Abstract
The DNA damage response comprises DNA repair, cell-cycle checkpoint control, and DNA damage-induced apoptosis that collectively promote genomic integrity and suppress tumorigenesis. Previously, we have shown that the Chk2 kinase functions independently of the Mre11 complex (Mre11, Rad50, and Nbs1) and ATM in apoptosis and suppresses tumorigenesis resulting from hypomorphic alleles of Mre11 or Nbs1. Based on this work, we have proposed that Chk2 limits the oncogenic potential of replication-associated DNA damage. Here we further address the role of Chk2 and damage-induced apoptosis in suppressing the oncogenic potential of chromosome breaks. We show that loss of Chk2 or a mutation in p53 (R172P), which selectively impairs its function in apoptosis, rescued the lethality of mice lacking Lig4, a ligase required for nonhomologous end-joining (NHEJ) repair of DNA double-strand breaks in G0/G1. In contrast to Lig4−/−p53−/− mice, Lig4−/−Chk2−/− and Lig4−/−p53R172P/R172P mice were not prone to organ-specific, rapid tumorigenesis. Although the severe NHEJ deficiency of Lig4−/− was a less potent initiator of tumorigenesis in the p53R172P/R172P and Chk2−/− backgrounds, where p53 cell-cycle functions are largely intact, even mild defects in the intra-S and G2/M checkpoints caused by mutations in Nbs1 are sufficient to influence malignancy in p53R172P/R172P mice. We conclude that the oncogenic potential of double-strand breaks resulting from NHEJ deficiency is highly restricted by nonapoptotic functions of p53, such as the G1/S checkpoint or senescence, suggesting that the particular facets of the DNA damage response required for tumor suppression are dictated by the proliferative status of the tumor-initiating cell.
Keywords: chromosome instability, kinase signaling, tumor suppressor
Following the detection of DNA lesions, the DNA damage response (DDR) coordinates DNA repair, cell-cycle checkpoints, and specialized programs, such as apoptosis and senescence, to prevent genomic instability (1). DDR genes frequently are mutated in chromosome instability and cancer predisposition syndromes, highlighting the role of the DDR in suppressing malignancy (2). Markers of DDR activation are observed readily in premalignant lesions, leading to the hypothesis that the DDR is an inducible barrier to tumorigenesis (3). Indices of DDR activation are reduced as tumors evolve, suggesting that selection against aspects of DNA damage signaling accompany tumor progression (1).
DNA double-strand breaks (DSBs) are physiologically important lesions, because they can be cytotoxic or cytostatic and generate oncogenic translocations (2, 4). Two major pathways of DSB repair are used in the cell: nonhomologous end-joining (NHEJ) and homology-directed repair (HDR). The use of HDR is cell cycle dependent, regulated by the activity of cyclin-dependent kinases and, in some cases, by the cell cycle-dependent expression or stability of required factors (5). HDR preferentially uses the sister chromatid as a template and thus is substantially less error-prone than NHEJ, which typically results in the deletion or addition of DNA sequences at the junction of repair. Deficiencies in both pathways have been linked to chromosomal instability, DNA damage sensitivity, and cancer predisposition, reflecting the hazards posed by DSBs (1).
The DDR is initiated by the recognition of DSBs that is mediated primarily by the Mre11 complex, consisting of Mre11, Rad50, and Nbs1. DSB engagement by the Mre11 complex is required for subsequent activation of ATM, an apical kinase in the DDR (2). ATM and related kinases are estimated to modify more than 700 substrates in response to DNA damage on consensus SQ/TQ sites (6, 7). Among these substrates are key regulators of cell-cycle checkpoints, DNA repair factors, and proteins that regulate apoptosis and senescence, accounting for the broad physiological impact of DDR functions (8).
Arguably one of the most important ATM substrates is the tumor suppressor p53. p53 controls the G1/S cell-cycle checkpoint and is required for both apoptosis and senescence in various cellular contexts (9). Deficiency of p53 results in severe predisposition to lymphoma in the mouse (10, 11). Mice harboring a separation-of-function mutation in p53, p53R172P, in which its apoptotic function is abolished but its influence on the G1/S checkpoint and the induction of senescence remain intact, have been established. The spectrum and kinetics of tumor development in p53R172P/R172P mice are markedly distinct from those of p53−/− mice (12). p53R172P/R172P mice have a late-onset tumor phenotype, exhibiting lymphomas and a variety of solid tumors, suggesting that senescence and G1/S checkpoint functions delay tumorigenesis in the context of apoptotic deficiency. Consistent with this view, the p53R172P allele retards the development of lymphomas resulting from Myc overexpression compared with p53 heterozygosity (13). Together, these data indicate that the apoptotic functions of p53 are not sufficient for its role as a tumor suppressor.
p53 functions are activated by DSBs induced in G1 cells, leading to the activation of apoptotic as well as nonapoptotic responses. Lig4−/− and Xrcc4−/− mice, which lack key components of the NHEJ machinery, exhibit perinatal lethality and are associated with high levels of DSBs in areas of the brain containing differentiated and largely nondividing cell populations (14, 15). p53 deficiency rescues the lethality of Lig4−/− and Xrcc4−/− mice, but Lig4−/− p53−/− and Xrcc4−/− p53−/− mice have rapid onset of pro– and pre–B-cell lymphomas as well as medulloblastomas with complete penetrance (16, 17). These data indicate that defective NHEJ-mediated repair of DSBs arising in G1 cells, such as those induced during antigen-receptor gene assembly and in differentiated CNS components, are highly oncogenic when p53 functions are lost (4).
Previously, we provided evidence that the Chk2 kinase suppressed the oncogenic potential of DNA replication-associated DNA damage through crosses with mice harboring mutations in Mre11 (Mre11ATLD1/ATLD1) and Nbs1 (Nbs1ΔB/ΔB) (18). This interpretation was based on the fact that those Mre11 complex alleles primarily affect the DDR in the S and G2 phases of the cell cycle and exhibit marked tumor predisposition in the context of Chk2 deficiency (19, 20). In contrast, Chk2 deficiency did not enhance tumorigenesis in PrkdcScid mice, in which DSBs induced in G1 cells during antigen-receptor gene assembly are not repaired (18). These data support the view that Chk2 tumor-suppressive activity may be relevant primarily for DNA damage that arises during S or G2 phase (18).
In this study, we examine genetic contexts in which defective apoptotic responses resulting from either p53 or Chk2 mutation are juxtaposed with DDR mutations that increase DNA damage in G1, S, or G2/M phases of the cell cycle. G1 DNA damage is enhanced in Lig4−/− mice. Unlike Lig4−/− p53−/− mice, Lig4−/− Chk2−/− and Lig4−/− p53R172P/R172P mice were not highly prone to the rapid development of lymphoma and medulloblastoma. Together, these outcomes suggest that the oncogenic potential of breaks generated by NHEJ deficiency is restricted by nonapoptotic p53 functions such as senescence or the imposition of the G1/S checkpoint. In contrast, Nbs1ΔC and Nbs1ΔB mutations, which impair the intra–S-phase and G2/M checkpoints, enhanced tumorigenesis in p53R172P/R172P mice. Combined with previous data regarding tumor predisposition in Nbs1ΔB/ΔB Chk2−/− mice, these results provide evidence for the tumor-suppressive influence of the intra-S and G2/M checkpoints. Collectively, the data presented indicate that the relative potency of apoptosis or senescence for tumor suppression is dependent on the DNA repair or cell-cycle checkpoint status of cancer-initiating cells.
Results
Nbs1 Mutations Influence Tumorigenesis in p53R172P/R172P Mice.
In p53-deficient mice, the DNA damage-dependent induction of apoptosis, senescence, and the G1/S checkpoint are lost. Because senescence and the G1/S checkpoint are intact in p53R172P/R172P mice, these animals provide a means to assess the importance of p53-dependent apoptosis for suppressing the oncogenic potential of DNA damage associated with DNA replication (12). We combined p53R172P/R172P with two Nbs1 mutants, Nbs1ΔB and Nbs1ΔC, which impair the response to DNA damage in S phase. The N terminus of Nbs1 contains a Forkhead-associated (FHA) and tandem Brca1 C-terminal (BRCT) phosphopeptide binding motifs, and the C terminus of Nbs1 contains a conserved domain that contributes to the physical interaction between the Mre11 complex and ATM (2, 21, 22). The Nbs1ΔC mutant lacks the C-terminal domain and exhibits an intra–S-phase checkpoint defect, as well as a defect in apoptosis, whereas the Nbs1ΔB mutant, lacking the FHA and first BRCT domain, is defective in both the intra-S and G2/M checkpoints but is competent for apoptosis (19, 23).
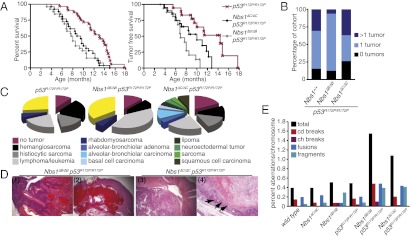
Relative to p53R172P/R172P alone, both p53R172P/R172P Nbs1ΔC/ΔC and p53R172P/R172P Nbs1ΔB/ΔB double mutants exhibited reduced survival, a more rapid onset of tumorigenesis, and alterations in tumor spectrum (Fig. 1 A–D and Tables S1–S7). These results reveal that the intra–S-phase checkpoint defects associated with Nbs1ΔC/ΔC decrease the latency of tumorigenesis. The reduction in latency was even more pronounced in Nbs1ΔB/ΔB p53R172P/R172P mutants than in Nbs1ΔC/ΔC p53R172P/R172P mutants, indicating that tumor suppression was impaired to a greater extent by defects in both the intra-S and G2/M checkpoints, present in cells from Nbs1ΔB/ΔB, than by defects in the intra-S checkpoint alone. We observed a marked increase in chromosome instability in cell cultures (Fig. 1E and Fig. S1) from both Nbs1ΔC/ΔC p53R172P/R172P and Nbs1ΔB/ΔB p53R172P/R172P animals as compared with single mutants, supporting the interpretation that the intra-S and G2/M checkpoints contribute to tumor suppression by promoting genomic integrity. This outcome is in contrast to the observation that tumors from p53R172P/R172P animals exhibited normal ploidy and few chromosomal aberrations, further underscoring the contributions of the intra-S and G2/M checkpoints to chromosome stability (12).
Fig. 1.
Mutations in Nbs1 influence tumor predisposition in p53R172P/R172P mice. (A) Overall and tumor-free survival in cohorts of p53R172P/R172P (n = 32/18), Nbs1ΔC/ΔC p53R172P/R172P (n = 26/21), and p53R172P/R172P Nbs1ΔB/ΔB (n = 26/17) double mutants. Mutations in Nbs1 lead to a reduction in the survival of p53R172P/R172P animals (P < 0.0001, p53R172P/R172P vs. p53R172P/R172P Nbs1ΔB/ΔB; P = 0.0002, p53R172P/R172P vs. p53R172P/R172P Nbs1ΔC/ΔC using the Mantel–Cox log rank test). (B) The percentage of mice in the cohort that were tumor free or that had one or more than one tumor. (C) The tumor spectrum and percentage of mice developing tumors in each genotype are graphed with uncharacterized tumors shown in yellow. More detailed information is provided in Tables S1–S7. (D) Histological examples of H&E-stained tumors in p53R172P/R172P Nbs1ΔB/ΔB or p53R172P/R172P Nbs1ΔC/ΔC mice. Shown from left to right are (1) a multicentric lymphoma (magnification: 40×); (2) hemangiosarcoma (magnification: 25×); (3) primitive neuroectodermal tumor in the cerebellum; and (4) vulva with squamous cell carcinoma (magnification: 100×) with arrows indicating infiltrative squamous cells. (E) Metaphase chromosome analysis of primary ear fibroblasts of the indicated genotype with percentages of chromatid (cd) and chromosome (ch) breaks, fusions, fragments, and total aberrations per chromosome. Examples are shown in Fig. S1.
Defective p53-Dependent Apoptosis Rescues the Viability of Lig4−/−.
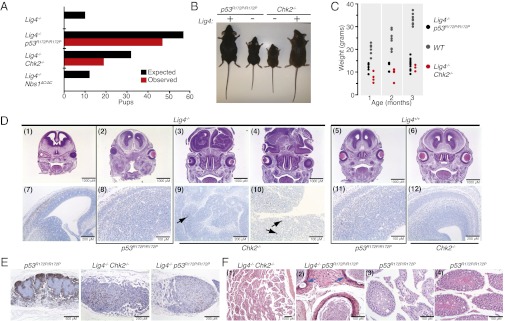
The combination of p53R172P/R172P mice with mutations affecting the Mre11 complex indicated that chromosomal instability associated with intra-S and G2/M checkpoint defects enhances tumorigenesis when apoptosis is impaired. The instability in those mutants is attributable primarily to impaired responses to DNA damage that arises during S phase as a result of DNA replication (24). Deficiency of DNA ligase 4 (Lig4−/−) severely impairs NHEJ, and Lig4−/− mice die in late embryogenesis. These mice exhibit extensive apoptosis in regions of the developing brain that contain ultimately postmitotic cells, including differentiated neurons (15). The lethality of Lig4−/− is rescued by the deletion of p53, and the rescued mice exhibit high-penetrance, early-onset medulloblastomas and B-cell lymphomas that exhibit gene duplications and recurrent translocations (17, 25, 26). To delineate the tumor-suppressive functions of apoptosis in response to DSBs in G1 cells, we crossed Lig4−/− mice with Nbs1ΔC/ΔC, Chk2−/−, and p53R172P/R172P mice and assessed the viability and development of the embryonic brain. The viability of Lig4−/− mice was rescued by Chk2 deficiency and the p53R172P allele, but Lig4−/− Nbs1ΔC/ΔC mice were inviable (Fig. 2 A and B and Table S8).
Fig. 2.
Apoptotic defects are sufficient to rescue the viability of Lig4-deficient animals. (A) Graphical representation of expected vs. observed number of pups based on Mendelian inheritance of alleles. Details of breeding schemes and numbers are provided in Table S8. (B) Representative images of 2-mo-old mice of the indicated genotypes. (C) Analysis of changes in pup weight of the indicated genotypes over 3 mo. (D) Immunohistochemical analysis of brain morphology in E13.5 embryos of the indicated genotypes. (Upper) H&E staining. (Lower) Cleaved caspase-3 staining. Arrows in panels 9 and 10 indicate rare clusters of caspase 3 positive cells. (E) B220 staining of mesenteric lymph nodes. (Left) Normal mesenteric lymph node of a p53R172P/R172P mouse. (Center and Right) Marked lymphoid atrophy/hypoplasia observed in all Lig4-null mice; genotypes are indicated. (F) Testicular pathology observed in 4/4 Lig4−/− Chk2−/− and 9/15 Lig4−/− p53R172P/R172P mice. (1) Degeneration of seminiferous tubules observed in Lig4−/− Chk2−/− mice between 3.2 and 7.1 (shown) mo. (2) Cross-section of 2.2-mo-old Lig4−/− p53R172P/R172P epididymis showing pleomorphic epithelial cells with karyomegaly (blue arrows) and vacuoles. (3) Variable loss of spermatogonia and primary and secondary spermatocytes observed in seminiferous tubules of the testes in a p53R172P/R172P mouse (age 14.8 mo). (4) Normal testes pathology in a p53R172P/R172P mouse (age 11.3 mo).
The degree of rescue in these settings, as inferred from size of the animals and level of CNS apoptosis, was correlated with the relative severity of the apoptotic defects in p53R172P/R172P and Chk2−/− mice. Lig4−/− p53R172P/R172P pups were less severely runted than Lig4−/− Chk2−/− and showed normal brain development and low levels of cleaved caspase-3 staining (Fig. 2 B–D; in D, subpanels 2 and 8 compared with subpanels 1 and 7). In contrast, gross morphological defects were evident in the brains of Lig4−/− Chk2−/− mice (Fig. 2D, subpanels 3 and 4 compared with subpanels 5 and 6). These brains contained limited areas of apoptotic cells, but, overall, Lig4−/− Chk2−/− double mutants were much less affected than Lig4−/− single mutants (Fig. 2D, subpanels 9 and 10 compared with subpanel 7). These results are consistent with data suggesting a role for Chk2 in genotoxic stress-induced checkpoint activation in the brain (27).
The phenotypic rescue observed in Lig4−/− p53R172P/R172P and Lig4−/− Chk2−/− double mutants was incomplete: Immune system development was not rescued (Fig. 2E), and both Lig4−/− Chk2−/− and, to a lesser extent, Lig4−/− p53R172P/R172P mice exhibited precocious degeneration of the seminiferous tubules (Fig. 2F). Cumulatively, these data indicate that the embryonic lethality of Lig4 deletion is dependent on apoptosis mediated by Chk2 and p53 and that the myriad pathologies evident are the consequences of impaired NHEJ-mediated DSB repair and the induction of p53-dependent cell-cycle regulatory programs.
Cell-Cycle Regulatory Functions of p53 Influence Tumorigenesis in Lig4−/− Animals.
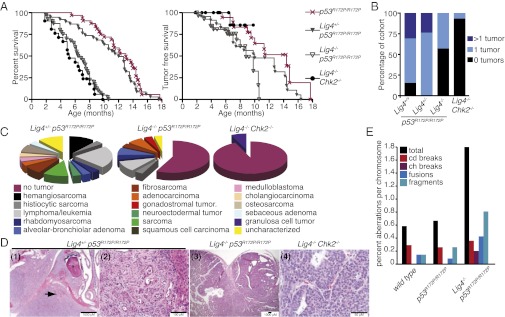
As noted previously, rescue of Lig4−/− viability by p53 deficiency results in rapid-onset pro–B-cell lymphoma and medulloblastoma with complete penetrance (16, 17). Viability of Lig4−/− mutants was rescued by p53R172P/R172P and Chk2−/−, but lifespan was reduced in both double mutants. Nevertheless, Lig4−/− Chk2−/− mice were not tumor prone, and Lig4−/− p53R172P/R172P mice died less often from tumors than did Lig4−/− p53−/− or p53R172P/R172P mice (Fig. 3 A–C) (12, 16). Microscopic analysis identified few lesions in a Lig4−/− Chk2−/− cohort, with only 1 of 15 mice exhibiting a granulosa cell tumor (Fig. 3C). In contrast to a previous report (28), the tumor spectrum of Lig4−/− p53R172P/R172P mice was similar to that of p53R172P/R172P mice, although the percentage of mice developing tumors was reduced relative to mice with the p53R172P/R172P mutation alone (Fig. 3 A–C and Table S4). The Lig4−/− p53R172P/R172P mice that did develop tumors did so more rapidly than did p53R172P/R172P mice, indicating that defective NHEJ may accelerate tumorigenesis through breakage-induced chromosomal rearrangements. Unlike Lig4−/− p53−/− mice, which succumb to tumors before 3 mo of age, Lig4−/− p53R172P/R172P mice had a longer median survival of 6.6 mo and developed few lymphomas and no medulloblastomas (Fig. 3C) (16, 17). Heterozygosity of Lig4, which has been shown to accelerate tumorigenesis in Ink4a/arf−/− mice, did not alter the kinetics of tumor development but altered the tumor spectrum compared with that of p53R172P/R172P mice (Fig. 3 A–D) (29).
Fig. 3.
The cell-cycle functions of p53 restrict Lig4-mediated tumorigenesis. (A) Overall and tumor-free survival of Lig4-deficient p53R172P/R172P (reproduced from Fig. 1A for a point of comparison; statistical analysis is presented in Tables S1–S7), p53R172P/R172P (n = 32/18), Lig4+/− p53R172P/R172P (n = 39/21), Lig4−/− p53R172P/R172P (n = 40/28), and Lig4−/− Chk2−/− (n = 21/15) mice. (B) The percentage of mice in the cohort that were tumor free or had one or more than one tumor. (C) Tumor spectrum and percentage of tumors observed in cohorts of the indicated genotypes. (D) Representative images of tumors from the indicated genotypes. (1) Osteosarcoma of the oral–nasal cavity with infiltration around the tooth (*) with erosion of the maxilla and osteoid formation (arrow) displacing the overlying soft tissue. (2) Cholangiosarcoma impinging upon the liver (right side). (3) Adenocarcinoma of the small intestine, Brunner’s glands. (4) Early-onset (6.6 mo) granulosa cell tumor of the ovary. (E) Metaphase chromosome analysis of primary embryo fibroblasts of the indicated genotype. Examples are shown in Fig. S1.
In p53-null backgrounds, it has been reported that loss of NHEJ leads to an increase in chromosomal translocations in both lymphomas and medulloblastomas (25, 26). We examined chromosomal spreads from primary fibroblasts derived from Lig4−/− p53R172P/R172P embryos to determine if increased genomic instability was evident. We found a marked increase in the number of chromosomal aberrations, particularly fusions and rearrangements, suggesting that a propensity for chromosomal translocations caused by canonical NHEJ deficiency may contribute to tumorigenesis in Lig4−/− p53R172P/R172P animals (Fig. 3E). Together these data indicate that a partial (Chk2−/−) or complete (p53R172P/R172P) apoptotic defect is sufficient to rescue the lethality of Lig4 deficiency but not to predispose the bearers to early-onset tumors of the CNS or immune system that are observed in the complete absence p53 function. Further, these results demonstrate that the DNA damage-induced activation of nonapoptotic p53-dependent signaling pathways can provoke substantial premature mortality in the absence of tumorigenesis.
Nbs1 and Chk2 Promote Apoptosis in Parallel Pathways.
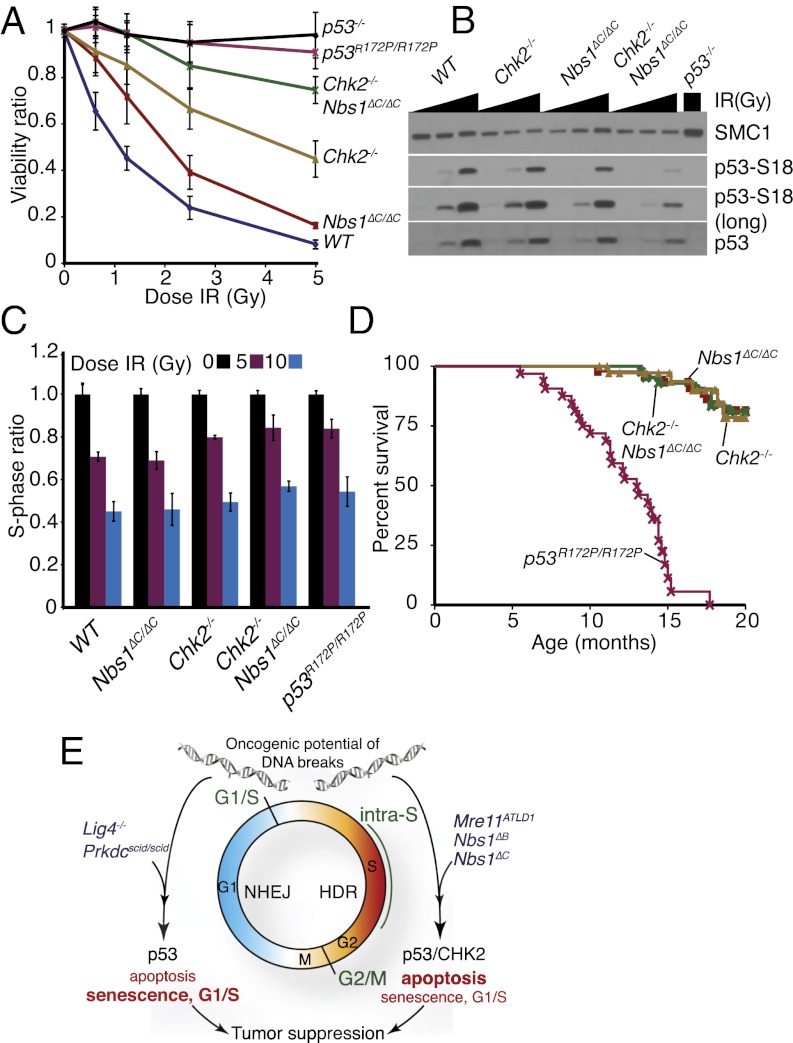
In previous work, we found that ATM and Chk2 independently influence p53-dependent apoptosis, indicated by the observation that the apoptotic defects in Atm−/− and Chk2−/− mutants were additive. In contrast, Nbs1ΔC/ΔC was largely epistatic with Atm−/−, consistent with their functioning in the same pathway (23). Because loss of Chk2 but not deletion of the C terminus of Nbs1 was sufficient to rescue Lig4 deficiency (Fig. 2A), we hypothesized that the Nbs1ΔC allele would influence apoptosis independently of Chk2. To address this possibility, we generated Nbs1ΔC/ΔC Chk2−/− double-mutant mice and cell cultures. The apoptotic defect of Nbs1ΔC/ΔC Chk2−/− was more severe than either single mutant and was similar in magnitude to that of p53−/− or p53R172P/R172P (Fig. 4A). Correlating with this defect, we observed reduced p53 phosphorylation and stabilization in Nbs1ΔC/ΔC Chk2−/− cells compared with either single mutant or wild-type controls (Fig. 4B). The strong additive phenotype of the Nbs1ΔC/ΔC Chk2−/− thymocytes indicated that Nbs1 and Chk2 functioned in parallel pathways and that p53-dependent apoptosis was severely impaired in response to ionizing radiation in these animals.
Fig. 4.
Defects in ionizing radiation-induced apoptosis are genetically separable from cell-cycle regulation. (A) Apoptosis assessed by flow cytometry of thymocytes of the indicated genotype 20 h after ionizing radiation treatment with a range of doses. Results are compiled from triplicate samples from at least two different animals of each genotype. (B) Western blotting of p53 serine 18 phosphorylation and total p53 levels following treatment with 0-, 0.5-, or 5-Gy ionizing radiation in primary cell cultures from the indicated genotypes. (C) G1/S checkpoint in early-passage MEF cultures. S-phase ratios (%BrdU+ cells in ionizing radiation or mock treated samples/average %BrdU+ in mock treated samples) are plotted. Results are the average of two to four experiments performed in triplicate for each genotype. Error bars denote SD. Results for individual cultures are shown in Fig. S2. (D) Cohort survival of Nbs1ΔC/ΔC (n = 45), Chk2−/− (n = 42), Nbs1ΔC/ΔC Chk2−/− (n = 40), and p53R172P/R172P (n = 32) mice over 20 mo. (E) Contributions of the DDR to tumor suppression. Mutations in NHEJ, such as the Prkdcscid or Lig4-null alleles, block DSB repair primarily in G1/G0-phase cells where HDR is not operative. The oncogenic potential of the resulting unrepaired breaks is suppressed by p53, with the cell-cycle functions (indicated in bold) having a primary influence. In S/G2-phase cells, the activation of apoptosis (indicated in bold) through p53 and Chk2 appears to be the primary mode of suppressing the oncogenic potential of DNA replication-associated DSBs, the levels of which are elevated by mutations in Mre11 or Nbs1 that impair the intra-S and G2/M checkpoints (green type).
We compared the apoptotic and cell-cycle checkpoint responses of Nbs1ΔC/ΔC Chk2−/− double mutants with those seen with the p53R172P/R172P mutation, which impairs apoptosis but retains the cell-cycle functions (12). We found that, like p53R172P/R172P, cultures of Nbs1ΔC/ΔC Chk2−/− cells had only a minor G1/S defect compared with wild type and a strong defect in ionizing radiation-induced apoptosis in thymocytes (Fig. 4 A and C and Fig. S2). As we have reported previously, a normal G1/S response was observed in mouse embryo fibroblasts (MEFs) from Nbs1ΔC/ΔC (Fig. 4C and Fig. S2) (23). Thus, Nbs1ΔC/ΔC Chk2−/− mice phenocopy p53R172P/R172P mice, exhibiting pronounced apoptotic defects but leaving ionizing radiation-dependent induction of the G1/S cell-cycle checkpoint intact.
Nbs1ΔC/ΔC Chk2−/− mice did not phenocopy the cancer susceptibility of p53R172P/R172P animals. p53R172P/R172P and Nbs1ΔC/ΔC Chk2−/− mice were followed over the course of 20 mo (Fig. 4D). Although tumors arose with a mean latency of ∼11 mo in p53R172P/R172P mice, as previously reported (12), tumorigenesis was not observed in the Nbs1ΔC/ΔC Chk2−/− cohort (Fig. 4D). These data likely indicate that p53 regulation induced by DNA damage is partially intact in Nbs1ΔC/ΔC Chk2−/− mice or that the p53R172P allele blunts the apoptotic response to a broader spectrum of lesions that promote tumorigenesis than the mutations in Nbs1 and Chk2, which are defective primarily in the response to DNA DSBs.
Discussion
With respect to the role of the DDR in tumor suppression, these data shed light on the relationship of apoptosis to cell-cycle regulation, on one hand, and to DSB repair, on the other. In either case, defects in the DNA damage-dependent induction of apoptosis, such as those evident in p53R172P/R172P and Chk2−/− mutations, are correlated with increased oncogenic potential. Nonapoptotic DDR signaling outcomes such as activation of the cell-cycle checkpoint mediated by the Mre11 complex and induction of senescence by p53 suppress that potential, as indicated by the fact that individual or combined loss of G1/S, intra-S, and G2/M checkpoint control enhance tumorigenesis in p53R172P/R172P mice (18, 30). This finding is particularly notable, because tumorigenesis in p53R172P/R172P mice is enhanced by the loss of p21 and the G1/S checkpoint or by the loss of Nbs1ΔC/ΔC and the intra-S checkpoint in the absence of major DNA repair defects per se.
DSB repair deficiency also enhances the oncogenic potential of apoptotic defects and appears to do so in a cell cycle-specific manner. This point is starkly illustrated by the observation that NHEJ defects, which are primarily relevant in G0/G1 cells, enhance tumorigenesis most efficiently when the G1/S checkpoint is impaired (e.g., in Lig4−/− p53−/− as compared with Lig4−/− p53R172P/R172P or Lig4−/− Chk2−/− mutants) (16). On the other hand, genetic analysis revealed that Chk2 suppresses tumorigenesis when G2/M checkpoint signaling and repair are defective but not in response to the absence of NHEJ (Fig. 3 and refs. 18, 31, and 32). The latter result is particularly striking, because it demonstrates that both the severity of the defect in apoptosis and the status of p53-dependent cell-cycle regulation moderate the influence of NHEJ deficiency on tumorigenesis. Hence, depending on the status of p53 signaling, chromosomal breakage arising from defective NHEJ can provoke tumorigenesis, tumor-free premature mortality, or a combination of both outcomes.
Both Nbs1ΔB and Nbs1ΔC alleles influence the tumor spectrum of p53R172P/R172P mutants, but only Nbs1ΔB promotes tumorigenesis in Chk2−/− mice, suggesting that particular functions of the Mre11 complex are important when apoptotic defects are less severe. A potential candidate for this activity is DSB resection, the conversion of dsDNA to ssDNA via nuclease activity (33). In yeast, DSB resection is required for both efficient G2/M checkpoint arrest and HDR, and this process has been proposed to underlie the DSB-induced activation of ATR and Chk1 in mammalian cells (34–36). Because the G2/M checkpoint and HDR are defective in Nbs1ΔB and other alleles that lead to tumorigenesis in mice lacking Chk2 but are intact in Nbs1ΔC mice, we hypothesize that defects in resection may promote the generation of oncogenic lesions that normally are eliminated by Chk2 activity through apoptosis or additional regulatory functions (18, 31, 37). Because the cell-cycle checkpoint defects of the Nbs1ΔC allele are restricted to S phase, these defects influence tumorigenesis only in the context of the more severe apoptotic defects of p53R172P/R172P mutations. We also cannot rule out the possibility that p53R172P and Nbs1ΔC alleles have additional defects in vivo that influence tumor growth. Considering this possibility, and given the diverse tumor spectrum, we predict that these additional functions are unlikely to be tissue specific.
Collectively, these data illustrate the interdependence of DSB repair, cell-cycle checkpoints, and apoptosis in the suppression of malignancy. These results are consistent with a model that in nondividing cells, which are dependent primarily on NHEJ for DSB repair, survival is dependent on apoptosis, but the suppression of oncogenesis relies heavily upon the imposition of cell-cycle regulation by p53 (Fig. 4E). On the other hand, dividing cells experiencing replicative stress and damage at replication forks primarily are dependent on both resection-based checkpoint and repair responses and on apoptosis to suppress the subsequent development of chromosomal instability and oncogenic lesions. Thus, we propose that the DDR barriers that cells must overcome before becoming initiators of tumorigenesis depend on their proliferative status. A more complete mechanistic understanding of the individual processes within the DDR involved in tumor suppression and of their interrelationships will be essential to identify functional biomarkers that will have predictive value in cancer predisposition, progression, and treatment.
Materials and Methods
Flow Cytometry.
Thymocytes were isolated from 7- to 9-mo-old animals and exposed ex vivo to the indicated doses of ionizing radiation. Twenty hours after ionizing radiation, cells were stained with Annexin-V FITC and propidium iodide (PI) (Becton Dickinson), and the percentage of double-negative (viable) cells was determined by flow cytometry and normalized to untreated cells (apoptotic ratio).
Checkpoint Analysis.
The G1/S checkpoint was assessed in early-passage (< p4) MEFs. Cells were exposed to the indicated doses of ionizing radiation using an X-ray cabinet and 14 h after treatment were pulse labeled with BrdU for 4 h and stained with anti–BrdU-FITC antibodies and PI (Becton Dickinson). The percentage of S-phase cells was determined by flow cytometry and normalized to untreated cells (S-phase ratio).
Immunohistochemistry.
Embryonic day (E) 13.5 embryos or moribund mice were euthanized, fixed in 10% (vol/vol) neutral-buffered formalin, paraffinized, and sectioned using standard methods. Serial sections were stained with H&E or antibodies to cleaved caspase-3 (Millipore).
Mice and Assessment of Tumor Predisposition.
Lig4−/−, Chk2−/−, p53R172P/R172P, p53−/−, and Atm−/− mice were obtained from P. McKinnon (St. Jude Childrens Research Hospital, Memphis, TN), T. Mak (Ontario Cancer Institute, Ontario, Canada), G. Lozano (The University of Texas MD Anderson Cancer Center, Houston, TX), T. Jacks (Massachusetts Institute of Technology, Boston, MA), and T. Wynshaw Boris (University of California, San Francisco, CA), respectively. Nbs1ΔB and Nbs1ΔC mice were described previously (19, 23). Mice were raised in a pathogen-free facility and genotyped by PCR (details upon request). Mice were maintained on a mixed 129/B6 background and monitored for signs of malignancy or distress three to five times per week. Any animals with palpable tumors or other signs of distress were euthanized and processed for pathological analysis. Appropriate National Institutes of Health guidelines for animal husbandry and treatment were followed.
Western Blotting.
Samples were lysed in TNG-150 buffer [50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1% Tween-20, 10% (vol/vol) NP40], separated by SDS/PAGE, and transferred to PVDF membrane (Millipore). Membranes were probed with antibodies for p53 (CM5; Vector), phospho-S15/18 p53 (Cell Signaling), or SMC1 (Abcam). Primary antibodies were detected with appropriate secondary antibodies conjugated to HRP (Jackson) and visualized by ECL (GE).
Supplementary Material
Acknowledgments
We thank Suzana Couto for assistance with pathological analysis and sample preparation, David Rossell and Evarist Planet of the Institute for Research in Biomedicine Biostatistics Unit for advice on statistical analysis, and members of the J.H.J.P. and T.H.S. laboratories for critical reading of the manuscript and discussions. S.S.F. is supported by the Memorial Sloan-Kettering Cancer Center Brain Tumor Center. J.H.J.P is supported by grants from the National Institutes of Health, the Geoffrey Beene Foundation, and the Goodwin Foundation. T.H.S. is a Ramon y Cajal investigator, supported by Grant SAF2009-10023 from the Ministerio de Ciencia e Innovacion, and was a Special Fellow of the Leukemia and Lymphoma Society.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1120476109/-/DCSupplemental.
References
- 1.Jackson SP, Bartek J. The DNA-damage response in human biology and disease. Nature. 2009;461:1071–1078. doi: 10.1038/nature08467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stracker TH, Petrini JH. The MRE11 complex: Starting from the ends. Nat Rev Mol Cell Biol. 2011;12:90–103. doi: 10.1038/nrm3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Halazonetis TD, Gorgoulis VG, Bartek J. An oncogene-induced DNA damage model for cancer development. Science. 2008;319:1352–1355. doi: 10.1126/science.1140735. [DOI] [PubMed] [Google Scholar]
- 4.Nussenzweig A, Nussenzweig MC. Origin of chromosomal translocations in lymphoid cancer. Cell. 2010;141:27–38. doi: 10.1016/j.cell.2010.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heyer WD, Ehmsen KT, Liu J. Regulation of homologous recombination in eukaryotes. Annu Rev Genet. 2010;44:113–139. doi: 10.1146/annurev-genet-051710-150955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matsuoka S, et al. ATM and ATR substrate analysis reveals extensive protein networks responsive to DNA damage. Science. 2007;316:1160–1166. doi: 10.1126/science.1140321. [DOI] [PubMed] [Google Scholar]
- 7.Mu JJ, et al. A proteomic analysis of ataxia telangiectasia-mutated (ATM)/ATM-Rad3-related (ATR) substrates identifies the ubiquitin-proteasome system as a regulator for DNA damage checkpoints. J Biol Chem. 2007;282:17330–17334. doi: 10.1074/jbc.C700079200. [DOI] [PubMed] [Google Scholar]
- 8.Ciccia A, Elledge SJ. The DNA damage response: Making it safe to play with knives. Mol Cell. 2010;40:179–204. doi: 10.1016/j.molcel.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iwakuma T, Lozano G. Crippling p53 activities via knock-in mutations in mouse models. Oncogene. 2007;26:2177–2184. doi: 10.1038/sj.onc.1210278. [DOI] [PubMed] [Google Scholar]
- 10.Donehower LA, et al. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature. 1992;356:215–221. doi: 10.1038/356215a0. [DOI] [PubMed] [Google Scholar]
- 11.Jacks T, et al. Tumor spectrum analysis in p53-mutant mice. Curr Biol. 1994;4:1–7. doi: 10.1016/s0960-9822(00)00002-6. [DOI] [PubMed] [Google Scholar]
- 12.Liu G, et al. Chromosome stability, in the absence of apoptosis, is critical for suppression of tumorigenesis in Trp53 mutant mice. Nat Genet. 2004;36:63–68. doi: 10.1038/ng1282. [DOI] [PubMed] [Google Scholar]
- 13.Post SM, et al. p53-dependent senescence delays Emu-myc-induced B-cell lymphomagenesis. Oncogene. 2010;29:1260–1269. doi: 10.1038/onc.2009.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao Y, et al. A critical role for DNA end-joining proteins in both lymphogenesis and neurogenesis. Cell. 1998;95:891–902. doi: 10.1016/s0092-8674(00)81714-6. [DOI] [PubMed] [Google Scholar]
- 15.Orii KE, Lee Y, Kondo N, McKinnon PJ. Selective utilization of nonhomologous end-joining and homologous recombination DNA repair pathways during nervous system development. Proc Natl Acad Sci USA. 2006;103:10017–10022. doi: 10.1073/pnas.0602436103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frank KM, et al. DNA ligase IV deficiency in mice leads to defective neurogenesis and embryonic lethality via the p53 pathway. Mol Cell. 2000;5:993–1002. doi: 10.1016/s1097-2765(00)80264-6. [DOI] [PubMed] [Google Scholar]
- 17.Lee Y, McKinnon PJ. DNA ligase IV suppresses medulloblastoma formation. Cancer Res. 2002;62:6395–6399. [PubMed] [Google Scholar]
- 18.Stracker TH, Couto SS, Cordon-Cardo C, Matos T, Petrini JH. Chk2 suppresses the oncogenic potential of DNA replication-associated DNA damage. Mol Cell. 2008;31:21–32. doi: 10.1016/j.molcel.2008.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Williams BR, et al. A murine model of Nijmegen breakage syndrome. Curr Biol. 2002;12:648–653. doi: 10.1016/s0960-9822(02)00763-7. [DOI] [PubMed] [Google Scholar]
- 20.Theunissen JW, et al. Checkpoint failure and chromosomal instability without lymphomagenesis in Mre11(ATLD1/ATLD1) mice. Mol Cell. 2003;12:1511–1523. doi: 10.1016/s1097-2765(03)00455-6. [DOI] [PubMed] [Google Scholar]
- 21.Falck J, Coates J, Jackson SP. Conserved modes of recruitment of ATM, ATR and DNA-PKcs to sites of DNA damage. Nature. 2005;434:605–611. doi: 10.1038/nature03442. [DOI] [PubMed] [Google Scholar]
- 22.You Z, Chahwan C, Bailis J, Hunter T, Russell P. ATM activation and its recruitment to damaged DNA require binding to the C terminus of Nbs1. Mol Cell Biol. 2005;25:5363–5379. doi: 10.1128/MCB.25.13.5363-5379.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stracker TH, Morales M, Couto SS, Hussein H, Petrini JH. The carboxy terminus of NBS1 is required for induction of apoptosis by the MRE11 complex. Nature. 2007;447:218–221. doi: 10.1038/nature05740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adelman CA, De S, Petrini JH. Rad50 is dispensable for the maintenance and viability of postmitotic tissues. Mol Cell Biol. 2009;29:483–492. doi: 10.1128/MCB.01525-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu C, et al. Unrepaired DNA breaks in p53-deficient cells lead to oncogenic gene amplification subsequent to translocations. Cell. 2002;109:811–821. doi: 10.1016/s0092-8674(02)00770-5. [DOI] [PubMed] [Google Scholar]
- 26.Yan CT, et al. XRCC4 suppresses medulloblastomas with recurrent translocations in p53-deficient mice. Proc Natl Acad Sci USA. 2006;103:7378–7383. doi: 10.1073/pnas.0601938103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Squatrito M, et al. Loss of ATM/Chk2/p53 pathway components accelerates tumor development and contributes to radiation resistance in gliomas. Cancer Cell. 2010;18:619–629. doi: 10.1016/j.ccr.2010.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van Nguyen T, Puebla-Osorio N, Pang H, Dujka ME, Zhu C. DNA damage-induced cellular senescence is sufficient to suppress tumorigenesis: A mouse model. J Exp Med. 2007;204:1453–1461. doi: 10.1084/jem.20062453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sharpless NE, et al. Impaired nonhomologous end-joining provokes soft tissue sarcomas harboring chromosomal translocations, amplifications, and deletions. Mol Cell. 2001;8:1187–1196. doi: 10.1016/s1097-2765(01)00425-7. [DOI] [PubMed] [Google Scholar]
- 30.Barboza JA, Liu G, Ju Z, El-Naggar AK, Lozano G. p21 delays tumor onset by preservation of chromosomal stability. Proc Natl Acad Sci USA. 2006;103:19842–19847. doi: 10.1073/pnas.0606343104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cao L, et al. ATM-Chk2-p53 activation prevents tumorigenesis at an expense of organ homeostasis upon Brca1 deficiency. EMBO J. 2006;25:2167–2177. doi: 10.1038/sj.emboj.7601115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fishler T, et al. Genetic instability and mammary tumor formation in mice carrying mammary-specific disruption of Chk1 and p53. Oncogene. 2010;29:4007–4017. doi: 10.1038/onc.2010.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mimitou EP, Symington LS. DNA end resection: Many nucleases make light work. DNA Repair (Amst) 2009;8:983–995. doi: 10.1016/j.dnarep.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mimitou EP, Symington LS. Sae2, Exo1 and Sgs1 collaborate in DNA double-strand break processing. Nature. 2008;455:770–774. doi: 10.1038/nature07312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhu Z, Chung WH, Shim EY, Lee SE, Ira G. Sgs1 helicase and two nucleases Dna2 and Exo1 resect DNA double-strand break ends. Cell. 2008;134:981–994. doi: 10.1016/j.cell.2008.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cimprich KA, Cortez D. ATR: An essential regulator of genome integrity. Nat Rev Mol Cell Biol. 2008;9:616–627. doi: 10.1038/nrm2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Niida H, et al. Cooperative functions of Chk1 and Chk2 reduce tumour susceptibility in vivo. EMBO J. 2010;29:3558–3570. doi: 10.1038/emboj.2010.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.