Abstract
The transfer of calcium from mother to milk during lactation is poorly understood. In this report, we demonstrate that parathyroid hormone–related protein (PTHrP) production and calcium transport in mammary epithelial cells are regulated by extracellular calcium acting through the calcium-sensing receptor (CaR). The CaR becomes expressed on mammary epithelial cells at the transition from pregnancy to lactation. Increasing concentrations of calcium, neomycin, and a calcimimetic compound suppress PTHrP secretion by mammary epithelial cells in vitro, whereas in vivo, systemic hypocalcemia increases PTHrP production, an effect that can be prevented by treatment with a calcimimetic. Hypocalcemia also reduces overall milk production and calcium content, while increasing milk osmolality and protein concentrations. The changes in milk calcium content, milk osmolality, and milk protein concentration were mitigated by calcimimetic infusions. Finally, in a three-dimensional culture system that recapitulates the lactating alveolus, activation of the basolateral CaR increases transcellular calcium transport independent of its effect on PTHrP. We conclude that the lactating mammary gland can sense calcium and adjusts its secretion of calcium, PTHrP, and perhaps water in response to changes in extracellular calcium concentration. We believe this defines a homeostatic system that helps to match milk production to the availability of calcium.
Introduction
The extracellular calcium-sensing receptor (CaR) was first cloned from the bovine parathyroid gland in 1993 (1). It is a G protein–coupled cell-surface receptor that binds calcium ions and allows cells to react to changes in the extracellular concentration of calcium (2–4). It is a member of the subgroup of the G protein–coupled receptor gene superfamily that also includes odorant, taste, and pheromone sensors (2). The CaR and other members of this subgroup appear to be descendants of nutrient or periplasmic binding proteins in bacteria (2, 5, 6).
In the parathyroids, the CaR regulates the secretion of parathyroid hormone (PTH) in response to changes in extracellular calcium (2–4). In addition, the CaR is prominently expressed in the kidney where it regulates the handling of calcium in the renal tubules (2, 7). The CaR also may participate in the regulation of bone turnover, renal production of 1,25-dihydroxyvitamin D, and gastrointestinal calcium absorption (2–4). By conferring the ability to sense calcium to these organs, the CaR is pivotal in orchestrating the body’s integrated calcium homeostasis.
The CaR has also been found in a wide variety of organs not involved in systemic calcium homeostasis (2–4). At these sites, the CaR has been implicated in the regulation of a number of cellular processes, such as ion and water transport, proliferation, differentiation, and apoptosis. One of the functions of the CaR at several of these nonparathyroid sites appears to be the regulation of the production of parathyroid hormone–related protein (PTHrP). PTHrP is a cytokine that was discovered as the cause of the clinical syndrome of humoral hypercalcemia of malignancy (8, 9). The PTH and PTHrP genes are related and were both derived from the duplication of a common ancestral gene (8). In contrast to PTH, which acts as a classic peptide hormone, PTHrP normally acts as a local paracrine or autocrine modulator of cellular function. However, both peptides bind to and activate the common type 1 PTH/PTHrP receptor (8). Therefore, if PTHrP does enter the circulation it can alter systemic calcium metabolism by mimicking the actions of PTH.
During lactation, the breast coordinates large fluxes of calcium from the maternal circulation into the milk (10, 11). The maternal adaptations to this demand for calcium are met at least in part by the mobilization of skeletal calcium stores through accelerated bone resorption (12). The lactating breast is also a prominent source of PTHrP (13, 14). Large quantities of PTHrP are secreted into milk. In addition, lactation appears to be the only time at which PTHrP normally enters the systemic circulation. It is secreted from the lactating breast and contributes to an increase in the rate of bone resorption (15, 16). The loss of bone mineral during lactation presumably helps to maintain a steady supply of calcium to the breast for the purposes of milk production. However, the mechanisms, if any, by which skeletal calcium mobilization and milk production are coordinated remain unclear. Furthermore, little is known of the molecular mechanisms used by mammary epithelial cells to regulate the transepithelial flux of calcium into milk.
Recently it has been shown that the CaR is expressed in the epithelial ducts of the normal human breast (17). Furthermore, it has been reported that the CaR can regulate the production of PTHrP by breast cancer cell lines in tissue culture (18). In this study, we examined the physiologic role of the CaR in the mammary glands of mice. We report that the mammary gland becomes a calcium-sensing organ during lactation and that it uses the CaR to adjust its production of PTHrP and its transport of calcium into milk.
Methods
RNase protection analysis.
Total RNA was isolated using TRIzol (Invitrogen Corp., Carlsbad, California, USA). A 318-bp CaR cDNA, representing nucleotides 227–545 of the mouse CaR mRNA sequence (GenBank accession number AF128842), was amplified from 1 μg of mouse kidney RNA by RT-PCR using SuperScript One-Step (Invitrogen Corp.). The primers 5′-ATGGTTTGGCTACTGTTTGG-3′ and 5′-GGATCCTAATACGACTCACTATAG GGAGGCAGAGCCTTGGAGACGGTGT-3′ were designed to add a T7 promoter sequence (underlined) downstream of the CaR cDNA in order to allow for in vitro transcription of an antisense RNA probe. The PTHrP probe template was a 349-bp AvrII-PvuII genomic fragment of the mouse PTHrP gene, while a 220-bp Sau3a-Sau3a fragment of the mouse cyclophilin gene was used as a loading control (19). Antisense riboprobes were synthesized by in vitro transcription (Riboprobe System; Promega Corp., Madison, Wisconsin, USA) in the presence of α-[32P]UTP (PerkinElmer Inc., Boston, Massachusetts, USA). RNase protection was carried out on total cellular RNA prepared from mammary glands using the RPA III kit (Ambion Inc., Austin, Texas, USA). Mouse kidney RNA was used as a positive control for CaR expression. Yeast RNA served as a negative control. Autoradiographs were scanned using Kodak Image Station 440 (Eastman Kodak Scientific Imaging Systems, Rochester, New York, USA), and band intensities were determined with Kodak 1D image analysis software (Eastman Kodak Scientific Imaging Systems).
Microarray analysis.
FVB mice were obtained from Taconic Farms (Germantown, New York, USA). Pregnancies were timed from the appearance of a vaginal plug (day 1); lactation day 1 was identified as the first day after parturition occurred. Bilateral fourth mammary glands were removed and the embedded lymph nodes were excised prior to tissue processing. Collected tissue samples were stored in RNAlater stabilization buffer (QIAGEN Inc., Valencia, California, USA) according to the manufacturer’s instructions. Total RNA was isolated from each tissue sample and purified following the QIAGEN RNA extraction/cleanup protocol. Purity, concentration, and integrity of total RNA from each sample were verified using a spectrophotometer and the RNA 6000 Nano Assay (Agilent Technologies, Palo Alto, California, USA). Nondegraded and sufficiently concentrated RNA was amplified, labeled with biotin (Enzo Biochem Inc., Farmingdale, New York, USA), and fragmented following the 2002 protocol for eukaryotic target preparation from Affymetrix Inc. (Santa Clara, California, USA). The labeled and fragmented cRNA products of the Affymetrix protocol were also verified for sample integrity and concentration using the RNA 6000 Nano Assay. Acceptable samples were hybridized to Affymetrix Mu74Av2 microarray chips. Raw data were gathered from scanned array chips using Affymetrix Microarray Suite (MAS) version 5.0. A global scaling strategy in MAS 5.0 was adopted to properly compare probe set intensity data across all scanned chips: the output from every chip array was scaled so that the trimmed mean signal (the mean of all signals, less the top and bottom 2% of intensity data) was centered on the same target intensity value. Compiled data were analyzed using GeneSpring microarray analysis software (Silicon Genetics, San Francisco, California, USA).
Immunofluorescence.
Formalin-fixed, paraffin-embedded mammary tissue from CD1 mice (Charles River Laboratories, Wilmington, Massachusetts, USA) was stained with the polyclonal anti-CaR antibody 4637 (20) or rabbit IgG (Vector Laboratories Inc., Burlingame, California, USA) at 10 μg/ml. Prior to staining, antigen retrieval was performed by trypsin treatment (21). Mammospheres (see below) were also stained by the protocol of Debnath et al. (22). The secondary antibody was Alexa 594–conjugated goat anti-rabbit IgG (Molecular Probes Inc., Eugene, Oregon, USA) used at 1:800. Immunofluorescent images were produced using a Zeiss LSM 510 confocal microscope (Carl Zeiss MicroImaging Inc., Thornwood, New York, USA).
PTHrP production in vitro.
Primary cultures of mammary epithelial cells were prepared from pregnant CD1 mice (day 11–14) as previously described (19). Cells were grown to confluence over 7 days in DMEM/F12 (Invitrogen Corp.) with 5% FBS (Atlanta Biologicals Inc., Atlanta, Georgia, USA), and the following lactogenic hormone mixture: 5 μg/ml insulin, 1 μg/ml hydrocortisone, and 3 μg/ml prolactin (Cambrex Corp., Walkersville, Maryland, USA). Cells were then incubated for 6 hours in DMEM (Invitrogen Corp.) supplemented with 0.2% BSA and 0.5 mM CaCl2 with or without: (a) additional CaCl2 at 2.5 mM or 5 mM, (b) the polycationic CaR agonist neomycin (300 μM), or (c) 1 mM CaCl2 with either the calcimimetic NPS R-467 (2.5 μM) or the less active control compound NPS S-467 (2.5 μM) (courtesy of NPS Pharmaceuticals, Salt Lake City, Utah, USA). PTHrP 1-86 was measured in conditioned medium using a two-site immunoradiometric assay (Diagnostic Systems Laboratories Inc., Webster, Texas, USA). PTHrP levels were corrected for the protein concentration in the conditioned media as determined by the Bradford method (Bradford reagent; Bio-Rad Laboratories Inc., Hercules, California, USA).
Dietary calcium restriction.
All experiments done were approved by Yale University’s Institutional Animal Care and Use Committee. Pregnant, age-matched CD1 female mice were purchased from Charles River Laboratories and allowed to deliver at our animal facility. All animals were 12–15 weeks old at the time of analysis. On the first day postpartum, mice were switched from the standard RMH3000 diet (Purina Mills Inc., Richmond, Indiana, USA) to the low-calcium diet or the control diet. Mice had access to food and tap water ad libitum. Mice received either the Test Diet Low-calcium Diet 5855 (0.01% calcium; PMI Nutrition International, Richmond, Indiana, USA) or the Test Diet Basal Diet 5775 (PMI Nutrition International) containing 0.6% calcium. At day 4 postpartum, some mice were implanted subcutaneously with Alzet miniature osmotic pumps, model 2002 (Durect Corp., Cupertino, California, USA), containing the calcimimetic NPS R-467 or its less active enantiomer, NPS S-467, at doses of 4 μmol/kg/d or 40 μmol/kg/d. The compounds were dissolved in DMSO and were diluted to the proper concentration with 45% 2-hydroxypropyl-β-cyclodextrin (Sigma-Aldrich, St. Louis, Missouri, USA).
On day 12 postpartum, mice were injected intraperitoneally with 0.1 mIU of oxytocin (Sigma-Aldrich) and manually milked. Blood was collected by cardiac puncture into syringes containing 50 μl PTHrP protease inhibitor cocktail (Nichols Institute Diagnostics, San Juan Capistrano, California, USA). Blood was centrifuged at 750 g for 10 minutes at 4°C, and plasma was removed, aliquoted, and stored at –70°C. Urine was collected prior to bleeding.
Measurements in vivo.
Milk PTHrP levels were measured using a two-site immunoradiometric assay specific for PTHrP 1-86 (Diagnostic Systems Laboratories Inc.). In order to stay within the range of this assay, milk was diluted 1:1,000 into mouse plasma (Hilltop Lab Animals, Scottsdale, Pennsylvania, USA). Plasma PTHrP levels were measured using a radioimmunoassay specific for PTHrP 1-34 (23). PTH was measured in plasma using a two-site immunoradiometric assay for rat PTH (Immutopics International LLC, San Clemente, California, USA). Plasma calcium and milk calcium (milk was diluted 1:100 in distilled water) were measured with an atomic absorptiometer (model 2380; PerkinElmer Inc.), and milk osmolality (milk was diluted 1:10 in distilled water) was measured with a Fiske Micro-Sample Osmometer, model 210 (Fiske Associates, Norwood, Massachusetts, USA), by the Clinical Chemistry Laboratory at Yale–New Haven Hospital. Milk protein concentrations were measured using the Bradford protein determination assay (Bio-Rad Laboratories Inc.).
Calcium transport in vitro.
To make mammospheres, primary mouse mammary epithelial cells were plated on Matrigel (Becton, Dickinson and Co., Franklin Lakes, New Jersey, USA) and grown for 7 days in DMEM/F12 with 5% FBS and lactogenic hormones. To verify the integrity of the tight junctions in the mammospheres, a modification of the procedure of Chen et al. (24) was used. Briefly, the mammospheres were incubated at room temperature for 30 minutes with 2 mg/ml sulfosuccinimidyl-6-(biotinamido)hexanoate (NHS-LC-biotin; Pierce Chemical Co., Rockford, Illinois, USA) in HBSS with either 1 mM CaCl2 and 1 mM MgCl2 or with 2.5 mM EGTA (to disrupt tight junctions). One-tenth volume of 500 mM glycine, pH 7.4, was added and incubation was continued for 30 minutes at room temperature to neutralize excess NHS-LC-biotin. The mammospheres were washed four times with PBS and fixed for 30 minutes in 4% paraformaldehyde (in PBS) at room temperature. Frozen sections of these mammospheres were blocked in 1% BSA for 1 hour at room temperature, and then stained with 6.7 μg/ml FITC-avidin (Molecular Probes Inc.) for 1 hour at room temperature. Cells were viewed using a Zeiss LSM 510 confocal microscope (Carl Zeiss MicroImaging Inc.).
To measure transepithelial transport of calcium into the lumen of the mammospheres, 0.25 μCi of 45Ca was added to each well of a 24-well plate in calcium-free DMEM (Invitrogen Corp.) supplemented with (a) 1.0, 5.0, or 10.0 mM CaCl2 or (b) 1.0 mM CaCl2 plus NPS R-467 or NPS S-467 (2.5 μM). After 16 hours at 37°C, the mammospheres were washed with PBS and then removed from the Matrigel using MatriSperse solution (Becton, Dickinson and Co.). To release the lumen contents, the mammospheres were incubated at 37°C for 15 minutes in 0.5 mM EGTA in PBS. The cells were removed by centrifugation at 1,000 g for 5 minutes, and 45Ca was measured by liquid scintillation. To examine the relationship between the regulation of PTHrP and calcium transport by the CaR in mammary epithelial cells, we performed these experiments in cells lacking the PTHrP gene. We made mammospheres from day 11–14 pregnant β-lactoglobulin–Cre/PTHrPlox/– (BLG-Cre/PTHrPlox/–) mice (15) that had previously undergone at least one complete reproductive cycle (pregnancy, lactation, and involution). In these mice, the BLG-Cre transgene is expressed specifically in mammary epithelial cells during lactation, resulting in deletion of the PTHrP gene and the complete absence of PTHrP expression in the mammary gland (15).
Results
CaR mRNA is expressed in the lactating mammary gland.
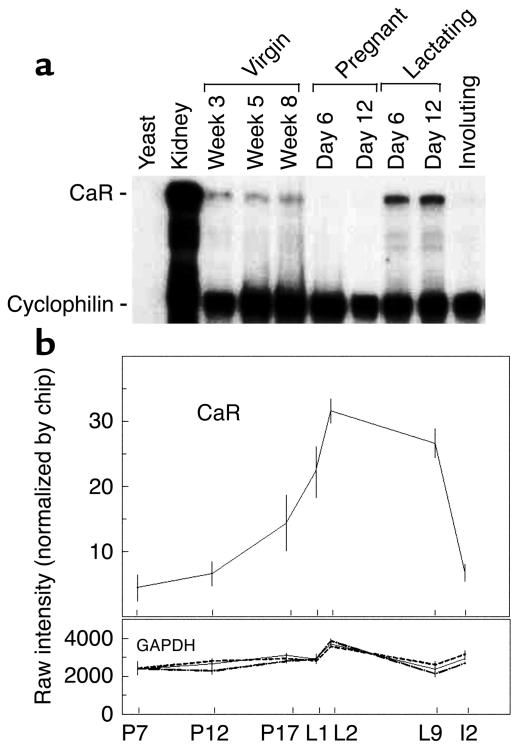
Normal mammary gland development proceeds through a series of well-defined stages that ultimately result in the milk-producing acinar structure present during lactation (25). To begin investigating a potential physiologic function of the CaR in mammary tissue, we examined the profile of CaR mRNA expression in whole mammary glands harvested from normal mice at various timepoints representative of the different stages of mammary development. As can be seen in Figure 1a, the CaR was expressed at low levels in the mammary glands of virgin mice at all timepoints tested. These include prepubertal glands at 3 weeks of age, midpubertal glands at 5 weeks of age, and early adult glands at 8 weeks of age. Expression of the receptor was downregulated during pregnancy and was upregulated to its highest levels of expression during lactation. Expression was again downregulated to very low levels in the involuting gland 2 days after withdrawal of the pups. In order to confirm independently that CaR expression was upregulated at the transition from pregnancy to lactation, we examined the profile of CaR transcripts within an oligonucleotide-based gene array database that profiles gene expression in the normal mammary gland during the transition from pregnancy to lactation. As shown in Figure 1b, levels of CaR transcripts began to rise several days before parturition and increased by approximately sixfold from midpregnancy to midlactation. As a reference, we show GAPDH expression, which was nearly constant in this tissue over the time period examined. Other markers of epithelial cell number, keratin 19 and claudin 7, did not change significantly between P17 and L2 (data not shown), suggesting that the changes in CaR expression were unlikely to be due to alterations in the cellular composition of the gland.
Figure 1.
CaR expression over the course of mammary gland development. (a) RNase protection analysis of CaR mRNA levels during mouse mammary gland development. Fifty micrograms of total RNA prepared from mouse mammary glands harvested at the timepoints noted in the figure was assayed. Five micrograms of mouse kidney RNA served as a positive control. Fifty micrograms of yeast RNA served as a negative control. (b) Profile of CaR transcript representation in an Affymetrix gene expression database. Each point represents the mean of four separate RNA samples prepared from mammary glands harvested at the indicated times. Error bars represent the SEM. P7, P12, and P17 represent days 7, 12, and 17 of pregnancy, respectively; L1, L2, and L9 represent days 1, 2, and 9 of lactation, respectively; I2 represents the second day of involution after weaning. The profile for GAPDH transcripts is provided for comparison.
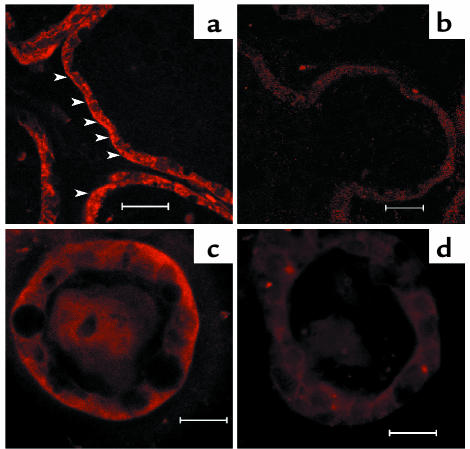
To define the cell type expressing the CaR and to determine CaR’s subcellular distribution, we performed immunofluorescent staining on tissue sections prepared from lactating glands. We also examined the pattern of CaR expression in normal mammary epithelial cells in three-dimensional culture. Mammary cells cultured in a laminin-rich extracellular matrix and in the presence of lactogenic hormones differentiate fully and form hollow spheres (mammospheres) that recapitulate the normal acinar structures of the lactating gland (26). As shown in Figure 2a, the CaR was located in the luminal epithelial cells of the lactating gland. Staining for the CaR was seen at the basolateral surface and in the cytoplasm. A similar pattern of staining was seen in epithelial cells within the mammospheres (Figure 2c). No specific staining was seen in tissues or cells when nonimmune IgG was substituted for the CaR antibody (Figure 2, b and d).
Figure 2.
Immunofluorescence staining for the CaR in mammary glands of lactating mice (a and b) and in mammospheres (c and d). Staining was done with 10 μg/ml anti-CaR antibody (a and c) or rabbit IgG (b and d), and images were produced using a Zeiss LSM 510 confocal microscope. Arrowheads in a indicate basolateral membrane staining. Scale bars: 20 μm.
CaR signaling regulates PTHrP production by mammary epithelial cells in vitro.
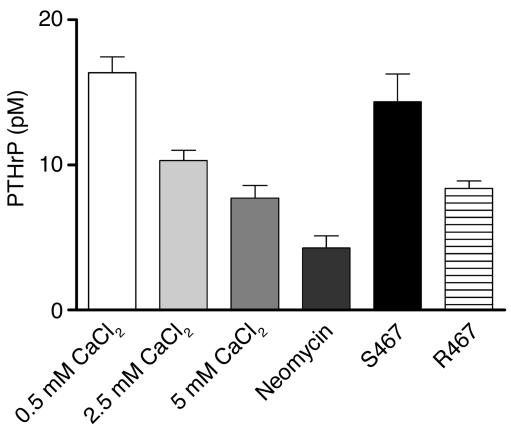
The increase in CaR expression in lactating versus pregnant mammary glands is similar to the pattern of PTHrP gene expression in the mammary gland (14). In addition, a previous report had demonstrated that calcium, acting through the CaR, could increase PTHrP secretion from cultured breast cancer cell lines (18). Thus, we next asked if the extracellular calcium concentration affected the secretion of PTHrP by normal mammary epithelial cells in culture. Primary mammary epithelial cells were grown on plastic dishes in media containing lactogenic hormones (insulin, hydrocortisone, and prolactin) and 1.05 mM calcium. Under these conditions, the cells expressed ample levels of CaR mRNA (data not shown). After the cells reached confluence (7 days in culture), the calcium content of the media was changed to 0.5 mM, 2.5 mM, or 5 mM for 6 hours and PTHrP concentrations in the media were measured. As shown in Figure 3, increasing concentrations of extracellular calcium led to a dose-dependent inhibition of PTHrP secretion from these cells. To determine whether the effect of calcium on PTHrP secretion was mediated by the CaR, similar experiments were carried out in the presence of neomycin and the calcimimetic compound NPS R467. NPS R467 is a small molecule, an allosteric activator of the CaR that sensitizes the receptor to lower concentrations of extracellular calcium. Another compound, NPS S467 (the enantiomer of NPS R467), with much reduced activity in this regard (27), is essentially inactive at equivalent doses and serves as a convenient control. As can be seen in Figure 3, 300 μM neomycin inhibited the secretion of PTHrP by mammary epithelial cells, as did NPS R467. However, NPS S467 did not. In an identical fashion, increasing levels of calcium, neomycin, and NPS R467 also inhibited PTHrP secretion from an immortalized but nontransformed mammary epithelial cell line, EpH4 cells (data not shown).
Figure 3.
PTHrP concentrations in conditioned media harvested from cultures of normal mammary epithelial cells exposed to the various concentrations of calcium noted on the graph. Cells were also exposed to 300 μM of neomycin and 2.5 μM of the calcimimetic NPS R467 or its less active isomer NPS S467. Bars represent the mean of three experiments; error bars represent the SEM. The differences between 0.5 mM and 2.5 mM or 5.0 mM CaCl2 were significant (P < 0.01 for 0.5 mM vs. 2.5 mM, P < 0.001 for 0.5 mM vs. 5.0 mM), but the difference between 2.5 and 5.0 mM was not. The differences between 0.5 mM CaCl2 and neomycin were significant (P < 0.001) as were the differences between NPS S467 and NPS R467 (P < 0.01).
CaR signaling regulates PTHrP production by mammary epithelial cells in vivo.
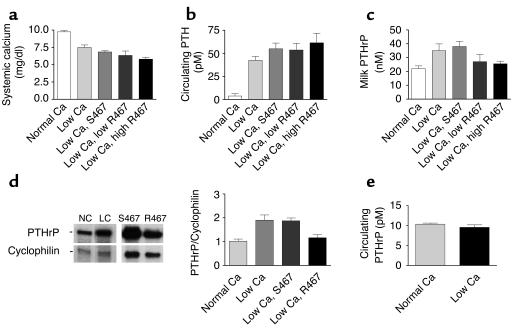
We hypothesized that the ability of the CaR to regulate PTHrP production by mammary epithelial cells would confer on the intact mammary gland the ability to sense systemic calcium and adjust PTHrP production in lactating mice. It has been previously reported that a low-calcium diet produces significant hypocalcemia in lactating rats (28). Therefore, we used dietary calcium restriction to manipulate serum calcium levels in lactating mice and measured the resultant production of PTHrP by the mammary glands. Mice were placed either on a low-calcium diet (0.01%) or normal chow (0.6% Ca) on the day of delivery and were allowed to lactate for 12 days before being sacrificed. As shown in Figure 4a, a low-calcium diet led to a significant reduction in the average plasma calcium from 9.7 mg/dl in controls to 7.5 mg/dl in mice on a calcium-restricted diet. As expected, this resulted in a significant increase in circulating PTH levels from 3.8 pM in the controls to 42.3 pM in the mice on a low-calcium diet (Figure 4b). To measure PTHrP production by the mammary gland, we assayed PTHrP concentrations in milk and PTHrP mRNA levels in the mammary glands. As seen in Figure 4c, placing lactating mice on a low-calcium diet increased the concentration of PTHrP in milk by 60 percent (22 nM in controls vs. 35 nM in calcium restricted mice). Calcium restriction also significantly increased the steady-state level of PTHrP mRNA in the mammary gland as assessed by RNase protection assay (Figure 4d). We also examined the circulating concentration of PTHrP in plasma from calcium-restricted or control mice (Figure 4e). We and others have previously shown that levels of PTHrP are increased in lactating versus virgin mice (29). However, despite the dramatic increases in PTHrP mRNA in the mammary gland and PTHrP concentrations in milk induced by calcium restriction, there were no calcium intake–dependent changes in circulating PTHrP levels as measured by a sensitive amino-terminal–specific radioimmunoassay. Thus, as predicted by our findings in cultured mammary epithelial cells in vitro, the lactating mammary gland responds to hypocalcemia by increasing PTHrP gene expression and secreting more PTHrP into milk.
Figure 4.
Plasma calcium, PTH, and PTHrP levels, milk PTHrP concentrations, and PTHrP mRNA expression levels in lactating mice fed a normal calcium diet, a low calcium diet, or a low calcium diet and treated with either NPS S467 or NPS R467. (a) Plasma calcium concentrations in mice fed either a normal-calcium diet (0.6%) or a low-calcium diet (0.01%). Mice on a low-calcium diet either received nothing (low Ca) or were infused with NPS S467 at 40 μmol/kg/d or with NPS R467 at doses of 4 μmol/kg/d (low R467) or 40 μmol/kg/d (high R467). Calcium restriction led to a significant decline in plasma calcium (P < 0.001, normal Ca vs. low Ca). The higher dose of NPS R467 led to a further significant decline in plasma calcium (P < 0.01, low Ca vs. low Ca plus high R467). (b) Plasma PTH in mice on normal or low-calcium diets with or without calcimimetic treatment. (c) Milk PTHrP concentrations in the same groups described above. Milk PTHrP concentrations were significantly different in groups given low Ca (P < 0.05) and low Ca plus S467 (P < 0.05) compared with the group on a normal diet. However, milk PTHrP concentrations in the mice treated with NPS R467 were not significantly different from baseline. (d) PTHrP mRNA levels in mammary glands of mice on a normal-calcium diet (NC), a low-calcium diet (LC), a low-calcium diet with NPS S467 treatment (40 μmol/kg/d), or a low-calcium diet with NPS R467 treatment (40 μmol/kg/d) assessed by RNase protection analysis. Forty micrograms of total RNA was assayed. The bar graph represents cumulative data from four animals per treatment normalized to cyclophilin. (e) Circulating plasma PTHrP levels in mice fed a normal-calcium diet or a low-calcium diet.
In order to determine whether the mammary effects of systemic hypocalcemia on mammary glands were mediated by the CaR, we next asked if they could be mitigated by infusing the calcimimetic agent NPS R467 into lactating mice on a low-calcium diet. Alzet minipumps were implanted in mice on day 4 after parturition and were programmed to deliver either NPS R467 or NPS S467 at doses of 4 μmol/kg or 40 μmol/kg per day. In preliminary experiments, treatment with NPS R467 at doses of 3.65 and 14.5 μmol/kg/d induced significant suppression of PTH levels (2.8 ± 0.5 pM and 2.5 ± 0.2 pM, respectively) compared with vehicle treatment (9.7 ± 0.8 pM) in virgin mice fed a low-calcium diet. As shown in Figure 4a, infusion of NPS R467 during calcium restriction led to a dose-dependent reduction in plasma calcium concentrations compared with that in mice receiving a low-calcium diet alone or mice receiving NPS S467. Interestingly, in contrast to what was observed in virgin mice, calcimimetic infusion did not suppress PTH levels during lactation (Figure 4b). However, stimulation of CaR signaling by infusion of NPS R467 (but not NPS S467) prevented the rise in milk PTHrP concentration (Figure 4c) and mammary gland PTHrP mRNA levels (Figure 4d) normally seen with calcium restriction. Therefore, the CaR mediates the changes in PTHrP gene expression and protein secretion that occur in response to hypocalcemia during lactation.
CaR signaling regulates milk composition and calcium transport into milk in vivo.
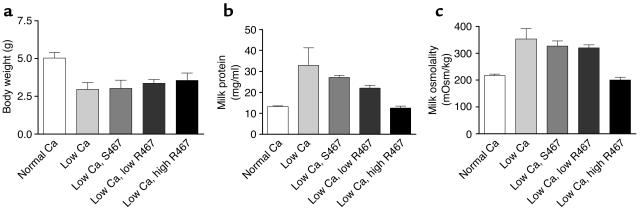
Lactating mice fed a calcium-restricted diet appeared to produce less milk that was more viscous than that of their counterparts fed a normal diet. Because the CaR has been shown to regulate water and bulk fluid transport in several epithelial cell types (2), this observation prompted us to ask if the CaR might affect these processes in mammary epithelial cells during lactation. Milking mice is imprecise, and milk production rates cannot be determined accurately in this manner. One alternative that has traditionally been used as an index of milk production is the growth rate of the pups being suckled (30). As seen in Figure 5a, maternal calcium restriction led to a pronounced reduction in the weight of the pups, suggesting that milk production was lower in these dams. Importantly, histological examination of the mammary glands from calcium-restricted mice did not show any changes suggestive of milk stasis and there was no epithelial apoptosis by TUNEL staining (data not shown). Therefore, the reduction in milk secretion was not the result of premature involution of the gland. We next measured the total protein concentration and osmolality of the milk (Figure 5, b and c). In addition to a reduction in overall milk production, calcium restriction led to significant increases in milk protein content and osmolality (Figure 5, b and c). As before, in order to determine whether these changes were mediated by the CaR, we infused either NPS R467 or NPS S467 into the mice fed a low-calcium diet. Figure 5b and Figure 5c show that NPS R467 but not NPS S467 lowered milk protein and osmolality, demonstrating that these effects are mediated by the CaR. Although there appeared to be a slight improvement in the average weight of pups suckling dams infused with the higher dose of NPS R467, these differences were not statistically significant. The effects of CaR signaling on milk protein concentration and osmolality (Figure 5) suggest that the CaR may contribute to the regulation of water transport into milk.
Figure 5.
Pup weight, milk protein concentration, and milk osmolality measured in lactating dams receiving a normal calcium diet or in calcium-restricted lactating mice treated with a calcimimetic or the control compound. (a) Average pup body weight on day 12 of lactation in litters suckling on dams fed a normal-calcium diet or a low-calcium diet with or without calcimimetic treatment. Mice received 40 μmol/kg/d of NPS S467 or one of two doses of NPS R467, 4 μmol/kg/d (low) or 40 μmol/kg/d (high). Pups were significantly smaller when mothers consumed a low-calcium diet. Calcimimetic treatment did not significantly increase pup weight. (b) Protein concentration of milk derived from the groups of mice described in a. Each bar represents the mean of three to nine samples; error bars represent the SEM. Milk protein was significantly higher in samples from mice on a low-calcium diet compared with mice on a normal-calcium diet (P < 0.001) or mice receiving NPS R467 (P < 0.01). However, the protein concentrations were not significantly different in milk from mice receiving NPS R467 compared with milk from those on a normal diet. (c) Milk osmolality in samples from the same groups described in a and b. Osmolality was significantly increased in milk from calcium-restricted mice (P < 0.001 vs. normal Ca) and mice receiving NPS S467 (P < 0.01 vs. normal Ca). The low dose of NPS R467 did not significantly decrease milk osmolality, but the high dose did (P < 0.001 for low Ca vs. low Ca plus high R467). mOsm, milliosmoles.
The CaR has a prominent role in regulating calcium handling by the renal tubules and may play a similar role in other epithelia (2, 7). Therefore, we asked whether CaR signaling might regulate the secretion of calcium into milk. The total calcium concentration of the milk did not change with calcium restriction (data not shown). However, much of the calcium in milk is complexed to proteins (10, 11), and when we corrected for the alterations in milk protein, there was a significant decline in the amount of calcium/protein in the milk derived from calcium-restricted dams (see Figure 6). As before, infusion of NPS R467 but not the control compound increased milk calcium content in a dose-dependent manner. However, calcimimetic treatment did not restore the calcium content of milk completely to baseline.
Figure 6.
Milk calcium content corrected for milk protein concentration in samples from mice fed either a normal-calcium diet or a low-calcium diet and either treated with NPS S467 or NPS R467 at two different doses. Bars represent the means of three to nine samples; error bars represent the SEM. Calcium restriction led to a significant decrease in the calcium content of the milk (P < 0.001 for normal Ca vs. low Ca). Treatment with the higher dose of NPS R467 significantly increased the calcium content of the milk compared with a low-calcium diet (P < 0.05); however it remained significantly lower than the calcium content in mice fed a normal diet (P < 0.05).
CaR signaling regulates calcium transport across mammary epithelial cells in vitro.
Although our data in vivo suggested that the CaR mediates the effects of calcium restriction on milk calcium content, we wanted to assess more directly whether the CaR could regulate the directional transport of calcium across mammary epithelial cells. As noted previously, mammary epithelial cells form hollow spheres if cultured in a three-dimensional basement membrane preparation in the presence of lactogenic hormones (26). Cells within these “mammospheres” fully differentiate, express milk proteins, and secrete a milk-like substance into the central lumen. We adapted this culture system to allow us to measure 45Ca accumulation from the media to the lumen of the mammospheres as an index of transcellular calcium transport. In order to ensure that 45Ca could not passively diffuse from the media into the lumen of the mammospheres, we first assayed the integrity of the mammosphere tight junctions using the technique of Chen and colleagues (24). Mammosphere cultures were treated with NHS-LC-biotin, a cell-impermeant marker that covalently crosslinks to membrane surfaces and that can freely diffuse through open paracellular channels. Sections through the mammospheres were then stained with fluorescently-tagged avidin to detect the bound NHS-LC-biotin. As seen in Figure 7a, in untreated cultures, NHS-LC-biotin bound only to the basolateral cell surfaces, demonstrating that the tight junctions were intact and that there was no paracellular leak of media contents into the lumens of these structures. In contrast, in the presence of EGTA (Figure 7b), the tight junctions became leaky and NHS-LC-biotin diffused from the media into the lumen, labeling the apical membranes of the cells.
Figure 7.
CaR-stimulated transcellular calcium transport in mouse mammary epithelial cells cultured on Matrigel. (a) Fluorescent micrograph of a section through a mammosphere incubated with NHS-LC-biotin and stained with fluorescein-tagged avidin. As one can see, NHS-LC-biotin added to the media is excluded from the lumen, documenting that there is no paracellular leak through the tight junctions between epithelial cells. Lu, lumen; am, apical membrane; bm, basolateral membrane. (b) Same as in a, except that in addition to NHS-LC-biotin, 2.5 mM EGTA was added to the media of the mammosphere cultures. In this instance, the tight junctions became leaky and the NHS-LC-biotin labeled both basolateral and apical membranes. (c) 45Ca accumulation within the lumens of mammospheres made from WT mice (white bars) or from BLG-Cre/PTHrPlox/– mice (black bars) cultured in 1, 5, or 10 mM CaCl2 or in 1 mM CaCl2 with 2.5 μM NPS S467 or NPS R467 added. Mammary epithelial cells from the BLG-Cre/PTHrPlox/– mice did not secrete PTHrP (not shown). As can be seen, extracellular calcium stimulates the accumulation of 45Ca in the lumen of mammospheres in a dose-dependent manner, regardless of the presence or absence of PTHrP. Likewise, stimulation of CaR signaling with NPS R467 led to a significant increase in the luminal accumulation of tracer compared with treatment with NPS S467 in both types of cells (WT, P < 0.0001; BLG-Cre/PTHrPlox–, P < 0.05). Each bar represents the mean of three experiments; error bars represent the SEM.
We next examined the ability of mammospheres to transport calcium into their lumens. Normal mammary epithelial cells were allowed to form spheres in culture, after which 45Ca was added to the media. After 16 hours of incubation, we found that 75% of the total mammosphere 45Ca was contained within the luminal contents (data not shown), demonstrating that the mammary epithelial cells in these structures are able to take up calcium from the media and transport it into the mammosphere lumens. In order to examine the effects of CaR signaling on this process, mammospheres were incubated with 45Ca in basal media and in the presence of varying concentrations of extracellular calcium or with 1 mM CaCl2 plus NPS R467 or NPS S467 at a concentration of 2.5 μM. As shown, increasing extracellular calcium concentrations caused a dose-dependent increase in the accumulation of 45Ca in the mammosphere lumens (Figure 7c, white bars). Likewise, treatment with the calcimimetic agent NPS R-467 approximately doubled the amount of 45Ca in the luminal contents of the mammospheres (Figure 7c), while treatment with NPS S467 had no effect. These data demonstrate that activation of the CaR on the basolateral surface of mammary epithelial cells can stimulate the directional transport of calcium into the lumen of mammospheres.
PTHrP has previously been shown to regulate the transfer of calcium across the placenta from mother to fetus. Because CaR signaling modulates both PTHrP production by mammary cells and transcellular calcium transport into milk, we asked if PTHrP might mediate the calcium-regulated changes in calcium transport that we observed in the mammary gland. In order to approach this question we made mammospheres from PTHrP-deficient mammary epithelial cells. We recently showed that by using the Cre-lox recombinase system, we could efficiently remove the PTHrP gene specifically from lactating mammary epithelial cells (15). To accomplish this we made use of the promoter for the BLG gene to express Cre within mammary cells during lactation. We harvested mammary epithelial cells from BLG-Cre/PTHrPlox/– mice and grew them as mammospheres. These cells no longer expressed PTHrP mRNA and secreted no PTHrP into the media of the cultures (data not shown). However, mammospheres made with these cells showed identical regulation of 45Ca transport by the CaR compared with the WT cells (Figure 7c, compare black bars with white bars). Therefore, PTHrP is not necessary for calcium transport into the lumen of mammospheres, and changes in PTHrP secretion do not mediate the changes in calcium transport in response to CaR signaling.
Discussion
Milk production is necessary for successful reproduction in mammals because milk provides all nutrients essential for neonatal growth, including calcium (10, 11). The lactating mammary gland mediates the transfer of large amounts of calcium from mother to offspring (10–12). These calcium fluxes pose unique challenges for systemic calcium metabolism in the mother as well as for cellular calcium dynamics in mammary epithelial cells. The maternal demands for calcium delivery to the gland are met by a combination of dietary calcium absorption, renal calcium conservation, and the mobilization of skeletal calcium reserves through increased rates of bone resorption (12). Once calcium arrives at the mammary gland, it must be transported up a steep concentration gradient into milk. Details of the regulation of this process remain incomplete, but calcium transport is thought to occur via a transcellular route involving the Golgi apparatus and secretory vesicles of the mammary epithelial cell (10, 11, 31, 32). It is not known how calcium crosses the basolateral surface of mammary cells, but once inside the cell it is rapidly transported from the cytoplasm into the Golgi apparatus through the actions of a calcium-ATPase. In the Golgi apparatus, most calcium becomes complexed with casein, phosphate, and citrate (33–35), and is secreted into milk in that form. The concentration of free calcium in milk is about 3 mM, and this calcium also is thought to enter via the Golgi apparatus, as the work of Linzell and Peaker (36) suggested that there is no direct apical transport of calcium into milk. Thus, calcium enters milk through the secretory pathway.
Our data demonstrate that the CaR participates in the control of milk secretion by regulating calcium (and perhaps water transport into milk) and by controlling mammary epithelial secretion of PTHrP. We have found that the CaR is expressed on mammary epithelial cells and that its expression varies with the stage of mammary gland development, being downregulated during pregnancy and upregulated during lactation. Stimulation of the calcium receptor in cultured mammary epithelial cells inhibits the secretion of PTHrP, and hypocalcemia secondary to dietary calcium restriction upregulates PTHrP production in the mammary gland in vivo. In addition, calcium restriction decreases the calcium content of milk, increases milk osmolality and protein concentration, and decreases overall milk production. These effects of calcium restriction are mediated by the mammary epithelial calcium receptor, for infusion of calcimimetic compounds prevents most of the changes noted above. Finally, stimulation of the basolateral calcium receptor directly increases transcellular calcium transport in mammary epithelial cells in vitro, an effect that does not depend on changes in PTHrP secretion. These data demonstrate that the mammary gland becomes a calcium-sensing organ during lactation and that it adjusts its secretion of PTHrP and calcium in response to changes in systemic calcium concentrations.
A number of groups have demonstrated that circulating PTHrP levels are elevated during lactation (12, 16, 29, 37–39), and it has been suggested that PTHrP might contribute to the mobilization of skeletal calcium reserves during this time. It has also been suggested that the PTHrP in milk may exert effects on the neonatal gut and/or neonatal calcium metabolism (8, 12). We recently reported that targeted deletion of the PTHrP gene from mammary epithelial cells leads to a reduction in systemic PTHrP levels, reduced bone turnover, and the preservation of bone mass in lactating mice (15). These data confirm that the lactating breast does, in fact, secrete PTHrP into the systemic circulation in order to influence systemic calcium and bone metabolism (15). Therefore, it is of considerable interest that the intact, lactating mammary gland adjusts PTHrP production in response to the systemic calcium concentration. This implies that PTHrP might act as part of a feedback loop that coordinates maternal and/or neonatal calcium metabolism with the amount of calcium available for milk production. However, despite the significant increases in PTHrP mRNA levels within the gland, we were not able to detect increased PTHrP concentrations in the circulation in the calcium-restricted mice. There are at least two potential explanations for the failure to detect increased systemic PTHrP levels. First, the systemic hypocalcemia and secondary hyperparathyroidism that resulted from dietary calcium restriction may have somehow overridden mammary secretion of PTHrP. Second, the levels of PTHrP in the circulation during lactation are near the threshold of sensitivity of the assays used, and therefore the assays may not be able to detect small but biologically meaningful changes in circulating levels of PTHrP. Another possibility is that the regulation of PTHrP levels in milk in response to changes in systemic calcium concentrations is meant to coordinate maternal and neonatal calcium metabolism in some way. Preliminary data demonstrate that pups consuming milk with different amounts of PTHrP have varying total body calcium content. Thus, by regulating mammary PTHrP production, the mammary CaR may act to coordinate calcium handling by the mammary gland with both maternal and neonatal systemic bone and calcium metabolism.
It is interesting that, although calcimimetic treatment modulated the production of PTHrP and calcium transport by the mammary glands during lactation, it did not suppress PTH secretion at the doses used in our experiments. However, pilot experiments did show that NPS R467 prevented the rise in PTH induced by a low-calcium diet in virgin mice. These observations are consistent with previous studies showing that PTH secretion was not suppressed when serum calcium was raised in lactating rats on a low-calcium diet (40). Furthermore, it has been shown that, when compared with cells from virgin rats, dispersed parathyroid cells cultured from lactating rats show alterations in both the calcium set-point for PTH secretion and the amount of nonsuppressible PTH produced (41). The relative nonsuppressible nature of PTH secretion during lactation might also contribute to the observed acceleration of bone resorption.
Our experiments in vivo suggested that calcium influences its own transport across mammary epithelial cells and into milk. In order to test this idea, we developed a model of transepithelial calcium transport in vitro that would be free of the potentially confounding systemic effects of hypocalcemia. Our data from cultured mammospheres confirm that activation of the basolateral CaR does indeed stimulate calcium transport across the mammary epithelium. In the kidney, the CaR regulates transcellular calcium transport in the cortical thick ascending limb (42) and in the distal convoluted tubule (43). In Madin-Darby canine kidney cells, a model of the distal convoluted tubule, it has been suggested that CaR activation inhibits basolateral calcium extrusion by inhibiting plasma membrane Ca-ATPase (PMCA) activity. Interestingly, it has been shown that the PMCA2b isoform is highly expressed in the mammary gland during lactation (44). Much more work regarding the molecular mechanisms of calcium entry and partitioning within mammary epithelial cells will be needed to understand the effects of CaR signaling on these processes. The mammosphere system should prove valuable in this effort.
We have shown that the lactating mammary gland is a calcium-sensing organ. In response to changes in extracellular calcium, it modulates its secretion of PTHrP, its transport of calcium, and perhaps also its transport of water. It would appear from our data that the CaR adjusts calcium transport and overall milk production as a function of the maternal supply of calcium. In this case, the CaR would be functioning as a nutrient sensor in a manner similar to the actions of its evolutionary ancestors, the bacterial periplasmic binding proteins (2, 5, 6). Given the metabolic costs of lactation, it would be an adaptive advantage for a mother to reduce the use of nutrients for milk production if her own supply became limiting. We propose that the mammary gland CaR acts as a component of a protective mechanism guarding against hypocalcemia during lactation. When calcium becomes limiting, reduced CaR signaling would lead to reductions in calcium secretion and milk production. This notion is supported by the further decline in systemic calcium levels in calcium-restricted mice treated with NPS R467, which effectively short-circuited this defense (see Figure 4a). We believe that this system may represent another mechanism to ensure that reproduction proceeds only when adequate resources are available.
Acknowledgments
We thank John Fox and Ed Nemeth at NPS Pharmaceuticals for helpful suggestions and for the generous donation of calcimimetic compounds. We are indebted to Jack Martin for measuring circulating PTHrP concentrations. We thank Arthur Broadus for valuable discussions and for a critical reading of the manuscript. This work was facilitated by core facilities of the Yale Core Center for Musculoskeletal Disorders (NIH AR-6032). This work was supported by NIH grants 5F32 DK-059719 to J. VanHouten and CA-94175 and DK-55501 to J. Wysolmerski.
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Nonstandard abbreviations used: calcium-sensing receptor (CaR); parathyroid hormone (PTH); parathyroid hormone–related protein (PTHrP); sulfosuccinimidyl-6-(biotinamido)hexanoate (NHS-LC-biotin); β-lactoglobulin (BLG).
References
- 1.Brown EM, et al. Cloning and characterization of an extracellular Ca(2+)-sensing receptor from bovine parathyroid. Nature. 1993;366:575–580. doi: 10.1038/366575a0. [DOI] [PubMed] [Google Scholar]
- 2.Brown EM, MacLeod RJ. Extracellular calcium sensing and extracellular calcium signaling. Physiol. Rev. 2001;81:239–297. doi: 10.1152/physrev.2001.81.1.239. [DOI] [PubMed] [Google Scholar]
- 3.Chattopadhyay N, Mithal A, Brown EM. The calcium-sensing receptor: a window into the physiology and pathophysiology of mineral ion metabolism. Endocr. Rev. 1996;17:289–307. doi: 10.1210/edrv-17-4-289. [DOI] [PubMed] [Google Scholar]
- 4.Brown EM. Extracellular Ca2+ sensing, regulation of parathyroid cell function, and role of Ca2+ and other ions as extracellular (first) messengers. Physiol. Rev. 1991;71:371–411. doi: 10.1152/physrev.1991.71.2.371. [DOI] [PubMed] [Google Scholar]
- 5.Conklin BR, Bourne HR. Homeostatic signals. Marriage of the flytrap and the serpent. Nature. 1994;367:22. doi: 10.1038/367022a0. [DOI] [PubMed] [Google Scholar]
- 6.Oh BH, et al. Three-dimensional structures of the periplasmic lysine/arginine/ornithine-binding protein with and without a ligand. J. Biol. Chem. 1993;268:11348–11355. [PubMed] [Google Scholar]
- 7.Quamme GA. Effect of hypercalcemia on renal tubular handling of calcium and magnesium. Can. J. Physiol. Pharmacol. 1982;60:1275–1280. doi: 10.1139/y82-187. [DOI] [PubMed] [Google Scholar]
- 8.Philbrick WM, et al. Defining the roles of parathyroid hormone-related protein in normal physiology. Physiol. Rev. 1996;76:127–173. doi: 10.1152/physrev.1996.76.1.127. [DOI] [PubMed] [Google Scholar]
- 9.Strewler GJ. The physiology of parathyroid hormone-related protein. N. Engl. J. Med. 2000;342:177–185. doi: 10.1056/NEJM200001203420306. [DOI] [PubMed] [Google Scholar]
- 10.Shennan DB, Peaker M. Transport of milk constituents by the mammary gland. Physiol. Rev. 2000;80:925–951. doi: 10.1152/physrev.2000.80.3.925. [DOI] [PubMed] [Google Scholar]
- 11.Horst RL, Goff JP, Reinhardt TA. Calcium and vitamin D metabolism during lactation. J. Mammary Gland Biol. Neoplasia. 1997;2:253–263. doi: 10.1023/a:1026384421273. [DOI] [PubMed] [Google Scholar]
- 12.Kovacs CS, Kronenberg HM. Maternal-fetal calcium and bone metabolism during pregnancy, puerperium, and lactation. Endocr. Rev. 1997;18:832–872. doi: 10.1210/edrv.18.6.0319. [DOI] [PubMed] [Google Scholar]
- 13.Budayr AA, et al. High levels of a parathyroid hormone-like protein in milk. Proc. Natl. Acad. Sci. U. S. A. 1989;86:7183–7185. doi: 10.1073/pnas.86.18.7183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thiede MA, Rodan GA. Expression of a calcium-mobilizing parathyroid hormone-like peptide in lactating mammary tissue. Science. 1988;242:278–280. doi: 10.1126/science.3175653. [DOI] [PubMed] [Google Scholar]
- 15.VanHouten JN, et al. Mammary-specific deletion of parathyroid hormone–related protein preserves bone mass during lactation. J. Clin. Invest. 2003;112:1429–1436. doi:10.1172/JCI200319504. doi: 10.1172/JCI19504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sowers MF, et al. Elevated parathyroid hormone-related peptide associated with lactation and bone density loss. JAMA. 1996;276:549–554. [PubMed] [Google Scholar]
- 17.Cheng I, et al. Identification and localization of the extracellular calcium-sensing receptor in human breast. J. Clin. Endocrinol. Metab. 1998;83:703–707. doi: 10.1210/jcem.83.2.4558. [DOI] [PubMed] [Google Scholar]
- 18.Sanders JL, et al. Extracellular calcium-sensing receptor expression and its potential role in regulating parathyroid hormone-related peptide secretion in human breast cancer cell lines. Endocrinology. 2000;141:4357–4364. doi: 10.1210/endo.141.12.7849. [DOI] [PubMed] [Google Scholar]
- 19.Dunbar ME, et al. Stromal cells are critical targets in the regulation of mammary ductal morphogenesis by parathyroid hormone-related protein. Dev. Biol. 1998;203:75–89. doi: 10.1006/dbio.1998.9029. [DOI] [PubMed] [Google Scholar]
- 20.Kifor O, et al. Reduced immunostaining for the extracellular Ca2+-sensing receptor in primary and uremic secondary hyperparathyroidism. J. Clin. Endocrinol. Metab. 1996;81:1598–1606. doi: 10.1210/jcem.81.4.8636374. [DOI] [PubMed] [Google Scholar]
- 21.Harlow, E., and Lane, D. 1999. Unmasking hidden epitopes with proteases. In Using antibodies: a laboratory manual. E. Harlow and D. Lane, editors. Cold Spring Harbor Laboratory Press. Cold Spring Harbor, New York, USA. 214.
- 22.Debnath J, Muthuswamy SK, Brugge JS. Morphogenesis and oncogenesis of MCF-10A mammary epithelial acini grown in three-dimensional basement membrane cultures. Methods. 2003;30:256–268. doi: 10.1016/s1046-2023(03)00032-x. [DOI] [PubMed] [Google Scholar]
- 23.Kovacs CS, Manley NR, Moseley JM, Martin TJ, Kronenberg HM. Fetal parathyroids are not required to maintain placental calcium transport. J. Clin. Invest. 2001;107:1007–1015. doi: 10.1172/JCI11321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen Y, Merzdorf C, Paul DL, Goodenough DA. COOH terminus of occludin is required for tight junction barrier function in early Xenopus embryos. J. Cell Biol. 1997;138:891–899. doi: 10.1083/jcb.138.4.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Daniel, C.W., and Silberstein, G.B. 1987. Postnatal development of the rodent mammary gland. In The mammary gland: development, regulation, and function. M.C. Neville and C.W. Daniel, editors. Plenum Press. New York, New York, USA. 3–36.
- 26.Barcellos-Hoff MH, Aggeler J, Ram TG, Bissell MJ. Functional differentiation and alveolar morphogenesis of primary mammary cultures on reconstituted basement membrane. Development. 1989;105:223–235. doi: 10.1242/dev.105.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nemeth EF, et al. Calcimimetics with potent and selective activity on the parathyroid calcium receptor. Proc. Natl. Acad. Sci. U. S. A. 1998;95:4040–4045. doi: 10.1073/pnas.95.7.4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garner SC, Peng TC, Hirsch PF, Boass A, Toverud SU. Increase in serum parathyroid hormone concentration in the lactating rat: effects of dietary calcium and lactational intensity. J. Bone Miner. Res. 1987;2:347–352. doi: 10.1002/jbmr.5650020412. [DOI] [PubMed] [Google Scholar]
- 29.VanHouten JN, Wysolmerski JJ. Low estrogen and high PTHrP levels contribute to accelerated bone resorption and bone loss in lactating mice. Endocrinology. 2003;144:5521–5529. doi: 10.1210/en.2003-0892. [DOI] [PubMed] [Google Scholar]
- 30.Garner SC, Boass A, Toverud SU. Parathyroid hormone is not required for normal milk composition or secretion or lactation-associated bone loss in normocalcemic rats. J. Bone Miner. Res. 1990;5:69–75. doi: 10.1002/jbmr.5650050111. [DOI] [PubMed] [Google Scholar]
- 31.Neville MC, Peaker M. The secretion of calcium and phosphorus into milk. J. Physiol. 1979;290:59–67. doi: 10.1113/jphysiol.1979.sp012759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Neville MC, Peaker M. Calcium fluxes in mouse mammary tissue in vitro: intracellular and extracellular pools. J. Physiol. (Lond.) 1982;332:497–517. doi: 10.1113/jphysiol.1982.sp014088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Virk SS, Kirk CJ, Shears SB. Ca2+ transport and Ca2+-dependent ATP hydrolysis by Golgi vesicles from lactating rat mammary glands. Biochem. J. 1985;226:741–748. doi: 10.1042/bj2260741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.West DW. Energy-dependent calcium sequestration activity in a Golgi apparatus fraction derived from lactating rat mammary gland. Biochim. Biophys. Acta. 1981;673:374–386. doi: 10.1016/0304-4165(81)90469-4. [DOI] [PubMed] [Google Scholar]
- 35.Neville MC, Selker F, Semple K, Watters C. ATP-dependent calcium transport by a Golgi-enriched membrane fraction from mouse mammary gland. J. Membr. Biol. 1981;61:97–105. doi: 10.1007/BF02007636. [DOI] [PubMed] [Google Scholar]
- 36.Linzell JL, Peaker M. Changes in mammary gland permeability at the onset of lactation in the goat: an effect on tight junctions? J. Physiol. 1973;230:13P–14P. [PMC free article] [PubMed] [Google Scholar]
- 37.Lippuner K, Zehnder HJ, Casez JP, Takkinen R, Jaeger P. PTH-related protein is released into the mother’s bloodstream during lactation: evidence for beneficial effects on maternal calcium-phosphate metabolism. J. Bone Miner. Res. 1996;11:1394–1399. doi: 10.1002/jbmr.5650111004. [DOI] [PubMed] [Google Scholar]
- 38.Yamamoto M, et al. Suckling-mediated increases in urinary phosphate and 3′,5′-cyclic adenosine monophosphate excretion in lactating rats: possible systemic effects of parathyroid hormone-related protein. Endocrinology. 1991;129:2614–2622. doi: 10.1210/endo-129-5-2614. [DOI] [PubMed] [Google Scholar]
- 39.Dobnig H, et al. Elevated parathyroid hormone-related peptide level after human gestation: relationship to changes in bone and mineral metabolism. J. Clin. Endocrinol. Metab. 1995;80:3699–3707. doi: 10.1210/jcem.80.12.8530622. [DOI] [PubMed] [Google Scholar]
- 40.Garner SC, Boass A, Toverud SU. Hypercalcemia fails to suppress elevated serum parathyroid hormone concentrations during lactation in rats. J. Bone Miner. Res. 1989;4:577–583. doi: 10.1002/jbmr.5650040417. [DOI] [PubMed] [Google Scholar]
- 41.Schultz VL, Boass A, Garner SC, Toverud SU. Altered regulation of parathyroid hormone secretion by calcium in pregnant and lactating rats. J. Bone Miner. Res. 1997;12:903–908. doi: 10.1359/jbmr.1997.12.6.903. [DOI] [PubMed] [Google Scholar]
- 42.Motoyama HI, Friedman PA. Calcium-sensing receptor regulation of PTH-dependent calcium absorption by mouse cortical ascending limbs. Am. J. Physiol. Renal Physiol. 2002;283:F399–F406. doi: 10.1152/ajprenal.00346.2001. [DOI] [PubMed] [Google Scholar]
- 43.Blankenship KA, et al. The calcium-sensing receptor regulates calcium absorption in MDCK cells by inhibition of PMCA. Am. J. Physiol. Renal Physiol. 2001;280:F815–F822. doi: 10.1152/ajprenal.2001.280.5.F815. [DOI] [PubMed] [Google Scholar]
- 44.Reinhardt TA, Horst RL. Ca2+-ATPases and their expression in the mammary gland of pregnant and lactating rats. Am. J. Physiol. 1999;276:C796–C802. doi: 10.1152/ajpcell.1999.276.4.C796. [DOI] [PubMed] [Google Scholar]