Abstract
Another person’s caress is one of the most powerful of all emotional social signals. How much the primary somatosensory cortices (SIs) participate in processing the pleasantness of such social touch remains unclear. Although ample empirical evidence supports the role of the insula in affective processing of touch, here we argue that SI might be more involved in affective processing than previously thought by showing that the response in SI to a sensual caress is modified by the perceived sex of the caresser. In a functional MRI study, we manipulated the perceived affective quality of a caress independently of the sensory properties at the skin: heterosexual males believed they were sensually caressed by either a man or woman, although the caress was in fact invariantly delivered by a female blind to condition type. Independent analyses showed that SI encoded, and was modulated by, the visual sex of the caress, and that this effect is unlikely to originate from the insula. This suggests that current models may underestimate the role played by SI in the affective processing of social touch.
Keywords: affective touch, sexual selection, gender, crossmodal perception, hot cognition
The exact same sensual caress can feel divine from a person we find attractive, and aversive from one we find repulsive (1, 2). Although a large literature documents the powerfully emotional nature of interpersonal touch and its significance in everyday life, we still lack a thorough understanding of how the brain processes this class of stimulus. In particular, we seek to know how somatosensory information in touch is integrated with the visual features (e.g., attractiveness) of the person giving the caress to produce emotional responses (2). Prevailing models of how the brain processes the sensory and affective properties of gentle touch have drawn predominantly on experiments that used inanimate objects with varying textures, rather than interpersonal touch (2, 3), or from the known neural organization of unmyelinated, C-tactile (CT) fibers in hairy skin (4–7), which respond specifically to light touch (8) and project to and activate the insula, but project to and inhibit the primary somatosensory cortex (SI) (9, 10). Differences in brain activation in the insula, anterior cingulate cortex (ACC), and orbitofrontal cortex (OFC) have been found according to stimulus type (e.g., a wooden rod vs. a velvet cloth) (11) and subjective ratings of emotion (12, 13). Thus, previous work has shown that SI primarily discriminates sensory properties (e.g., location, pressure, texture), whereas the insula, together with the ACC and the OFC, primarily discriminate affective/emotional properties (i.e., perceived pleasantness) (7, 11, 14, 15). Although some models propose an interaction between these systems, they propose that SI’s role in affective processing is probably indirect: (i) SI is modulated by sensory properties of touch, such as the velocity [e.g., 3 cm/s vs. 30cm/s (16)] or location [e.g., arm vs. palm (17)], that happen also to modulate the pleasantness of the caress by differentially recruiting CT fibers; or (ii) affective properties are first processed in the insula, which in turn modulates responses in SI/secondary somatosensory cortex (SII) (7). Theoretical frameworks thus far would therefore argue that SI should play no role in discriminating affective properties of touch beyond its somatosensory properties on the skin, or only as a result from modulation by the insula (7), and predict that blood oxygenation level-dependent (BOLD) activation within SI should not differentiate between being caressed by a person we find visually attractive and one we do not—as long as the caress is physically identical on the skin. This prediction finds indirect evidence from McCabe et al. (18). In this study, a cream was rubbed on participants’ arms while text on the screen suggested that the cream was “thin” or “rich.” In fact, the same cream was applied in all cases, ensuring that the cutaneous afferent to the brain remained constant. The cognitive manipulation led participants to find the rich cream 20% more pleasant than the thin cream, and led to more activation in the OFC for the rich condition, but no significant differences were measured in SI.
Indirect evidence, however, sheds doubt on the assumption that SI would only process sensory properties of the somatosensory input. SI is sensitive to aspects of touch processing beyond simple “bottom-up” somatosensory properties (19–22), and it shows a trend in discriminating pleasant from neutral touch when stimuli differ in tactile properties (11, 14, 16, 17). Differential SI activation has been reported also while participants view painful somatosensory stimulation to another person (refs. 22–31, but see ref. 32). However, it remains unclear whether these vicarious tactile responses in SI convey information about the valence of a touch: Ebisch et al. (33) compared the vision of an actor being slapped vs. caressed and found stronger activity in the caress condition in SI, but the difference was not significant. Others have shown that viewing the hand actions of other people (34–41) activates the hand region of SI, showing that visual information can drive SI activation when that information directly depicts proprioceptive stimulation in another person (40). A recent study showed that seeing someone else haptically explore different objects triggered patterns of activity in SI that contain information about the tactile properties of the objects (42). Also, viewing touch delivered at 3 cm/s is perceived as more pleasant and activates SI more than seeing touch delivered at 30 cm/s (16). Finally, the fact that SI is not activated by projections from unmyelinated CT afferents (10) does not preclude the possibility that SI processes pleasant interpersonal touch. Some interpersonal touches are pleasant without having the properties that activate CT afferents (2), and SI receives information from thick fibers that sense tactile properties (e.g., velocity, pressure) that could be processed to recognize a pleasant caress. However, none of these considerations yet demonstrate that SI encodes affective/social properties of touch, above and beyond sensory differences. In particular, they do not indicate whether, for the response in SI, who you believe is caressing you matters.
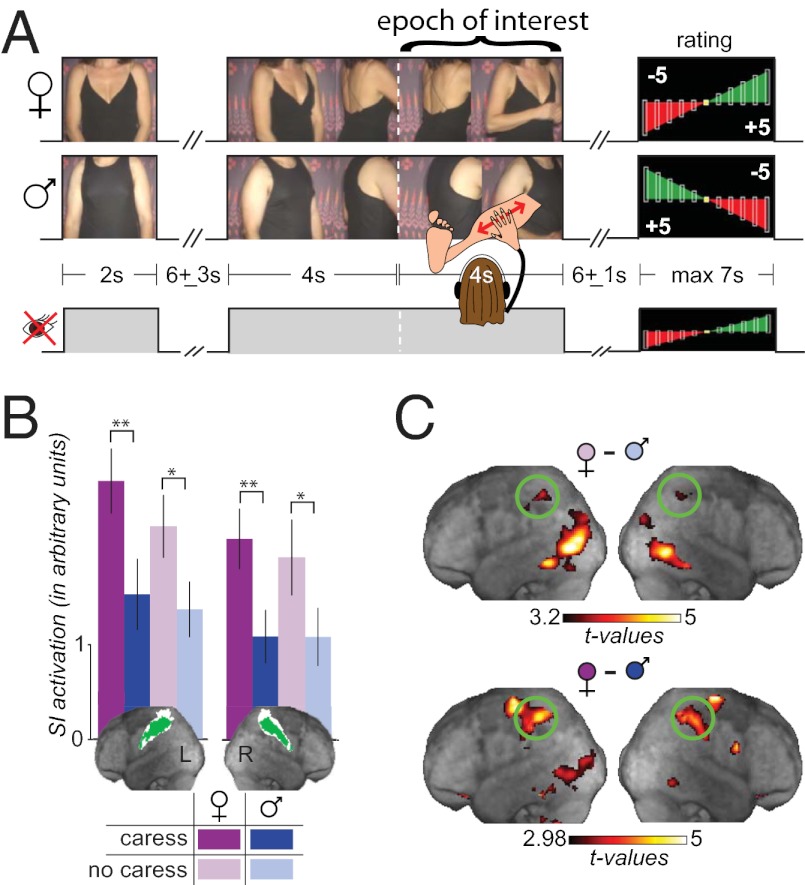
We used functional MRI (fMRI) to investigate whether the visually perceived identity of a caresser might result in differential activation to a cutaneously invariant stimulus in SI. Heterosexual male participants experienced a sensual caress on their legs while viewing film clips, synchronized with the caress, that depicted either an attractive woman (i.e., visually female caress) or a less attractive man (i.e., visually male caress; Fig. 1A). Three video epochs composed a trial of each condition: a 2-s epoch showing the male or female actor standing motionless beside the scanner in which the subject was lying (epoch 1), followed, after a brief jittered interval of a black screen, by a 4-s epoch showing the actor turning toward the subject’s legs with the intent to perform the caress (epoch 2) and, immediately after, a final 4-s epoch showing the actor leaning forward to caress the legs of the subject and then returning to the original position (epoch 3). None of these epochs actually showed the subject’s leg being caressed; the visual stimulus only suggested that this action was being performed (Fig. 1A). Unbeknownst to the participants, all caresses were actually delivered by the female actor/experimenter, who was blind to the condition played in the video clip. Additional conditions involved the same visual stimuli without delivery of the caress (i.e., visually female no-caress and visually male no-caress conditions). Finally, to localize the somatosensory representation of the legs, participants were caressed on their legs while viewing a gray screen (i.e., localizer). In our study, we recruited only heterosexual male participants to maximize the emotional difference between the caress conditions, as heterosexual men find the caress of the opposite sex more desirable, and of the same sex more undesirable (43–45), than heterosexual women do. To provide a uniform and salient context, participants were asked to place themselves in a mindset in which a caress would signal that the man or woman “was coming on to them” (e.g., at a party or beach). We then looked for spatially restricted visual-sex discrimination in the brain via three complementary and independent analysis methods: (i) a region-of-interest (ROI) analysis in SI, (ii) a general linear model to localize differences in activation at the whole-brain level, and (iii) multivariate pattern classification using the searchlight technique (46), also at the whole-brain level, to identify localized multivoxel differences in brain activation patterns.
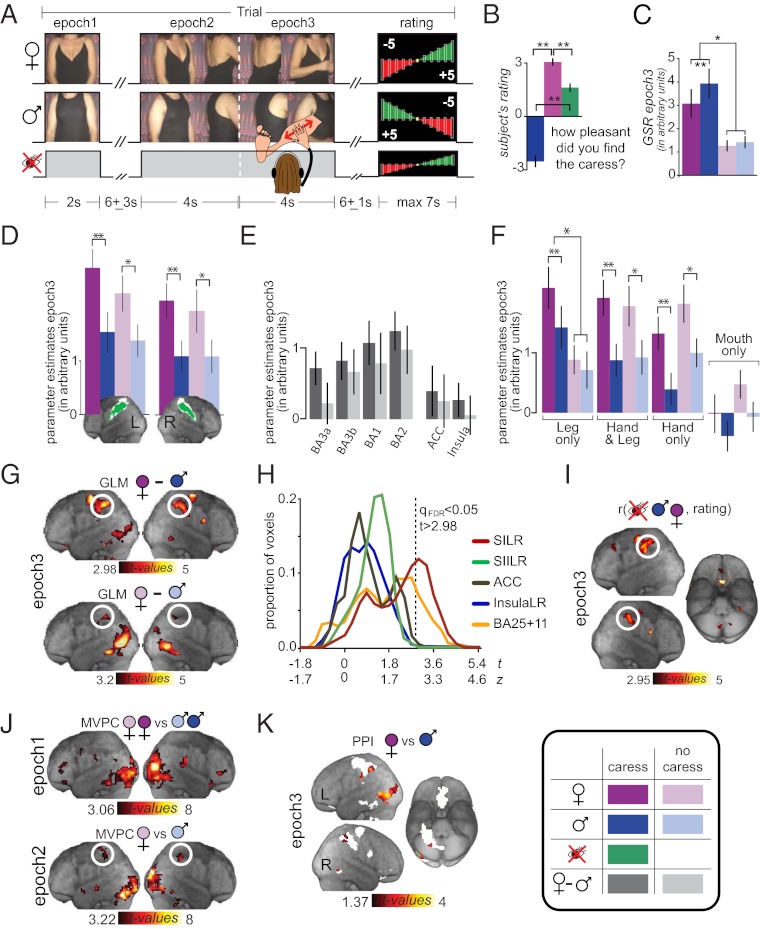
Fig. 1.
(A) Paradigm. (B) Average rating of visually male, female and localizer caress. (Inset: Color code for the entire figure.) (C) GSR (n = 10) as function of condition in arbitrary units. (D) Average activation in SI ROI as function of hemisphere and condition; white regions on the renders show the location of SI according to the Anatomy Toolbox; green voxels within SI significantly activated during the localizer. (E) Visual SI modulation (visually female minus visually male) separately for caress and no-caress as a function of ROI. (F) SI activation as a function of condition and somatotopic subregion. (E and F) The two hemispheres were averaged. (G) GLM in epoch 3 for visually female minus visually male, separately for caress and no-caress. (H) Effect size distribution (as t value or z-value) as a function of ROI. The dashed vertical line represents the significance threshold for a whole-brain FDR correction. (I) Correlation analysis between trial-by-trial pleasantness rating and BOLD activation. (J) Multivoxel pattern classification, visually female vs. visually male no-caress, using searchlights as a function of epoch. White circles in G, I, and J indicate clusters including SI. (K) PPI between activation in SI ROI and visually female vs. visually male caress inclusively masked (white mask) with activations of G. (C–F) Error bars represent SEM (*P < 0.05 and **P < 0.01). (G, J, and K) Threshold q < 0.05 using FDR over the entire brain; (I) threshold uncorrected P < 0.005 (results also survive q < 0.05 using FDR for the volume of SI).
Results
All participants gave the male actor a negative rating on sexual attractiveness (mean rating ± SD, −2.58 ± 2.24 on a scale from −5 to 5) and the female actor positive ratings (3.14 ± 1.7). During the actual fMRI study, all participants found the visually male caress significantly less pleasant (male, −2.53 ± 1.38; female, 3.05 ± 0.87; P < 0.002; Fig. 1B) and also significantly more emotionally arousing [from stable galvanic skin responses (GSRs) available for 10 participants, P < 0.001; Fig. 1C] than the visually female caress. This demonstrates that the experimental design effectively created differences in the emotional perception of the caresses and affective differences between conditions arose solely from the different visual stimuli, as all caresses were delivered by the same person, who was blind to the condition. We defined the SI ROI separately within each hemisphere by including all voxels of the cytoarchitectonic maximum probability maps of SI, which were also significantly activated during our localizer. We then measured the mean BOLD response within these ROIs during epoch 3 (Fig. 1D), which is when visual and tactile information come together in the caress conditions. A 2 × 2 × 2 repeated-measures ANOVA with two hemispheres (left and right), two caress conditions (caress and no caress), and two genders (visually female, visually male) revealed only a main effect of sex [F(1,17) = 27, P < 0.0001]. This indicates that both the right and left SI responded more in conditions in which participants thought they were caressed by a female than by a male. On the contrary, the absence of a main effect of caress [F(1,17) = 0.8, P > 0.38], and of an interaction of caress by sex [F(1,17) = 0.29, P > 0.6], suggests that in the SI ROI as a whole the visually triggered expectation of a caress in the no-caress condition was sufficient to create a response similar to that in the caress condition. Repeating this analysis separately for each of the four cytoarchitectonic subdivisions of SI revealed a rostrocaudal gradient with the effect strongest in Brodmann area (BA) 2 and weakest in BA3a (Fig. 1E).
To determine if these effects were confined to the leg representation of SI (representing the sensation of the caress on the participant’s leg) or extended to the hand representation [vicariously representing the proprioceptive and tactile sensations of the actor in the movie while the actor caresses the participant’s leg (34–41)], we functionally divided SI into sectors representing the leg, hand, and mouth (Fig. S1). This analysis revealed (i) that the leg representation discriminates visual sex in the caress (but not in the no-caress condition) and (ii) the hand representation and the extensive region of overlap responding to both leg and hand discriminate visual sex in the caress and no-caress condition (Fig. 1F). The leg region responded more strongly to the caress than the no-caress conditions (P < 0.01), but the hand and the overlap of leg and hand regions did not (both P > 0.42). This suggests that visual sex modulated the sensations on the participant’s legs only when a caress was actually delivered and modulated the simulation of the sensations in the actor’s hand whenever the movies were seen (whether a caress was delivered). The same analysis failed to reveal a significant effect of visual sex in the mouth region. This confirms that visual sex modulated SI activity in a manner specific to the perceived action and received caress.
To explore whether discrimination of visual sex was spatially confined to SI, we complemented the ROI analyses with a whole-brain GLM analysis by using planned contrasts. We found that few other clusters exhibited sensitivity to sex (with visually female greater than visually male; no significant activations survived in any regions for the opposite contrast), both during the period of actual caress and the corresponding epoch of the no-caress conditions [epoch 3; false-discovery-rate corrected (qFDR) q < 0.05; Fig. 1G; Fig. S2 shows an example of single subjects]. The SI activation was centered in BA2 and BA1, as determined by the Anatomy Toolbox (Table S1), confirming the results of our ROI analysis. Other areas that showed a similar modulation included the premotor, inferior parietal, intraparietal cortices, and visual association areas. In addition, of the three brain regions most often associated with affective touch, the OFC, but not the ACC or insula, differentiated visual sex in the GLM. To examine if the lack of effect in ACC or insula was caused by a lack of power, we repeated the GLM with more lenient thresholds in the ACC (focusing on the ventral portion, for affective signal discrimination) and insula (q < 0.05, FDR-corrected for the volume of the ACC or insula), but still found no significant effects. The same was true for ROI analyses (Fig. 1F and Fig. S3) in the same regions. To examine more quantitatively whether some voxels in the insula or ACC may have shown sex effects as strong as in SI, we extracted visual sex effect sizes from all voxels in SI, SII, insula, ACC, and OFC and compared the distributions (Fig. 1H). Only the OFC, but not the SII, insula, or ACC, closely matched SI in how strongly voxels were sensitive to visual sex. Comparing the proportion of significant voxels (t > 2.98) in each region by using χ2 tests revealed that this proportion was larger in SI than in any other region, with the OFC showing the second largest proportion, exceeding that seen in SII, insula, and ACC.
To examine whether trial-by-trial activation magnitude in SI in response to a caress was correlated with affective processing, we additionally pooled the BOLD data from all epoch 3 in which a caress occurred (i.e., visually female and male caress and localizer condition) and correlated trial-by-trial activation with trial-by-trial pleasantness rating. Results indicated that the BOLD signal in SI across caress conditions was positively correlated with reported pleasantness (Fig. 1I and Table S1). Importantly, because these analyses combined conditions with complex visual stimuli (e.g., visually female and male caress) and without complex visual stimuli (e.g., localizer), they support the notion that SI activation tracked the participants’ affective valuation of the caress, and not just the visual features of the movies shown to participants.
During the anticipatory periods (whole-brain analyses on epochs 1 and 2), the only regions that significantly discriminated visual sex were the occipital lobe and the lingual gyrus in the temporal lobe (FDR-corrected q < 0.05; Table S1). To explore the presence of more subtle differences in brain activation during the anticipation of the caress, we used a multivoxel pattern classification analysis of visual sex, with the searchlight technique, during epochs 1 and 2. This revealed an area in SI that was sensitive to sex during epoch 2 but not epoch 1 (Fig. 1J and Table S1), including voxels of BA1 and BA2 that also responded during actual caress. Thus, the ability of SI to distinguish visual sex extends to the close anticipation of that touch. Of the other areas normally associated with affective touch, the insula did not discriminate visual sex significantly, the OFC did so only during epoch 1, and the ACC did so only during epoch 2 (Fig. 1J and Table S1).
To identify if any of the brain regions that showed more activation during the visually female than the visually male epoch 3 might be the source of the visual modulation of SI, we performed a psychophysiologic interaction (PPI) analysis. This analysis revealed that subregions of the visual and posterior parietal cortex augmented their functional connectivity with SI during the visually female caress compared with visually male caress epoch 3 (FDR-corrected q < 0.05; Fig. 1K and Table S1). The same was not true for the OFC or the premotor regions, even lowering the threshold to an uncorrected P value < 0.05.
Discussion
We experimentally decoupled the affective significance of a caress from its cutaneous sensory properties by showing participants a male or female engaged in the caress, which was actually always performed by the same woman. We found a large and robust modulation of activation within SI by the perceived sex, across three different types of analysis. Of the brain regions more classically associated with affective processing of touch (insula, ACC, and OFC), only the OFC was significantly modulated by visual sex, even when lenient, small volume-corrected analyses were used for the ACC and insula. The size of the sex effect (i.e., female vs. male) was larger in SI than in SII, insula, and ACC. Moreover, we found evidence for differential SI activation even in the 4 s preceding the moment when touch would have occurred (i.e., epoch 2 no-caress condition), when participants had only seen the person who would perform the caress start to turn toward their legs, but no cutaneous stimulus had yet been delivered. Our somatotopic analysis finally revealed that the leg region of SI showed significant sex effects when the caress was present but not when it was absent. Taken together, our findings provide evidence that SI integrates visual and tactile information during the processing of interpersonal touch and that SI is sensitive to the sex of a caresser and to the concomitant perceived pleasantness of her/his sensual touch. Because the sex of the caresser changed the affective valence of the caress, and because activation in SI also correlated with trial-by-trial pleasantness ratings of the caress when the sex of the caresser was not included in the analysis, prevailing models may have underestimated the role played by SI in socially relevant affective processing. The most parsimonious interpretation of our results may thus be that SI participates in encoding the affective valence of touch in at least some socially relevant contexts, perhaps especially those that have implications for sexual selection. It is important to acknowledge that future work will be necessary to dissect how exactly our manipulation led SI activation to correlate with affective valence. Because our experiment used only visual stimuli, it remains unclear whether auditory manipulations (e.g., the voice of a male or female or a textual description of the caresser) would have led to similar effects. Our data also cannot rule out effects of putatively expected tactile differences between female and male conditions, even though actual tactile properties were identical. For example, a visually male caresser might trigger the expectation of a caress that differs in sensory properties on the skin (e.g., rougher touch applied with more pressure) compared with the visually female caress. However, we show that, at least in certain contexts, SI does bias the representation of a caress in ways consistent with heterosexual males’ trial-by-trial reported preferences. This dovetails with recent findings that caresses delivered at 3 cm/s are rated as more pleasant and result in increased SI activation compared with those delivered at 30 cm/s (16), and also that caresses on the arm are rated as more pleasant than those on the palm (17).
We also found segregation within SI according to its cytoarchitectonic and somatotopic subdivisions. Cytoarchitectonically, SI is composed of four distinct brain regions: BA3a and BA3b, which are often jointly referred to as BA3, as well as BA1 and BA2. Within these regions, the effect of perceived sex of the caresser was strongest in BA2 and BA1 and weakest in BA3. This is consistent with anatomical connectivity between these subregions (40). BA3 receives its main inputs from the thalamus and has strong reciprocal cortical connections only with M1 and other somatosensory brain regions. BA3 is, therefore, the most direct “primary” somatosensory cortex. In contrast, BA2 is more of a tactile association area. It receives somatosensory input from BA3 but has reciprocal connections with cortical regions that combine visual, auditory, and somatosensory information (47–51) and respond to complex visual stimuli: the fundus of the intraparietal sulcus (ventral intraparietal area) responding to the sight of touch (52), the inferior parietal lobule (areas PF/PFG in particular) and the premotor cortex responding to the sight of actions (48, 51), and finally the ACC and insula responding to the sight of emotions (47, 53). Electrophysiologically controlled tracer injections in the limb representation of BA2 have not revealed direct connections with the OFC (50), making OFC an unlikely source of direct information to BA2 [but weak direct connections may exist between the tongue representation of SI and the OFC for integration of texture and taste (54, 55)]. In the context of our experiment, BA2 could thus receive tactile information from lower-level somatosensory cortices and transformed visual information about the actions and attractiveness of the person giving the sensual caress from the inferior parietal lobule, the intraparietal sulcus, the premotor cortex, the insula, and the ACC. Our effect size comparison showed that the insula and the ACC are unlikely sources of such information, as these regions, although strongly activated by all caresses, did not differentiate visual sex significantly or as strongly as SI. Our data are consistent with some of the remaining anatomical routes: in addition to BA2 and BA1, comparing the visually female and visually male conditions revealed a network of occipital, inferior parietal, intraparietal, and premotor cortices, all of which could therefore contribute transformed visual information to SI through their direct anatomical connections. Our PPI analysis further constrains these routes by showing that, of the regions showing increased activation during the visually female compared with the visually male caress condition, only two, the visual cortex and the posterior parietal lobe, displayed the augmented functional connectivity that would be expected from a region that modulates SI across the sex conditions. As BA2 has anatomical connections with the posterior parietal lobe but not the visual cortex (47–51, 56), the posterior-parietal lobe is the most likely source of visual information to SI.
Visual information sent to BA2 could then be transmitted back to BA1 and BA3, explaining the gradient of the decreasing sex effect from BA2 to BA1 and BA3. This would be in line with the notion that visual information in the somatosensory cortex flows in the direction opposite to that of the normal somatosensory information flow (40, 42). Our finding that the higher association levels of SI (BA2 and BA1) showed the greatest response to combined somatosensory and visual information is also in line with recently reviewed data showing that seeing the actions and somatic pains of others also activates SI along a similar caudorostral gradient, with the strongest activations found in BA2 (40). Also, only BA2 increases activation during visual and tactile shape recognition (57). Finally, the sensitivity to perceived sex shown in the present study is also compatible with evidence that actual (58) or virtual lesions (59) encompassing SI can impair the capacity to visually perceive the affect of others.
Activation in SI has often been considered to depend on peripheral somatosensory stimuli. In our experiment, however, the sensitivity of SI to visual information was not found to be restricted to periods of actual somatic stimulation: the hand region of SI and the region of overlap between the hand and leg representation were also more active at the moment in which participants expected to be touched by a female, compared with a male (i.e., epoch 3, no-caress), but did not receive such stimulation. An increasing number of experiments (reviewed in ref. 40) also observed activation in SI in the absence of peripheral somatosensory input while participants view the actions of others (35–39, 41), or the hands or feet of others in painful situations (refs. 23–29, but see ref. 32). Activation in SI in the absence of touch has also been observed in highly empathic individuals that experience mirror-touch synesthesia (60, 61). SI activation has also been observed in the absence of touch during hypnotic suggestion (19), while participants recognize visually presented shapes (57), while monkeys view patterns that have been associated with tactile stimuli (62), and while participants imagine being touched (63, 64). Finally, SI activation patterns have also been shown to differ while observing other individuals haptically explore different objects (42) or be caressed (16). By analogy to theories conceptualizing the premotor cortices as “simulating” actions in the absence of overt movement during action observation and motor imagery (65), SI could therefore be said to simulate somatosensory states in the absence of actual tactile or proprioceptive input. A relevant question is then what our differential activation in SI reflects (i) a difference in simulating the participant’s own sensations on the leg while being supposedly caressed by a female or a male or (ii) a difference between simulating the proprioceptive and/or tactile input that the female and male caresser would experience on his/her hand while caressing the participant.
The results of our somatotopic ROI analysis suggest a combination of both (i) and (ii). First, the region of SI that only responded to a caress on the hand showed more activation in visually female than male conditions, regardless of whether the caress was actually delivered. In accord with many hand-action observation experiments reporting activation in the hand region of SI (35–39, 41), this effect is likely a result of simulation of the proprioceptive and tactile sensations of the supposed caresser’s hand. That such activation is higher while viewing the female actor is novel, but dovetails with the fact that, from a motivational point of view, the female actor’s touch is more desirable. Similar findings suggesting that simulation processing is modulated by motivation can be found in recent work in which stronger premotor activations were seen for hungry, compared with satiated, participants while they viewed a hand grasping a food item (66).
Second, the region of SI responding only to a caress on the leg was modulated by visual sex in the caress conditions only, and responded more when the caress occurred than when it did not. This suggests that the SI representation of the leg sensation of being caressed is modulated by perception of who is giving the caress (female or male), but only when a caress actually occurs. This supports the notion that the reported effects are caused by integration between tactile and visual information, against accounts based on anticipated tactile differences (i.e., tactile imagery) alone. Although other studies have shown that imagining touch on a body part activates the SI representation of that body part (64), if our SI activation differences in the leg region were simply caused by participants’ tactile imagery, such differences would have been equally strong in touch and no-touch conditions. Two further arguments speak against differential tactile imagery accounting for the SI effects we report here: the few participants who reported feeling sensory differences between the supposedly female and male caress reported that the male caress was rougher. However, studies comparing touch with gratings of different coarseness failed to find robust differences in SI activation (7, 33), and studies comparing a softer and lighter touch with velvet compared with a coarser and stronger one with wood found the latter to cause larger activation in SI (11, 14). Thus, if tactile imagery of “rougher” male hands were driving our effect, we would expect to see larger SI activation for the male vs. female condition, which is the opposite of what we actually observed. Instead, just as being hungry seems to magnify the representation of grasping an apple in premotor cortices (66), and satiation can reduce the response to chocolate in the OFC (67), the pleasantness/desirability of a caress, as driven in our experiment from visual information, modulates the response of SI to the caress. That SI activation shows a trial-by-trial correlation with pleasantness ratings over three conditions with different visual signals (i.e., visually male caress, visually female caress, and the gray-screen localizer touch) provides further evidence for this account. It also seems unlikely that our SI effect is a result of sexual arousal triggered by the sight of the attractive woman: even explicit visual erotica fail to consistently trigger activation in the hand or leg region of SI (68).
Three lines of reasoning also suggest that our effect differs from traditional tactile attention. First, unpleasant stimuli typically trigger stronger orienting responses than pleasant stimuli, and this was seen in our study via the GSRs recorded during scanning. Our participants were more emotionally aroused during visually male, compared with visually female, conditions. This would predict stronger attention to and subsequent processing of the visually male condition. In contrast, the BOLD response in SI was actually stronger for the visually female condition than the visually male condition (even when only data from the 10 participants for whom GSR was measured were analyzed). Second, deliberate selective visual attention toward a particular body part is known to have significant effects on the representation of that body part in SII, but no effects, or at least smaller effects, in SI (69–71). In contrast, we found a significant effect of visual sex on SI but not SII, and our effect size comparison confirmed that the effect of visual sex was stronger in SI than SII (Fig. 1H). Finally, deliberate selective visual attention is associated with the caudal superior parietal lobe (72, 73), but this region was not significantly involved in our PPI analysis.
Further insight into the affective modulation of interpersonal touch is provided by examining the epoch in which sex had effects. An overall significant sex-dependent increase in activation in SI was limited to the period during which touch actually occurred or should have occurred (epoch 3). During the earlier phases of each trial SI was not more active while viewing a female than a male. This shows that the sex-dependent SI activation difference is not a nonspecific visual modulation, but one tightly linked to processing a real or expected tactile stimulus. Despite a lack of overall activation in earlier phases of a trial, pattern classification showed that the pattern of SI activation in epoch 2 distinguishes the female and male actors. Visual information in epoch 2 therefore could prepare SI for processing the caress differentially depending on sex, but this modulation was of pattern rather than of overall amplitude until the caress actually occurred in epoch 3.
Importantly, although our data argue for including SI in models of affective processing of social touch, they should not be interpreted as arguing against the role of the OFC, insula, and ACC in processing gentle touch. Indeed, we found OFC, insula, and ACC all to be activated by our sensual caress, as predicted by these models. In the case of the insula, this is particularly compatible with the CT-afferent input it receives (4, 5, 10), which is optimally triggered by the kind and velocity (∼8 cm/s) of gentle touch we delivered (8). The lack of difference in BOLD signal between visual genders in the insula should also be interpreted with care, as it does not exclude an involvement of this region in the affective response to both these stimuli as they have similarly strong valence, if one considers the absolute value of the pleasantness ratings, and the BOLD signal in this region is augmented by both positively and negatively valenced social stimuli (e.g., ref. 74). In addition, the OFC was sensitive to visual sex (with effect sizes similar to those in SI), in agreement with the affective valuation often associated with this region (11–13). Previous models have associated the medial OFC with the monitoring and memory of the reward value of reinforcers (75), and the medial orbital gyrus specifically with the positive value of erotic as opposed to monetary reinforcers (76). In agreement with these findings, we found more activation in the medial OFC for the rewarding visually female condition. Significantly more activation for the visually male condition was not found in any brain region in our study. This suggests that the visually female caress was therefore processed as a reinforcer, but the male caress was not processed as a salient punishment. Considering the connections of the OFC with regions involved in motor control (75), and the deficits in decision-making occurring after OFC lesions (75), one might speculate that the difference in OFC response to the two genders would serve to influence a decision to approach or withdraw from the caresser during mate selection.
Finally, if SI is sensitive to affect, why have previous studies failed to show this? First, most studies were not aiming at studying the role of SI, and therefore applied their pleasant stimuli with less pressure than their neutral stimuli (11, 14). In such designs, the effect of pleasantness in SI is overshadowed by the sensitivity of this area to pressure. A similar problem might apply to the study of Ebisch et al., who found a trend toward the vision of a caress causing more SI activation than the vision of a hit (33), again with differences in sensory properties prevailing, as a hit involves greater pressure than a caress. Also, two recent studies found SI activation to be stronger for a more pleasant caress (16, 17). Although these studies are therefore compatible with SI participating in the affective coding of a caress, because their more pleasant caresses also differed in sensory properties (i.e., velocity/location) from their less pleasant caress, the differences in SI activation observed in those studies were not attributed to affective processing. By keeping the cutaneous stimuli constant, our study circumvented these issues. Second, the only other study that kept cutaneous stimulation constant varied pleasantness by only 20% by suggesting that one cream was thin (rating of ∼1 on a scale from −2 to 2) and the other was rich (∼1.2 rating) (18). In contrast, our study leveraged what might be a key mechanism in sexual selection, namely visual sex. This resulted in varying reported pleasantness by >200% in our study (male, −2.53; female, +3.05 on a rating scale from −5 to 5). Such a powerful manipulation, drawing on evolutionarily relevant processes, may have been critical in revealing SI’s participation in affective processing of social touch.
In conclusion, the present study reveals an important role for the SI in social touch. We found that visual information about the person giving a sensual caress modulates tactile input as early as SI. This modulation in overall activation level in SI, as detected by using our ROI and GLM analyses, is precisely timed to the moment the actual tactile stimulation occurs or should occur (epoch 3 but not epochs 1 or 2). However, the SI modulation is foreshadowed by a change in activity pattern, detectable by using multivoxel pattern classification in the 4 s preceding the likely onset of the caress. The visually triggered modulation of touch showed trial-by-trial correlation with pleasantness ratings and was significant within SI but not in the insula or ACC, areas previously thought to mediate this effect (14, 15, 63), and it seems to be independent of the modulation in the OFC, as suggested by a lack of functional connectivity between OFC and SI in our experiment. By showing that SI, a region classically associated with the processing of sensory properties of touch, has activity correlated with pleasantness, our data suggest that sensory and affective properties of touch are processed by partially overlapping neural systems. By doing so, our data shed further doubt on the appropriateness of distinguishing brain regions involved in sensory and affective tactile processing. Future experiments should manipulate the pleasantness of a sensual caress using other modalities (e.g., the voice or a verbal description of the caresser) and include homo- and heterosexual men and women to further dissect the SI modulation we observed. Finally, interfering with SI activity in a similar paradigm (i.e., via transcranial magnetic stimulation) could determine if the effects reported here indicate a causal role for SI in evaluating the affective significance of touch.
Materials and Methods
Participants.
Eighteen healthy, self-reported heterosexual, white male volunteers (mean age, 26.2 y; range, 21–31y) with normal or corrected-to-normal vision and no history of neurological or psychiatric disorders were recruited from outside the California Institute of Technology community to participate in the experiment. All subjects were informed about the content of the study and signed an informed consent form. The experiment was approved by the institutional review board at the California Institute of Technology and was in accordance with the Declaration of Helsinki.
Design, Stimuli, and Procedure.
The experimental design was dictated by two main requirements: (i) the need for consistent tactile stimuli and (ii) the need to create an emotionally salient, socially relevant situation.
Accordingly, at the start of the experiment subjects were introduced to two actors (the same individuals later shown in the video clips): an attractive woman, wearing a black evening dress and high heels, who behaved in a warm and friendly manner; and a man, wearing a black tank top and jeans, who behaved in a more distant manner (Fig. 1). The actors’ clothing was matched for color and similar in coverage of the arms and torso. Participants were led to believe that, during the experiment, they would be caressed on the lower legs by each of these two actors. They were told that a direct view throughout the experiment was not possible, so they would watch the two actors through goggles connected to a closed circuit video camera, which would allow the torso and arms of the actors and the side of the scanner to be seen in real time. Subjects were told that the camera would not show their own legs or the hands of the actors touching the legs, and that, for the purpose of the experiment, they would not be able to see the video feed throughout, but only at specific times (epochs 1, 2, and 3). They were further told that they would only be caressed on half of the trials. This therefore generated four experimental conditions: visually female caress, visually female no-caress, visually male caress, and visually male no-caress. Subjects were asked to imagine they were looking for a date in a social situation of their choice; a situation in which experiencing a sensual caress on the legs would evoke a strong emotional response. Debriefing revealed the two most frequently used scenarios were parties (at home or in a bar) or being on the beach. Care was taken to cover the bore of the scanner with a patterned fabric to make it impossible for the subjects to see their legs or the actors caressing them and to facilitate participants’ projection into the requested socially relevant scenario. During scanning, participants were actually shown prerecorded videos of the two actors, and the actual caress was always given by the same actor (the woman). The female actor received audio cues through headphones to match the timing of the caress to the appearance of the movement on the video clips, but was blind to the experimental condition (i.e., whether each particular caress was part of a male, female, or localizer trial).
On half the trials involving videos showing the actors, the videos of the caress were played but no caress was administered (the actor moved her hands above the subject’s legs without touching them). Participants were told that these conditions were necessary for experimental aims. Finally, in a fifth condition (the localizer trials), the participants viewed a blank, gray screen, which was always accompanied by a caress. In these trials, participants were asked to simply feel the caress, without reflecting about whether a man or woman touched them. This condition served to map brain regions involved in processing the tactile properties of the stimulus.
We used an event-related design. Every trial was composed of three video epochs followed by a rating (Fig. 1). Epoch 1 was a 2-s clip showing the male or female actor standing still beside the scanner, epoch 2 was a 4-s clip showing the actor turning toward the subject’s legs with the intent to perform the caress, and epoch 3 was a 4-s clip showing the actor leaning forward to caress the legs of the subject and then returning to the original position. The experiment involved five different conditions as follows, and the same condition was never presented more than twice in a row.
i) Visually female no-caress: epoch 1 showed the female actor standing motionless beside the scanner, epoch 2 showed her rotating toward the participant’s legs, and epoch 3 showed her leaning forward while moving her arms in a caressing motion, then returning to the original position. No caress occurred. Participants were asked to enter a rating of 0 on all trials for which they felt no caress.
ii) Visually female caress: this was the same as in the visually female no-caress condition, but both the participant’s legs were softly caressed during epoch 3. Participants were instructed to use the rating scale to report the pleasantness of the caress at the end of each trial.
iii) Visually male no-caress: this was the same as in the visually female no-caress condition, but showed the male actor.
iv) Visually male caress: this was the same as in the visually female caress condition, but showed the male actor.
v) Localizer: in the localizer condition, instead, epochs 1 to 3 were replaced by a gray screen. A caress was always given and the participants were instructed to use the rating scale to report the pleasantness of the caress. Participants were asked to avoid guessing the sex of the actor giving the caress.
All caresses were administered to the shin and calf of both legs, moving from the knees toward the ankles, with a touch velocity of ∼8 cm/s. The precise location and kinematics of the caresses were varied from trial to trial so the caresses remained novel and pleasant throughout the experiment, thus minimizing habituation to the delivered touch. The fact that the actor delivering the caress was blind to the conditions ensured that the variation given to the caress was not condition-dependent.
The participants were asked to rate the pleasantness of the caress by means of a visual analog scale ranging from +5 (a very pleasant caress; Fig. 1, green rectangle) to −5 (a very unpleasant caress; Fig. 1, red rectangle). In a random half of the trials, the positive values were presented on the right side of the screen and, in the other half, they were on the left, such that participants could not plan their response movement before the appearance of the scale. Participants indicated their rating by means of an MRI-compatible track ball mouse (HH-TRK-1; Current Designs) and had a maximum of 7 s to complete each rating.
The video clips were recorded with a digital video camera (video camcorder DCR-VX2100; Sony) and with the actors always standing on one side of the scanner. Half the clips where then flipped during editing (AdobePremiere; Adobe Systems) to show the actors on the other side. Each actor was filmed delivering 12 different caresses. Half the movies were randomly assigned to the caress condition, the other half to the no-caress condition. Each movie was only shown once to each participant. Clips were presented using Presentation (www.neuro-bs.com) and projected by using MRI-compatible LCD goggles (Resonance Technology).
Three trials of each condition were presented in each run (i.e., 15 trials total per run). In 16 of 18 participants, four runs were collected (12 repetitions per condition per participant), whereas, for two participants, technical problems allowed the acquisition of only three runs (nine repetitions per condition per participant). On average, 340 functional volumes were acquired in each run.
After completion of scanning, participants rated the sexual attractiveness of the two actors on a scale from −5 to +5, with +5 being “a partner with whom one would really desire sexual relations.” During debriefing, the participants were asked if they believed that they had actually been caressed by the two actors throughout the experiment and that the videos were fed live. All participants reported “yes” to both questions.
Neuroimaging Data Acquisition.
Data were acquired on a Siemens 3.0-T Trio MRI scanner using an eight-channel head coil. Whole-brain T1-weighted anatomical images were collected at a resolution of 1 × 1 × 1 mm3. Functional images were collected with a gradient-echo T2*-weighted echoplanar images with BOLD contrast by using an interleaved, ascending image sequence (repetition time, 2 s; echo time, 30 ms; field of view, 192 mm; 34 slices at 4-mm thickness; and 64 × 64 voxels, resulting in a voxel size of 3 × 3 × 4 mm3).
Neuroimaging Data Preprocessing.
Data were preprocessed by using SPM2 (http://www.fil.ion.ucl.ac.uk/spm/software/spm2). Echoplanar imaging (EPI) from all sessions were slice-time corrected and realigned to the first volume of the first run. Head motions never exceeded the acquired voxel size (3 × 3 × 4).
High-quality T1 images were coregistered to the mean EPI image and segmented. The gray matter segment of each participant was postprocessed by using MRIcro to remove tissue that was inaccurately classified as gray matter. The coregistered and cleaned gray matter segment was normalized (with 1 × 1 × 1-mm voxel size) onto the Montreal Neurological Institute gray matter template and the resulting normalization parameters applied to all EPI images (2 × 2 × 2 voxel size for the GLM, 3 × 3 × 3 for the multivoxel pattern classification analysis). EPI images were smoothed using an isotropic Gaussian kernel [full width at half maximum (FWHM)] of 10 × 10 × 10 mm for the GLM analyses and ROI analyses (unless otherwise specified). Multivoxel pattern analyses used a voxel-multiple smoothing kernel with FWHM of 9 × 9 × 9 mm. This choice of spatial smoothing is compatible with the aims of multivariate analyses (77, 78).
Mass Univariate GLM Analysis.
For each individual, data were analyzed voxel by voxel by applying a GLM on the smoothed normalized data. All conditions were modeled by using a boxcar function convolved with a canonical hemodynamic response function. Six additional predictors of no interest were modeled to account for translation and rotation along the three possible dimensions as determined during the realignment procedure.
Specifically, we used the following predictors of interest:
First, a boxcar function starting at the beginning of epoch 1 and lasting for its 2-s duration was used. Three different predictors were used to model the appearance of the female, the male, and the gray square.
Second, a boxcar function starting at the beginning of epoch 2 and lasting for 4 s was used. This corresponds to the part of the movie showing the actor rotating toward the participant’s leg (or the gray screen for the localizer). Five different predictors were used to model this phase, one for each of the five experimental conditions. Because after convolution with the hemodynamic response function, epoch 2 and epoch 3 predictors overlap in time, activation related to the experience of the caress (epoch 3 of caress conditions) could “contaminate” parameter estimates for epoch 2 of caress trials. The use of separate epoch 2 predictors for caress and no-caress condition thus isolates epoch 2 of no-caress conditions from potential effects of caress experience during epoch 3 of caress trials.
Third, a boxcar function starting at the beginning of epoch 3 and lasting for 4 s was used. This corresponds to the part of the movie showing the actor leaning forward, as for caressing the participant’s leg, and returning at the original position (or the gray screen for the localizer). In three of the five trial types, this corresponded to the time of the actual caress. Five different predictors were used to model this phase, one for each of the five experimental conditions.
Fourth, a boxcar function starting at the beginning of the rating period until the participant’s response, or with a duration of 7 s (maximum time allowed for the response), whichever occurred first, was used. Two different predictors were used, one for ratings in the no-caress conditions (participants were asked to select the yellow square in the middle of the screen corresponding to a rating of 0 rather than provide a rating), and one for the rating following the caress conditions (participants were asked to indicate how pleasant they found the caress).
For each participant, the following contrasts were calculated:
i) visually female caress epoch 2 minus visually male caress epoch 2;
ii) visually female caress epoch 3 minus visually male caress epoch 3;
iii) visually female no-caress epoch 2 minus visually male no-caress epoch 2;
iv) visually female no-caress epoch 3 minus visually male no-caress epoch 3; and
v) visually female epoch 1 minus visually male epoch 1.
Epochs 2 and 3 of each trial are temporally autocorrelated. Accordingly, contrasts (i) and (ii) were not tested separately by using t tests at the second (i.e., group) level, but entered in a single one-way ANOVA with correction for nonsphericity. The same was done for contrasts (iii) and (iv). These two ANOVAs were additionally masked with the mean gray matter segment of our participants, thresholded at 0.35. Contrast (v) was analyzed at the second level by means of one-sample t tests. All results are reported with a threshold of an FDR-corrected q < 0.05. Although we expected a priori for the more pleasant female condition to lead to stronger activation than the male condition, the reverse of contrasts (i) to (v) (male minus female) were also calculated, but never led to significant results.
ROI Analysis.
We defined, separately for each hemisphere, the caress-sensitive voxels within SI by using the localizer condition. Specifically, we compared the parameter estimates from epoch 3 of the localizer against rest (FDR-corrected q < 0.05). To link activations measured in the ROI analysis to particular cytoarchitectonic regions, we then identified the voxels within this thresholded localizer map that fell within anatomical maps of SI (jointly in Fig. 1D and separately in Fig. 1F for BA1, BA2, BA3a, and BA3b in the Anatomy Toolbox for SPM; http://www.fz-juelich.de/inm/inm-1/DE/Forschung/_docs/SPMAnatomyToolbox/SPMAnatomyToolbox_node.html), separately for the left and right hemispheres. Marsbar (http://marsbar.sourceforge.net/) was then used to estimate parameter estimates in these regions during epoch 3 (in which visual and tactile information come together in the caress conditions) by applying the same GLM used for the voxel-wise analysis in SPM to the mean signal in each of the ROIs.
The parameter estimates of epoch 3 were then analyzed by using a repeated-measures ANOVA with the following factors and their interactions: hemisphere, caress (i.e., caress and no-caress conditions), and sex (i.e., visually female and male). We focused our interest on the main effect of sex and its interactions with the remaining factors.
The same procedure was applied for the ACC and insula ROIs, with the exception that the anatomical ROIs were defined according to the Wake Forest University PickAtlas (www.ansir.wfubmc.edu) because they are not yet available in the Anatomy Toolbox.
Searchlight Multivoxel Pattern Classification.
To explore the possibility that changes in the pattern of neural activation might occur as a function of visual sex before the actual caress occurred, we extracted parameter estimate images for each epoch of each trial by using SPM by fitting a GLM, using a separate predictor for each epoch of each trial. The parameter estimate images were further processed (in R, version 2.8.0; R Foundation for Statistical Computing) to remove voxels (from all subjects) with zero variance in any individual subject. Within each searchlight, we attempted to classify the sex using the data from epochs 1 and 2. The latter was done only for cases in which the subject did not receive a caress (i.e., visually female no-caress vs. visually male no-caress). This ensured that BOLD activation resulting from the tactile input of the caress could not contaminate the estimates of the response in the earlier epoch. The searchlight analysis was performed in each subject individually using pyMVPA (79) with a linear kernel support vector machine, the cost parameter fixed at 1, and scaling the voxels to zero mean and unit variance within each run (80, 81). Partitioning was done on the runs (three- or fourfold cross-validation, depending on the number of runs for the participant); there were no missing examples within runs, so the number of examples in all classifications was balanced for all subjects (three per run of each sex). Each searchlight had a diameter of 6 mm (although the use of smoothed images means that some information from a wider area will enter each searchlight), and the analysis was performed by using a whole-brain mask.
The second-level analysis was performed in SPM2 (Wellcome Department of Imaging Neuroscience). A one-tailed t test was used on each voxel of the searchlight accuracy map for each subject, masked with the mean gray matter mask of the subjects, to determine if the accuracy of the sphere centered at that voxel was significantly greater than chance across subjects. FDR was used for multiple-comparisons correction, with a cluster size of 10 and threshold (q) set at <0.05. Before performing the second-level analysis, the searchlight accuracy maps were smoothed at 6 mm FWHM to improve anatomical alignment between subjects; similar results were found with unsmoothed accuracy maps.
PPI Analysis.
To identify which regions responding more to visually female than male caress could be the sources of our visual modulation of SI, we performed a PPI analysis (82). We extracted the time course for the right and left cluster of SI that resulted from the contrast visually female caress epoch 3 minus visually male caress epoch 3 for each participant. This was done by creating two binary ROI images as the intersection of this contrast at the group level and the cytoarchitectonic definition of SI in the right and left hemisphere, respectively. For each participant, the same contrast was then opened at a more permissive threshold (P < 0.05 or P < 0.5 if P < 0.05 led to no active voxels in the ROI), and the eigenvalues summarizing the activation of all of the significant voxels within the two ROIs were extracted. A PPI GLM was then fitted separately for each of the two ROIs and each participant. It included the activation of the ROI, a condition vector containing 1 during visually female epoch 3 and −1 during visually male epoch 3, and the interaction of these two predictors, as defined previously (82) and implemented in SPM2. At the group level, a two-hemisphere one-way ANOVA was used to compare the parameter estimates of all the participants against zero. Only voxels in which visually female epoch 3 was above visually male epoch 3 in the group were included in this analysis (i.e., explicitly masked with this contrast) to directly test which of the areas in this contrast could have provided visual information to SI. We used a global null conjunction to identify voxels that show evidence of augmenting their connectivity with the right or the left SI, and used a threshold of an FDR-corrected q < 0.05.
GSR.
GSR from the eccrine glands in the left hand was measured during fMRI scanning (GSR100C amplifier, MP150 psychophysiological acquisition unit, disposable EL509 snap electrodes; Biopac) as described in Carter (83). Measurements from 10 participants yielded clean data for analyses. The remainder were unusable as a result of electrodes becoming detached during recording. Data from each trial, starting with the beginning of epoch 1 and lasting until 15 s after the onset of epoch 3, were linearly detrended and numerically zeroed by subtracting the minimum GSR value. Each participant’s maximal GSR value, across all trials, was used to normalize that participant’s data. GSR values were then summed (to calculate the area under the curve) from the beginning of epoch 3 for 15 s, averaged across repetitions of the same condition and divided by 1,000.
Somatotopic ROI Analysis.
Four additional Caucasian male volunteers (mean age, 35y) were scanned (same acquisition and data-preprocessing parameters as in the main experiment) while the experimenter bilaterally caressed their lower legs (shin and calf) or the dorsal part of their hands for 4 s. The participants viewed a dot that was gray during the baseline (6 ± 2 s), and turned red 4 s before the caress. This 4-s warning period is comparable to epoch 2 of the main experiment, during which the turning of the actor predicted the upcoming caress, and will be called leg epoch 2 or hand epoch 2. It was followed by the 4-s caress (comparable with epoch 3 of the main experiment), during which the dot progressively shrunk to indicate the duration of the caress (leg or hand epoch 3). A single run was collected for each participant (20 repetitions of each condition). Data were analyzed voxel-by-voxel by using a GLM with, for each participant, separate boxcar functions for leg epoch 2, hand epoch 2, leg epoch 3, and hand epoch 3, all convolved with the hemodynamic response function and six predictors for translations and rotations along the three possible dimensions. Because of the small number of individuals, data from the four participants were included in a single model (fixed-effect analysis), but results are very similar to those from a study with 16 participants and random-effects analyses (84). Because of the known overlap between the representation of different effectors (84), three contrasts were generated:
i) Leg only: Leg epoch 3 > baseline AND NOT (hand epoch 3 > baseline)
ii) Leg and hand: Leg epoch 3 > baseline AND hand epoch 3 > baseline
iii) Hand only: Hand epoch 3 > baseline AND NOT (leg epoch 3 > baseline).
By using ImCalc, we then intersected these three contrasts (at an uncorrected P < 0.001) with the anatomical maps of SI (Anatomy Toolbox for SPM; BA3a plus BA3b plus BA1 plus BA2) separately for the left and right hemispheres, yielding our ROIs (coronal slices shown in Fig. S1). We then extracted the parameter estimates in these regions during epoch 3 of the main experiment as in Fig. S2, and averaged the parameters for the right and left hemisphere because the effect of visual sex did not differ across hemispheres. An additional fourth mouth ROI was created to investigate the specificity of the effect of visual sex to the hand and leg representations. This ROI was defined in a previous experiment, in which 16 participants explore an object with their mouth, hand, or feet (84), and was intersected inclusively with the anatomical maps of SI and exclusively with the combination of the above mentions ROIs [mouth only: mouth ROI (84) AND SI AND NOT (leg only OR leg and hand OR hand only)]. The use of ROIs defined in four individuals to analyze data from a different group of participants is valid in SI because variance in the localization of body parts in SI across individuals [∼5 mm (85)] is small compared with the distance between the representations of different body parts in the same region (20 mm between our left leg only and hand only ROI, and 23 mm between the same ROIs in the right hemisphere).
Correlation of BOLD with Pleasantness Rating.
For all caress conditions, participants rated the pleasantness of each caress in the scanner. For two participants, these ratings were lost because of software errors. For the remaining 16, we examined whether BOLD signals during epoch 3 were correlated, on a trial-by-trial basis, with pleasantness ratings. At the subject level, we modified the GLM described in Mass Univariate GLM Analysis by combining epoch 3 of the conditions visually female caress, visually male caress, and localizer into a single epoch 3 caress predictor, and adding the mean-corrected, trial-by-trial pleasantness ratings as a parametric modulator to this predictor. The parameter estimates for this parametric modulator were then averaged across runs. At the second, group, level, we then compared the averaged modulator against zero to localize regions in which the BOLD response to the caress tracks the reported pleasantness of that caress (Fig. 1I).
Effect Size Analysis.
As in ROI Analysis, we anatomically defined SI (Anatomy Toolbox BA3a, BA3b, BA1, and BA2), SII (OP1-4), insula, and ACC (PickAtlas), but did not restrict the analysis to voxels activated by the localizer. For each voxel, the t-value for the contrast of visually female caress minus visually male caress in epoch 3 was extracted from the GLM, and the distribution of these effect sizes for each ROI is shown in the histogram of Fig. 1H. t Values were also converted to Z-values by using spm_t2x(t,34) in SPM. To compare SI effect sizes with those in the OFC, we also created an anatomical ROI for the medial OFC including BA11 and BA25 (PickAtlas). Because the entire OFC extends far beyond these two regions and would be much larger than the other ROIs, only BA11 and BA25 were selected because they overlap with the OFC cluster showing increased activation to the female condition compared with the male condition. Results were very similar for the right and left hemisphere, and we therefore combined results from both hemispheres.
Supplementary Material
Acknowledgments
This work was supported by a Marie Curie Excellence Grant of the European Commission (to C.K.); a Vidi Grant of the Netherlands Science Foundation (NWO) (to C.K.); a Veni Grant from NWO (to V.G.); Scripps College Faculty Research Grants (to M.L.S.); National Institutes of Health Grants (to R.A.); the Gordon and Betty Moore Foundation (R.A.); and the Tamagawa University Global Centers of Excellence Program of the Japanese Ministry of Education, Culture, Sports, and Technology (R.A.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Author Summary on page 9688 (volume 109, number 25).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1113211109/-/DCSupplemental.
References
- 1.Dunbar RI. The social role of touch in humans and primates: Behavioural function and neurobiological mechanisms. Neurosci Biobehav Rev. 2010;34:260–268. doi: 10.1016/j.neubiorev.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 2.Gallace A, Spence C. The science of interpersonal touch: an overview. Neurosci Biobehav Rev. 2010;34:246–259. doi: 10.1016/j.neubiorev.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 3.Guest S, et al. Sensory and affective judgments of skin during inter- and intrapersonal touch. Acta Psychol (Amst) 2009;130:115–126. doi: 10.1016/j.actpsy.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 4.Olausson H, et al. Functional role of unmyelinated tactile afferents in human hairy skin: Sympathetic response and perceptual localization. Exp Brain Res. 2008;184:135–140. doi: 10.1007/s00221-007-1175-x. [DOI] [PubMed] [Google Scholar]
- 5.Olausson H, et al. Unmyelinated tactile afferents signal touch and project to insular cortex. Nat Neurosci. 2002;5:900–904. doi: 10.1038/nn896. [DOI] [PubMed] [Google Scholar]
- 6.Olausson H, Wessberg J, Morrison I, Mcglone F, Vallbo Ã. The neurophysiology of unmyelinated tactile afferents. Neurosci Biobehav Rev. 2008;34:185–191. doi: 10.1016/j.neubiorev.2008.09.011. [DOI] [PubMed] [Google Scholar]
- 7.Morrison I, Löken LS, Olausson H. The skin as a social organ. Exp Brain Res. 2010;204:305–314. doi: 10.1007/s00221-009-2007-y. [DOI] [PubMed] [Google Scholar]
- 8.Löken LS, Wessberg J, Morrison I, McGlone F, Olausson H. Coding of pleasant touch by unmyelinated afferents in humans. Nat Neurosci. 2009;12:547–548. doi: 10.1038/nn.2312. [DOI] [PubMed] [Google Scholar]
- 9.Craig AD. Pain mechanisms: Labeled lines versus convergence in central processing. Annu Rev Neurosci. 2003;26:1–30. doi: 10.1146/annurev.neuro.26.041002.131022. [DOI] [PubMed] [Google Scholar]
- 10.Olausson HW, et al. Unmyelinated tactile afferents have opposite effects on insular and somatosensory cortical processing. Neurosci Lett. 2008;436:128–132. doi: 10.1016/j.neulet.2008.03.015. [DOI] [PubMed] [Google Scholar]
- 11.Rolls ET, et al. Representations of pleasant and painful touch in the human orbitofrontal and cingulate cortices. Cereb Cortex. 2003;13:308–317. doi: 10.1093/cercor/13.3.308. [DOI] [PubMed] [Google Scholar]
- 12.Lovero KL, Simmons AN, Aron JL, Paulus MP. Anterior insular cortex anticipates impending stimulus significance. Neuroimage. 2009;45:976–983. doi: 10.1016/j.neuroimage.2008.12.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rolls ET, Grabenhorst F, Parris BA. Warm pleasant feelings in the brain. Neuroimage. 2008;41:1504–1513. doi: 10.1016/j.neuroimage.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 14.Francis S, et al. The representation of pleasant touch in the brain and its relationship with taste and olfactory areas. Neuroreport. 1999;10:453–459. doi: 10.1097/00001756-199902250-00003. [DOI] [PubMed] [Google Scholar]
- 15.Mcglone F, Vallbo A, Olausson H, Loken L, Wessberg J. Discriminative touch and emotional touch. Can J Exp Psychol Rev. 2007;61:173–183. doi: 10.1037/cjep2007019. [DOI] [PubMed] [Google Scholar]
- 16.Morrison I, Björnsdotter M, Olausson H. Vicarious responses to social touch in posterior insular cortex are tuned to pleasant caressing speeds. J Neurosci. 2011;31:9554–9562. doi: 10.1523/JNEUROSCI.0397-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gordon I, et al. Brain mechanisms for processing affective touch. Hum Brain Mapp. 2011 doi: 10.1002/hbm.21480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCabe C, Rolls ET, Bilderbeck A, McGlone F. Cognitive influences on the affective representation of touch and the sight of touch in the human brain. Soc Cogn Affect Neurosci. 2008;3:97–108. doi: 10.1093/scan/nsn005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schulz-Stübner S, et al. Clinical hypnosis modulates functional magnetic resonance imaging signal intensities and pain perception in a thermal stimulation paradigm. Reg Anesth Pain Med. 2004;29:549–556. doi: 10.1016/j.rapm.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 20.Derbyshire SWG, Whalley MG, Stenger VA, Oakley DA. Cerebral activation during hypnotically induced and imagined pain. Neuroimage. 2004;23:392–401. doi: 10.1016/j.neuroimage.2004.04.033. [DOI] [PubMed] [Google Scholar]
- 21.Rainville P, Duncan GH, Price DD, Carrier B, Bushnell MC. Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science. 1997;277:968–971. doi: 10.1126/science.277.5328.968. [DOI] [PubMed] [Google Scholar]
- 22.Bufalari I, Aprile T, Avenanti A, Di Russo F, Aglioti SM. Empathy for pain and touch in the human somatosensory cortex. Cereb Cortex. 2007;17:2553–2561. doi: 10.1093/cercor/bhl161. [DOI] [PubMed] [Google Scholar]
- 23.Costantini M, Galati G, Romani GL, Aglioti SM. Empathic neural reactivity to noxious stimuli delivered to body parts and non-corporeal objects. Eur J Neurosci. 2008;28:1222–1230. doi: 10.1111/j.1460-9568.2008.06406.x. [DOI] [PubMed] [Google Scholar]
- 24.Jackson PL, Brunet E, Meltzoff AN, Decety J. Empathy examined through the neural mechanisms involved in imagining how I feel versus how you feel pain. Neuropsychologia. 2006;44:752–761. doi: 10.1016/j.neuropsychologia.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 25.Jackson PL, Meltzoff AN, Decety J. How do we perceive the pain of others? A window into the neural processes involved in empathy. Neuroimage. 2005;24:771–779. doi: 10.1016/j.neuroimage.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 26.Lamm C, Decety J. Is the extrastriate body area (EBA) sensitive to the perception of pain in others? Cereb Cortex. 2008;18:2369–2373. doi: 10.1093/cercor/bhn006. [DOI] [PubMed] [Google Scholar]
- 27.Lamm C, Meltzoff AN, Decety J. How do we empathize with someone who is not like us? A functional magnetic resonance imaging study. J Cogn Neurosci. 2009;22:362–376. doi: 10.1162/jocn.2009.21186. [DOI] [PubMed] [Google Scholar]
- 28.Lamm C, Nusbaum HC, Meltzoff AN, Decety J. What are you feeling? Using functional magnetic resonance imaging to assess the modulation of sensory and affective responses during empathy for pain. PLoS ONE. 2007;2:e1292. doi: 10.1371/journal.pone.0001292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morrison I, Downing PE. Organization of felt and seen pain responses in anterior cingulate cortex. Neuroimage. 2007;37:642–651. doi: 10.1016/j.neuroimage.2007.03.079. [DOI] [PubMed] [Google Scholar]
- 30.Avenanti A, Bolognini N, Maravita A, Aglioti SM. Somatic and motor components of action simulation. Curr Biol. 2007;17:2129–2135. doi: 10.1016/j.cub.2007.11.045. [DOI] [PubMed] [Google Scholar]
- 31.Valeriani M, et al. Seeing the pain of others while being in pain: A laser-evoked potentials study. Neuroimage. 2008;40:1419–1428. doi: 10.1016/j.neuroimage.2007.12.056. [DOI] [PubMed] [Google Scholar]
- 32.Morrison I, Lloyd D, di Pellegrino G, Roberts N. Vicarious responses to pain in anterior cingulate cortex: Is empathy a multisensory issue? Cogn Affect Behav Neurosci. 2004;4:270–278. doi: 10.3758/cabn.4.2.270. [DOI] [PubMed] [Google Scholar]
- 33.Ebisch SJ, et al. Differential involvement of somatosensory and interoceptive cortices during the observation of affective touch. J Cogn Neurosci. 2011;23:1808–1822. doi: 10.1162/jocn.2010.21551. [DOI] [PubMed] [Google Scholar]
- 34.Caspers S, Zilles K, Laird AR, Eickhoff SB. ALE meta-analysis of action observation and imitation in the human brain. Neuroimage. 2010;50:1148–1167. doi: 10.1016/j.neuroimage.2009.12.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dinstein I, Hasson U, Rubin N, Heeger DJ. Brain areas selective for both observed and executed movements. J Neurophysiol. 2007;98:1415–1427. doi: 10.1152/jn.00238.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Filimon F, Nelson JD, Hagler DJ, Sereno MI. Human cortical representations for reaching: Mirror neurons for execution, observation, and imagery. Neuroimage. 2007;37:1315–1328. doi: 10.1016/j.neuroimage.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gazzola V, Keysers C. The observation and execution of actions share motor and somatosensory voxels in all tested subjects: Single-subject analyses of unsmoothed fMRI data. Cereb Cortex. 2009;19:1239–1255. doi: 10.1093/cercor/bhn181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gazzola V, Rizzolatti G, Wicker B, Keysers C. The anthropomorphic brain: the mirror neuron system responds to human and robotic actions. Neuroimage. 2007;35:1674–1684. doi: 10.1016/j.neuroimage.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 39.Grèzes J, Armony JL, Rowe J, Passingham RE. Activations related to “mirror” and “canonical” neurones in the human brain: an fMRI study. Neuroimage. 2003;18:928–937. doi: 10.1016/s1053-8119(03)00042-9. [DOI] [PubMed] [Google Scholar]
- 40.Keysers C, Kaas JH, Gazzola V. Somatosensation in social perception. Nat Rev Neurosci. 2010;11:417–428. doi: 10.1038/nrn2833. [DOI] [PubMed] [Google Scholar]
- 41.Turella L, Erb M, Grodd W, Castiello U. Visual features of an observed agent do not modulate human brain activity during action observation. Neuroimage. 2009;46:844–853. doi: 10.1016/j.neuroimage.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 42.Meyer K, Kaplan JT, Essex R, Damasio H, Damasio A. Seeing touch is correlated with content-specific activity in primary somatosensory cortex. Cereb Cortex. 2011;21:2113–2121. doi: 10.1093/cercor/bhq289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Andersen JF, Andersen PA, Lustig MW. Opposite sex touch avoidance: A national replication and extension. J Nonverbal Behav. 1987;11:89–109. [Google Scholar]
- 44.Crawford CB. Effects of sex and sex roles on avoidance of same- and opposite-sex touch. Percept Mot Skills. 1994;79:107–112. doi: 10.2466/pms.1994.79.1.107. [DOI] [PubMed] [Google Scholar]
- 45.McDaniel E, Andersen PA. International patterns of interpersonal tactile communication: A field study. J Nonverbal Behav. 1998;22:59–75. [Google Scholar]
- 46.Kriegeskorte N, Bandettini P. Analyzing for information, not activation, to exploit high-resolution fMRI. Neuroimage. 2007;38:649–662. doi: 10.1016/j.neuroimage.2007.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hutchison WD, Davis KD, Lozano AM, Tasker RR, Dostrovsky JO. Pain-related neurons in the human cingulate cortex. Nat Neurosci. 1999;2:403–405. doi: 10.1038/8065. [DOI] [PubMed] [Google Scholar]
- 48.Keysers C, et al. Audiovisual mirror neurons and action recognition. Exp Brain Res. 2003;153:628–636. doi: 10.1007/s00221-003-1603-5. [DOI] [PubMed] [Google Scholar]
- 49.Maunsell JH, van Essen DC. The connections of the middle temporal visual area (MT) and their relationship to a cortical hierarchy in the macaque monkey. J Neurosci. 1983;3:2563–2586. doi: 10.1523/JNEUROSCI.03-12-02563.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pons TP, Kaas JH. Corticocortical connections of area 2 of somatosensory cortex in macaque monkeys: A correlative anatomical and electrophysiological study. J Comp Neurol. 1986;248:313–335. doi: 10.1002/cne.902480303. [DOI] [PubMed] [Google Scholar]
- 51.Rozzi S, et al. Cortical connections of the inferior parietal cortical convexity of the macaque monkey. Cereb Cortex. 2006;16:1389–1417. doi: 10.1093/cercor/bhj076. [DOI] [PubMed] [Google Scholar]
- 52.Ishida H, Nakajima K, Inase M, Murata A. Shared mapping of own and others’ bodies in visuotactile bimodal area of monkey parietal cortex. J Cogn Neurosci. 2010;22:83–96. doi: 10.1162/jocn.2009.21185. [DOI] [PubMed] [Google Scholar]
- 53.Keysers C, Gazzola V. Expanding the mirror: Vicarious activity for actions, emotions, and sensations. Curr Opin Neurobiol. 2009;19:666–671. doi: 10.1016/j.conb.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 54.Barbas H. Anatomic organization of basoventral and mediodorsal visual recipient prefrontal regions in the rhesus monkey. J Comp Neurol. 1988;276:313–342. doi: 10.1002/cne.902760302. [DOI] [PubMed] [Google Scholar]
- 55.Carmichael ST, Price JL. Sensory and premotor connections of the orbital and medial prefrontal cortex of macaque monkeys. J Comp Neurol. 1995;363:642–664. doi: 10.1002/cne.903630409. [DOI] [PubMed] [Google Scholar]
- 56.Rolls ET. Convergence of sensory systems in the orbitofrontal cortex in primates and brain design for emotion. Anat Rec A Discov Mol Cell Evol Biol. 2004;281:1212–1225. doi: 10.1002/ar.a.20126. [DOI] [PubMed] [Google Scholar]
- 57.Stilla R, Sathian K. Selective visuo-haptic processing of shape and texture. Hum Brain Mapp. 2008;29:1123–1138. doi: 10.1002/hbm.20456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Adolphs R, Damasio H, Tranel D, Cooper G, Damasio AR. A role for somatosensory cortices in the visual recognition of emotion as revealed by three-dimensional lesion mapping. J Neurosci. 2000;20:2683–2690. doi: 10.1523/JNEUROSCI.20-07-02683.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pitcher D, Garrido L, Walsh V, Duchaine BC. Transcranial magnetic stimulation disrupts the perception and embodiment of facial expressions. J Neurosci. 2008;28:8929–8933. doi: 10.1523/JNEUROSCI.1450-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Banissy MJ, Ward J. Mirror-touch synesthesia is linked with empathy. Nat Neurosci. 2007;10:815–816. doi: 10.1038/nn1926. [DOI] [PubMed] [Google Scholar]
- 61.Blakemore SJ, Bristow D, Bird G, Frith C, Ward J. Somatosensory activations during the observation of touch and a case of vision-touch synaesthesia. Brain. 2005;128:1571–1583. doi: 10.1093/brain/awh500. [DOI] [PubMed] [Google Scholar]
- 62.Zhou YD, Fuster JM. Neuronal activity of somatosensory cortex in a cross-modal (visuo-haptic) memory task. Exp Brain Res. 1997;116:551–555. doi: 10.1007/pl00005783. [DOI] [PubMed] [Google Scholar]
- 63.Olivetti Belardinelli M, et al. An fMRI investigation on image generation in different sensory modalities: The influence of vividness. Acta Psychol (Amst) 2009;132:190–200. doi: 10.1016/j.actpsy.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 64.Yoo SS, Freeman DK, McCarthy JJ, 3rd, Jolesz FA. Neural substrates of tactile imagery: A functional MRI study. Neuroreport. 2003;14:581–585. doi: 10.1097/00001756-200303240-00011. [DOI] [PubMed] [Google Scholar]
- 65.Jeannerod M. Neural simulation of action: A unifying mechanism for motor cognition. Neuroimage. 2001;14:S103–S109. doi: 10.1006/nimg.2001.0832. [DOI] [PubMed] [Google Scholar]
- 66.Cheng Y, Meltzoff AN, Decety J. Motivation modulates the activity of the human mirror-neuron system. Cereb Cortex. 2007;17:1979–1986. doi: 10.1093/cercor/bhl107. [DOI] [PubMed] [Google Scholar]
- 67.Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008;58:306–324. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kühn S, Gallinat J. A quantitative meta-analysis on cue-induced male sexual arousal. J Sex Med. 2011;8:2269–2275. doi: 10.1111/j.1743-6109.2011.02322.x. [DOI] [PubMed] [Google Scholar]
- 69.Burton H, Sinclair RJ, McLaren DG. Cortical network for vibrotactile attention: A fMRI study. Hum Brain Mapp. 2008;29:207–221. doi: 10.1002/hbm.20384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Burton H, Sinclair RJ. Attending to and remembering tactile stimuli: A review of brain imaging data and single-neuron responses. J Clin Neurophysiol. 2000;17:575–591. doi: 10.1097/00004691-200011000-00004. [DOI] [PubMed] [Google Scholar]
- 71.Drevets WC, et al. Blood flow changes in human somatosensory cortex during anticipated stimulation. Nature. 1995;373:249–252. doi: 10.1038/373249a0. [DOI] [PubMed] [Google Scholar]
- 72.Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- 73.Behrmann M, Geng JJ, Shomstein S. Parietal cortex and attention. Curr Opin Neurobiol. 2004;14:212–217. doi: 10.1016/j.conb.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 74.Jabbi M, Swart M, Keysers C. Empathy for positive and negative emotions in the gustatory cortex. Neuroimage. 2007;34:1744–1753. doi: 10.1016/j.neuroimage.2006.10.032. [DOI] [PubMed] [Google Scholar]
- 75.Kringelbach ML. The human orbitofrontal cortex: Linking reward to hedonic experience. Nat Rev Neurosci. 2005;6:691–702. doi: 10.1038/nrn1747. [DOI] [PubMed] [Google Scholar]
- 76.Sescousse G, Redouté J, Dreher JC. The architecture of reward value coding in the human orbitofrontal cortex. J Neurosci. 2010;30:13095–13104. doi: 10.1523/JNEUROSCI.3501-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kamitani Y, Sawahata Y. Spatial smoothing hurts localization but not information: Pitfalls for brain mappers. Neuroimage. 2010;49:1949–1952. doi: 10.1016/j.neuroimage.2009.06.040. [DOI] [PubMed] [Google Scholar]
- 78.Kriegeskorte N, Cusack R, Bandettini P. How does an fMRI voxel sample the neuronal activity pattern: Compact-kernel or complex spatiotemporal filter? Neuroimage. 2010;49:1965–1976. doi: 10.1016/j.neuroimage.2009.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hanke M, et al. PyMVPA: A Unifying approach to the analysis of neuroscientific data. Front Neuroinform. 2009;3:3. doi: 10.3389/neuro.11.003.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Etzel JA, Gazzola V, Keysers C. Testing simulation theory with cross-modal multivariate classification of fMRI data. PLoS ONE. 2008;3:e3690. doi: 10.1371/journal.pone.0003690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pereira F, Mitchell T, Botvinick M. Machine learning classifiers and fMRI: A tutorial overview. Neuroimage. 2009;45(1 suppl):S199–S209. doi: 10.1016/j.neuroimage.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Friston KJ, et al. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6:218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- 83.Carter RM. Pasadena, CA: California Institute of Technology; 2006. Explicit and implicit processes in human aversive conditioning. PhD dissertation. [Google Scholar]
- 84.Gazzola V, Aziz-Zadeh L, Keysers C. Empathy and the somatotopic auditory mirror system in humans. Curr Biol. 2006;16:1824–1829. doi: 10.1016/j.cub.2006.07.072. [DOI] [PubMed] [Google Scholar]
- 85.Nelson AJ, Chen R. Digit somatotopy within cortical areas of the postcentral gyrus in humans. Cereb Cortex. 2008;18:2341–2351. doi: 10.1093/cercor/bhm257. [DOI] [PubMed] [Google Scholar]