Abstract
FLORICAULA (FLO) of Antirrhinum and LEAFY (FLY) of Arabidopsis regulate the formation of floral meristems. To examine whether same mechanisms control floral development in distantly related species such as grasses, we isolated RFL, FLO-LFY homolog of rice, and examined its expression and function. Northern analysis showed that RFL is expressed predominantly in very young panicle but not in mature florets, mature leaves, or roots. In situ hybridization revealed that RFL RNA was expressed in epidermal cells in young leaves at vegetative growth stage. After the transition to reproductive stage, RFL RNA was detected in all layers of very young panicle including the apical meristem, but absent in the incipient primary branches. As development of branches proceeds, RFL RNA accumulation localized in the developing branches except for the apical meristems of the branches and secondary branch primordia. Expression pattern of RFL raised a possibility that, unlike FLO and LFY, RFL might be involved in panicle branching. Transgenic Arabidopsis plants constitutively expressing RFL from the cauliflower mosaic virus 35S promoter were produced to test whether 35S-RFL would cause similar phenotype as observed in 35S-LFY plants. In 35S-RFL plants, transformation of inflorescence meristem to floral meristem was rarely observed. Instead, development of cotyledons, rosette leaves, petals, and stamens was severely affected, demonstrating that RFL function is distinct from that of LFY. Our results suggest that mechanisms controlling floral development in rice might be diverged from that of Arabidopsis and Antirrhinum.
Gramineae is a large and variable family. Many features of flower development and mature architecture of grass flowers and inflorescences are distinct from those of dicots. Although our knowledge on the genetic network governing initiation and morphogenesis of flowers increased significantly in the last several years, little is known about molecular mechanisms controlling floral development in grass species. Understanding grass flower development may offer general insights into the genetic and developmental bases of morphological evolution among the plant species. Rice (Oryza sativa L.) has several advantages that make it a good candidate as a model species to study molecular basis of grass flower development (1). Rice is a diploid species with a small genome (430 Mb/haploid), and analyses of the rice genome and cDNAs have rapidly progressed (2, 3). Moreover, transgenic rice plants can be relatively easily produced by Agrobacterium-mediated transformation (4).
Genetic and molecular studies with two dicot plants, Antirrhinum and Arabidopsis, have shown that the genetic network controlling flower development is conserved at least in the two dicot species (5–7). After the transition from vegetative to reproductive development, floral meristems are initiated by the action of a set of genes called floral meristem identity genes. Among them, FLORICAULA (FLO) of Antirrhinum and its Arabidopsis counterpart LEAFY (LFY) seem to play the most important role for the establishment of floral fate. In strong flo and lfy mutant plants, flowers are transformed into inflorescence shoots (8, 9). FLO/LFY encode putative transcription factors that do not show significant homology to any known genes (8, 9).
To understand molecular mechanisms controlling floral meristem initiation and inflorescence structure of rice we have isolated RFL, the FLO/LFY homolog of rice, and analyzed its expression and function. We found that the function of RFL is distinct from that of LFY. Our analysis on RFL expression showed that it is unlikely that RFL is absolutely required for floral initiation in rice. Instead, the expression pattern of RFL suggests its possible involvement in panicle branching. Our results show that the findings obtained from Arabidopsis and Antirrhinum may not be simply applied as a general model to other distantly related species such as grasses.
MATERIALS AND METHODS
Construction and Screening of cDNA Library.
To construct a cDNA library, total RNA was isolated from young panicles of rice (Oryza sativa L., cv. Toride 1). Poly(A)+ mRNA was purified from the total RNA by using Magnesphere streptavidin paramagnetic particles (Promega). Double-stranded cDNA was synthesized according to the protocol of supplier (Pharmacia) and cloned into ZAPII vector (Stratagene).
Primers used to amplify rice FLO/LFY-like genes by the PCR were designed based on highly conserved sequences found in FLO/LFY. The 5′ primer was 5′-TAC/TATA/CAAC/TAAA/GCCA/G/C/TAAA/GATG-3′ and the 3′ primer was 5′-AGCC/TTG/TGTG/TGGG/C/AACA/GTACCA-3′. Genomic DNA of rice cv. Toride 1 was used as templates for the PCR. The PCR product of 235 bp was cloned into pGEM-T (Promega) and used as a probe for screening a cDNA library and Southern blot analysis.
Approximately 106 plaques were screened with a gel-purified radiolabeled probe. Hybridization and washes were carried out by standard protocol. The plasmids containing positive cDNA were rescued in vivo from phages according to the manufacturer’s protocol (Stratagene). Both strands of the cDNA were sequenced by an automated sequencer (Applied Biosystems 373A) by using dideoxy-cycle sequencing protocol.
DNA Isolation and Southern Blot Analysis.
DNA was isolated from young leaves of rice cv. Toride 1 according to the method described previously (10). Two micrograms of the genomic DNA was digested with restriction enzymes and electrophoresed. A 235-bp fragment amplified by PCR was labeled with digoxygenin and used as a probe. Hybridization and washing were carried out as described in the protocol (Boehringer Mannheim).
RNA Isolation and Northern Blot Analysis.
RNA was isolated from various tissues by a published method (11). Twenty micrograms of total RNA was separated by electrophoresis. The gel was blotted to nylon membrane (Hybond N+) and hybridized with radiolabeled probe.
In Situ Hybridization.
Full-length RFL cDNA cloned into pBS(SK+) was linearized and used as a template to produce digoxygenin-labeled antisense RNA probe. Hybridization, washing, and detection were carried out according to Kouchi et al. (12).
Arabidopsis Transformation.
A 1.5-kb RFL cDNA in pBS(SK+) was digested by XbaI and EcoRI and subsequently cloned into pIG121-Hm (13) to produce 35S-RFL (accession no. AB005620). The 35S-RFL construct was introduced to Arabidopsis, ecotype Columbia, by vacuum infiltration (14). T1 generation seeds were sown on MS medium containing 2% sucrose, 0.8% agar, and 30 mg/liter kanamycin. Kanamycin-resistant plants were transferred to soil and grown further in a glass house at 24°C under long day conditions (14 h light/10 h dark).
RESULTS
Isolation of RFL.
The FLO/LFY homolog was obtained by screening a cDNA library prepared from young rice panicles. The longest clone was named as RFL and used for further analysis. RFL cDNA is 1.5 kb and encodes an ORF of 379 aa (Fig. 1). Deduced amino acid sequence of RFL has a higher homology in the C-terminal half than in the N-terminal half. Overall identity of RFL to FLO and LFY were 48% and 44%, respectively. Southern blot analysis revealed that RFL exists as a single-copy gene in rice (data not shown). Comparison between RFL cDNA and genomic sequence revealed that the number and position of introns are precisely conserved in RFL, FLO, and LFY (data not shown).
Figure 1.
Comparison of amino acid sequences encoded by RFL, FLO, and LFY. Conserved amino acids are shown by asterisks.
Expression Pattern of RFL During Panicle Development.
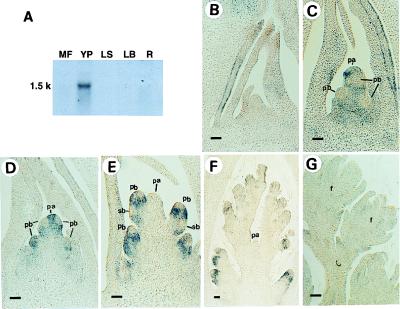
Northern analysis showed that RFL is expressed predominantly in young panicles but not in mature florets, leaves, or roots (Fig. 2A). Temporal and spatial expression patterns of RFL RNA were further examined by in situ hybridization.
Figure 2.
Distribution of RFL RNA in rice plants. (A) Northern blot analysis. MF, mature florets; YP, young panicles; LS, leaf sheaths; LB, leaf blades; R, roots. (B–G) RFL expression analyzed by in situ hybridization. (B) A vegetative shoot apex. (C and D) A young panicle at primary branch primordia differentiation stage. (E) A young panicle at secondary branch primordia differentiation stage. (F) A developing panicle. Four primary branches at various developmental stages in a panicle are shown. The oldest primary branch (arrow) is in the floret differentiation stage. (G) Developing florets. All floral organis have developed by this stage. pb, primary branch; sb, secondary branch; pa, panicle apex; f, floret. (Bar = 50 μm.)
A vegetative shoot apex produces leaves distichously. After the transition to reproductive growth, a young panicle apex produces several bracts of the panicle. Primary branches grow out from the axil of each bract. Secondary branches are produced from the primary branches. These branches terminate in a single-flowered spikelet (15).
Before transition to reproductive growth, RFL RNA was detected in epidermal cells at the marginal region in young leaves but not in the vegetative shoot apical meristem or stem tissue (Fig. 2B). After the transition, RFL RNA expression was observed in all layers of the very young panicle producing primary branch primordia but was absent in the primary branch differentiation sites (Fig. 2 C and D). As development of branches proceeded, RFL RNA accumulation was localized in the developing branches except for the apical meristem of the branches (Fig. 2 E and F). The apical meristem of the panicle axis loses its activity and degenerates after the production of several primary branch primordia, leaving a scar on the main rachis (15). The RFL RNA expression was observed in the vegetative apical meristem at a very early stage of panicle development (Fig. 2 B and C), then it started to diminish in the corpus of the panicle axis at the middle stage of primary branch differentiation (Fig. 2D). After all the primordia of primary branches had initiated, RFL RNA disappeared entirely from the main axis of the panicle (Fig. 2 E and F). Down-regulation of the RFL expression was observed again in incipient or developing secondary branches (Fig. 2E). No detectable level of RFL RNA expression was found in developing panicles after the branch formation stage (Fig. 2 F and G). Control sections hybridized with the sense RFL RNA probe gave no signal above background (data not shown).
Ectopic Expression of RFL in Arabidopsis.
Ectopic expression of Arabidopsis LFY by cauliflower mosaic virus (CaMV) 35S promoter caused transformation of lateral and main inflorescence shoots into floral meristems (16). To examine whether expression of RFL gene product in Arabidopsis would result in the phenotype similar to that of 35S-LFY plants, we made transgenic Arabidopsis plants containing 35S-RFL.
T2 plants of 10 independent transgenic lines were grown in a glass house to examine phenotypic alterations. Generally, the phenotype observed in 35S-RFL was different from that reported in the 35S-LFY plants. We found transformation of inflorescence meristems into flower meristem only in 2 cases out of 100 plants derived from the 10 lines, and the observed terminal flower was extremely abnormal, as shown in Fig. 3A. This suggests that RFL possesses similar function with LFY to a very limited extent. We observed defects in both vegetative and reproductive growth in nine lines, which was not found in 35S-LFY transgenic plants. Six lines showed highly abnormal morphology, and three were less severely affected. One line did not show any morphological difference from wild-type plants, and this line was not analyzed further. Cotyledons of 35S-RFL plants of all the nine lines were cup-shaped and narrower than those of wild-type plants (Fig. 3 F–I). Cosegregation of this cotyledon phenotype and kanamycin resistance was genetically confirmed in all nine lines (data not shown). Rosette leaves were also affected in all nine lines (Fig. 3 J and K). Curling of rosette leaves was commonly observed. The 35S-RFL transgenic plants with severe phenotypes did not have distinct petioles, and plant size was significantly reduced (Fig. 3J). The severity of phenotypes in cotyledons correlated well with the curled and wrinkled rosette leaves and shorter petioles. Flowers of 35S-RFL plants were also affected. Petals and stamens in 35S-RFL plants were shorter than those in wild-type plants (Fig. 3 B–E).
Figure 3.
Transgenic Arabidopsis plants transformed with 35S-RFL. (A) A 35S-RFL plant with an abnormal terminal flower. (B–D) Flowers with short petals and short stamens in 35S-RFL plants (B and D) and wild-type flowers (C and E). A sepal and a petal were removed from a flower in D and E. (F–H) Seedlings of 35S-RFL (F and H) and wild-type (G and I) plants. (J–L) Rosette leaves in 35S-RFL plants (J and K) and a wild-type plant at 19 days (L). (Bar = 5 mm.)
DISCUSSION
We found significant differences in RFL expression pattern from that of FLO and LFY (8, 9). FLO and LFY are expressed in the floral meristem at very early stages of development, consistent with their roles in the initiation of floral meristems. In contrast, RFL RNA accumulation was observed in young panicles and developing branches much earlier than the initiation of floral meristem. It should be noted that RFL expression has been excluded from the apical meristem of the branches since the initiation of the branch primordia. These meristems are to be converted into floral meristems to form a terminal flower at the top of each primary branch. These demonstrate that initiation of floral meristems takes place without detectable levels of RFL RNA accumulation. From this it appears unlikely that RFL is required for initiation of floral meristems in rice.
Interestingly, RFL RNA accumulation is down-regulated in the cells determined to initiate inflorescence branch primordia. Similar patterns of down-regulation of RNA expression has been reported for KNOTTED 1 (KN 1) gene of maize (17) and SHOOTMERISTEMLESS (STM) (18) gene of Arabidopsis. KN 1 and STM RNAs are expressed in vegetative shoot meristems and are down-regulated in the incipient and developing leaves. Arabidopsis seedlings homozygous for recessive mutations of STM exhibit failure to develop and maintain shoot apical meristem. Although the role of the wild-type KN 1 gene product is unknown, gain-of-function mutations of the KN 1 cause extra cell division in leaf blade resulting in outgrowth or knot formation. Transgenic plants constitutively expressing KN 1 developed ectopic meristems (19). From these studies, it has been proposed that the KN 1 and KN 1 STM gene products are involved in the maintenance of the indeterminate state of meristems, and that down-regulation of their expression leads to the initiation of determinant lateral organs. Based on the analogy of KN 1 and STM expression, we speculate that RFL may play a role in pattern formation of inflorescence architecture by maintaining an undifferentiated state of cells in the meristems and/or repressing differentiation. An alternative interpretation is that the RFL expression is a prerequisite for the initiation of the branch primordium. Identification of loss-of-function phenotypes should help elucidate the exact role of RFL during panicle development.
We showed that the coding sequence from the RFL gene has a very limited ability to act as a developmental switch to initiate floral meristems compared with LFY. The main shoot and all lateral shoots were converted to solitary flowers in transgenic Arabidopsis expressing LFY under the control of CaMV 35S promoter (16). In contrast, transformation of main or lateral inflorescence shoots to floral meristems was rarely observed in Arabidopsis containing 35S-RFL. Furthermore, plants containing 35S-RFL showed a variety of morphological abnormalities in vegetative organs in contrast to the absence of abnormal phenotypes in the LFY mutant plants or 35S-LFY Arabidopsis plants (9). Lee et al. (20) reported lobed leaves in transgenic Arabidopsis carrying 35S-UFO, and this phenotype required the presence of functional LFY. They also reported that a low level of LFY RNA expression is present in young leaf primordia of Arabidopsis. Their results and ours suggest that LFY plays an unknown role in the development of leaves. Expression of the RFL but not LFY may disturb the function of endogenous LFY function in leaves. It will be interesting to see whether FLO/LFY homologs from other grass species cause similar phenotypes as 35S-RFL when ectopically expressed in Arabidopsis. Although conservation of sequences indicates that RFL and FLO/LFY arose from the common ancestral gene, diversification of their functions as well as regulation of their expression have occurred during evolution.
Acknowledgments
We thank Koji Goto of Kyoto University for providing Arabidopsis seeds and help in Arabidopsis transformation.
Footnotes
Data deposition: The sequence reported in this paper was deposited in the GenBank database (accession no. AB005620).
References
- 1.Izawa T, Shimamoto K. Trends Plant Sci. 1996;1:95–99. [Google Scholar]
- 2.Kurata N, Nagamura Y, Yamamoto K, Harushima Y, Sue N, Wu J, Antonio B A, Shomura A, Shimizu T, Lin S-Y, et al. Nat Genet. 1994;8:365–372. doi: 10.1038/ng1294-365. [DOI] [PubMed] [Google Scholar]
- 3.Sasaki T, Song J, Koga-Ban Y, Matsui E, Fang F, Higo H, Nagasaki H, Hori M, Miya M, Murayama-Kayano E, et al. Plant J. 1994;6:615–624. doi: 10.1046/j.1365-313x.1994.6040615.x. [DOI] [PubMed] [Google Scholar]
- 4.Hiei Y, Ohta S, Komari T, Kumashiro T. Plant J. 1994;6:271–282. doi: 10.1046/j.1365-313x.1994.6020271.x. [DOI] [PubMed] [Google Scholar]
- 5.Coen E S, Meyerowitz E M. Nature (London) 1991;353:31–37. doi: 10.1038/353031a0. [DOI] [PubMed] [Google Scholar]
- 6.Weigel D, Meyerowitz E M. Cell. 1994;78:203–209. doi: 10.1016/0092-8674(94)90291-7. [DOI] [PubMed] [Google Scholar]
- 7.Weigel D. Annu Rev Genet. 1995;29:19–39. doi: 10.1146/annurev.ge.29.120195.000315. [DOI] [PubMed] [Google Scholar]
- 8.Coen E S, Romero J M, Doyle S, Elliott R, Murphy G, Carpenter R. Cell. 1990;63:1311–1322. doi: 10.1016/0092-8674(90)90426-f. [DOI] [PubMed] [Google Scholar]
- 9.Weigel D, Alvarez J, Smyth D R, Yanofsky M F, Meyerowitz E M. Cell. 1992;69:843–859. doi: 10.1016/0092-8674(92)90295-n. [DOI] [PubMed] [Google Scholar]
- 10.Dellaporta S J, Wood J, Hicks J B. Plant Mol Biol Rep. 1983;1:19–21. [Google Scholar]
- 11.Chomczynski P, Sacchi N. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 12.Kouchi H, Sekine M, Hata S. Plant Cell. 1995;7:1143–1155. doi: 10.1105/tpc.7.8.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Akama K, Shiraishi H, Ohta S, Nakamura K, Okada K, Shimura Y. Plant Cell Rep. 1992;12:7–11. doi: 10.1007/BF00232413. [DOI] [PubMed] [Google Scholar]
- 14.Bechtold N, Eliss J, Pelletier G. C R Acad Sci. 1993;316:1194–1199. [Google Scholar]
- 15.Greyson R I, editor. The Development of Flowers. New York: Oxford Univ. Press; 1994. [Google Scholar]
- 16.Weigel D, Nilsson O. Nature (London) 1995;377:495–500. doi: 10.1038/377495a0. [DOI] [PubMed] [Google Scholar]
- 17.Jackson D, Veit B, Hake S. Development. 1994;120:405–413. [Google Scholar]
- 18.Long J A, Moan E I, Medford J I, Barton M K. Nature (London) 1996;379:66–69. doi: 10.1038/379066a0. [DOI] [PubMed] [Google Scholar]
- 19.Sinha N R, Williams R E, Hake S. Genes Dev. 1993;7:787–795. doi: 10.1101/gad.7.5.787. [DOI] [PubMed] [Google Scholar]
- 20.Lee I, Wolfe D S, Nilsson O, Weigel D. Curr Biol. 1997;7:95–104. doi: 10.1016/s0960-9822(06)00053-4. [DOI] [PubMed] [Google Scholar]