Abstract
HDL is a major atheroprotective factor, but the mechanisms underlying this effect are still obscure. HDL binding to scavenger receptor-BI has been shown to activate eNOS, although the responsible HDL entities and signaling pathways have remained enigmatic. Here we show that HDL stimulates NO release in human endothelial cells and induces vasodilation in isolated aortae via intracellular Ca2+ mobilization and Akt-mediated eNOS phosphorylation. The vasoactive effects of HDL could be mimicked by three lysophospholipids present in HDL: sphingosylphosphorylcholine (SPC), sphingosine-1-phosphate (S1P), and lysosulfatide (LSF). All three elevated intracellular Ca2+ concentration and activated Akt and eNOS, which resulted in NO release and vasodilation. Deficiency of the lysophospholipid receptor S1P3 (also known as LPB3 and EDG3) abolished the vasodilatory effects of SPC, S1P, and LSF and reduced the effect of HDL by approximately 60%. In endothelial cells from S1P3-deficient mice, Akt phosphorylation and Ca2+ increase in response to HDL and lysophospholipids were severely reduced. In vivo, intra-arterial administration of HDL or lysophospholipids lowered mean arterial blood pressure in rats. In conclusion, we identify HDL as a carrier of bioactive lysophospholipids that regulate vascular tone via S1P3-mediated NO release. This mechanism may contribute to the vasoactive effect of HDL and represent a novel aspect of its antiatherogenic function.
Introduction
Injury of the vascular endothelium is a critical event in the pathogenesis of atherosclerosis. The endothelium has pleiotropic physiological activities that are directly or indirectly involved in atheroprotection: it regulates the adhesion and extravasation of leukocytes, modulates the proliferation of VSMCs, contributes to the maintenance of nonthrombogenic surfaces, and regulates vasomotor tone (1). Production of NO is believed to be integral to many of these functions. Consequently, dysfunction of the endothelium due to limited NO availability accelerates recruitment of macrophages into the vascular wall, promotes thrombosis, and impairs vasodilation in response to various stimuli (2, 3). Impairment of endothelial vasodilator functions has been demonstrated in subjects with coronary heart disease even in the absence of clinical symptoms (4). In animals on a high-cholesterol diet, progressive deterioration of endothelium-dependent relaxation could also be observed, which could be reversed by supplementation with L-arginine, an NO precursor (5).
Numerous epidemiological and clinical studies have documented an inverse relationship between HDL levels and the progression of atherosclerosis. However, the mechanisms by which HDL exerts its powerful antiatherogenic effect are still not entirely clear. The endothelium is a key target of HDL action: HDL ameliorates the inhibitory effects of oxidized LDL on vascular reactivity, and endothelium-dependent vasorelaxation is directly associated with HDL levels (reviewed in ref. 6). However, it remains unclear whether the vasoactive effects of HDL result from its specific interaction with the endothelium or rather reflect its ability to prevent LDL oxidation and increase the antioxidant capacity of the plasma. Recently, HDL has been shown to stimulate NO release through activation of eNOS via interaction with the scavenger receptor-BI (SR-BI) (7, 8). However, the HDL component known to bind to SR-BI, apoAI, had no effect, which leaves the identity of the HDL-associated entities responsible for its vasodilatory effect, as well as the signaling pathways involved, still enigmatic.
In this study, we provide evidence that HDL-associated lysophospholipids mediate the vasodilatory effect of HDL via Akt-mediated activation of eNOS both in vitro and in isolated mouse and rat aortae. We further show that HDL and the lysophospholipids induce vasodilation in rats in vivo. In addition, we identify the lysophospholipid receptor S1P3 as an integral component of HDL- and lysophospholipid-mediated vasodilation.
Methods
Cell culture and isolation of neonatal vascular endothelial cells from the murine heart.
Human umbilical vein endothelial cells (HUVECs) were isolated from human umbilical cords and cultured in RPMI 1640 supplemented with 15% calf serum, 0.4% bovine pituitary brain extract (GIBCO BRL, Karlsruhe, Germany), and 50 μg/ml heparin. Murine neonatal heart endothelial cells were isolated as previously described (9). Briefly, ten mouse pups at the age of 2 days were decapitated and the hearts were collected. The hearts were washed in isolation buffer (116 mmol/l NaCl, 20 mmol/l HEPES, pH 7.0, 1 mmol/l NaH2PO4, 5 mmol/l KCl, 0.8 mmol/l MgSO4, and 5.5 mmol/l glucose) and minced with sterile razor blades. The hearts were digested with a solution of 10 ml 0.2% collagenase B and 0.005% DNase (Roche Applied Science, Mannheim, Germany) in RPMI 1640 medium and incubated for 45 minutes at 37°C with occasional shaking. After incubation, the solution was pipetted up and down five to ten times to disperse the tissue. The supernatant was transferred into a fresh tube to pellet the cells (200 g for 10 minutes). The pellet was resuspended in 2 ml 40% Percoll (vol/vol; Amersham Biosciences Europe, Freiburg, Germany) in PBS and overlaid consecutively with 25% Percoll (vol/vol) and 2 ml PBS. The gradient was centrifuged at 400 g for 15 minutes. Endothelial cells located at the interphase of 25% and 40% Percoll were removed, washed with PBS, and cultured in endothelial cell medium on gelatin-coated dishes. The cells express endothelium-specific surface molecules such as vascular endothelial cadherin and vWF, as demonstrated by FACS analysis.
Isolation of lipoproteins, apolipoproteins, HDL lipid, HDL protein, and lysophospholipids.
HDL (d = 1.125–1.210 g/ml) was isolated from human plasma as described (10). Lipid-free apoAI was isolated by reverse-phase HPLC as previously described (11). To obtain the lipid fraction of HDL, 0.1 ml of native HDL (10 g/l) was diluted with 0.9 ml water, adjusted to pH 3.0 with sulfuric acid, and subsequently mixed with 1.0 ml acetonitrile. After addition of 0.5 g NaCl, the sample was centrifuged for 5 minutes at 800 g. The upper organic phase (arbitrarily called the HDL lipid fraction) was dried, dissolved in ethanol, and rapidly injected into PBS while vortexing. This fraction was used for cell stimulation in amounts indexed to the original HDL concentration. Sphingosylphosphorylcholine (SPC), sphingosine-1-phosphate (S1P), and lysosulfatide (LSF) were purchased from Sigma-Aldrich Chemie GmbH (Taufkirchen, Germany).
Western blotting.
HUVECs were lysed in 0.18 mol/l Tris-HCl, 0.15 mol/l NaCl, 10% (vol/vol) NP-40, 5% (vol/vol) sodium deoxycholate, 1% (vol/vol) SDS, 50 mmol/l NaF, 1 mmol/l EGTA, 1 mmol/l orthovanadate, and Complete protease inhibitor cocktail (Roche Applied Science). Cell lysates (50 μg) were subjected to SDS-PAGE and proteins were transferred to PVDF membranes. After blocking in 5% nonfat dry milk in Tris-buffered saline for 1 hour, the membranes were incubated overnight with specific antibodies. The antibody to eNOS was purchased from Pharmingen (San Diego, California, USA). Anti–phospho-Akt and anti–phospho-eNOS antibodies for Western blotting were from New England Biolabs Inc. (Schwalbach, Germany).
Metabolic labeling with [32P]orthophosphate and immunoprecipitation of eNOS.
Confluent HUVECs in 10-cm dishes were incubated in phosphate-free RPMI containing 0.1 mCi/ml of [32P]orthophosphate (ICN Biomedicals Inc., Eschwege, Germany) at 37°C for 4 hours and then stimulated with agonists. Cells were then washed twice with ice-cold PBS and scraped into 0.5 ml lysis buffer containing 1% Triton X-100, 50 mmol/l Tris-HCl pH 7.5, 150 mmol/l NaCl, 2 mmol/l EDTA, 8 mmol/l EGTA, 25 mmol/l NaF, 10 mmol/l Na4P2O7, 1 mmol/l Na3VO4, 5 μg/ml leupeptin, 5 μg/ml pepstatin, 5 μg/ml aprotinin, and 0.5 mmol/l PMSF. For immunoprecipitation, 2 μg monoclonal anti-eNOS antibody was incubated with 500 μg cell lysate in a total volume of 500 μl overnight followed by incubation with protein G–sepharose (40 μl slurry) for 2 hours. After extensive washing (five times), beads were taken up in fourfold SDS sample buffer and boiled, and the proteins were separated by SDS-PAGE and transferred to PVDF membranes. Phosphorylation of eNOS was analyzed by autoradiography, after which Western blotting for eNOS was performed to ensure equal amounts of immunoprecipitated protein.
Fluorescence microscopy.
For detection of intracellular NO generation, the NO-sensitive fluorescence dye DAF-2DA (Merck Biosciences, Schwalbach, Germany) was applied as described (12). Briefly, HUVECs (1.2 × 105 cells) were plated on gelatin-coated coverslips (diameter 12 mm) and incubated for 120 minutes in RPMI containing 1% FCS (vol/vol). DAF-2DA was added for the final 30 minutes of incubation. Cells were washed and stimulated with HDL or lysophospholipids for 10 minutes in the presence or absence of inhibitors. Reactions were stopped by fixing the cells in 2% paraformaldehyde (vol/vol) for 5 minutes at 4°C. Coverslips were examined with a fluorescence microscope equipped with an excitation filter (470–490 nm), a dichroic mirror (505 nm), and an emission filter (515 nm).
Arterial relaxation studies.
The direct effects of HDL, SPC, S1P, and LSF on arterial relaxation were evaluated in 2-mm rings of thoracic aortae from 3-month-old female Wistar rats (Charles River Laboratories, Sulzfeld, Germany), 3-month-old female C57BL/J6 mice (Charles River Laboratories), eNOS-null male mice and WT littermates (A. Gödecke), and S1P3-null mice and WT littermates (J. Chun.) (13, 14). The wall tension of the vasculature was measured in rat and mouse aortas using established methodology (15). Aortae from both species displayed reliable NO-related responses. Following equilibration and submaximal precontraction with phenylephrine (PE) (1 × 10–6 mol/l), relaxation to 1 × 10–5 mol/l acetylcholine was tested to confirm the integrity of the endothelium. After washing, rings were again contracted with PE and the direct effects of HDL, various fractions of HDL compounds, SPC, S1P, and LSF were assessed. At the end of the experiment, the relaxation response to acetylcholine was confirmed. Selected studies were performed in rings treated with nitro-L-arginine methylester (L-NAME; 50 μmol/l), SKF-525A (50 μmol/l), or indomethacin (10 μmol/l) added 10 minutes before PE exposure. The maintenance of functional smooth muscle cell integrity after manipulation was confirmed by evaluation of endothelium-independent relaxation to sodium nitroprusside (1 × 10–6 mol/l). All animal experiments were approved by the Landesamt für Gesundheit, Ernährung und technische Sicherheit Berlin (LAGETSI) ethics committee.
Measurement of mean arterial blood pressure after intra-arterial administration of HDL and lysophospholipids.
Mean arterial blood pressure (MAP) was measured in WKY rats (300 g, 16 weeks old) as previously described (16). Vascular tone was elevated by intravenous administration of endothelin (500 ng + 1,000 ng/h per 300-g rat). HDL (1 mg), SPC (200 nmol), S1P (200 nmol), and LSF (200 nmol) were administered intra-aortically via the left carotid artery. Acetylcholine (200 nmol) was applied to control for intact endothelial function.
Immunohistochemistry.
Aortic segments from Wistar rats were mounted in a small vessel myograph as described above and perfused with a medium containing 0.5 mg/ml HDL for 10 minutes. Samples were fixed immediately in buffered 4% formalin, embedded in paraffin, and stained with hematoxylin and eosin. Immunostaining was performed with a polyclonal phospho–Ser1177-eNOS antibody (Upstate, Hamburg, Germany; 1:100) overnight at 4°C after wet autoclaving of the sections with 0.01 mol/l citrate buffer (pH 6.0) for 10 minutes. Detection was performed using a mouse anti-rabbit bridging antibody (1:125; 30 minutes at room temperature) followed by a monoclonal mouse APAAP complex (1:100; 60 minutes at room temperature). The enzyme reaction was developed in fuchsine solution containing levamisole and counterstained with hematoxylin.
Determination of lysophospholipids in HDL.
S1P levels were determined as described (17). Briefly, 1 ml methanol containing 2.5 μl concentrated HCl was added to 100 μl of HDL suspension (13.1 mg HDL per 1 ml buffer). Dihydro-S1P (50 pmol) was added, and lipids were extracted by addition of 1 ml chloroform and 200 μl NaCl (4 mol/l). For alkalization, 100 μl NaOH (3 mol/l) was added. The alkaline aqueous phase was transferred into a siliconized glass tube, and the organic phase was re-extracted with 0.5 ml methanol, 0.5 ml NaCl (1 mol/l), and 50 μl NaOH (3 mol/l). The aqueous phases were combined, acidified with 100 μl concentrated HCl, and extracted twice with 1.5 ml chloroform. The organic phases were evaporated, and the dried lipids were dissolved in 275 μl of a mixture of methanol 0.07 mol/l K2HPO4 (9:1). A derivatization mixture of 10 mg o-phthaldialdehyde, 200 μl ethanol, 10 μl 2-mercaptoethanol, and 10 ml boric acid (3%, vol/wt) was prepared and adjusted to pH 10.5 with KOH. Twenty-five microliters of the derivatization mixture was added to the resolved lipids and this was incubated for 15 minutes at room temperature. The derivatives were analyzed with a Merck-Hitachi LaChrom HPLC system (Merck-Hitachi, Darmstadt, Germany) using an RP 18 Kromasil column (Chromatographie Service GmbH, Langerwehe, Germany). Separation was done with a gradient of methanol and K2HPO4 (0.07 mol/l) (17). The recovery of S1P was calculated using dihydro-S1P as a standard (17). To verify that no other substances were coincidentally overlapping the S1P peak, samples were incubated with alkaline phosphatase, which effectively cleaved the phosphate groups from S1P and dihydro-S1P. Treatment of standard and HDL samples with this enzyme resulted in an almost 90% decrease of S1P and dihydro-S1P peaks (data not shown). For digestion experiments with alkaline phosphatase, 50 units of enzyme in 450 μl of buffer containing 200 mmol/l Tris-HCl, pH 4.5, and 75 mmol/l MgCl2 in glycine (2 mol/l, pH 9.0) were added to the combined alkaline aqueous phases of the lipid extraction and incubated for 30 minutes at 37°C. Then, 100 μl of concentrated HCl was added and lipids were extracted twice with 1.5 ml chloroform.
To measure SPC content in HDL, 20 μl of isolated HDL (10 mg protein/ml) was diluted with 400 μl PBS (0.01 mmol/l) and applied to a 100-mg Isolute HCX-Q SPE column (Separtis GmbH, Grenzach-Wyhlen, Germany). After the column was washed with 1 ml PBS/methanol (80:20, vol/vol), SPC was eluted with 500 μl methanol containing 1% HCl, which was then evaporated to dryness. The dried sample was dissolved in 100 μl methanol with 10 μl of OPA reagent (0.5 mg o-phthaldialdehyde, 1 ml methanol, 100 μl mercaptoethanol, 900 μl of 0.4 mol/l borate buffer, pH 8.5) and incubated at room temperature for 15 minutes. After evaporation, the sample was dissolved in methanol and 10 μl was injected on a Kontron model 522 liquid chromatograph interfaced with an SFM 25 fluorescence detector (Kontron AG, Neufahrn, Germany). The excitation and emission wavelength of the detector was set at 335 nm and 420 nm, respectively. Reverse-phase chromatography was carried out on a 3-μm Spherisorb ODS2 column (100 mm length × 6.6 mm internal diameter) (Waters GmbH, Eschborn, Germany) with a flow rate of 0.5 ml/min and an eluent composition of methanol-tetrahydrofuran-acetate buffer, pH 4.8 (85:10:5, vol/vol/vol).
Determination of intracellular Ca2+ concentration.
Intracellular Ca2+ concentration ([Ca2+]i) measurements were performed using the Ca2+-sensitive fluorescence probe Fura2-AM according to established methods (18). Briefly, HUVECs (1 × 106 cells/ml) were loaded with Fura2-AM (5 μmol/l) for 60 minutes at 37°C. The fluorescence intensity was recorded at 37°C using the F2000 spectrofluorophotometer (Hitachi Ltd., Tokyo, Japan) with alternate excitation wavelengths of 340 nm and 380 nm (bandwidth, 5 nm) and an emission wavelength of 510 nm (bandwidth, 5 nm). [Ca2+]i was calculated as previously described (18).
Results
HDL-induced vasodilation is dependent on eNOS in vitro and in aortic segments.
To examine the effect of HDL on vascular tone, we added HDL to PE-precontracted rings of rat thoracic aortae. HDL (0.5 mg/ml) caused a direct 45% relaxation of the PE-precontracted rings: PE alone induced a maximal vasoconstriction of 14.0 ± 1.0 mN, which HDL was able to vasodilate by 6.3 ± 0.7 mN; n = 14, P < 0.01 (Figure 1a). This relaxation by HDL was abolished by pretreatment with the eNOS antagonist L-NAME, while indomethacin, an inhibitor of cyclooxygenase, and SKF-525A, an inhibitor of cytochrome P-450–dependent arachidonate metabolism, had no effect (n = 6 each; Figure 1b). To examine whether HDL has a direct effect on NO generation in vitro, we incubated HUVECs with the NO-sensitive fluorescence dye DAF-2DA and stimulated them with 0.5 mg/ml HDL for 10 minutes (Figure 1c). This resulted in a substantial increase in NO-dependent fluorescence intensity, which was abolished by pretreatment with 10 μmol/l L-NAME (Figure 1c). The vasodilatory effect of HDL was concentration-dependent, with a maximum at 0.5 mg/ml (Figure 1d) and an EC50 of 8.6 ± 0.5 μg/ml. The effect was also dependent on the presence of an intact endothelium, as it was abolished after endothelial denudation (data not shown). The HDL concentrations used were in the range of those in vivo (0.8–1.2 mg/ml HDL protein is the approximate equivalent of 40–70 mg HDL cholesterol/dl).
Figure 1.
HDL induces vasodilation in aortae from rats and mice in an eNOS-dependent manner. (a) Thoracic aortic rings from WKY rats were precontracted with PE (1 × 106 mol/l, arrows), and direct relaxation responses to HDL (0.5 mg/ml) or HDL and L-NAME (50 μmol/l) were evaluated. Shown are representative tracings from one experiment of 16. (b) Cumulative findings (mean ± SEM) for maximal relaxation in response to 0.5 mg/ml HDL in the presence of L-NAME (50 μmol/l), SKF-525A ((SKF, 50 μmol/l), or indomethacin (Indo, 10 μmol/l) (n = 6 each). *P < 0.01 vs. HDL. (c) HUVECs loaded with DAF-2DA were stimulated with 0.5 mg/ml HDL in the absence or presence of L-NAME. Cells were fixed and fluorescence was evaluated under a fluorescence microscope. Shown are representative results (n = 5). (d) Dose response of the vasodilatory effect of HDL (n = 3). (e) Thoracic aortic rings from WT 129/C57BL/6 mice and eNOS–/– mice were precontracted with PE, and direct relaxation responses to HDL (0.5 mg/ml) were measured. Shown are representative tracings from one experiment of six.
To test for involvement of eNOS in the HDL-induced vasorelaxing effect, we assessed the response of PE-precontracted thoracic aortae from eNOS knockout (eNOS–/–) mice to HDL. While aortae from WT and eNOS–/– mice responded similarly to preconstriction with PE (6.8 ± 0.8 mN vs. 7.5 ± 1.1 mN, respectively; n = 12; no significant difference), HDL (0.5 mg/ml) caused relaxation only in aortae from WT mice and not in aortae from eNOS–/– mice (Figure 1e): relaxation in response to HDL was 4.8 ± 0.5 mN (n = 6) (P < 0.01 vs. PE) in WT mice in contrast to 0.1 ± 0.3 mN (n = 6) (no significant difference vs. PE) in eNOS–/– mice (Figure 1e).
HDL induces vasodilation via Akt-stimulated eNOS phosphorylation in endothelial cells and in aortic segments.
As we have recently shown that HDL induces Akt activation (11) and Akt can activate eNOS via phosphorylation of Ser1177 (19, 20), we examined whether HDL-induced Akt activation in HUVECs results in eNOS phosphorylation and NO generation. Incubation of cells with 0.5 mg/ml HDL after metabolic labeling with [32P]orthophosphate substantially increased 32P incorporation into immunoprecipitated eNOS (Figure 2a). Preincubation with 10 μmol/l LY294002, a selective inhibitor of Akt activation by PI3K, substantially decreased both Ser473-Akt phosphorylation and 32P-eNOS amounts induced by HDL (Figure 2a). Phosphorylation of eNOS at Ser1177 was examined with a phosphospecific anti-eNOS antibody and revealed maximal phosphorylation 30 minutes after stimulation with 0.5 mg/ml HDL, while maximal Ser473-Akt phosphorylation occurred after 10 minutes (Figure 2a).
Figure 2.
HDL induces NO release and vasodilation via Akt-mediated eNOS phosphorylation in endothelial cells and in aortic segments. (a) Left panel: [32P]orthophosphate-labeled HUVECs were stimulated with HDL (0.5 mg/ml) in the presence or absence of LY294002 (10 μmol/l). Immunoprecipitated eNOS (ip) was analyzed by autoradiography (n = 3), and amounts of immunoprecipitated protein were detected by Western blotting. Phosphorylation of Akt at Ser473 was determined in cell lysates with a phosphospecific antibody (n = 5). Right panel: Time-dependence of HDL-induced eNOS and Akt phosphorylation at Ser1177 and Ser473, respectively, as analyzed by densitometry (n = 3). (b) Following precontraction of thoracic aortic rings from WKY rats with PE (1 × 10–6 mol/l, arrows), direct relaxation responses to HDL (0.5 mg/ml) in the absence or presence of LY294002 (10 μmol/l) were evaluated. Shown are original tracings from one experiment of eight. (c) Aortic segments perfused with 0.5 mg/ml HDL were fixed and immunostained for phospho-Ser1177-eNOS. Arrows indicate phospho-eNOS staining in the endothelial lining (original magnification, ×200). (d) Fura2-AM–loaded HUVECs were stimulated with 1 mg/ml HDL in the presence or absence of BAPTA-2AM (20 μmol/l) or Ni2+ (5 mM). [Ca2+]i was measured by fluorescence spectroscopy. Original tracings from representative experiments were superimposed for comparison. (e) HUVECs loaded with DAF-2DA and preincubated with BAPTA-2AM (20 μmol/l) or Ni2+ (5 mmol/l) were stimulated with HDL (1 mg/ml) and observed under a fluorescence microscope. Shown are representative results for one experiment of three.
To directly assess the role of Akt activation and eNOS phosphorylation in HDL-induced vasorelaxation, the effects of HDL on PE-precontracted rat aortae were examined in the presence of LY294002. Pretreatment with LY294002 potently reduced the vasodilating effect of HDL by 62% (HDL alone: 6.3 ± 0.8 mN, n = 8, vs. HDL + LY294002: 2.4 ± 0.6 mN, n = 8; P < 0.01) (Figure 2b). In the same aortic segments, immunohistochemistry with an antibody against phospho–Ser1177-eNOS revealed increased eNOS phosphorylation in the endothelial lining after treatment with HDL (Figure 2c).
Elevation of intracellular Ca2+ concentration is an important mechanism of eNOS activation and has been shown to precede Akt-dependent eNOS activation and NO-dependent vasorelaxation (21–23). As HDL has been reported to either increase or to have no effect on [Ca2+]i in endothelial cells in different studies (24, 25), we examined the role of calcium in HDL-induced NO generation. Incubation of HUVECs with HDL led to a substantial elevation of intracellular Ca2+ in HUVECs, leading to an increase of 94.5 ± 15.5 nmol/l (n = 6) (Figure 2d). In the presence of the intracellular Ca2+ chelator BAPTA-2AM (20 μmol/l) and the nonselective blocker of Ca2+ channels Ni2+ (5 mmol/l), the increase of HDL-induced [Ca2+]i was substantially inhibited (11.6 ± 5.6 and 32.3 ± 4.7 nmol/l, respectively, n = 6 each) (Figure 2d). When NO formation in response to HDL was measured by DAF-2DA fluorescence, HDL-dependent NO generation was suppressed after pretreatment of the cells with either BAPTA-2AM (20 μmol/l) or Ni2+ (5 mmol/l) (Figure 2e), suggesting that HDL induces NO formation in a Ca2+-dependent fashion.
HDL and the HDL-associated lysophospholipids SPC, S1P, and LSF induce vasorelaxation in aortic segments in vitro and in adult rats in vivo.
HDLs are complex molecules that contain several biologically active proteins and lipids. To determine HDL entities responsible for the HDL vasodilating effect, we tested several different components isolated from HDL for their ability to decrease PE-induced constriction of aortic segments. Neither the total protein fraction isolated from HDL (0.5 mg/ml) nor the major apolipoprotein of HDL, apoAI, in its purified form (0.1 mg/ml), had any effect. In contrast, the lipid fraction isolated from HDL (equivalent to 0.5 mg/ml of native lipoproteins) had a potent vasodilatory effect that was comparable to that of native HDL (Figure 3a). This effect was completely abolished in aortas pretreated with 10 μmol/l L-NAME (Figure 3a). The major lipid components of HDL such as cholesterol and sphingomyelin had either no effect or even induced vasoconstriction (as was the case for phosphatidylcholine) (Figure 3a), suggesting that other lipids contained in this fraction are responsible for the vasodilatory effect.
Figure 3.
HDL-associated lysophospholipids induce vasorelaxation in isolated arteries and NO production and eNOS phosphorylation in endothelial cells. (a) Following precontraction of thoracic aortic rings from WKY rats with PE (1 0 10–6 mol/l), measurements were taken of direct relaxation responses to HDL (0.5 mg/ml), the HDL-lipid fraction (Lipid, equivalent to 0.5 mg/ml HDL), the HDL protein fraction (Protein, equivalent to 0.5 mg/ml HDL), apoAI (0.1 mg/ml), cholesterol (Chol, 10 μmol/l), phosphatidylcholine (PC, 10 μmol/l), and sphingomyelin (Sm, 10 μmol/l). Cumulative findings (mean ± SEM) for maximal relaxation in eight experiments are shown (*P < 0.01 vs. HDL). (b) HPLC profile of S1P and dihydro-S1P separated on a reverse-phase C18 column after a two-step lipid extraction and derivatization with o-phthaldialdehyde (upper left panel) is shown beside a representative HPLC chromatogram of HDL after addition of dihdro-S1P (50 pmol) before extraction procedures (upper right panel). HPLC chromatogram of o-phthaldialdehyde derivatives of SPC: 0.5 μmol SPC standard (lower left panel) and HDL (lower right panel). arb U, arbitrary units. (c) Following precontraction of thoracic aortic rings from WT 129/C57BL/6 mice (WT) or eNOS–/– mice with PE (1 × 10–6 mol/l), direct relaxation responses to HDL (0.5 mg/ml), SPC (10 μmol/l), LSF (10 μmol/l), and S1P (10 μmol/l) were tested. Cumulative findings (mean ± SEM) for maximal relaxation in eight experiments are shown (*P < 0.01 vs. WT). (d) Following precontraction of thoracic aortic rings from WKY rats with PE, direct relaxation responses to different doses of SPC, S1P, and LSF were measured. Cumulative findings (mean ± SEM) for maximal relaxation in eight experiments are shown.
Recently we demonstrated that HDL serves as a carrier for bioactive lysophospholipids such as SPC and LSF, and that these compounds protect HUVECs against apoptosis through activation of Akt (11, 18). Another lysophospholipid present in HDL is S1P (26), which is also a potent Akt activator (21, 22, 27). Before testing the effect of these lysophospholipids on vasorelaxation, we quantitatively determined the amounts of SPC and S1P in HDL using HPLC after a two-step lipid extraction and derivatization with o-phthaldialdehyde (Figure 3b). The total amounts of SPC and S1P were 290 ± 20 pmol/mg and 287 ± 17 pmol/mg HDL protein (n = 3), respectively (Figure 3b).
We then tested the effect of all three lysophospholipids on the vascular contractile response. All three substances, SPC, S1P, and LSF (10 μmol/l each), exerted a potent vasorelaxing effect in PE-precontracted aortae from WT mice that was comparable to the effect of native HDL (Figure 3c). As with HDL, the vasorelaxing effect was completely absent in aortae from eNOS–/– mice (Figure 3c). All three lysophospholipids (SPC, S1P, LSF) induced a dose-dependent vasodilation in isolated aortae with a similar EC50 [–log/mol] (SPC = 7.7 ± 0.1; S1P = 7.6 ± 0.4 ; LSF = 7.5 ± 0.1) (Figure 3d).
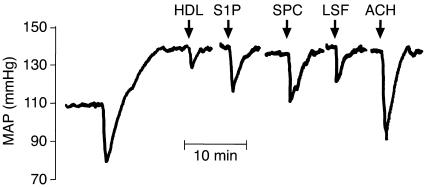
To test whether HDL and the lysophospholipids contained in HDL have an effect in vivo, we measured the influence of HDL, SPC, S1P, and LSF on MAP in Wistar rats. To achieve an initial increase in MAP, endothelin was infused intravenously (500 ng bolus + 1,000 ng/h per 300-g rat). This resulted in a significant rise in MAP, from 110.0 ± 6.2 mmHg to 139.7 ± 7.2 mmHg (n = 12). Intra-arterial administration of 1 mg HDL significantly reduced MAP by 9.9 ± 2.1 mmHg, while intra-arterial administration of 200 nmol LSF, SPC, or S1P significantly reduced MAP by 15.9 ± 4.3 mmHg, 20.9 ± 5.4 mmHg, and 17.6 ± 4.9 mmHg, respectively (Figure 4).
Figure 4.
Vasodilatory effects of HDL, SPC, S1P, and LSF in vivo. Original tracing of MAP in a rat with endothelin-induced elevation of MAP (500 ng bolus + 1,000 ng/h intravenously) after intra-aortic injection (arrows) of HDL (1 mg), S1P (200 nmol), SPC (200 nmol), LSF (200 nmol), or acetylcholine (ACH, 200 nmol).
The lysophospholipid receptor S1P3 mediates eNOS activation and vasorelaxation induced by HDL and HDL-associated lysophospholipids: loss of vasorelaxation in S1P3-deficient mice.
We next investigated the effect of SPC, S1P, and LSF on eNOS phosphorylation and NO generation in HUVECs. In Western blots using phosphospecific antibodies, there was a marked Ser1177 phosphorylation of eNOS and Ser473 phosphorylation of Akt by 0.5–10 μM SPC, S1P, and LSF, with a maximum occurring between 15 and 30 minutes after stimulation (Figure 5a). All three lysophospholipids potently enhanced NO-dependent fluorescence in DAF-2DA–loaded HUVECs (Figure 5b), revealing NO generation by all three agents. The effect was completely abolished after pretreatment with 10 μmol/l L-NAME (data not shown). Moreover, addition of SPC, S1P, or LSF (10 μmol/l each) to endothelial cells led to an increase in [Ca2+]i by 85 ± 5.4, 82.3 ± 6.7, and 91.3 ± 6.8 nmol/l (n = 3 each), respectively. The effects of SPC, S1P, and LSF on Akt and eNOS phosphorylation as well as on Ca2+ mobilization were concentration-dependent (Figure 5, a and d).
Figure 5.
Lysophospholipid signaling mediates HDL-, SPC-, S1P-, and LSF-induced Akt and eNOS phosphorylation as well as [Ca2+]i increase. (a) HUVECs were stimulated with HDL (0.5 mg/ml) or with 10 μmol/l each (left) or 0.5–5 μmol/l each (right) SPC, S1P, and LSF, with or without preincubation with 100 ng/ml PTX for 16 hours. “PTX control” indicates PTX treatment alone. Cell lysates were analyzed for phospho–Ser473-Akt (p-Ser473-Akt) and phospho–Ser1177-eNOS by Western blotting. Loading controls for total eNOS and total Akt content are shown. All results are representative of one experiment of three. (b) HUVECs loaded with DAF-2DA were stimulated with SPC, S1P, and LSF (10 μmol/l each) and observed under a fluorescence microscope. Shown are representative results for one experiment of three. (c) Fura2-AM–loaded HUVECs were stimulated with SPC, S1P, and LSF (10 μmol/l each), and [Ca2+]i was measured by fluorescence spectroscopy. Original tracings from representative experiments were superimposed for comparison. (d) Concentration dependence of [Ca2+]i increase in HUVECs stimulated with SPC, S1P, and LSF measured as described in c.
SPC and LSF are structurally and biologically related to S1P, a lysophospholipid that plays an important role in a number of fundamental cellular processes (28). S1P exerts its physiological effects by activating its cognate high-affinity G protein–coupled receptors S1P1 through S1P 5 (S1P1–5), resulting in the activation of different subsets of heterotrimeric G proteins including Gq, Gi/o, and G12/13 (29–31). SPC has been shown to act on several of these receptors as well. To test whether G protein–coupled receptor signaling by HDL and HDL-associated lysophospholipids is involved in their effects on NO generation and vasodilation, we preincubated HUVECs with pertussis toxin (PTX), an irreversible Gi activation blocker, prior to stimulation with HDL, SPC, S1P, and LSF. PTX (100 ng/ml) almost completely prevented phosphorylation of Akt at Ser473 and eNOS at Ser1177, respectively, 10 minutes after stimulation by HDL and each of three lysophospholipids (Figure 5a).
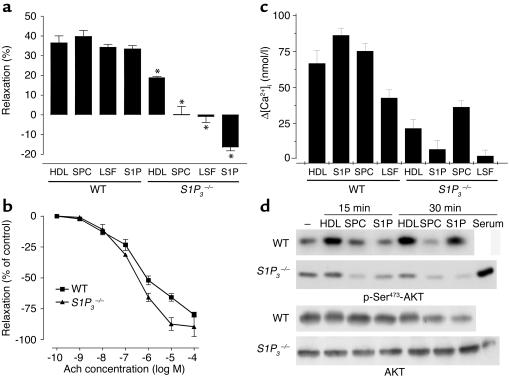
To test the role of lysophospholipid S1P1–5 receptors in mediating the vasodilatory effects of HDL and the HDL-associated lysophospholipids, we made use of mice deficient in one of the two major S1P receptors in endothelial cells, S1P3 (the other major receptor, S1P1, is embryonically lethal). We tested the effects of HDL, SPC, S1P, and LSF on vasodilation in PE-precontracted aortae from S1P3 knockout mice (13, 14); the vasodilatory effect of HDL was inhibited by 57% in S1P3-deficient mice (3.4 ± 0.4 mN; n = 4; P < 0.01) compared with WT mice (7.9 ± 1.3 mN; n = 4; P < 0.01) (Figure 6a), while the vasodilation induced by SPC, S1P, and LSF, respectively, was completely abolished in S1P3-deficient mice (Figure 6a). Basal contractile responses to PE (10 μmol) in WT and S1P3-deficient mice were similar (5.7 ± 0.9 mN vs. 5.9 ± 1.1 mN; n = 6 each). To exclude a generally impaired relaxation in S1P3-deficient animals, we performed experiments with acetylcholine and sodium nitroprusside over the complete range of their vasoactive concentrations in WT and S1P3–/– mice and observed no impaired relaxation (Figure 6b). Thus, we could exclude impaired relaxation in response to all vasorelaxants as a general S1P3–/– phenotype. Therefore, it appears that HDL-dependent vasodilation is mediated to approximately 60% by S1P3, while vasodilation induced by SPC, LSF, and S1P is mediated entirely through the S1P3 receptor.
Figure 6.
Role of S1P3 in the vasorelaxation induced by HDL and HDL-associated lysophospholipids. (a) Following precontraction of thoracic aortic rings from WT and S1P3–/– mice with PE (1 × 10–6 mol/l), direct relaxation responses to HDL (0.5 mg/ml), SPC (10 μmol/l), LSF (10 μmol/l), and S1P (10 μmol/l) were tested. Cumulative findings (mean ± SEM) for maximal relaxation in eight studies are shown (*P < 0.01 vs. same treatment in WT animals). (b) Vasorelaxation in response to different acetylcholine (Ach) concentrations in aortic rings from WT mice compared with S1P3–/– mice. (c) Fura2-AM–loaded mouse cardiac endothelial cells from WT and S1P3–/– mice were stimulated with HDL (1 mg/ml) or SPC, LSF, and S1P (10 μM each), respectively. [Ca2+]i was measured by fluorescence spectroscopy. Bar graph shows increases in [Ca2+]i calculated from three separate determinations (n = 3). (d) Mouse cardiac endothelial cells from WT and S1P3–/– mice were stimulated with HDL (1 mg/ml), SPC (10 μM), or S1P (10 μM), and Akt phosphorylation after 15 and 30 minutes was measured using phosphospecific antibodies. In S1P3–/– endothelial cells, Akt phosphorylation was also measured after stimulation with serum for 30 minutes as a control to exclude an overall inability of the cells to activate Akt.
To test the effect of the S1P3 lysophospholipid receptor on [Ca2+]i increase and Akt phosphorylation in endothelial cells in vitro, we isolated cardiac endothelial cells from WT and S1P3 knockout mice. The [Ca2+]i elevation in S1P3-deficient endothelial cells in response to HDL and SPC was reduced by more than 50%, while that of S1P and LSF was completely abolished (Figure 6c). Similarly, Akt phosphorylation in response to HDL, SPC, and S1P was severely reduced in S1P3-deficient endothelial cells compared with WT cells (Figure 6d).
A recent study has shown a vasodilatory effect of female HDL but not of male HDL, and has implicated HDL-associated estradiol for this difference. Therefore we isolated individual HDL fractions from 11 male (32.3 ± 7.4 years old) and 8 female (30.6 ± 4.8 years old, no contraceptives) nonsmoking, healthy subjects and tested their effect on PE-precontracted WT mouse aortae. Both male and female HDL (0.1 mg/ml) had a pronounced vasodilatory effect in WT-mice without any significant gender difference (male HDL: 5.8 ± 1.0 mN [48.0% ± 9.0% of maximal PE-induced vasoconstriction], n = 11; female HDL: 7.0 ± 0.7 mN [58.6% ± 6.1% of maximal PE-induced vasoconstriction], n = 8) (Figure 7, a and c). We also observed no difference in the extent of Akt phosphorylation by male and female HDL (data not shown). In S1P3–/– mice, the vasodilatory effect of both male and female HDL was substantially reduced but without significant difference: male HDL: 2.9 ± 0.6 mN (23.6% ± 4.8% of maximal PE-induced vasoconstriction), n = 11; female HDL: 3.8 ± 0.5 mN (31.7% ± 4.1% reduction of maximal PE-induced vasoconstriction, n = 8) (Figure 7, b and c). This corresponds to a reduction of vasodilation of 49.0% ± 16.5% for male HDL and 45.3% ± 12.8% for female HDL in S1P3–/– mice compared with WT mice. We also measured the S1P content in HDL from 11 male (33 ± 7.5 years old) and 7 female (29 ± 4.3 years old) subjects (triplicate determinations) and did not observe any statistically significant difference: male HDL contained 300 ± 30 pmol S1P/mg protein; female HDL contained 456 ± 56 pmol S1P/mg protein (Figure 7d).
Figure 7.
Male and female HDLs are equally potent at inducing vasodilation, and their effect is similarly reduced in S1P3–/– mice. Gender-specific S1P content in HDL. (a and b) Original tracings from (a) WT mice and (b) S1P3–/– mice of relaxation responses to male and female HDL (0.1 mg/ml), respectively, following precontraction of thoracic aortic rings with PE (1 × 10–6 mol/l, arrows). (c) Cumulative findings (mean ± SEM) for maximal relaxation of male (n = 11) and female (n = 8) HDL in WT and S1P3–/– mice (*P < 0.05 vs. WT). There was no significant difference between male and female HDL by Mann-Whitney U test. (d) Quantification of S1P in male (n = 11) and female HDL (n = 7).
Discussion
HDL-mediated vasodilation is entirely dependent on eNOS and partially on Akt.
Several clinical studies have demonstrated a close association between plasma levels of HDL and endothelium-dependent flow-mediated dilation (32). Recently, administration of reconstituted HDL was shown to restore abnormal endothelial function in hypercholesterolemic men (33). Several mechanisms underlying the effects of HDL on endothelial reactivity have been suggested, including synthesis of vasorelaxing prostanoids such as prostaglandin E2 and/or I2 by provision of arachidonic acid (34) as well as activation of eNOS via SR-BI (7). In our study, inhibitors of enzymes converting arachidonic acid to its vasoactive derivatives failed to inhibit HDL-induced vasorelaxation in isolated aortic segments. In contrast, HDL-induced vasorelaxation was completely abolished after pretreatment with L-NAME, an inhibitor of eNOS, and was absent in aortas from eNOS-deficient animals. Thus, the effects of HDL on endothelial reactivity in our study were exclusively mediated by stimulation of NO production via eNOS. The HDL concentrations we used are physiologically relevant, as 0.8–1.2 mg/ml HDL protein is equivalent to approximately 40–70 mg HDL cholesterol/dl.
NO release from the endothelium in response to VEGF, estrogens, and IGF-1 is mediated by phosphorylation of eNOS at Ser1177 by Akt, while protein kinase A phosphorylates eNOS in response to laminar shear stress (35–38). We previously reported that HDL protects endothelial cells against apoptosis by activating Akt (11) and have identified, in the present study, Ser1177 in eNOS as a phosphorylation target of HDL-mediated Akt activation in HUVECs in vitro as shown for other endothelial cells (8). Both eNOS phosphorylation and NO-dependent arterial vasorelaxation in our study were attenuated after inhibition of PI3K with LY294002, suggesting that HDL activation of the PI3K/Akt pathway leads to eNOS activation and NO production. However, inhibition of the PI3K/Akt pathway did not completely abolish HDL-mediated vasodilation in isolated arteries (although it reduced it by more than 50%), suggesting that other, Akt-independent pathways of eNOS activation by HDL exist. Activation of eNOS is primarily achieved by a rise in intracellular Ca2+ concentration, and the increase in [Ca2+]i has been previously demonstrated to precede or even to be a prerequisite of Akt-dependent eNOS activation and NO-dependent vasorelaxation (21–23). Our observations that HDL stimulates [Ca2+]i increase and that the inhibition of HDL-induced NO production can be achieved by interfering with Ca2+ mobilization strongly argue that this may be another mechanism by which HDL regulates NO production.
HDL and its lysophospholipid content are physiologically relevant for vasodilation in vivo.
HDLs are complex molecules known to induce a multitude of intracellular signals for which different components of HDL have been made responsible. In the present study, we demonstrate that three lysophospholipids contained in HDL, SPC, S1P, and LSF potently relaxed PE-precontracted aortae through activation of eNOS, while the HDL protein fraction or purified apoAI had no effect. The lysophospholipid concentrations effective in inducing vasodilation are likely to be provided in vivo by plasma HDL: according to our data, one mg of HDL contains 287 ± 17 pmol S1P (similar to previously published values; ref. 26) and 290 ± 20 pmol SPC. In dose-response curves performed in isolated arteries, 50% of the maximal relaxation is achieved in the range between 0.1 and 1 μM of the individual lysophospholipid. The physiological concentration of 1 mg/ml HDL used in our study corresponds to approximately 0.58 nM bioactive, similarly effective lysophospholipids (S1P and SPC together). When injected in vivo, 1 mg HDL and 200 nmol of each lysophospholipid induced a significant decrease in MAP. In vivo, additional aspects may also be relevant: (a) several of the vasoactive lysophospholipids contained in HDL would work together in achieving vasodilation, and (b) the S1P3 receptor would encounter the whole HDL particle with its total load of lysophospholipids, resulting in a much higher biologically active concentration at the single receptor level compared with lysophospholipids in solution.
The vasodilatory effect of HDL is partially mediated by its S1P, SPC, and LSF content acting via the S1P3 lysophospholipid receptor.
Bioactive lysophospholipids such as S1P and SPC mediate a variety of physiological processes by binding to their cognate G protein–coupled receptors from the lysophospholipid receptor family (28–31, 39). Among these processes, angiogenesis and promotion of endothelial cell survival have been shown to depend on the activation of Akt. Recently, S1P was demonstrated to induce Akt activation, eNOS phosphorylation, and NO production in endothelial cells (21, 22, 27). Both S1P and SPC interact with several lysophospholipid receptors such as S1P1, S1P 3, and S1P5, which are all expressed in endothelial cells (40–42). These receptors couple to trimeric G proteins, including GI (29–31, 43). In addition, SPC has been reported to activate three other G protein–coupled receptors, the ovarian cancer G protein–coupled receptor-1 (OGR-1), GPR4, and G2A, and shares these with lysophosphatidylcholine, although the ligand identities of these receptors is currently ambiguous (44). The effects of HDL and HDL-associated lysophospholipids on Akt activation, eNOS activation, and NO release in HUVECs were inhibited by PTX, suggesting the involvement of a PTX-sensitive Gi protein in vitro. In isolated aortic segments, the vasodilatory effects of SPC, LSF, and S1P were completely abrogated in aortae from S1P3-deficient animals, suggesting that this particular lysophospholipid receptor completely mediates the vasodilatory effect of all three compounds. As the increase in [Ca2+]i and Akt phosphorylation were either completely abolished (S1P and LSF) or substantially reduced (SPC), both major mechanisms of eNOS activation appear to be mediated by S1P3. This is in agreement with two other studies that have implicated S1P3 in Akt activation and NO production, respectively (45, 46). The failure of S1P to increase [Ca2+]i and activate phosphatidylinositol-specific phospholipase C, another target of HDL-triggered intracellular signaling (18), has been reported previously for embryonic fibroblasts from S1P3-deficient mice (47). Thus, we suggest that the specific part of the vasodilatory effect of HDL that was lost in S1P3-deficient animals (∼50%) is attributable to its SPC, S1P, and LSF content acting via the S1P3 receptor.
It is crucial to emphasize that there is a pronounced difference in the arterial response to lysophospholipids that depends on whether the artery has been precontracted (as in our case by PE) or not. In the first case, S1P and SPC induce vasodilation, as supported by all our data. In the latter case (effect on basal arterial tone), S1P and SPC induce vasoconstriction (data not shown), which is in line with several publications on S1P in the literature (48, 49). The vasodilatory effect on precontracted aortae was much more pronounced than the vasoconstrictive effect on nonprecontracted aortae (∼8 mN vs. ∼1 mN; Figure 6a and data not shown). Most importantly, the vasoconstrictive effect was independent of S1P3, as it was not different between aortae from WT and S1P3 knockout animals (data not shown). The further augmentation of vasoconstriction by S1P in PE-precontracted aortae from S1P3–/– or eNOS–/– mice (Figures 6a and 3c) suggests that S1P has a dual action: it contracts arteries under basal conditions (but not via S1P3) and mediates vasodilation (via S1P3) in the case of increased arterial tone. This appears to be mediated by at least two different pathways: one impacting on endothelial cells (as in our study) and another on VSMCs, and these effects may differ among different vascular beds (48).
Alternative and putatively cooperative mechanisms of HDL-induced eNOS activation.
In contrast to the complete loss of eNOS activation by lysophospholipids in S1P3-deficient animals, the vasodilatory response to HDL, although substantially inhibited (by ∼60%), was not completely abrogated. Other HDL-mediated effects, besides resulting in eNOS and/or Akt activation, may account for the remaining part of HDL-induced eNOS-dependent vasodilation. Shaul and coworkers have shown that HDL-induced eNOS activation is critically dependent on the binding of HDL to the SR-BI (7), and that Src, Akt, and MAPK signaling via SR-BI contribute to eNOS activation (8). However, the authors reported that the major SR-BI protein ligand of HDL, apoAI, failed to activate eNOS (similar to results of our study), while an antibody against the cytoplasmic C-terminus of SR-BI inhibited the ability of SR-BI to stimulate eNOS (8). Lysophospholipids are amphipathic molecules, and their ability to diffuse freely between HDL and the cell surface may be limited. Therefore, the interaction of HDL with SR-BI could provide the necessary spatial proximity for SPC, S1P, and LSF to effectively stimulate S1P3. A model of SR-BI and S1P3 cooperation in HDL-induced signaling is proposed in Figure 8. However, it is important to note that our data neither exclude direct activation of S1P3 via lysophospholipids within the HDL complex nor indirect mechanisms of S1P production/release, which could also activate S1P3.
Figure 8.
Model of HDL-induced eNOS activation and vasodilation by the lysophospholipid receptor S1P3. PI-PLC, phosphatidylinositol-specific phospholipase C. A-I, apoAI; N and C, amino- and caboxy-terminus of SR-BI.
In contrast to studies suggesting a role for Akt in eNOS activation by HDL, Smart and coworkers recently described that HDL binding to SR-BI stimulated eNOS via an increase in intracellular ceramide without an increase of [Ca2+]I or Akt activation (24). In a different study, the same authors showed that female but not male HDL activated eNOS and promoted vasodilation, which they attributed to HDL-associated estradiol (50). In our study, we did not observe any differences in the vasodilatory effect between male and female HDL, and there was no gender difference in the S1P content. Possible explanations may be the different source of arteries used (femoral arteries vs. aortae in our study) and the different precontraction stimulus (5-hydroxytryptamine vs. PE in our study). This may also contribute to the substantial differences in both the magnitude of vasodilation by HDL and EC50 values. A number of studies strongly support an important role of estrogens in vasodilation. However, the estradiol concentrations shown to be effective in a physiologically relevant range (10–7 to 10–8 mol/l) are several orders of magnitude higher than the concentrations of HDL-associated estradiol sufficient for maximal vasodilation in the study by Smart et al. Clearly, several mutually nonexclusive mechanisms may account for the vasodilatory effects of HDL under different experimental conditions. Interestingly, recent findings suggest that plasma membrane estrogen receptors activate eNOS via Akt (51) and that they are biologically coupled to eNOS through GαI (52). This has led to the suggestion that crosstalk exists between estrogen receptors and a G protein–coupled receptor that signals via Gαi (53). An excellent candidate emerging from our data would be the S1P3 lysophospholipid receptor.
The observation that S1P3 acts as a functional HDL receptor and that lysophospholipids mediate an important part of its vasodilatory effect raises exciting questions about the role of lysophospholipid receptors in the antiatherogenic activity exerted by HDL. Several potentially antiatherogenic effects have been attributed to lysophospholipid receptor signaling in vitro such as preservation of endothelial cell integrity, induction of endothelial cell migration, and eNOS activation (54). However, little information is available on lysophospholipid receptor regulation and function in human atherosclerosis and in animal models of the disease. Furthermore, no studies on the lysophospholipid content of HDL in patients with atherosclerosis or coronary artery disease have been conducted to date. Such studies may help elucidate the role of lysophospholipids and their receptors in HDL-mediated atheroprotection and reveal new perspectives in the prevention and treatment of clinical atherosclerosis.
Acknowledgments
We gratefully acknowledge the technical assistance of K. Kloke and M. Wolters, the isolation of cardiac endothelial cells by A. Sokoll, and electron microscopy by W. Völker. This study was supported by Interdisziplinäres Klinisches Forschungszentrum Münster (project grants A11, to B. Levkau; and B10, to M. Schäfers), Sonderforschungsbereich 556 (project grant B6, to B. Levkau), and the National Institute of Mental Health (J. Chun and I. Ishii).
Footnotes
See the related Commentary beginning on page 509.
Jerzy-Roch Nofer and Markus van der Giet contributed equally to this work.
Conflict of interest: The authors have declared that no conflict of interest exists.
Nonstandard abbreviations used: scavenger receptor-BI (SR-BI); human umbilical vein endothelial cell (HUVEC); sphingosylphosphorylcholine (SPC); sphingosine-1-phosphate (S1P); lysosulfatide (LSF); phenylephrine (PE); nitro-l-arginine methyl ester (l-NAME); mean arterial blood pressure (MAP); intracellular Ca2+ concentration ([Ca2+]i); pertussis toxin (PTX).
References
- 1.Toborek M, Kaiser S. Endothelial cell functions. Relationship to atherogenesis. Basic Res. Cardiol. 1999;94:295–314. doi: 10.1007/s003950050156. [DOI] [PubMed] [Google Scholar]
- 2.Loscalzo J. Nitric oxide insufficiency, platelet activation, and arterial thrombosis. Circ. Res. 2001;88:756–762. doi: 10.1161/hh0801.089861. [DOI] [PubMed] [Google Scholar]
- 3.Cohen RA. The role of nitric oxide and other endothelium-derived vasoactive substances in vascular disease. Prog. Cardiovasc. Dis. 1995;38:105–128. doi: 10.1016/s0033-0620(05)80002-7. [DOI] [PubMed] [Google Scholar]
- 4.Harrison DG, Ohara Y. Physiologic consequences of increased vascular oxidant stresses in hypercholesterolemia and atherosclerosis: implications for impaired vasomotion. Am. J. Cardiol. 1995;75:75B–81B. doi: 10.1016/0002-9149(95)80018-n. [DOI] [PubMed] [Google Scholar]
- 5.Candipan RC, Wang BY, Buitrago R, Tsao PS, Cooke JP. Regression or progression. Dependency on vascular nitric oxide. Arterioscler. Thromb. Vasc. Biol. 1996;16:44–50. doi: 10.1161/01.atv.16.1.44. [DOI] [PubMed] [Google Scholar]
- 6.Nofer JR, et al. HDL and arteriosclerosis: beyond reverse cholesterol transport. Atherosclerosis. 2002;161:1–16. doi: 10.1016/s0021-9150(01)00651-7. [DOI] [PubMed] [Google Scholar]
- 7.Yuhanna IS, et al. High-density lipoprotein binding to scavenger receptor-BI activates endothelial nitric oxide synthase. Nat. Med. 2001;7:853–857. doi: 10.1038/89986. [DOI] [PubMed] [Google Scholar]
- 8.Mineo C, Yuhanna IS, Quon MJ, Shaul PW. High density lipoprotein-induced endothelial nitric-oxide synthase activation is mediated by Akt and MAP kinases. J. Biol. Chem. 2003;278:9142–9149. doi: 10.1074/jbc.M211394200. [DOI] [PubMed] [Google Scholar]
- 9.Lodge PA, Haisch CE, Thomas FT. A simple method of vascular endothelial cell isolation. Transplant. Proc. 1992;24:2816–2817. [PubMed] [Google Scholar]
- 10.Havel RJ, Eder H, Bragdon J. The distribution and chemical composition of ultracentrifugally isolated lipoproteins from human plasma. J. Clin. Invest. 1955;34:1345–1353. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nofer JR, et al. Suppression of endothelial cell apoptosis by high density lipoproteins (HDL) and HDL-associated lysophospholipids. J. Biol. Chem. 2001;276:34480–34485. doi: 10.1074/jbc.M103782200. [DOI] [PubMed] [Google Scholar]
- 12.Sugimoto K, Fujii S, Takemasa T, Yamashita K. Detection of intracellular nitric oxide using a combination of aldehyde fixatives with 4,5-diaminofluorescein diacetate. Histochem. Cell Biol. 2000;113:341–347. doi: 10.1007/s004180000151. [DOI] [PubMed] [Google Scholar]
- 13.Ishii I, et al. Selective loss of sphingosine 1-phosphate signaling with no obvious phenotypic abnormality in mice lacking its G protein-coupled receptor, LPB3/EDG-3. J. Biol. Chem. 2001;276:33697–33704. doi: 10.1074/jbc.M104441200. [DOI] [PubMed] [Google Scholar]
- 14.Ishii I, et al. Marked perinatal lethality and cellular signaling deficits in mice null for the two sphingosine 1-phosphate receptors, S1P2/LPB2/EDG-5 and S1P3/LPB3/EDG-3. J. Biol. Chem. 2002;277:25152–25159. doi: 10.1074/jbc.M200137200. [DOI] [PubMed] [Google Scholar]
- 15.Tepel M, et al. Diadenosine polyphosphates’ action on calcium and vessel contraction. Am. J. Hypertens. 1997;10:1404–1410. doi: 10.1016/s0895-7061(97)80305-6. [DOI] [PubMed] [Google Scholar]
- 16.Schluter H, et al. Diadenosine phosphates and the physiological control of blood pressure. Nature. 1994;367:186–188. doi: 10.1038/367186a0. [DOI] [PubMed] [Google Scholar]
- 17.Ruwisch L, Schafer-Korting M, Kleuser B. An improved high-performance liquid chromatographic method for the determination of sphingosine-1-phosphate in complex biological materials. Naunyn Schmiedebergs Arch. Pharmacol. 2001;363:358–363. doi: 10.1007/s002100000365. [DOI] [PubMed] [Google Scholar]
- 18.Nofer JR, et al. Activation of phosphatidylinositol-specific phospholipase C by HDL-associated lysosphingolipid. Involvement in mitogenesis but not in cholesterol efflux. Biochemistry. 2000;39:15199–15207. doi: 10.1021/bi001162a. [DOI] [PubMed] [Google Scholar]
- 19.Dimmeler S, et al. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature. 1999;399:601–605. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- 20.Fulton D, et al. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature. 1999;399:597–601. doi: 10.1038/21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Igarashi J, Bernier SG, Michel T. Sphingosine 1-phosphate and activation of endothelial nitric-oxide synthase: differential regulation of Akt and MAP kinase pathways by EDG and bradykinin receptors in vascular endothelial cells. J. Biol. Chem. 2001;276:12420–12426. doi: 10.1074/jbc.M008375200. [DOI] [PubMed] [Google Scholar]
- 22.Tanimoto T, Jin ZG, Berk BC. Transactivation of vascular endothelial growth factor (VEGF) receptor Flk-1/KDR is involved in sphingosine 1-phosphate-stimulated phosphorylation of Akt and endothelial nitric-oxide synthase (eNOS) J. Biol. Chem. 2002;277:42997–43001. doi: 10.1074/jbc.M204764200. [DOI] [PubMed] [Google Scholar]
- 23.Dantas AP, Igarashi J, Michel T. Sphingosine 1-phosphate and control of vascular tone. Am. J. Physiol. 2003;284:H2045–H2052. doi: 10.1152/ajpheart.01089.2002. [DOI] [PubMed] [Google Scholar]
- 24.Li XA, et al. High density lipoprotein binding to scavenger receptor, Class B, type I activates endothelial nitric-oxide synthase in a ceramide-dependent manner. J. Biol. Chem. 2002;277:11058–11063. doi: 10.1074/jbc.M110985200. [DOI] [PubMed] [Google Scholar]
- 25.Honda HM, et al. High-density lipoprotein increases intracellular calcium levels by releasing calcium from internal stores in human endothelial cells. Atherosclerosis. 1999;143:299–306. doi: 10.1016/s0021-9150(98)00302-5. [DOI] [PubMed] [Google Scholar]
- 26.Kimura T, et al. Sphingosine 1-phosphate may be a major component of plasma lipoproteins responsible for the cytoprotective actions in human umbilical vein endothelial cells. J. Biol. Chem. 2001;276:31780–31785. doi: 10.1074/jbc.M104353200. [DOI] [PubMed] [Google Scholar]
- 27.Morales-Ruiz M, et al. Sphingosine 1-phosphate activates Akt, nitric oxide production, and chemotaxis through a Gi protein/phosphoinositide 3-kinase pathway in endothelial cells. J. Biol. Chem. 2001;276:19672–19677. doi: 10.1074/jbc.M009993200. [DOI] [PubMed] [Google Scholar]
- 28.Levade T, et al. Sphingolipid mediators in cardiovascular cell biology and pathology. Circ. Res. 2001;89:957–968. doi: 10.1161/hh2301.100350. [DOI] [PubMed] [Google Scholar]
- 29.Yang AH, Ishii I, Chun J. In vivo roles of lysophospholipid receptors revealed by gene targeting studies in mice. . Biochim. Biophys. Acta. 2002; 1582:197–203. doi: 10.1016/s1388-1981(02)00172-5. [DOI] [PubMed] [Google Scholar]
- 30.Fukushima N, Ishii I, Contos JJA, Weiner JA, Chun J. Lysophospholipid receptors. Ann. Rev. Pharmacol. Toxicol. 2001;41:507–534. doi: 10.1146/annurev.pharmtox.41.1.507. [DOI] [PubMed] [Google Scholar]
- 31.Chun J, et al. International Union of Pharmacology. XXXIV. Lysophospholipid receptor nomenclature. Pharmacol. Rev. 2002;54:265–269. doi: 10.1124/pr.54.2.265. [DOI] [PubMed] [Google Scholar]
- 32.O’Connell BJ, Genest J., Jr High-density lipoproteins and endothelial function. Circulation. 2001;104:1978–1983. doi: 10.1161/hc3901.096667. [DOI] [PubMed] [Google Scholar]
- 33.Spieker LE, et al. High-density lipoprotein restores endothelial function in hypercholesterolemic men. Circulation. 2002;105:1399–1402. doi: 10.1161/01.cir.0000013424.28206.8f. [DOI] [PubMed] [Google Scholar]
- 34.Fleisher LN, Tall AR, Witte LD, Miller RW, Cannon PJ. Stimulation of arterial endothelial cell prostacyclin synthesis by high density lipoproteins. J. Biol. Chem. 1982;257:6653–6655. [PubMed] [Google Scholar]
- 35.Boo YC, et al. Shear stress stimulates phosphorylation of endothelial nitric-oxide synthase at Ser1177 by Akt-independent mechanisms: role of protein kinase A. J. Biol. Chem. 2002;277:3388–3396. doi: 10.1074/jbc.M108789200. [DOI] [PubMed] [Google Scholar]
- 36.Zachary I, Gliki G. Signaling transduction mechanisms mediating biological actions of the vascular endothelial growth factor family. Cardiovasc. Res. 2001;49:568–581. doi: 10.1016/s0008-6363(00)00268-6. [DOI] [PubMed] [Google Scholar]
- 37.Haynes MP, et al. Membrane estrogen receptor engagement activates endothelial nitric oxide synthase via the PI3-kinase-Akt pathway in human endothelial cells. Circ. Res. 2000;87:677–682. doi: 10.1161/01.res.87.8.677. [DOI] [PubMed] [Google Scholar]
- 38.Michell BJ, et al. The Akt kinase signals directly to endothelial nitric oxide synthase. Curr. Biol. 1999;9:845–848. doi: 10.1016/s0960-9822(99)80371-6. [DOI] [PubMed] [Google Scholar]
- 39.Xu Y. Sphingosylphosphorylcholine and lysophosphatidylcholine: G protein-coupled receptors and receptor-mediated signal transduction. . Biochim. Biophys. Acta. 2002; 1582:81–88. doi: 10.1016/s1388-1981(02)00140-3. [DOI] [PubMed] [Google Scholar]
- 40.Lee MJ, et al. Vascular endothelial cell adherens junction assembly and morphogenesis induced by sphingosine-1-phosphate. Cell. 1999;99:301–312. doi: 10.1016/s0092-8674(00)81661-x. [DOI] [PubMed] [Google Scholar]
- 41.McGiffert C, Contos JJ, Friedman B, Chun J. Embryonic brain expression analysis of lysophospholipid receptor genes suggests roles for s1p(1) in neurogenesis and s1p(1–3) in angiogenesis. FEBS Lett. 2002;531:103–108. doi: 10.1016/s0014-5793(02)03404-x. [DOI] [PubMed] [Google Scholar]
- 42.Zhang G, Contos JJA, Weiner JA, Fukushima N, Chun J. Comparative analysis of three murine G-protein coupled receptors activated by sphingosine-1-phosphate. Gene. 1999;227:89–99. doi: 10.1016/s0378-1119(98)00589-7. [DOI] [PubMed] [Google Scholar]
- 43.Meyer zu Heringdorf D, Himmel HM, Jakobs KH. Sphingosylphosphorylcholine-biological functions and mechanisms of action. . Biochim. Biophys. Acta. 2002; 1582:178–189. doi: 10.1016/s1388-1981(02)00154-3. [DOI] [PubMed] [Google Scholar]
- 44.Ishii, I., Fukushima, N., Ye, X., and Chun, J. 2004. Lysophospholipid receptors: signaling and biology. Ann. Rev. Biochem. In press. [DOI] [PubMed]
- 45.Banno Y, et al. Involvement of phospholipase D in insulin-like growth factor-I-induced activation of extracellular signal-regulated kinase, but not phosphoinositide 3-kinase or Akt, in Chinese hamster ovary cells. Biochem. J. 2003;369:363–368. doi: 10.1042/BJ20021368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kwon YG, et al. Sphingosine 1-phosphate protects human umbilical vein endothelial cells from serum-deprived apoptosis by nitric oxide production. J. Biol. Chem. 2001;276:10627–10633. doi: 10.1074/jbc.M011449200. [DOI] [PubMed] [Google Scholar]
- 47.Ishii I, et al. Marked perinatal lethality and cellular signaling deficits in mice null for the two sphingosine 1-phosphate (S1P) receptors, S1P(2)/LP(B2)/EDG-5 and S1P(3)/LP(B3)/EDG-3. J. Biol. Chem. 2002;277:25152–25159. doi: 10.1074/jbc.M200137200. [DOI] [PubMed] [Google Scholar]
- 48.Salomone S, et al. S1P(3) receptors mediate the potent constriction of cerebral arteries by sphingosine-1-phosphate. Eur. J. Pharmacol. 2003;469:125–134. doi: 10.1016/s0014-2999(03)01731-x. [DOI] [PubMed] [Google Scholar]
- 49.Ohmori T, et al. Sphingosine 1-phosphate induces contraction of coronary artery smooth muscle cells via S1P2. Cardiovasc. Res. 2003;58:170–177. doi: 10.1016/s0008-6363(03)00260-8. [DOI] [PubMed] [Google Scholar]
- 50.Gong M, et al. HDL-associated estradiol stimulates endothelial NO synthase and vasodilation in an SR-BI-dependent manner. J. Clin. Invest. 2003;111:1579–1587. doi:10.1172/JCI200316777. doi: 10.1172/JCI16777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Haynes MP, et al. Src kinase mediates phosphatidylinositol 3-kinase/Akt-dependent rapid endothelial nitric-oxide synthase activation by estrogen. J. Biol. Chem. 2003;278:2118–2123. doi: 10.1074/jbc.M210828200. [DOI] [PubMed] [Google Scholar]
- 52.Wyckoff MH, et al. Plasma membrane estrogen receptors are coupled to endothelial nitric-oxide synthase through Galpha(i) J. Biol. Chem. 2001;276:27071–27076. doi: 10.1074/jbc.M100312200. [DOI] [PubMed] [Google Scholar]
- 53.Chambliss KL, Shaul PW. Estrogen modulation of endothelial nitric oxide synthase. Endocr. Rev. 2002;23:665–686. doi: 10.1210/er.2001-0045. [DOI] [PubMed] [Google Scholar]
- 54.Tamama K, Okajima F. Sphingosine 1-phosphate signaling in atherosclerosis and vascular biology. Curr. Opin. Lipidol. 2002;13:489–495. doi: 10.1097/00041433-200210000-00004. [DOI] [PubMed] [Google Scholar]