Abstract
Nerve impulse activity produces both developmental and adult plastic changes in neural networks. For development, however, its precise role and the mechanisms involved remain elusive. Using the classic model of synapse competition and elimination at newly formed neuromuscular junctions, we asked whether spike timing is the instructive signal at inputs competing for synaptic space. Using a rat strain whose soleus muscle is innervated by two nerves, we chronically evoked different temporal spike patterns in the two nerves during synapse formation in the adult. We found that asynchronous activity imposed upon the two nerves promotes synapse elimination, provided that their relative spikes are separated by 25 ms or more; remarkably, this elimination occurs even though an equal number of spikes were evoked in the competing axons. On the other hand, when spikes are separated by 20 ms or less, activity is perceived as synchronous, and elimination is prevented. Thus, in development, as in adult plasticity, precise spike timing plays an instructive role in synaptic modification.
In the adult brain, plastic changes in the strength of synaptic connections between excitable cells occur as a result of experience and contribute to shaping its architecture during development. Many of these changes are known to be tightly linked to the pattern of action potential firing. Temporal spike correlation in pre- and postsynaptic cells strengthens or weakens synapses during development (1–3) and in cellular models of learning (long-term potentiation and depression) (4, 5). Two well known paradigms for these phenomena are Hebb’s postulate (6) and spike-timing-dependent plasticity (STDP) (7–10). A classic model to investigate synaptic modification is the elimination of input that occurs at developing neuromuscular junctions (NMJs) as a result of competition among the motor nerve terminals (11–14). Elimination of input is widespread in the developing peripheral nervous system and CNS; at the NMJ, where it was identified (13), it spans the first 2 wk of postnatal life in rodents, during which time the innervation of each muscle fiber changes from innervation by the collaterals of different motor neurons (polyneuronal innervation) to innervation by only one collateral (mononeuronal innervation). Examples of this peculiar end result also are observed in the CNS (e.g., the innervation of Purkinje cells by climbing fibers) (15). Activity influences this process, but its precise role remains controversial. Briefly, some studies emphasize the overall neuromuscular activity, others emphasize the differences in the amount of activity in competing inputs, and still other studies emphasize activity-independent factors (16–27). (We explore this controversy in more detail in the Discussion.)
Differences in activity generally have been tested by comparing active inputs with inputs completely inactivated by conduction block. However, the insight gained from these experiments is limited because all competing motor terminals are active during normal development. Thus, essential information about competition between active inputs is lacking, with the exception that, in our recent investigations with synchronous activation of inputs, we found a marked stabilization of polyneuronal innervation (19, 28) rather than an acceleration of synapse elimination as previously reported (23, 26). These results prompted us to test the consequences of different firing patterns of competing motor terminals, thus investigating the importance of the timing of action potentials in competing nerves. We compared synchronous and asynchronous patterns of activity using an equal number of stimuli per day. A second motivation was the finding that the spikes of different motor units of a given muscle recorded in vivo in newborn rats are tightly synchronized at postnatal days (P)1–3 but quickly desynchronize a few days later, just before the bulk of synapse elimination (29–31).
For our experimental model we used the soleus muscle of adult rats of the AO strain, with its peculiar innervation by two nerves. After crushing these nerves reinnervate the muscle and recapitulate normal development. The experimental plan requires establishing in vivo, over a period of 2–3 wk, a central chronic conduction block (to eliminate spontaneous activity) and peripheral electrical stimulation of the two nerves independently from each other. Ideally, the experiments would be done in newborn mice or rats, but their small size and dependence on the mother made this approach impractical. On the other hand, the adult crushing–reinnervation paradigm has been used extensively for studying synapse elimination and is perfectly suited for these experiments. The regenerating axons quickly reinnervate the original synaptic sites, where multiple inputs from different motor neurons converge on the same acetylcholine receptor aggregate (32, 33); the inputs initially intermingle with one another (34), but eventually the process disposes of all but one input (33, 35, 36), as occurs during normal development (14, 37).
Results
Asynchronous Activity Promotes Elimination Between Competing Neuromuscular Inputs.
We studied activity-dependent synaptic competition using the in vivo model of reinnervation of adult soleus muscles in the AO strain of rats after the crushing of their two nerves (soleus and aberrant) (Fig. 1A). We distinguish three territories in reinnervated muscles: (i) all myofibers reinnervated only by the soleus axons, mono- or polyneuronally (referred to as soleus “same-nerve” field); (ii) all myofibers reinnervated only by the aberrant axons (aberrant “same-nerve” field); and (iii) their region of overlap, characterized by a variable number of myofibers polyneuronally innervated by the two axon types (“both-nerves” field) (Materials and Methods). Initially, a number of reinnervated muscle fibers are innervated polyneuronally, but, as a result of competition and synapse elimination, they become singly innervated within a few weeks (14).
Fig. 1.
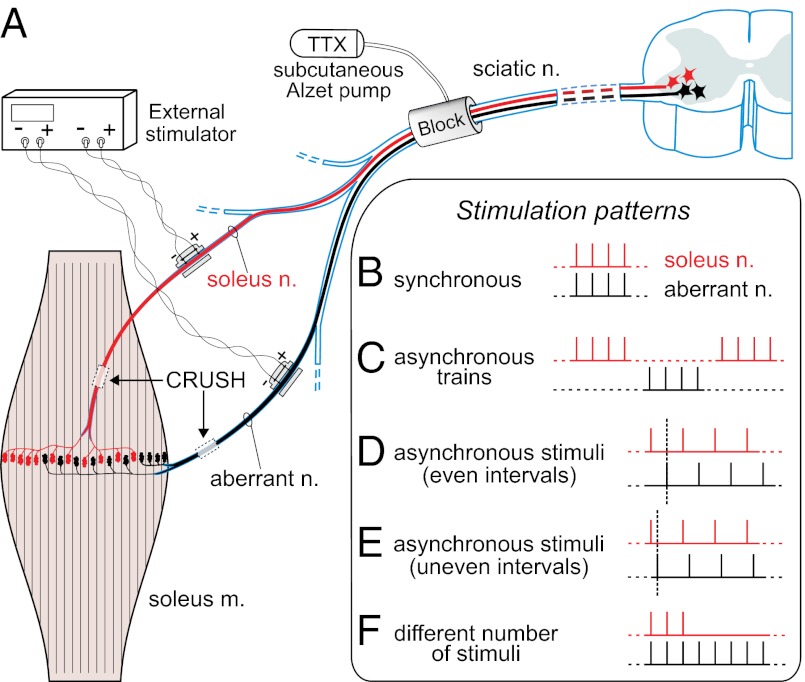
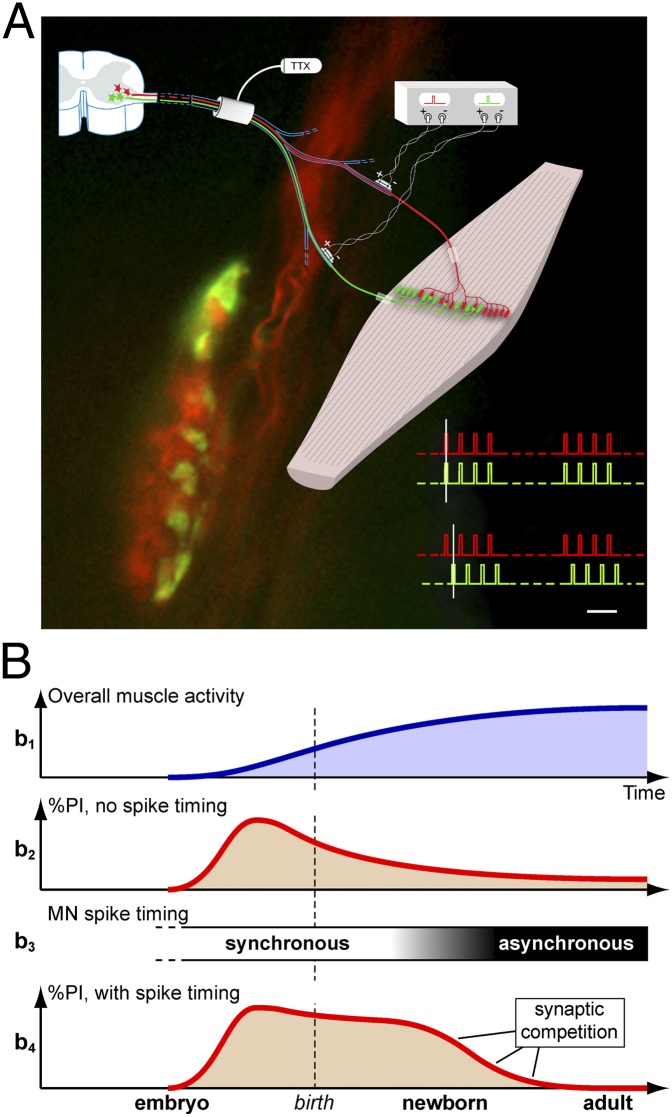
Experimental plan and patterns of electrical stimulation. (A) Schematic of the placement of chronic conduction blocking, nerve crushing, and chronic stimulating electrodes of soleus and aberrant nerves. Shown are fields of reinnervation after crushing. (B–F) Different timings of stimuli trains. Each nerve receives one train of eight 100-μs pulses every 11 s, 62.836 pulses per day (except in F, see below); train pulse frequency is 20 Hz (except for 10 Hz in E and a subpattern of D). (B) Synchronous trains and stimuli. (C) Asynchronous trains (i.e., alternating trains, with equally spaced intervals). (D) Synchronous trains and asynchronous stimuli, with even intervals between the stimuli to the two nerves (50 and 25 ms, respectively, for the two subpatterns of 10- and 20-Hz pulse frequency). (E) Synchronous trains and asynchronous stimuli, with uneven intervals (20 and 80 ms); frequency 10 Hz. (F) Two series consisting of three versus eight and five versus 12 stimuli in the trains (each nerve received 20-Hz trains every 11 s; total numbers per day are given in Materials and Methods and other details in Results). Dashed vertical lines in D and E help visualize the timing relationship between stimuli to the two nerves.
To investigate how the timing of activity per se influences synaptic competition between the soleus and aberrant motor terminals, we applied an equal number of stimuli per day to the nerves in two groups of animals. In one group, the action potentials in the two nerves fired synchronously; in the other group they fired asynchronously (Fig. 1 B–D and Materials and Methods). The stimulations lasted 7–10 d (mean, 8.2 d). A chronic conduction block always was used to suppress the spontaneous motoneuronal activity (Fig. 1). To estimate synaptic competition, we quantified the proportion of muscle fibers in the both-nerves territory that retained polyneuronal innervation from the two nerves (detected through their evoked endplate potentials, EPPs) relative to all innervated fibers in the same territory.
In the group in which the soleus and aberrant nerves fired asynchronously, we observed a dramatic, ∼threefold decrease in the proportion of polyinnervation in the both-nerves region compared with the group in which the competing nerves fired synchronously (Fig. 2A). These results demonstrate that synchronous firing among the competing inputs of the two nerves stabilizes polyneuronal innervation, whereas asynchronous firing among competing inputs promotes competition and synapse elimination. Fig. 2A shows pooled results from experiments with various degrees of asynchrony: in the experiment (four rats) in which asynchrony was highest the two nerves were stimulated regularly in an alternate pattern (Fig. 1C). In a pattern that still was asynchronous, albeit of lesser degree, the trains were delivered to the two nerves in phase but with a shift of the individual stimuli to one nerve relative to the other (Fig. 1D): In one subset of this experiment (three rats), the pulse frequency in the train was 10 Hz, and the shift was 50 ms; in another subset (five rats), the pulse frequency in the train was 20 Hz, and the shift was 25 ms.
Fig. 2.
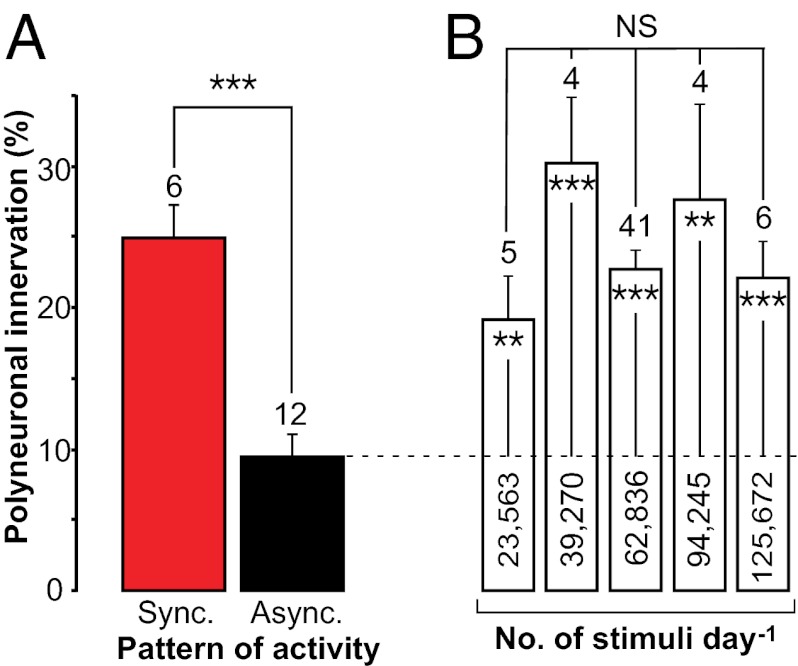
Equal amounts of synchronous and asynchronous spike activity have different effects on synapse elimination. (A) Effects of chronic electrical stimulation on level of polyneuronal innervation at NMJs reinnervated by soleus and aberrant axons (both-nerves fields) under the conditions shown in Fig. 1A. A synchronous paradigm (Sync) (six muscles; pattern in Fig. 1B) induces higher polyinnervation than three asynchronous paradigms pooled together (Async) (12 muscles: four with asynchronous trains; three with asynchronous stimuli, 50-ms interval; five with asynchronous stimuli, 25-ms interval; patterns shown in Fig. 1 C and D). Results are presented as mean ± SEM; the number of muscles is shown above the columns. The 144 fibers innervated from both nerves in the synchronous group correspond to 24.9 ± 2.35% of all innervated fibers of the both-nerves territory, whereas the 336 fibers in the asynchronous group correspond to only 9.4 ± 1.55%; ***P < 0.0005. (B) Persistence of high polyinnervation level with increasing amounts of activity evoked with chronic electrical stimulation (all synchronous paradigms). Number of stimuli per day is shown inside columns. For each level of imposed activity, data from soleus and aberrant same-nerve fields are pooled together; for the column of 62,836 stimuli, synchronous both-nerves fields are used also. P = NS for differences between columns [F(4,55) = 1.2, P = 0.323, ANOVA]; **P < 0.01 and ***P < 0.0005 for differences between each column and the asynchronously activated muscles shown in A (the mean of the latter is indicated by the dashed line across columns).
Some considerations are important in evaluating this result. First, although synchronous and asynchronous stimulation evoked the same number of action potentials in each nerve, the muscle fibers innervated by both nerves (both-nerves territory) received twice as many synaptic potentials with asynchronous stimulation as with synchronous stimulation. To determine whether the decrease in the proportion of polyinnervated fibers with asynchronous stimulation simply was a result of increased muscle activation, we stimulated the two nerves synchronously and increased the number of action potentials over a fourfold range (23,563–94,245 per day). There was no tendency for a decrease in polyinnervation with increased synchronous stimulation (Fig. 2B); the proportion remained significantly greater than that of the asynchronously activated myofibers (dashed line). Furthermore, even 125,672 spikes per day (Fig. 2B) transmitted to myofibers in the asynchronous stimuli/uneven intervals series (see Fig. 4A, column 3) (i.e., a number equal to those transmitted to the asynchronously activated myofibers of Fig. 2A) induce high levels of polyneuronal innervation. Therefore, we conclude that the reduction in polyinnervation with asynchronous stimulation is not a result of increased muscle activity.
Fig. 4.
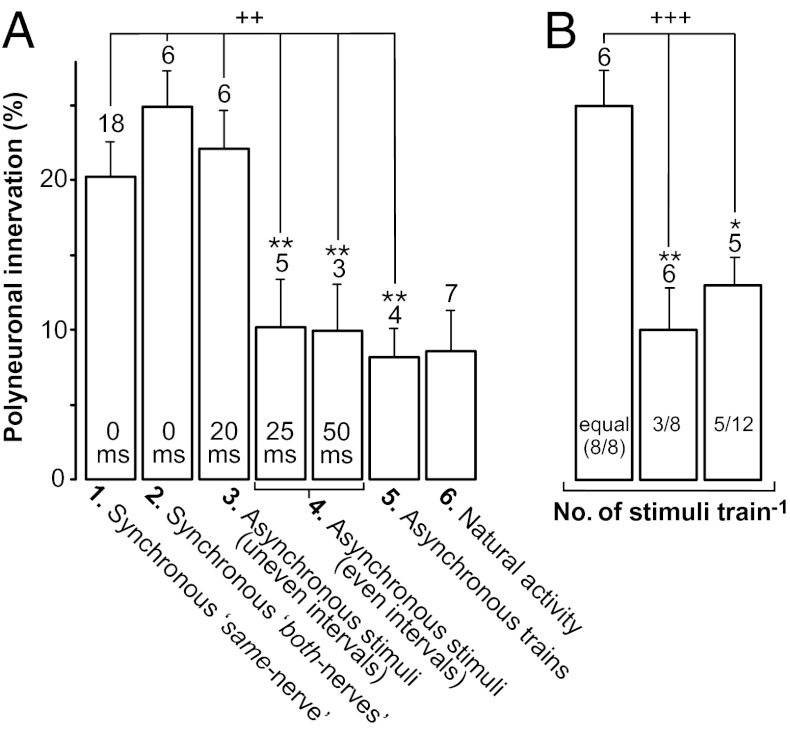
Specific paradigms of asynchronous spike activity and time window of effects of synchrony. (A) Polyinnervation levels of various muscle groups, in order (left to right) of increasing delay between spikes in the competing (aberrant versus soleus) inputs. Data reveal a time window (zero to 20–25 ms) within which the timing of these spikes is sensed as not competition-promoting, thus maintaining polyinnervation. Column 1: same-nerve fields of the asynchronously activated muscles of Fig. 2A (labeled 0 ms interval); Column 2: Both-nerves fields of synchronously activated muscles of Fig. 2A (also 0 ms); Column 3: both-nerves fields of the asynchronous/uneven interval group of 20–80 ms (labeled 20 ms, pattern in Fig. 1E), definable as synchronous-like according to its effects; Column 4: both-nerves fields of the asynchronous/even interval groups (two columns: 25 ms/20 Hz and 50 ms/10 Hz, pattern in Fig. 1D); Column 5: asynchronous trains (pattern in Fig. 1C); Column 6: natural activity. ++P < 0.002 for differences between indicated columns [F(5,36) = 4.9; P = 0.0016, ANOVA]. **P < 0.01 for the individual comparisons relative to the synchronous both-nerves group (column 2). Values of columns 1 and 3 are not significantly different from column 2. (B) Polyinnervation levels in both-nerves regions of two new series of muscles in which the soleus and aberrant nerve received different numbers of stimuli (three versus eight, and five versus 12, and vice-versa) in each train, the stimuli in common being synchronous (pattern in Fig. 1F). Data from soleus and aberrant fields with the same number of stimuli are pooled together. Comparison with the synchronously activated both-nerves fields of Fig. 2A, where the two nerves received an equal number of stimuli (eight/eight, for each train), shows that the different quantity of stimuli promotes competition. +++P < 0.0001 for differences between the indicated columns [F(2,18) = 17.7; P = 0.00006, ANOVA], *P < 0.05 and **P < 0.01 for the individual comparisons. Note that the same-nerve fields of this group with different numbers of stimuli, characterized by various amounts of imposed activity, are shown in Fig. 2B. In both A and B, number of muscles is given above columns. In B, for three vs. eight stimuli (3/8 column), three soleus and three aberrant nerves were the more stimulated nerve; for five vs. 12 stimuli, one soleus and four aberrant nerves were the more stimulated nerve. For fields of natural activity n = 7 instead of 9 shown in Fig. 3B (white circles), because here the cutoff of 12 innervated fibers is applied to the same-nerve fields (Materials and Methods).
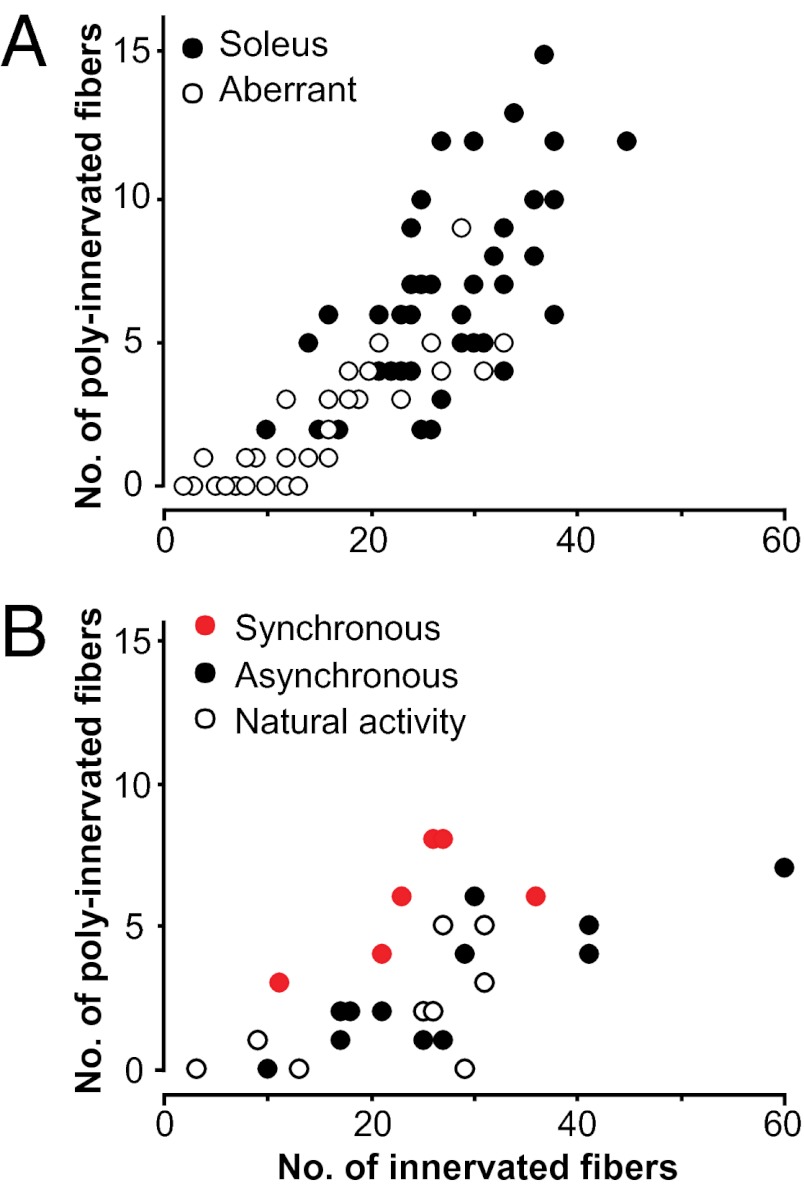
The fields of innervation of the two nerves are quite variable in size from muscle to muscle. Therefore, a second question is whether the size of the fields could influence the proportion of polyneuronal innervation. This question is relevant from two standpoints, one relative to the same-nerve fields and the other to both-nerves fields. With regard to the same-nerve fields, Fig. 3A plots the number of polyneuronally innervated fibers against the innervated fibers in each field (soleus or aberrant). They increase together linearly (r = 0.83; aberrant fields have fewer poly-innervated fibers than soleus fields, because of their smaller dimensions), the proportion remaining roughly stable at ∼1/4. Thus we can treat the same-nerve fields as one pool, as done in Fig. 4A, column 1, where they represent a crucial internal control (see Materials and Methods for further details). With regard to the both-nerves fields, Fig. 3B shows that there was no difference in size between synchronously (red circles) and asynchronously (black circles) activated fields, thus eliminating the possibility of a bias that would have occurred had the asynchronously stimulated preparations contained a significantly lower number of innervated fibers.
Fig. 3.
Sizes of fields of innervation of soleus and aberrant nerves. (A) Plot of the number of polyneuronally innervated fibers versus the total number of innervated fibers per muscle (same-nerve fields) shows positive correlation for both soleus and aberrant axons (r = 0.83). (B) The same plot for synchronous and asynchronous both-nerves fields (data already shown in Fig. 2A). The difference in the number of innervated fibers in the two groups was not significant (P = 0.53): asynchronous, mean 28 ± 4.0, range 10–60; synchronous, mean 24 ± 3.4, range 11–36. Also displayed in B are nine regions [three both-nerves and six same-nerve (three aberrant nerves and three soleus nerves)] from three control muscles in which no block or stimulation was applied, with the reinnervating axons maintaining their natural activity.
Time Window of 20 ms Defines the Effects of Synchrony.
The conclusion that asynchronous but not synchronous activity promotes competition is strengthened further by our results from the same-nerve territories. In the muscles of Fig. 2A in which the both-nerves zone was activated asynchronously by the soleus and aberrant nerves and had a low amount of polyinnervation (black column), the same-nerve regions served as valuable internal controls because they had been activated synchronously. These regions, which we refer to as “synchronous same-nerve regions” (Fig. 4A, column 1) had a significantly higher level of polyinnervation (20.17 ± 2.08%, n = 18) than did the both-nerves region and were not significantly different from the same-nerve fields of the synchronously activated muscles of Fig. 2A (25.84 ± 2.8%, n = 10; P = 0.11; not shown). These same-nerve data also are in agreement with our previous findings with electrical stimulation of a single nerve reinnervating the soleus muscle, where either a foreign nerve reinnervated an ectopic region (19) or the original one reinnervated the old endplates (28), all being synchronous paradigms.
Fig. 4A also displays three separate groups receiving asynchronous paradigms (these groups already have been presented and pooled together in Fig. 2A): column 4, asynchronous stimuli/even intervals (subdivided into two columns: 25 ms and 20 Hz and 50 ms and 10 Hz, i.e., the pattern in Fig. 1D) and column 5, asynchronous trains (the pattern in Fig. 1C). Although the number in each of these groups is much smaller, their level of polyneuronal innervation remains significantly lower than that of the synchronous groups (columns 1 and 2).
To explore further the time window of synchrony, we also tested a 20- to 80-ms paradigm (Fig. 4A, column 3) of asynchronous stimuli/uneven intervals, 10 Hz (i.e, the pattern in Fig. 1E; 8–10 d duration, mean 8.5 d). This paradigm resulted in high polyneuronal innervation, a level comparable with the synchronous both-nerves group of column 2, and thus also can be defined as a synchronous-like paradigm. Finally, Fig. 4A, column 6 shows the low level of polyneuronal innervation in three muscles (in three separate rats) in which no block or stimulation was applied; in these muscles the reinnervating motor axons maintained their endogenous activity (n = 7, resulting from three both-nerves and four same-nerve zones pooled together).
Different Numbers of Stimuli to Soleus and Aberrant Nerves Activate Competition.
Thus far identical numbers of stimuli were delivered to each of the two competing nerves. Next, we asked whether competition would be affected if we evoked different numbers of action potentials in the two nerves, a condition that occurs physiologically during development when synapse elimination is underway. In one group we stimulated one nerve with three stimuli per train and the other nerve with eight stimuli per train (the first pulses being synchronous; Fig. 1F); this pattern was delivered for 9 d on average (range 8–10 d). In another group, one nerve was stimulated with five stimuli per train, and the other nerve was stimulated with 12 stimuli per train, for 13 d (range 12–14 d). Both groups were composed of two subsets of three to four rats in which one nerve or the other received more stimuli (other parameters were the same as in previous paradigms). We found that the proportion of polyinnervation in the both-nerves zone of the 3/8 and 5/12 groups, was significantly lower than the proportion of polyinnervation in the both-nerves zone of muscles with equal number of synchronous stimuli (Fig. 4B). This demonstrates that different numbers of action potentials in the two nerves promote competition. Moreover, in a subset of muscle fibers in which polyneuronal innervation was still present, the EPP amplitude of the more activated competitor was statistically larger than that evoked by the less activated one (21 of 30 pairs in the 5/12 series; P = 0.028, χ2 test; four of the aberrant nerves and one soleus nerve was the more stimulated nerves. In the 3/8 series, the difference was not significant).
Nerve Terminals Compete for the Same Synaptic Space.
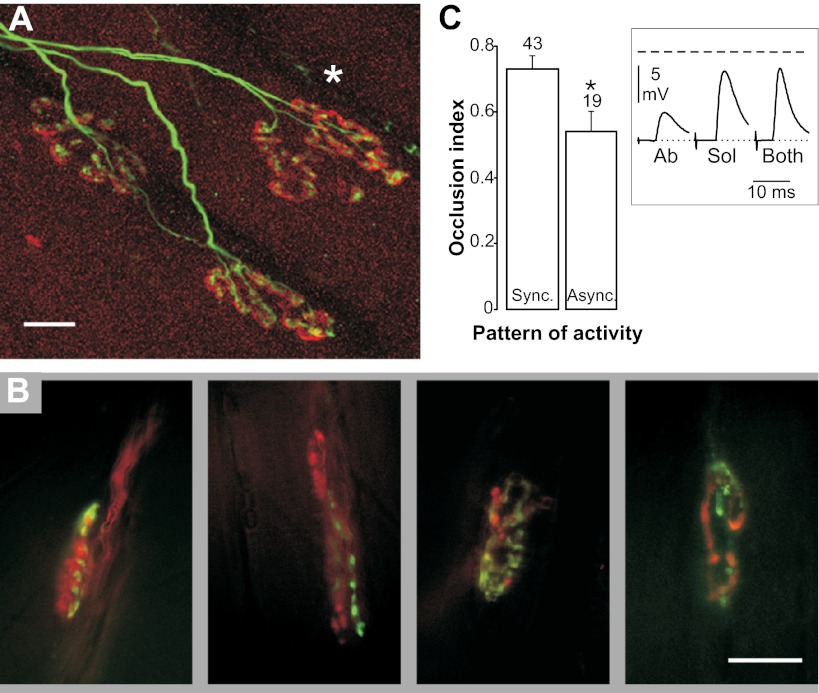
To demonstrate that the reinnervating soleus and aberrant nerve terminals are indeed competing for the same synaptic space, as they do during development, we visualized terminals in two ways, by confocal microscopy and activity-dependent staining with styryl dyes (FM1-43 and FM4-64). In confocal images nerve endings always were seen to converge on the same acetylcholine receptor (AChR) aggregate (Fig. 5A; 206 NMJs, three muscles). Activity-dependent staining with styryl dyes revealed terminals of the two nerves converging on the same endplate, each occupying multiple, intermingled small regions (Fig. 5B; four examples selected from several similar NMJs observed in three muscles). This interdigitation of sites occupied by the two nerves is similar to that observed during development (37) and after reinnervation in the adult (34). Furthermore, using electrophysiology, we showed that the EPPs evoked from the two nerves had the same time course (rise+half relaxation time: 3.9 ± 0.17 ms in the soleus nerve and 3.9 ± 0.18 ms in the aberrant nerve; n = 51 pairs).
Fig. 5.
Convergent polyneuronal innervation, interdigitation between soleus and aberrant axon terminals, and EPP occlusion. (A) Confocal image of dually innervated endplates [upper synapse (*) and also, by terminal sprouting, the lower one to the right]; soleus and aberrant axons stimulated synchronously for 10 d. (B) Examples of interdigitating terminals of aberrant (green) and soleus (red) reinnervating axons detected by fluorescence microscopy after activity-dependent uptake of styryl dyes (11 d of synchronous stimulation). (C) Inset: example of EPPs occlusion recorded at a reinnervated endplate, evoked by single-shock stimulation of the soleus nerve (Sol), the aberrant nerve (Ab), or both. The dashed line indicates the nonlinear summation (NLS) level (Materials and Methods). The voltage difference between the NLS expected peak amplitude and the measured amplitude of combined EPPs gives an estimate of the amount of occlusion, formalized as the occlusion index [the ratio between the above indicated difference and the maximum possible difference (the latter being the NLS expected peak amplitude minus the larger EPP amplitude)]. (C) The mean level of the occlusion index for synchronous (Sync) and asynchronous (Async) data indicates a significantly higher occlusion in the synchronous group (*P = 0.0116). The number of endplates is stated above columns. (Scale bars: 10 μm.)
The observed convergence and interdigitation prompted us to investigate the effects of simultaneous stimulation of soleus and aberrant nerves on EPP amplitudes in the final acute experiment. Fig. 5C, Inset shows that the two EPPs occluded one another, as reported for polyneuronal innervation of developing (38) and reinnervated adult (34) mammalian NMJs. Furthermore, it is known that occlusion is associated with intimate contact (38) and intermingling (34) between terminals, although the underlying mechanism remains speculative. Interestingly, the occlusion of the EPPs in the asynchronously activated both-nerves fields was significantly smaller than that in the synchronously activated fields (Fig. 5C), a result consistent with a segregating action of asynchronous activity. Such segregation has been shown to be the step preceding synapse elimination (37).
Discussion
Several studies have investigated how nerve impulse activity affects competition among multiple inputs converging on the same target. Here we demonstrate that the outcome depends critically upon the firing pattern in the competing axons. We show that asynchronous firing enhances competition, leading to synapse elimination and mononeuronal innervation, whereas synchronous firing diminishes competition and prolongs polyneuronal innervation. The effect is robust when the synchronous group is compared with groups that received different types of asynchronous stimulation (Fig. 4A). Importantly, enhanced competition, as measured by low polyneuronal innervation, occurs with both asynchronous trains and synchronous trains with asynchronous stimuli, the latter simulating a physiological pattern of activity that occurs late in development and in adults (30).
Our work on the NMJ may have general applicability to competition observed elsewhere in the nervous system. For example, our paradigms of asynchronous trains and asynchronous stimuli reproduce conditions known to promote competition between visual cortical connections, such as alternate eye closure and strabismus, respectively (1). Furthermore, our NMJ results are consistent with those of Stryker and Strickland (39) who also used asynchronous stimulation to investigate visual cortical connections.
In many studies on competition, researchers have focused on the amount of activity by investigating how active inputs compete against completely inactivated ones. Relevantly, we show that imposing an equal amount of activity, i.e., the same number of stimuli per day, results in dramatically different levels of polyneuronal innervation depending on whether activity is synchronous or asynchronous; this result indicates the importance of timing. In fact, differences in the amount of activity (see Fig. 4B) can be seen as a special case of asynchrony, because if the overall number of action potentials differs between competitors, then some spikes in the more active input must occur at a time when the less active input is silent, even if some of the spikes coincide in time. Our experiments went further by testing different intervals and revealed that the competition is activated or clearly accelerated only when the interval of asynchrony is longer than ∼20 ms (Fig. 4A). Given two (or more) competing inputs on a common target that fire repetitively during polyneuronal innervation in adult reinnervated muscles, some competition is activated every time a spike invades one motor terminal while the other is electrically silent (a single event), with advantage for one or the other terminal. Clearly this event reverses repeatedly during repetitive asynchronous firing, but the situation is one of instability, as revealed by the specific effects of asynchronous stimulation.
In the single event described above, our findings using asynchronous stimulation in theory would be equally compatible with an advantage obtained by the active or by the inactive competitor (Hebbian and non-Hebbian mechanisms, respectively). Most studies investigating competition under different amounts of activity (generally, active vs. totally inactive inputs) indicate that active terminals outcompete inactive terminals. These studies include several in which one of two inputs reinnervating adult rat lumbrical muscle fibers was electrically silenced by tetrodotoxin (TTX); in such cases, the winner in synapse elimination is the active input (24, 40, 41). Similarly, a study using a genetic approach to disrupt the gene for choline acetyltransferase in a select number of motor neurons showed conclusively that inactive terminals are eliminated at developing NMJs (18). In addition, experiments using electrical stimulation in newborn mice showed that more active terminals outcompete less active ones (25). Furthermore, partial blocking of the adult NMJ with α-bungarotoxin (αBTx) also demonstrates an advantage in favor of the active part of a synapse (16). Similarly in agreement, although on a much faster time scale, are results in Xenopus nerve-muscle cocultures indicating that tetanic stimulation of one of two neurons innervating the same myofiber produces an immediate functional suppression of the nonactivated NMJ (42). A recent study in rodent extraocular muscles also supports activity, indicating a marked delay in synapse elimination in the levator palpebrae in accord with its delayed onset of activity (43). Finally, in our study we also tested greater amounts of stimulation to one nerve than to the other and found that the more active terminals outcompete the less active ones.
To our knowledge, there is only one study indicating that inactive inputs at the NMJ prevail in competition (20). This study, which used a TTX conduction block of a subset of soleus motor axons in newborn rabbits, based its conclusions on the amplitudes of motor unit twitch contractions; unfortunately, the study did not include any direct electrophysiological and/or morphological measurements. The indirect measure of competition used raised doubts about possible complex effects of chronic inactivity (24, 44) that were resolved only partly in a following paper (45). Theoretical justifications for both outcomes have been suggested: That there is an early advantage for the more active inputs and a late advantage for the less active inputs (17, 46). However, it must be stressed that Buffelli et al. (18) conclusively demonstrated that in newborn mice the inactivated terminals are the ones eliminated. All in all, it seems safe to conclude that activity is the winning feature for competing NMJ inputs.
Further Considerations on the Role of the Timing of Spike Activity in Competing Inputs.
The effects of our synchronous/asynchronous paradigms on synapse elimination conform to the Hebbian postulate of synaptic plasticity, in that inputs that fire coordinately with postsynaptic cells are consolidated (6), and those that do not are eliminated (3). In particular, the protective effect of synchrony on convergent soleus and aberrant inputs is Hebbian in character. This result is clear-cut, but it appears to be at odds with a study demonstrating that electrical stimulation of motor nerves in newborn rats speeds up elimination (26). We addressed this apparent discrepancy in a previous study on elimination at ectopic or original synapses (19, 28). Briefly, this paradox has to do with the distinction between postsynaptic activity per se and the differential activity of presynaptic inputs.
In general, overall postsynaptic activity influences muscle innervation mainly during embryonic life, somewhat earlier than the period of synapse elimination. Muscle fibers release chemical signals, such as neurotrophins, that have a permissive role in motor neuron survival and a stimulating effect on axonal growth and sprouting (47–55). Thus, the target-produced signal calls for innervation, but once innervation is achieved, the signal is down-regulated in response to the evoked activity (26, 47, 50, 54, 56, 57). The same signal is up-regulated again in adult muscle by paralysis or partial denervation, inducing nerve sprouting and polyneuronal innervation (19, 58, 59). In all likelihood, this up-regulation is why paralysis prolongs polyneuronal innervation during postnatal development (60–62). According to the above scenario, increasing postsynaptic activity by electrical stimulation in newborn animals down-regulates the muscle signal and speeds up synapse elimination (23, 26, 63). On the other hand, in our preparations asynchronous spontaneous activity of motor neurons is blocked by TTX in the sciatic nerve, and this blocking unmasks the purely stabilizing effect on polyneuronal inputs of synchronous activity by distal electrical stimulation.
In our comparison between synchronous and asynchronous patterns, the soleus muscle is fully active, thus removing the confounding influence of the sprouting signal from muscle. Also, the concept and the effects of synchronous activity as an Hebbian paradigm, as shown by us here and previously (19, 28), cannot be considered equivalent to that of synchronous inactivity [complete paralysis, a paradigm not used here, powerfully stimulates polyneuronal innervation (19, 28, 62)], because in the latter case it is the up-regulation of the muscle sprouting signal that comes into play.
Of particular interest is our result identifying a critical interval between impulses in the competing axons: At an interval shorter than 20 ms, impulses are perceived as synchronous, and no competition occurs, whereas at intervals ≥25 ms impulses are perceived as asynchronous, and competition is activated. This value agrees well with those found in several models of activity-dependent synaptic plasticity in the CNS (8). In addition, it corresponds to the width of the peak of cross-correlation (5–25 ms) between the spikes of different motor units (soleus and tibialis anterior) recorded in rats immediately after birth (30). This synchrony also is seen in vitro in the mouse spinal cord at P1 (64) but switches to the adult desynchronized pattern shortly afterwards (30). Because this change in firing pattern precedes the bulk of synapse elimination, it strengthens the present conclusion that the transition from synchronous to asynchronous firing at competing terminals plays an important role in the physiology of polyneuronal innervation and synapse elimination. Moreover, reduced gap junctional coupling causes desynchronization and synapse elimination (31).
Our results further demonstrate that having a different number of spikes in competing inputs promotes synapse elimination, with inputs carrying fewer spikes being eliminated (Fig. 4B). This effect is well explained by the same mechanism of asynchrony. A related question is whether a terminal that systematically receives spikes that precede those of its competitor during repetitive spike trains also would be granted a competitive advantage, even if the two terminals receive an equal number of spikes. Exploring the time course in detail and using asynchronous patterns with uneven intervals should provide answers to this question and further elaborate the role of STDP in synapse elimination. In visual cortical slices, the timing of the first spike in each burst (pre- versus postsynaptic) dominates in synaptic plasticity (strengthening or weakening) (65), a phenomenon that also could apply to the developmental case of competing inputs firing asynchronously at NMJs and in CNS.
By showing the importance of the temporal aspect of firing, our results further elucidate the role of activity during synapse competition in development. For example, when two identified neurons compete at several myofibers, the same neuron wins at all NMJs (66). The instructive role of activity provides a simple explanation for this striking finding, because the activity pattern in a given neuron is the same at all its terminals.
Other Possible Factors Affecting Synapse Competition.
Our conclusions do not preclude the possibility that, in addition to the timing of activity, other factors contribute to synapse elimination, such as a decrease in synaptic strength or activity-independent factors. With respect to synaptic strength, it has been shown that terminals undergoing elimination exhibit a reduction of synaptic efficacy (18, 67); this reduction is certainly a necessary step along the process of elimination but also might be its cause, a distinction that should be investigated further. Regarding activity-independent factors, partial-denervation experiments revealed an intrinsic tendency of motor terminals to withdraw during postnatal development, indicating that motor neurons cannot permanently maintain the initial large size of their innervation fields (68, 69). This substantial withdrawal in rat and mouse soleus muscles is noncompetitive in nature and is separate from the competitive process of synapse elimination.
Activity-independent synapse elimination has been reported in doubly innervated rat IV deep lumbrical muscle (21, 34, 70). In the most recent study, after crushing of the sural nerve (SN), denervated fibers were reinnervated quickly by sprouting of the intact lateral plantar nerve (LPN). As the SN grew back to muscle fibers, a TTX sciatic nerve block was applied, making both the LPN and the SN inactive. When the SN reached the targets, it competed successfully with a number of LPN terminals, demonstrating that synapse elimination occurred in the absence of action potentials. This model suggests an activity-independent mechanism of competition. However, a more likely interpretation is that the already expanded LPN motor units become strongly stimulated to expand further in response to sprouting factors released by the entirely paralyzed muscle (58). Irrespective of how many of these sprouts succeed in forming new synapses, the LPN motor units reach their limits of arborization and withdraw from some of the muscle fibers; the SN motor units, on the other hand, did not need to withdraw and only expand. This process is another example of the limited capacity of neurons to maintain an enlarged innervation territory permanently (68, 69).
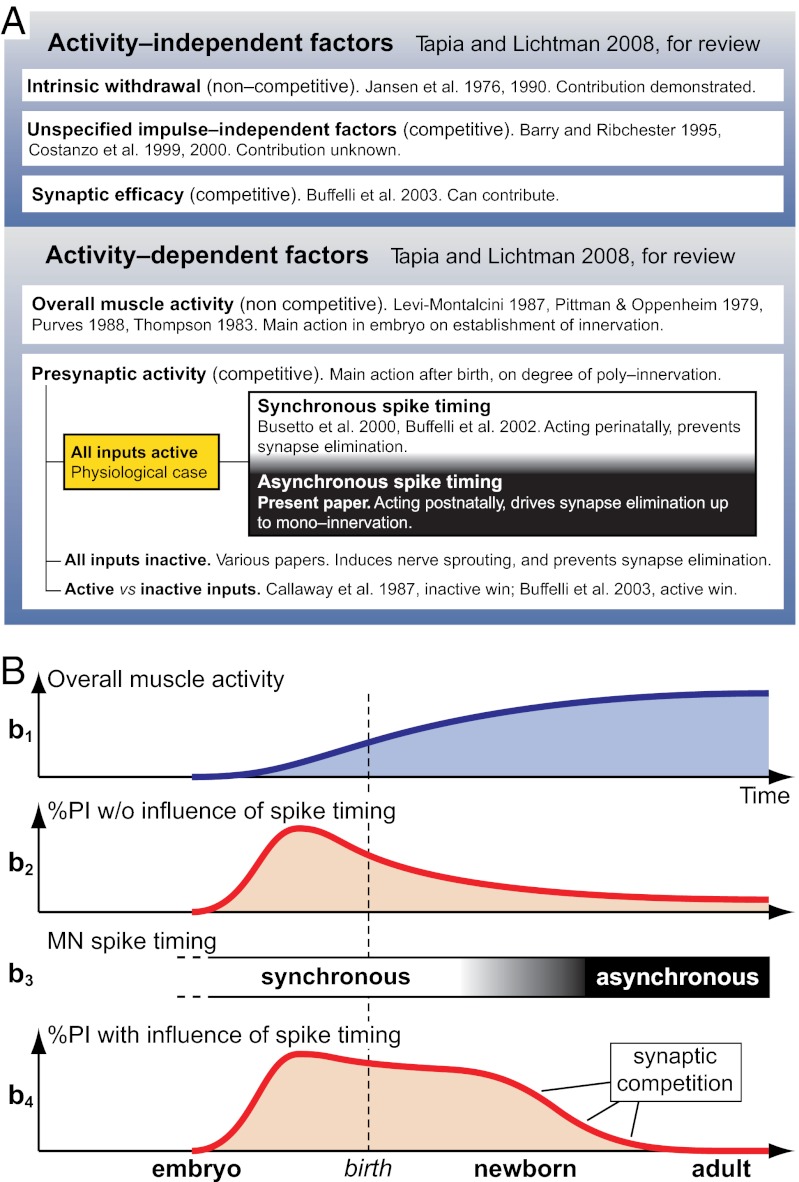
All the factors discussed above are synthesized graphically in Fig. 6. Fig. 6A is a schematic showing activity-independent and activity-dependent factors with emphasis on the physiological role of presynaptic spike-timing. Fig. 6B graphically focuses on the two activity-dependent mechanisms: (i) the overall muscle activity, which is important in the early part of the establishment of neuromuscular innervations, and (ii) the presynaptic spike-timing mechanism, which is essential for synapse competition.
Fig. 6.
Factors involved in synapse elimination. (A) Factors known or proposed in the literature to affect synapse elimination, and their relationship with present results on the role of spike-timing (the complete list of citations is found in the Discussion). (B) Relationship between the two main activity-dependent mechanisms (overall activity and spike timing) affecting the entire developmental time course of neuromuscular innervation. b1, overall muscle activity. b2, influence of overall activity alone on the level of polyneuronal innervation (PI). Zero value on ordinate indicates monoinnervation; zero value on abscissa indicates onset of embryonic development. b3, relative timing of spike activity between competing inputs, with physiological transition from synchrony to asynchrony. b4, combined influence of overall activity and spike timing on PI level. MN, motoneuron.
To conclude, various factors can contribute to the withdrawal of nerve terminals from muscle and to synapse elimination. However, only an activity-dependent mechanism based on timing, as shown here, seems to assure that at the end of the competitive process all polyneuronal innervation is eliminated and a single motor terminal invariably remains on each myofiber, a physiologically essential feature of adult NMJs and motor units.
Materials and Methods
Animals, Surgery, and Chronic Experiments.
All procedures for animal handling were authorized by the Istituto Superiore di Sanita’ and the Ministry of Health of Italy. In the AO strain of rats (200–400 g) (Harlan) the soleus muscles frequently are innervated by two nerves, the soleus (a branch of the lateral gastrocnemius) and the aberrant, coming from the plantar nerve and forming NMJs in the same central region as the soleus axons; adult myofibers, as in normal strains, are innervated by only one axon, either soleus or aberrant (Fig. 1A) (69). The soleus nerve has a greater participation in muscle innervation, because it has more motor units; however, they are comparable in size to those of the aberrant nerve (69). During reinnervation after crushing, polyneuronal innervation develops transiently, recapitulating development. This innervation occurs from axons of the same nerve [as in singly innervated adult muscles after crushing (33)] and also from both nerves, as demonstrated here, allowing independent stimulation of the competing inputs during synapse elimination. A centrally placed block of nerve conduction (Fig. 1A) prevents arrival of the spontaneous asynchronous patterns of adult motor neurons at the competing terminals (30, 71, 72); other effects of the block are described below. The block is created by perfusing the sciatic nerve with TTX (7.8–8.4 μg per day) using a 2ML4 osmotic pump (Alzet, Durect) placed in the animal (73).
Under Equithesin anesthesia (9.7 mg/mL sodium pentobarbital, 42.5 mg/mL chloral hydrate, administered i.p. at 0.2–0.4 mL/100 g body weight ) the two nerves are crushed on one side at a distance ∼3 mm from the muscle entry point. Reinnervation starts 2–3 d later and proceeds rapidly thereafter, being completed by 2.5–3 wk after crushing (74), the time of the final acute experiment. In the same operation, chronic stimulating cuffs are placed ipsilaterally around the lateral gastrocnemius nerve (containing the soleus axons) and the plantar nerve (containing the aberrant axons), several millimeters central to the crush (Fig. 1A). The cuffs are custom-made short silicone tubings (i.d. 0.6/o.d. 1.1 mm, length 2.6 mm) (MED-6382; NuSil Technology), inside which the bare ends of two seven-stranded insulated stainless steel wires (the anode and cathode, 1.5 mm apart) (AISI316; Advent) protrude slightly. The wires are led under the skin to the animal’s back and are left there while the TTX perfusion system is put in place. After recovery from anesthesia, the rats are allowed to move freely in their cages while soleus muscle reinnervation occurs in the presence of paralysis that initially enhances polyneuronal innervation and intermingling of the soleus and aberrant territories in the muscle, before stimulation begins. At day 9, the rats are anesthetized again briefly, and the wires coming from the electrodes are connected to an external stimulator (Master 8; A.M.P.I.) through a tether and an electrical swivel (Chatam). Stimuli are supramaximal for motor axons: First the threshold (Th) voltage for eliciting contractile responses is determined, and then a voltage of 3× Th is used for chronic stimulation (19). The appropriate responses are foot extension for soleus axons (stimulated nerve: lateral gastrocnemius) and plantar flexion of the digits for aberrant axons (stimulated nerve: plantar). These contractions are used throughout the chronic in vivo experiment as monitors of the correct functioning of the stimulating electrodes and can be evoked since the initial operation, because the crush is applied only to the soleus and the aberrant nerves. The chronic stimulation starts at day 9, when reinnervation of the soleus muscle by the two nerves is well developed, and continues for the following 7–14 d. During this period the correct supramaximal level of the stimuli is checked two or three times per day using the 3× Th criterion: Th values remained very stable, within ± 2% of the initial one. In some rats, however, one response or the other disappeared because of mechanical damage, leading to termination of the experiment. Stimulus diffusion from one nerve to the other never occurred at 3× Th strength and appeared only at 6–10× Th or not at all within the end-scale voltage of the stimulator.
Criteria Used for Choosing Chronic Stimulation Patterns.
In theory, testing temporal aspects of firing involves varying the intervals between stimuli to competing axons, frequencies inside trains, overall number of stimuli per day, and combinations of these factors. Because several weeks were required to test each pattern in vivo in a given animal, we concentrated on patterns essential for answering the basic questions: (i) whether asynchrony and synchrony have differing effects on polyneuronal innervation, and (ii) if so, the order of magnitude of the temporal window thus emerging (seconds, hundreds, or tens of milliseconds). Another constraint was not to exceed the frequency of 20 Hz inside trains; this frequency is physiological for soleus motor neurons.
In all except one of the chronic stimulation protocols identical patterns and number of stimuli were applied to the two nerves: 100-μs pulses in eight-pulse (10–20 Hz) trains every 11 s, for 62,836 pulses per day; however, the relative pattern of stimuli was either synchronous or asynchronous, as outlined in Fig. 1 B–E. Only one group received different numbers of stimuli in the trains to the two nerves (Fig. 1F): either three versus eight or five versus 12, the first stimuli being synchronous and the frequency in each train 20 Hz; all other parameters were the same as in the other patterns. The number of stimuli per day for this unequal-number group was 23,563 for three-stimuli trains, 39,270 for five-stimuli trains, and 94,245 for 12-stimuli trains.
Electrophysiology and Mapping of the Muscle.
Of the ∼250 rats operated on initially, 145 could be used in the chronic stimulation experiment; rats with aberrant nerves that were too thin or bilaterally absent were discarded. Only 48 rats completed the chronic in vivo period of 15–22 d described above, most failures being caused by wire disconnections or mechanical damage.
After 7–14 d of chronic stimulation (or 8–11 d in nonstimulated controls, during which natural motoneuronal activity resumed), soleus muscles were isolated in vitro with both nerves and were perfused with oxygenated physiological solution (19, 28). Intracellular recordings were made with 3M KCl pipettes from first-layer fibers, from edge to edge over the entire surface of nerve’s entrance, following the central endplate band. Mono- and/or polyneuronal innervation from each nerve and from both nerves was determined by graded intensities of stimulation. Large-amplitude EPPs were recorded under 2–4 μM μ-conotoxin (Peptide Institute), a selective blocker of muscle voltage-dependent Na+ channels (75). Occasionally, small concentrations of curare were added because of residual μ-conotoxin resistance after the initial denervation (76). In mapping the muscle, one first meets a region where the fibers are innervated only by the aberrant nerve, mono- or polyneuronally. Then one encounters a region that is innervated by both nerves; some fibers are innervated by one nerve, some by the other (again mono- or polyneuronally), and some fibers by both nerves. Finally, one enters a region innervated only by the soleus nerve. We distinguished three fields, identified as both-nerves, aberrant same-nerve, and soleus same-nerve. The same-nerve field comprises the entire population of myofibers innervated by each nerve; the both-nerves field is their region of overlap. We considered both-nerves to be a field with a good intermingling of the two axonal types and characterized it as one in which each fiber innervated by one nerve is flanked on either side by no more than three fibers innervated by the other nerve. In more than half the muscles there was only one such region; in a minority of muscles there were two or, rarely, three. As regards the share of muscle innervation, of 1,534 fibers reinnervated in 39 muscles, 64% were from the soleus nerve, and 36% were from the aberrant nerve. A small number of the same-nerve fields (essentially aberrant nerves) had no polyinnervated fibers, and this occurrence coincided with small fields of innervation (Fig. 3A). Because the absence of polyinnervated fibers was a consequence of insufficient reinnervation, we applied a cutoff of 12 reinnervated fibers to these fields; thus we included in the same-nerve fields of Fig. 4A (column 1) those with ≥13 reinnervated fibers. Finally, the comparability of same-nerve and both-nerves regions across different groups of data was good, because they had a similar number of fibers in the three main groups of our results: high polyneuronal innervation (synchronous and synchronous-like, 12 muscles), low polyneuronal innervation (asynchronous, 12 muscles) and different quantity (11 muscles) (see Results). For example, the both-nerves regions had the following values, in the three groups, respectively: 29 ± 2.4 fibers per muscle (mean ± SEM), 28 ± 3.9, and 33 ± 4.0; difference not significant.
To estimate the amount of occlusion of simultaneously evoked soleus and aberrant EPPs (see Results and Fig. 5C, Inset), we calculated the level of nonlinear summation according to McLachlan and Martin (77). We then determined the occlusion index from many endplates (Fig. 5C) and, as expected, found values ranging from 0 to 1. However, we also found a few values exceeding these limits because of uncontrolled fluctuations in the resting membrane potential; we reduced 10 values to 1 and increased two values to 0 (from a total of 62 values).
Morphology.
In some muscles NMJs were visualized with confocal microscopy (Zeiss LSM 510). AChRs were stained with Alexa 594-conjugated α-BTx (red); axons were stained with anti-neurofilament and anti-SV2 Alexa 488 antibodies (green) (28). Other muscles were studied with fluorescence microscopy after double vital staining of endplates for soleus (red) and aberrant endings (green) with activity-dependent uptake of FM4-64 and FM1-43 aminostyryl dyes (Molecular Probes), respectively, as described (70, 78). Soleus muscles with their two nerves were placed in a small chamber with oxygenated physiological solution and incubated with 5 μM FM1-43 and curare (10−5 g/mL). The aberrant nerve was stimulated for 10 min with 100-μs supramaximal pulses in 360-pulse (20 Hz) trains every 20 s. After washing for 30 min, the procedure was repeated while the soleus nerve was stimulated under 5 μM FM4-64 and curare. Following a final 30 min washing, fresh muscles were mounted in a Sylgard-coated (Dow-Corning) Petri dish under oxygenated physiological solution for imaging (BX51WI; Olympus). Images were acquired with a Retiga Exi digital camera (Q-Imaging) and processed with Photoshop.
Statistics.
Data are expressed as mean ± SEM. P < 0.05 was considered significant (two-tail unpaired t test, one-way ANOVA, and χ2 test).
Note Added in Proof.
During revision of this ms., an article appeared in the February, 2012 issue of Nature Neuroscience [Zhang et al. (2012) Visual map development depends on the temporal pattern of binocular activity in mice. 15:298–307] presenting results similar to ours in the visual system, including the finding of a time window of a few tens of ms for the effects of synchrony. This speaks for a developmental mechanism highly conserved across the nervous system.
Acknowledgments
We thank Prof. Linda Cooper of McGill University for comments on earlier drafts of our manuscript, Mr. Marco Veronese for technical assistance in preparing the figures, and Dr. Lorenzo Cangiano for help in analyzing occlusion data and in planning Fig. 6. This work was supported by a grant from the Italian Ministry of Education, Universities and Research (to A.C.) and by Research Projects of National Interest 2006 Grant 2006055835, Italy (to G.B.).
Footnotes
The authors declare no conflict of interest.
See Author Summary on page 9690 (volume 109, number 25).
*This Direct Submission article had a prearranged editor.
References
- 1.Hubel DH, Wiesel TN. Binocular interaction in striate cortex of kittens reared with artificial squint. J Neurophysiol. 1965;28:1041–1059. doi: 10.1152/jn.1965.28.6.1041. [DOI] [PubMed] [Google Scholar]
- 2.Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science. 1996;274:1133–1138. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- 3.Stent GS. A physiological mechanism for Hebb’s postulate of learning. Proc Natl Acad Sci USA. 1973;70:997–1001. doi: 10.1073/pnas.70.4.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bliss TV, Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol. 1973;232:331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malenka RC, Bear MF. LTP and LTD: An embarrassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 6.Hebb D. The Organization of Behavior. New York: Wiley; 1949. [Google Scholar]
- 7.Bi GQ, Poo MM. Synaptic modifications in cultured hippocampal neurons: Dependence on spike timing, synaptic strength, and postsynaptic cell type. J Neurosci. 1998;18:10464–10472. doi: 10.1523/JNEUROSCI.18-24-10464.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dan Y, Poo MM. Spike timing-dependent plasticity: From synapse to perception. Physiol Rev. 2006;86:1033–1048. doi: 10.1152/physrev.00030.2005. [DOI] [PubMed] [Google Scholar]
- 9.Magee JC, Johnston D. A synaptically controlled, associative signal for Hebbian plasticity in hippocampal neurons. Science. 1997;275:209–213. doi: 10.1126/science.275.5297.209. [DOI] [PubMed] [Google Scholar]
- 10.Markram H, Lübke J, Frotscher M, Sakmann B. Regulation of synaptic efficacy by coincidence of postsynaptic APs and EPSPs. Science. 1997;275:213–215. doi: 10.1126/science.275.5297.213. [DOI] [PubMed] [Google Scholar]
- 11.Bennett MR, Pettigrew AG. The formation of synapses in striated muscle during development. J Physiol. 1974;241:515–545. doi: 10.1113/jphysiol.1974.sp010670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown MC, Jansen JKS, Van Essen DC. Polyneuronal innervation of skeletal muscle in new-born rats and its elimination during maturation. J Physiol. 1976;261:387–422. doi: 10.1113/jphysiol.1976.sp011565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Redfern PA. Neuromuscular transmission in new-born rats. J Physiol. 1970;209:701–709. doi: 10.1113/jphysiol.1970.sp009187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tapia JC, Lichtman JW. Synapse elimination. In: Squire LR, et al., editors. Fundamental Neuroscience. 3rd Ed. New York: Elsevier; 2008. pp. 469–490. [Google Scholar]
- 15.Mariani J, Changeux JP. Ontogenesis of olivocerebellar relationships. II. Spontaneous activity of inferior olivary neurons and climbing fiber mediated activity of cerebellar Purkinje cells in developing rats. J Neurosci. 1981;1:703–709. doi: 10.1523/JNEUROSCI.01-07-00703.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Balice-Gordon RJ, Lichtman JW. Long-term synapse loss induced by focal blockade of postsynaptic receptors. Nature. 1994;372:519–524. doi: 10.1038/372519a0. [DOI] [PubMed] [Google Scholar]
- 17.Barber MJ, Lichtman JW. Activity-driven synapse elimination leads paradoxically to domination by inactive neurons. J Neurosci. 1999;19:9975–9985. doi: 10.1523/JNEUROSCI.19-22-09975.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buffelli M, et al. Genetic evidence that relative synaptic efficacy biases the outcome of synaptic competition. Nature. 2003;424:430–434. doi: 10.1038/nature01844. [DOI] [PubMed] [Google Scholar]
- 19.Busetto G, Buffelli M, Tognana E, Bellico F, Cangiano A. Hebbian mechanisms revealed by electrical stimulation at developing rat neuromuscular junctions. J Neurosci. 2000;20:685–695. doi: 10.1523/JNEUROSCI.20-02-00685.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Callaway EM, Soha JM, Van Essen DC. Competition favouring inactive over active motor neurons during synapse elimination. Nature. 1987;328:422–426. doi: 10.1038/328422a0. [DOI] [PubMed] [Google Scholar]
- 21.Costanzo EM, Barry JA, Ribchester RR. Competition at silent synapses in reinnervated skeletal muscle. Nat Neurosci. 2000;3:694–700. doi: 10.1038/76649. [DOI] [PubMed] [Google Scholar]
- 22.Jennings C. Developmental neurobiology. Death of a synapse. Nature. 1994;372:498–499. doi: 10.1038/372498a0. [DOI] [PubMed] [Google Scholar]
- 23.O’Brien RAD, Ostberg AJ, Vrbová G. Observations on the elimination of polyneuronal innervation in developing mammalian skeletal muscle. J Physiol. 1978;282:571–582. doi: 10.1113/jphysiol.1978.sp012482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ribchester RR, Taxt T. Motor unit size and synaptic competition in rat lumbrical muscles reinnervated by active and inactive motor axons. J Physiol. 1983;344:89–111. doi: 10.1113/jphysiol.1983.sp014926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ridge RM, Betz WJ. The effect of selective, chronic stimulation on motor unit size in developing rat muscle. J Neurosci. 1984;4:2614–2620. doi: 10.1523/JNEUROSCI.04-10-02614.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thompson WJ. Synapse elimination in neonatal rat muscle is sensitive to pattern of muscle use. Nature. 1983;302:614–616. doi: 10.1038/302614a0. [DOI] [PubMed] [Google Scholar]
- 27.Zito K. The flip side of synapse elimination. Neuron. 2003;37:1–2. doi: 10.1016/s0896-6273(02)01182-0. [DOI] [PubMed] [Google Scholar]
- 28.Favero M, Buffelli M, Cangiano A, Busetto G. The timing of impulse activity shapes the process of synaptic competition at the neuromuscular junction. Neuroscience. 2010;167:343–353. doi: 10.1016/j.neuroscience.2010.01.055. [DOI] [PubMed] [Google Scholar]
- 29.Buffelli M, Busetto G, Bidoia C, Favero M, Cangiano A. Activity-dependent synaptic competition at mammalian neuromuscular junctions. News Physiol Sci. 2004;19:85–91. doi: 10.1152/nips.01464.2003. [DOI] [PubMed] [Google Scholar]
- 30.Buffelli M, Busetto G, Cangiano L, Cangiano A. Perinatal switch from synchronous to asynchronous activity of motoneurons: Link with synapse elimination. Proc Natl Acad Sci USA. 2002;99:13200–13205. doi: 10.1073/pnas.202471199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Personius KE, Chang Q, Mentis GZ, O’Donovan MJ, Balice-Gordon RJ. Reduced gap junctional coupling leads to uncorrelated motor neuron firing and precocious neuromuscular synapse elimination. Proc Natl Acad Sci USA. 2007;104:11808–11813. doi: 10.1073/pnas.0703357104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ribchester RR. Co-existence and elimination of convergent motor nerve terminals in reinnervated and paralysed adult rat skeletal muscle. J Physiol. 1993;466:421–441. [PMC free article] [PubMed] [Google Scholar]
- 33.Rich MM, Lichtman JW. In vivo visualization of pre- and postsynaptic changes during synapse elimination in reinnervated mouse muscle. J Neurosci. 1989;9:1781–1805. doi: 10.1523/JNEUROSCI.09-05-01781.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Costanzo EM, Barry JA, Ribchester RR. Co-regulation of synaptic efficacy at stable polyneuronally innervated neuromuscular junctions in reinnervated rat muscle. J Physiol. 1999;521:365–374. doi: 10.1111/j.1469-7793.1999.00365.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gorio A, Carmignoto G, Finesso M, Polato P, Nunzi MG. Muscle reinnervation—II. Sprouting, synapse formation and repression. Neuroscience. 1983;8:403–416. doi: 10.1016/0306-4522(83)90188-4. [DOI] [PubMed] [Google Scholar]
- 36.McArdle JJ. Complex end-plate potentials at the regenerating neuromuscular junction of the rat. Exp Neurol. 1975;49:629–638. doi: 10.1016/0014-4886(75)90048-5. [DOI] [PubMed] [Google Scholar]
- 37.Gan WB, Lichtman JW. Synaptic segregation at the developing neuromuscular junction. Science. 1998;282:1508–1511. doi: 10.1126/science.282.5393.1508. [DOI] [PubMed] [Google Scholar]
- 38.Betz WJ, Chua M, Ridge RM. Inhibitory interactions between motoneurone terminals in neonatal rat lumbrical muscle. J Physiol. 1989;418:25–51. doi: 10.1113/jphysiol.1989.sp017827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stryker MP, Strickland SL. Physiological segregation of ocular dominance bars depends on the pattern of afferent electrical activity. Invest Ophthalmol Vis Sci. 1984;25(Suppl.):278. [Google Scholar]
- 40.Ribchester RR. Activity-dependent and -independent synaptic interactions during reinnervation of partially denervated rat muscle. J Physiol. 1988;401:53–75. doi: 10.1113/jphysiol.1988.sp017151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ribchester RR, Taxt T. Repression of inactive motor nerve terminals in partially denervated rat muscle after regeneration of active motor axons. J Physiol. 1984;347:497–511. doi: 10.1113/jphysiol.1984.sp015078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lo YJ, Poo MM. Activity-dependent synaptic competition in vitro: Heterosynaptic suppression of developing synapses. Science. 1991;254:1019–1022. doi: 10.1126/science.1658939. [DOI] [PubMed] [Google Scholar]
- 43.Fox MA, Tapia JC, Kasthuri N, Lichtman JW. Delayed synapse elimination in mouse levator palpebrae superioris muscle. J Comp Neurol. 2011;519:2907–2921. doi: 10.1002/cne.22700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ribchester RR. Competitive elimination of neuromuscular synapses. Nature. 1988;331:21–22. doi: 10.1038/331021a0. [DOI] [PubMed] [Google Scholar]
- 45.Callaway EM, Soha JM, Van Essen DC. Differential loss of neuromuscular connections according to activity level and spinal position of neonatal rabbit soleus motor neurons. J Neurosci. 1989;9:1806–1824. doi: 10.1523/JNEUROSCI.09-05-01806.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stollberg J. Synapse elimination, the size principle, and Hebbian synapses. J Neurobiol. 1995;26:273–282. doi: 10.1002/neu.480260211. [DOI] [PubMed] [Google Scholar]
- 47.Dahm LM, Landmesser LT. The regulation of synaptogenesis during normal development and following activity blockade. J Neurosci. 1991;11:238–255. doi: 10.1523/JNEUROSCI.11-01-00238.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gautam M, et al. Defective neuromuscular synaptogenesis in agrin-deficient mutant mice. Cell. 1996;85:525–535. doi: 10.1016/s0092-8674(00)81253-2. [DOI] [PubMed] [Google Scholar]
- 49.Hamburger V, Levi-Montalcini R. Proliferation, differentiation and degeneration in the spinal ganglia of the chick embryo under normal and experimental conditions. J Exp Zool. 1949;111:457–501. doi: 10.1002/jez.1401110308. [DOI] [PubMed] [Google Scholar]
- 50.Levi-Montalcini R. The nerve growth factor 35 years later. Science. 1987;237:1154–1162. doi: 10.1126/science.3306916. [DOI] [PubMed] [Google Scholar]
- 51.Misgeld T, et al. Roles of neurotransmitter in synapse formation: Development of neuromuscular junctions lacking choline acetyltransferase. Neuron. 2002;36:635–648. doi: 10.1016/s0896-6273(02)01020-6. [DOI] [PubMed] [Google Scholar]
- 52.Nguyen QT, Parsadanian AS, Snider WD, Lichtman JW. Hyperinnervation of neuromuscular junctions caused by GDNF overexpression in muscle. Science. 1998;279:1725–1729. doi: 10.1126/science.279.5357.1725. [DOI] [PubMed] [Google Scholar]
- 53.Oppenheim RW, von Bartheld CS. Programmed cell death and neurotrophic factors. In: Squire LR, et al., editors. Fundamental Neuroscience. 3rd Ed. New York: Elsevier; 2008. pp. 438–468. [Google Scholar]
- 54.Purves D. Body and Brain: A Trophic Theory of Neural Connections. Cambridge, MA: Harvard Univ Press; 1988. [DOI] [PubMed] [Google Scholar]
- 55.Poo MM. Neurotrophins as synaptic modulators. Nat Rev Neurosci. 2001;2:24–32. doi: 10.1038/35049004. [DOI] [PubMed] [Google Scholar]
- 56.Pittman R, Oppenheim RW. Cell death of motoneurons in the chick embryo spinal cord. IV. Evidence that a functional neuromuscular interaction is involved in the regulation of naturally occurring cell death and the stabilization of synapses. J Comp Neurol. 1979;187:425–446. doi: 10.1002/cne.901870210. [DOI] [PubMed] [Google Scholar]
- 57.Snider WD, Lichtman JW. Are neurotrophins synaptotrophins? Mol Cell Neurosci. 1996;7:433–442. doi: 10.1006/mcne.1996.0031. [DOI] [PubMed] [Google Scholar]
- 58.Betz WJ, Caldwell JH, Ribchester RR. Sprouting of active nerve terminals in partially inactive muscles of the rat. J Physiol. 1980;303:281–297. doi: 10.1113/jphysiol.1980.sp013285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brown MC. Sprouting of motor nerves in adult muscles: A recapitulation of ontogeny. Trends Neurosci. 1984;7:10–14. [Google Scholar]
- 60.Benoit P, Changeux JP. Consequences of tenotomy on the evolution of multiinnervation in developing rat soleus muscle. Brain Res. 1975;99:354–358. doi: 10.1016/0006-8993(75)90036-0. [DOI] [PubMed] [Google Scholar]
- 61.Brown MC, Holland RL, Hopkins WG. Restoration of focal multiple innervation in rat muscles by transmission block during a critical stage of development. J Physiol. 1981;318:355–364. doi: 10.1113/jphysiol.1981.sp013869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Thompson WJ, Kuffler DP, Jansen JKS. The effect of prolonged, reversible block of nerve impulses on the elimination of polyneuronal innervation of new-born rat skeletal muscle fibers. Neuroscience. 1979;4:271–281. doi: 10.1016/0306-4522(79)90088-5. [DOI] [PubMed] [Google Scholar]
- 63.Nelson PG, Fields RD, Yu C, Liu Y. Synapse elimination from the mouse neuromuscular junction in vitro: A non-Hebbian activity-dependent process. J Neurobiol. 1993;24:1517–1530. doi: 10.1002/neu.480241106. [DOI] [PubMed] [Google Scholar]
- 64.Tresch MC, Kiehn O. Synchronization of motor neurons during locomotion in the neonatal rat: Predictors and mechanisms. J Neurosci. 2002;22:9997–10008. doi: 10.1523/JNEUROSCI.22-22-09997.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Froemke RC, Dan Y. Spike-timing-dependent synaptic modification induced by natural spike trains. Nature. 2002;416:433–438. doi: 10.1038/416433a. [DOI] [PubMed] [Google Scholar]
- 66.Kasthuri N, Lichtman JW. The role of neuronal identity in synaptic competition. Nature. 2003;424:426–430. doi: 10.1038/nature01836. [DOI] [PubMed] [Google Scholar]
- 67.Colman H, Nabekura J, Lichtman JW. Alterations in synaptic strength preceding axon withdrawal. Science. 1997;275:356–361. doi: 10.1126/science.275.5298.356. [DOI] [PubMed] [Google Scholar]
- 68.Jansen JK, Fladby T. The perinatal reorganization of the innervation of skeletal muscle in mammals. Prog Neurobiol. 1990;34:39–90. doi: 10.1016/0301-0082(90)90025-c. [DOI] [PubMed] [Google Scholar]
- 69.Thompson W, Jansen JK. The extent of sprouting of remaining motor units in partly denervated immature and adult rat soleus muscle. Neuroscience. 1977;2:523–535. doi: 10.1016/0306-4522(77)90049-5. [DOI] [PubMed] [Google Scholar]
- 70.Barry JA, Ribchester RR. Persistent polyneuronal innervation in partially denervated rat muscle after reinnervation and recovery from prolonged nerve conduction block. J Neurosci. 1995;15:6327–6339. doi: 10.1523/JNEUROSCI.15-10-06327.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Burke RE. Physiology of motor units. In: Engel AG, Franzini-Armstrong C, editors. Miology. New York: McGraw Hill; 1994. pp. 464–484. [Google Scholar]
- 72.Rothwell J. Control of Human Voluntary Movement. 2nd Ed. London: Chapman and Hall; 1994. [Google Scholar]
- 73.Pasino E, et al. Effects of long-term conduction block on membrane properties of reinnervated and normally innervated rat skeletal muscle. J Physiol. 1996;497:457–472. doi: 10.1113/jphysiol.1996.sp021780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Buffelli M, Pasino E, Cangiano A. Paralysis of rat skeletal muscle equally affects contractile properties as does permanent denervation. J Muscle Res Cell Motil. 1997;18:683–695. doi: 10.1023/a:1018687923929. [DOI] [PubMed] [Google Scholar]
- 75.Cruz LJ, et al. Conus geographus toxins that discriminate between neuronal and muscle sodium channels. J Biol Chem. 1985;260:9280–9288. [PubMed] [Google Scholar]
- 76.Gonoi T, Ohizumi Y, Nakamura H, Kobayashi J, Catterall WA. The Conus toxin geographutoxin IL distinguishes two functional sodium channel subtypes in rat muscle cells developing in vitro. J Neurosci. 1987;7:1728–1731. doi: 10.1523/JNEUROSCI.07-06-01728.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.McLachlan EM, Martin AR. Non-linear summation of end-plate potentials in the frog and mouse. J Physiol. 1981;311:307–324. doi: 10.1113/jphysiol.1981.sp013586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Betz WJ, Mao F, Bewick GS. Activity-dependent fluorescent staining and destaining of living vertebrate motor nerve terminals. J Neurosci. 1992;12:363–375. doi: 10.1523/JNEUROSCI.12-02-00363.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]