp1 dephosphorylation of Cdc25 after metaphase also does not mediate Cdc25 degradation. It is worth noting that Cdc25-13A-GFP localization was indistinguishable from the localization of Cdc25-GFP at all cell cycle stages.
We also examined the abundance and phosphostatus of Cdc25-13A and Cdc25 during mitotic progression by immunoblotting. Cells were arrested in prometaphase, released, and sampled at intervals. Although the phosphorylation level of Cdc25 and Cdc25-13A decreased, Cdc25-13A was less phosphorylated initially and dephosphorylated faster in both clp1+ and clp1Δ strains (Fig. S2C), suggesting that kinases and phosphatases other than Cdk1 and Clp1 modulate Cdc25 phosphostatus in mitosis, and it is possible that kinases/phosphatases responsible for regulating Cdc25 during checkpoint and stress responses (22) contribute to this phosphoregulation. Neither Cdc25 nor Cdc25-13A decreased in abundance as cells exited mitosis, confirming that Cdk1-mediated phosphorylation is not involved in protecting Cdc25 from degradation (Fig. S2C). Thus, although clp1Δ cells have elevated Cdc25 levels (17, 18, 20), this finding must be an indirect effect of Clp1 on another cellular process.
Cdk1 Phosphorylation Activates Cdc25.
We next examined if Cdk1 phosphorylation directly enhances Cdc25 activity using a previously described assay (17, 20). Immunoprecipitated Cdc25 or Cdc25-13A from prometaphase-arrested cells was tested for its ability to stimulate inactive Cdk1-Cdc13 in protein lysates derived from interphase cells. Cdk1 activation was measured using the exogenous substrate, histone H1. Compared with Cdc25-13A, Cdc25 stimulated significantly higher histone H1 phosphorylation by Cdk1 (Fig. 3 C and D). Thus, we conclude that Cdk1 phosphorylation does not affect Cdc25 abundance but directly activates Cdc25.
Multisite Phosphorylation of Cdc25 by Cdk1 Contributes to Cdc25 Activity.
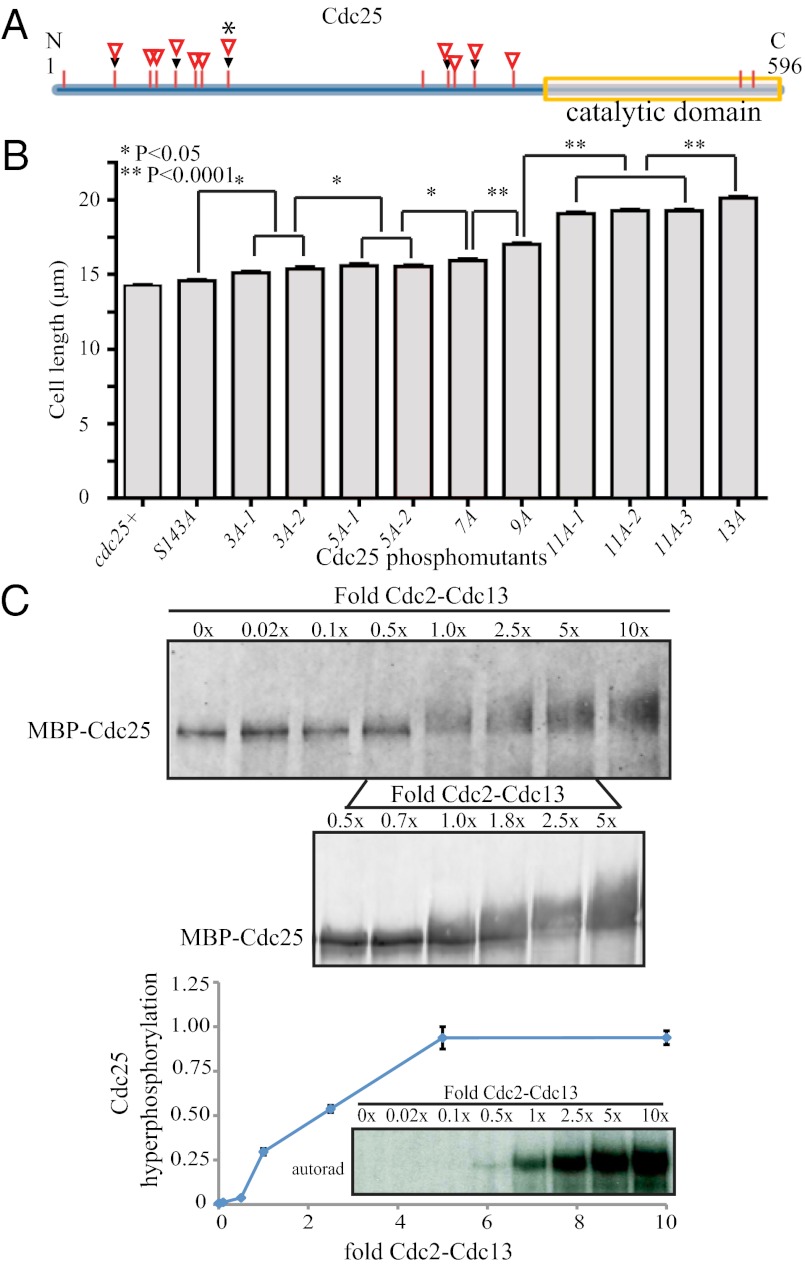
To examine if there are preferred Cdk1 sites on Cdc25 responsible for its activation, we performed an in vitro Cdk1 kinase assay followed by 2D tryptic peptide mapping and found one major and multiple other Cdc25 phosphopeptides (Fig. S4A). By LC-MS/MS analysis, Ser143 was identified as a prominent phosphosite (Fig. 4 A and B). When S143 was mutated to nonphosphorylable alanine to produce Cdc25-S143A, the major phosphopeptide was eliminated from the 2D map (Fig. S4A). We replaced cdc25+ with cdc25-S143A and found that this mutation did not significantly change septation length (14.6 ± 0.1 μm) compared with WT (14.2 ± 0.1 μm) (Fig. 4B and Fig. S4C). This finding indicated that, although S143 is a favored phosphosite in vitro, it is not necessary for efficient Cdc25 activation in vivo. Next, we mutated five sites (cdc25-5A) identified from in vitro Cdc25 phosphorylation or variations of these sites in clusters of three mutations (cdc25-3A-1 and cdc25-3A-2) to alanines and replaced endogenous cdc25+ with these mutants (Fig. 4 A and B and Fig. S4C). These mutations increased cell length only by 0.9–1.3 μm, showing that loss of these sites is not sufficient to reduce Cdc25 activity significantly. Mutating five other in vivo Cdk1 sites on Cdc25 identified by MS (cdc25-5A-2) (Fig. 4 A and B, Figs. S1 and S4C, and Table S1) also did not increase cell length to a large extent (15.5 ± 0.2 μm) (Fig. 4B). Thus, Cdk1 does not seem to have preferred sites of phosphorylation for Cdc25 activation.
Fig. 4.
Activation of Cdc25 by Cdk1 multisite phosphorylation. (A) Schematic of Cdc25 with S/T-P phosphorylation sites identified by MS indicated. Black triangles indicate sites identified from in vitro phosphorylation reactions; open red triangles indicate sites identified on Cdc25 purified from prometaphase-arrested cells. The asterisk indicates Ser143, a prominent in vitro Cdk1 phosphorylation site. (B) Cell lengths at septation of the indicated cdc25 phosphomutant alleles were determined, and SEM is shown. (C) Recombinant MBP-Cdc25 was incubated in vitro with varying levels of active Cdk1-CyclinB (Cdc2-Cdc13). Proteins were separated by SDS-PAGE, and Cdc25 was visualized by anti-Cdc25 antibody (Top and Middle). Cdc25 hyperphosphorylation was assessed by autoradiography, measured by scintillation counting, and presented as a percentage of maximal phosphorylation. Data from three independent experiments were averaged, and the SEM is presented (Bottom).
Cdk1 phosphorylation could activate Cdc25 by a stepwise mechanism, in which a specific number of phosphorylations is necessary for full Cdc25 activation or a progressive mechanism, in which Cdc25 is gradually activated as it becomes more highly phosphorylated. Furthermore, phosphorylation could occur in an ordered or disordered manner (8, 11, 23). To differentiate between these possibilities, we created more Cdc25 mutants, decreasing two available Cdk1 phosphosites in each subsequent phosphomutant (cdc25-7A to cdc25-11A) (Fig. S4C) and immunoprecipitated endogenously V5-tagged Cdc25 proteins. The extent of Cdc25 SDS-PAGE phosphoshift decreased progressively as phosphosites were eliminated (Fig. S4B). However, the mutation of particular sites did not seem to preclude phosphorylation at other sites (Fig. S4B), suggesting that phosphorylation is disordered, although we cannot rule out that a preferred order exists in WT Cdc25. For these mutants, the change in cell length between subsequent phosphomutants was initially small; however, cell length increased more with additional mutations (Fig. 4B), suggesting gradual Cdc25 activation with an increasing number of phosphorylations.
Cdc25 phosphorylation may also occur by a distributive mechanism, in which each phosphorylation on Cdc25 requires a separate Cdk1 binding and unbinding event, as modeled in silico (7, 10). Alternatively, Cdk1 can act as a priming kinase for subsequent processive phosphorylation by itself (24). Using an in vitro kinase assay in which the amount of Cdk1 was varied, we found that intermediate levels of Cdk1 yielded partially phosphoshifted Cdc25, indicating the existence of partially phosphorylated Cdc25 isoforms (Fig. 4C). Because processive phosphorylation would yield small amounts of fully shifted protein even at low Cdk1 concentrations, we conclude that Cdc25 phosphorylation by Cdk1 is most likely distributive.
Mathematical Model of Cdc25 Activation and Mitotic Entrance.
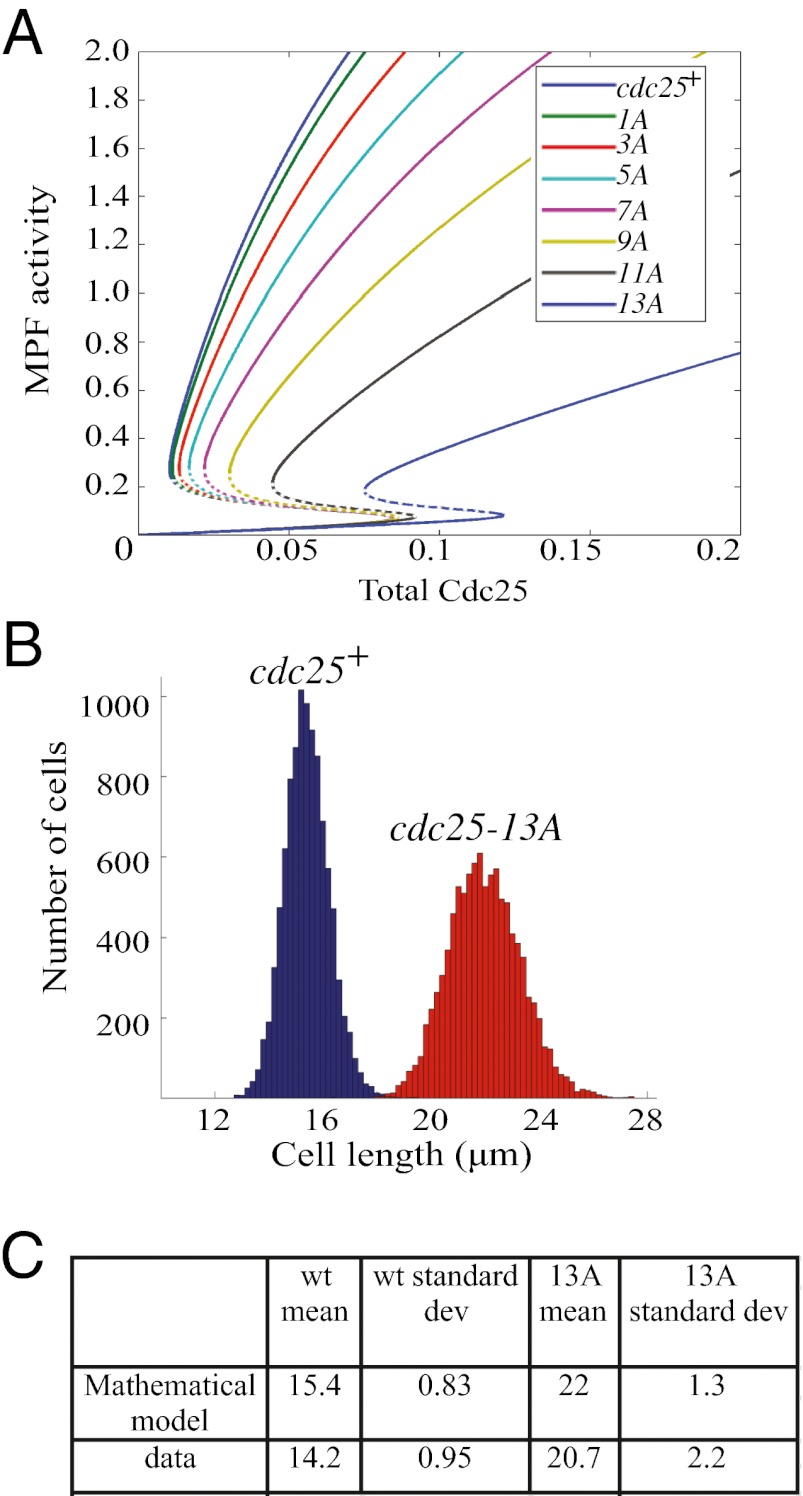
The contribution of Cdk1 phosphorylation on Cdc25 to the bistability of the G2/M transition was previously modeled (10). We modified this model by assuming that Cdk1 phosphorylates Cdc25 in a cooperative, disordered manner (SI Materials and Methods). Furthermore, we assumed a linear increase in Cdc25 activity with increasing Cdk1 phosphorylation, which results in an ultrasensitive response of Cdc25 to active Cdk1 [referred to as mitosis-promoting factor (MPF)] (Fig. S5A). These assumptions lead to a bistable response of MPF to Cdc25 levels, which is shown by S-shaped curves in Fig. 5A. The S-shaped curve is composed of three branches: top and bottom branches correspond to stable mitosis and interphase steady states, respectively, with a middle branch representing unstable steady states (Fig. 5A, dotted branch). During interphase, MPF activity rises slowly as Cdc25 concentration increases. When Cdc25 levels reach the end of the lower branch, the Cdk1-Cdc25-Wee1 feedback loops engage to fully activate Cdk1 and abruptly switch cells into mitosis (the top branch on the curve). Once in mitosis, MFP activity is high, and the system stays in mitosis until the Cdc25 level drops below a lower threshold than was required for mitotic entry, at which point the system transitions abruptly to interphase (Fig. 5A). In this system, there is a threshold level of Cdc25 required to reach mitosis. Because Cdc25 levels increase as the cell grows, we assume that this Cdc25 threshold for MPF activation is proportional to size at mitotic entry. Using this model, we tested mathematically how decreasing available phosphosites on Cdc25 would alter mitotic entry. Fig. 5A shows that, as the number of potential phosphosites decreases, the Cdc25 threshold for MPF activation increases, implying that mitotic entry is delayed and cell size is increased. This observation fits our experimental data showing increased cell lengths at septation in Cdc25 phosphomutants (Fig. 4B and Fig. S5B). The mathematical model also predicts a significant decrease in both Cdc25 activity and ultrasensitivity as the number of phosphosites on Cdc25 is reduced (Fig. S5 A and B).
Fig. 5.
Mathematical model of Cdc25 activation. (A) MPF activity as a function of total levels of Cdc25 for cdc25+ and different cdc25 phosphomutants. Dotted lines show unstable transition states. (B) Model-predicted distribution of cell lengths for WT and cdc25-13A cells. For each cell type, 104 cells (parameter sets) were generated by randomly varying model parameters. (C) The mean and SD for the mathematical model and actual data are shown.
To see if our model could predict the variation in cell lengths for cdc25-13A at mitotic entrance (Fig. 2), we generated the size distribution for 104 cells at mitotic entrance for cdc25+ and cdc25-13A cells by randomly varying model parameters (SI Materials and Methods). Indeed, not only is the modeled cdc25-13A average size larger than WT cells, but importantly, the distribution of sizes in the mutant is much wider than WT (Fig. 5 B and C). This prediction could account for part of the experimental variation that we observed (Fig. 2), and emphasizes the role of the Cdk1-Cdc25 positive feedback loop in controlling the abruptness of the mitotic switch.
Finally, we used our model to explore the contribution of disrupting Cdc25 phosphorylation (Cdc25-13A) in mitotic entrance mutants (cdc2-L7, cdc2-33, cdc13-117, clp1Δ, cdr1Δ, and cdr2Δ) (Fig. S5C). The strong consensus between experimental data and calculated lengths of mitotic entrance mutants (Fig. S5 A–C) emphasizes the contribution of Cdc25 multisite phosphorylation in the abruptness of mitotic entrance within the dynamic of multiple protein interactions.
Cytokinesis Timing Is Controlled by Clp1 Dephosphorylation of Cdc25.
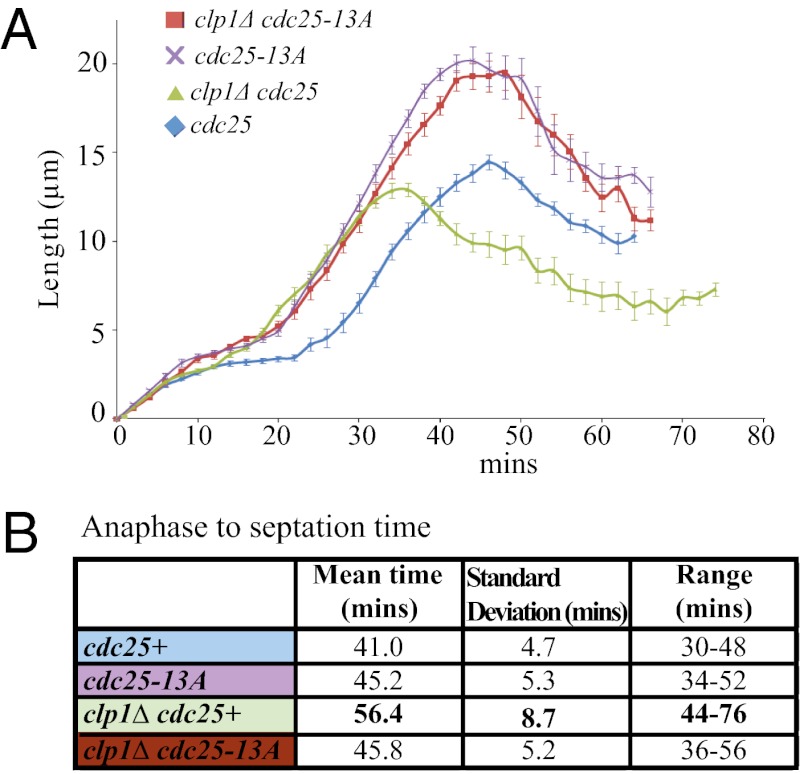
Retention of Cdk1 phosphorylation on Cdc25 in clp1Δ cells delays Cdk1 inactivation (17). To define which steps of mitotic exit are altered as a result, we monitored mitotic progression until septation in clp1+ cdc25-GFP, clp1+ cdc25-13A-GFP, clp1Δ cdc25-GFP, and clp1Δ cdc25-13A-GFP cells by measuring the distance between SPBs (marked by Sid4-RFP) as an indication of spindle length. S. pombe has three phases of spindle dynamics (23). In phase 1, a 2- to 2.5-μm spindle is formed. In phase 2, spindle length is maintained while chromosomes align and begin to separate (metaphase and anaphase A). Phase 3 corresponds to anaphase B, where the spindle elongates. After phase 3, the mitotic spindle collapses, and cells divide.
Because Clp1 dephosphorylates Cdc25 during anaphase (17, 18), we measured the time in and after phase 3 to assess the contribution of Cdc25 dephosphorylation. Phase 3 was significantly longer in cdc25-13A-GFP cells for both clp1+ and clp1Δ strains (25.6 ± 1.1 and 24.6 ± 1.2 min, respectively) compared with cdc25-GFP cells (22.0 ± 1.3 and 21.4 ± 2.2 min, respectively) (Fig. 6A). This change reflects the longer cell septation length (19.5 ± 1.0 μm for clp1+ cdc25-13A-GFP and 20.2 ± 0.8 μm for clp1Δ cdc25-13A-GFP cells) and time required for the spindle to fully elongate compared with clp1+ cdc25-GFP and clp1Δ cdc25-GFP cells (14.5 ± 0.4 and 12.9 ± 0.4 μm, respectively). In clp1Δ cdc25-GFP cells, time from mitotic spindle collapse at the end of phase 3 to septation was at least 10 min longer compared with all other strains, which were not significantly different between one another (Fig. 6A); this finding confirmed that Clp1 dephosphorylation of Cdc25 plays an important role in controlling the time to cell division. Eliminating Cdk1 phosphorylation in cdc25-13A cells restored normal timing of this cell cycle phase (Fig. 6A).
Fig. 6.
Clp1 dephosphorylation of Cdc25 is vital for proper mitotic exit. (A) The distances between SPBs for 10 cells per strain were measured from the beginning of mitosis until the time of septation at 2-min intervals and averaged. SEM is indicated for each time point. Mean, SD, and range of total time from anaphase to septation for each strain were calculated (B).
To probe if Clp1 and Cdc25 are involved in a double negative feedback loop that contributes to the abruptness of cytokinesis, we calculated the variation of time from anaphase onset to cell septation (mitotic exit) in individual cells. If a feedback loop exists, then removing the ability of Clp1 to dephosphorylate Cdc25 in clp1Δ cells should reduce the synchrony of mitotic exit. Indeed, we found that clp1Δ cdc25+ cells had a significantly larger range in mitotic exit times compared with all other strains (Fig. 6B). Thus, our data indicate that Clp1 and Cdc25 are in a double negative feedback loop that regulates the precise timing of cytokinesis, and this finding may explain the low but reproducible rate of cytokinetic failure in clp1Δ cells (25, 26).
Discussion
In this study, we used S. pombe as an in vivo model to explore the interactions between Cdk1, Clp1, and Cdc25. In vivo systems provide volume constraint, organelle compartmentalization, and precise activation of cell cycle checkpoints, variables that cannot be accounted for in the cell extract systems used thus far to study bistability in mitotic control. We show in vivo that Cdk1 phosphorylates Cdc25 on at least 11 and probably, 13 sites and that this multisite disordered phosphorylation activates Cdc25 and is crucial for the precision of the mitotic entrance switch and as a result, the maintenance of constant cell length and size.
Division lengths and growth rates for undisturbed S. pombe cells are remarkably invariant (27). However, cdc25-13A cells have increased division length variance, a mark of a disturbed mitotic switch. Although size differences in a population could be caused by subpopulations with distinct but stable mitotic switches, our single-cell analysis showed that mitotic entry was less predictable in each cdc25-13A cell irrespective of the mother’s division length. Thus, we show that, when the Cdk1-Cdc25 positive feedback loop is removed, it manifests as decreased precision in mitotic timing. Whether reduced mitotic entry precision in cdc25-13A cells is caused by a more stochastic but sharp mitotic switch or graded Cdk1 activity creating difficulty in executing a specific function (e.g., SPB separation) remains to be explored.
Our study shows that Cdk1 likely phosphorylates Cdc25 in a disordered and distributive manner, confirming previous in silico models postulating that only a distributive multisite phosphorylation mechanism can result in ultrasensitivity in protein phosphorylation (10). Although a preferred Cdk1 phosphorylation site on huWee1 is important for its degradation at mitosis (28), whether a specific Cdk1 site on Cdc25 is important to activate Cdc25 has not been systematically studied. The relative ease of genetic manipulation in S. pombe allowed us to study the effect on mitotic entry by eliminating subsets of Cdk1 sites on Cdc25. Our data provide no evidence of selective requirements for individual sites; rather, we propose that all 13 Cdk1 phosphosites on Cdc25 contribute equally to mitotic entry by the ultrasensitive phosphorylation and subsequent activation of Cdc25.
In all species, Cdc25 proteins have at least 6 and up to 13 possible Cdk1 phosphosites according to the minimal Cdk1 consensus sequence, allowing for the possibility of ultrasensitive Cdc25 activation by multisite phosphorylation. In humans, three Cdc25 isoforms with different numbers of potential Cdk1 phosphosites contribute to mitotic entrance (29). Thus, different numbers of available phosphosites combined with varying localization may provide temporal control in ultrasensitive activation of different Cdc25 isoforms. Beside multisite phosphorylation, other mechanisms also may contribute to the ultrasensitive responses of Cdc25 to Cdk1, such as competition among Cdk1 substrates and protein phosphoisoforms for enzyme binding (6, 30) and regulation of opposing enzymes like Clp1 (31).
We also suggest that there is a double negative feedback loop between Clp1 and Cdc25 operating at mitotic exit. Our work confirms and extends previous work (17) showing that Clp1 dampens Cdk1 activity by dephosphorylating and inactivating Cdc25. Clp1 facilitates proper contractile ring dynamics and timely cytokinesis by antagonizing Cdk1 phosphorylations that negatively regulate the septation initiation network (SIN) and antagonize contractile ring formation (26, 32–35). Our data show that abrogation of Clp1-Cdc25 feedback reduces the abruptness of cytokinesis. Therefore, unlike S. cerevisiae Cdc14, which facilitates the mitotic exit switch at the metaphase–anaphase transition (13, 14, 36), S. pombe Clp1 controls the abruptness of the mitotic exit switch primarily by promoting proper SIN and contractile ring activity.
Materials and Methods
Immunoprecipitations and Immunoblots.
Whole-cell lysates were prepared in supplemented (1 mM PMSF, 1 mM benzamidine) Nonidet P-40 buffer as described (17). Lysates were immunoprecipitated with anti-V5 antibody for Cdc25-V5. Immunocomplexes were mixed with SDS sample buffer and boiled before separation by 8% acrylamide SDS-PAGE. They were visualized by immunoblot using anti-Cdc25 (gift from Paul Russell, Scripps Research Institute, La Jolla, CA) or anti-Cdk1 (PSTAIRE; Invitrogen) antibodies.
In Vitro Kinase and Cdc25 Activity Assays.
Recombinant MBP fusion proteins were produced and purified from Escherichia coli. Recombinant Cdk1-Cdc13 (Cdk1-CyclinB in S. pombe) was purified from baculovirus-infected insect cells. In vitro Cdk1 kinase and Cdc25 phosphatase assays were performed as previously described (17, 20) (SI Materials and Methods).
Microscopy.
Details on microscopy settings and analytical methods are in SI Materials and Methods.
Mathematical Model.
Details are in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Dr. Paul Russell for sharing the anti-Cdc25 antibody and the K.L.G. laboratory members for technical advice, comments on the manuscript, and useful discussion. L.X.L. and this work were supported by National Institutes of Health Grant GM068786 and T32 GM07347 for the Vanderbilt Medical-Scientist Training Program. B.N. is supported by the European Community’s 7th Framework Programmes (UniCellSys and MitoSys). K.L.G. is an Investigator of The Howard Hughes Medical Institute.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1201366109/-/DCSupplemental.
References
- 1.Morgan DO. The Cell Cycle: Principles of Control. London: New Science Press; 2007. [Google Scholar]
- 2.Lindqvist A, Rodríguez-Bravo V, Medema RH. The decision to enter mitosis: Feedback and redundancy in the mitotic entry network. J Cell Biol. 2009;185:193–202. doi: 10.1083/jcb.200812045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Novák B, Tyson JJ. Numerical analysis of a comprehensive model of M-phase control in Xenopus oocyte extracts and intact embryos. J Cell Sci. 1993;106:1153–1168. doi: 10.1242/jcs.106.4.1153. [DOI] [PubMed] [Google Scholar]
- 4.Pomerening JR, Kim SY, Ferrell JE., Jr Systems-level dissection of the cell-cycle oscillator: Bypassing positive feedback produces damped oscillations. Cell. 2005;122:565–578. doi: 10.1016/j.cell.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 5.Pomerening JR, Sontag ED, Ferrell JE., Jr Building a cell cycle oscillator: Hysteresis and bistability in the activation of Cdc2. Nat Cell Biol. 2003;5:346–351. doi: 10.1038/ncb954. [DOI] [PubMed] [Google Scholar]
- 6.Kim SY, Ferrell JEJ., Jr Substrate competition as a source of ultrasensitivity in the inactivation of Wee1. Cell. 2007;128:1133–1145. doi: 10.1016/j.cell.2007.01.039. [DOI] [PubMed] [Google Scholar]
- 7.Trunnell NB, Poon AC, Kim SY, Ferrell JE., Jr Ultrasensitivity in the Regulation of Cdc25C by Cdk1. Mol Cell. 2011;41:263–274. doi: 10.1016/j.molcel.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Novák B, Tyson JJ. Design principles of biochemical oscillators. Nat Rev Mol Cell Biol. 2008;9:981–991. doi: 10.1038/nrm2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferrell JE, Xiong W. Bistability in cell signaling: How to make continuous processes discontinuous, and reversible processes irreversible. Chaos. 2001;11:227–236. doi: 10.1063/1.1349894. [DOI] [PubMed] [Google Scholar]
- 10.Domingo-Sananes MR, Novak B. Different effects of redundant feedback loops on a bistable switch. Chaos. 2010;20:045120. doi: 10.1063/1.3526967. [DOI] [PubMed] [Google Scholar]
- 11.Yang L, MacLellan WR, Han Z, Weiss JN, Qu Z. Multisite phosphorylation and network dynamics of cyclin-dependent kinase signaling in the eukaryotic cell cycle. Biophys J. 2004;86:3432–3443. doi: 10.1529/biophysj.103.036558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pomerening JR. Positive-feedback loops in cell cycle progression. FEBS Lett. 2009;583:3388–3396. doi: 10.1016/j.febslet.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holt LJ, Krutchinsky AN, Morgan DO. Positive feedback sharpens the anaphase switch. Nature. 2008;454:353–357. doi: 10.1038/nature07050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.He E, et al. System-level feedbacks make the anaphase switch irreversible. Proc Natl Acad Sci USA. 2011;108:10016–10021. doi: 10.1073/pnas.1102106108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stegmeier F, Amon A. Closing mitosis: The functions of the Cdc14 phosphatase and its regulation. Annu Rev Genet. 2004;38:203–232. doi: 10.1146/annurev.genet.38.072902.093051. [DOI] [PubMed] [Google Scholar]
- 16.Mocciaro A, et al. Vertebrate cells genetically deficient for Cdc14A or Cdc14B retain DNA damage checkpoint proficiency but are impaired in DNA repair. J Cell Biol. 2010;189:631–639. doi: 10.1083/jcb.200910057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wolfe BA, Gould KL. Fission yeast Clp1p phosphatase affects G2/M transition and mitotic exit through Cdc25p inactivation. EMBO J. 2004;23:919–929. doi: 10.1038/sj.emboj.7600103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Esteban V, et al. A role for the Cdc14-family phosphatase Flp1p at the end of the cell cycle in controlling the rapid degradation of the mitotic inducer Cdc25p in fission yeast. J Cell Sci. 2004;117:2461–2468. doi: 10.1242/jcs.01107. [DOI] [PubMed] [Google Scholar]
- 19.Wolfe BA, McDonald WH, Yates JR, 3rd, Gould KL. Phospho-regulation of the Cdc14/Clp1 phosphatase delays late mitotic events in S. pombe. Dev Cell. 2006;11:423–430. doi: 10.1016/j.devcel.2006.07.016. [DOI] [PubMed] [Google Scholar]
- 20.Kovelman R, Russell P. Stockpiling of Cdc25 during a DNA replication checkpoint arrest in Schizosaccharomyces pombe. Mol Cell Biol. 1996;16:86–93. doi: 10.1128/mcb.16.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fantes PA. Control of cell size and cycle time in Schizosaccharomyces pombe. J Cell Sci. 1977;24:51–67. doi: 10.1242/jcs.24.1.51. [DOI] [PubMed] [Google Scholar]
- 22.Langerak P, Russell P. Regulatory networks integrating cell cycle control with DNA damage checkpoints and double-strand break repair. Philos Trans R Soc Lond B Biol Sci. 2011;366:3562–3571. doi: 10.1098/rstb.2011.0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kõivomägi M, et al. Cascades of multisite phosphorylation control Sic1 destruction at the onset of S phase. Nature. 2011;480:128–131. doi: 10.1038/nature10560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nabeshima K, et al. Dynamics of centromeres during metaphase-anaphase transition in fission yeast: Dis1 is implicated in force balance in metaphase bipolar spindle. Mol Biol Cell. 1998;9:3211–3225. doi: 10.1091/mbc.9.11.3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cueille N, et al. Flp1, a fission yeast orthologue of the s. cerevisiae CDC14 gene, is not required for cyclin degradation or rum1p stabilisation at the end of mitosis. J Cell Sci. 2001;114:2649–2664. doi: 10.1242/jcs.114.14.2649. [DOI] [PubMed] [Google Scholar]
- 26.Trautmann S, et al. Fission yeast Clp1p phosphatase regulates G2/M transition and coordination of cytokinesis with cell cycle progression. Curr Biol. 2001;11:931–940. doi: 10.1016/s0960-9822(01)00268-8. [DOI] [PubMed] [Google Scholar]
- 27.Sveiczer A, Novak B, Mitchison JM. The size control of fission yeast revisited. J Cell Sci. 1996;109:2947–2957. doi: 10.1242/jcs.109.12.2947. [DOI] [PubMed] [Google Scholar]
- 28.Watanabe N, et al. M-phase kinases induce phospho-dependent ubiquitination of somatic Wee1 by SCFbeta-TrCP. Proc Natl Acad Sci USA. 2004;101:4419–4424. doi: 10.1073/pnas.0307700101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boutros R, Lobjois V, Ducommun B. CDC25 phosphatases in cancer cells: Key players? Good targets? Nat Rev Cancer. 2007;7:495–507. doi: 10.1038/nrc2169. [DOI] [PubMed] [Google Scholar]
- 30.Georgi AB, Stukenberg PT, Kirschner MW. Timing of events in mitosis. Curr Biol. 2002;12:105–114. doi: 10.1016/s0960-9822(01)00662-5. [DOI] [PubMed] [Google Scholar]
- 31.Ferrell JE., Jr Feedback regulation of opposing enzymes generates robust, all-or-none bistable responses. Curr Biol. 2008;18:R244–R245. doi: 10.1016/j.cub.2008.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guertin DA, Chang L, Irshad F, Gould KL, McCollum D. The role of the sid1p kinase and cdc14p in regulating the onset of cytokinesis in fission yeast. EMBO J. 2000;19:1803–1815. doi: 10.1093/emboj/19.8.1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roberts-Galbraith RH, et al. Dephosphorylation of F-BAR protein Cdc15 modulates its conformation and stimulates its scaffolding activity at the cell division site. Mol Cell. 2010;39:86–99. doi: 10.1016/j.molcel.2010.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dischinger S, Krapp A, Xie L, Paulson JR, Simanis V. Chemical genetic analysis of the regulatory role of Cdc2p in the S. pombe septation initiation network. J Cell Sci. 2008;121:843–853. doi: 10.1242/jcs.021584. [DOI] [PubMed] [Google Scholar]
- 35.Clifford DM, et al. The Clp1/Cdc14 phosphatase contributes to the robustness of cytokinesis by association with anillin-related Mid1. J Cell Biol. 2008;181:79–88. doi: 10.1083/jcb.200709060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.López-Avilés S, Kapuy O, Novák B, Uhlmann F. Irreversibility of mitotic exit is the consequence of systems-level feedback. Nature. 2009;459:592–595. doi: 10.1038/nature07984. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.