Abstract
Glucocorticoids have potent immunosuppressive properties, but their effects are often modulated by the conditions prevailing in the local immune milieu. In this study we determined whether the action of glucocorticoids is influenced by the degree of signaling during T cell activation. We found that dexamethasone (Dex) effectively suppressed T cell receptor–induced (TCR-induced) proliferation of naive CD4+ T cells, through a mechanism involving downregulation of c-Fos expression and inhibition of activator protein-1 (AP-1), nuclear factor of activated T cells (NF-AT), and NF-κB transcriptional activity. However, enhancement of TCR signaling by CD28- or IL-2–mediated costimulation abrogated the suppressive effect of Dex on c-Fos expression and AP-1 function and restored cellular proliferation. The amount of signaling through the MAPK pathway was critical in determining the effect of Dex on T cell activation. In particular, costimulatory signaling via MAPK kinase (MEK) and extracellular signal–regulated kinase (ERK) was essential for the development of T cell resistance to Dex. Selective blockade of MEK/ERK signal transduction abolished the costimulation-induced resistance. In contrast, transmission of IL-2 signals via STAT5 and CD28 signals via NF-κB remained inhibited by Dex. These results imply that the immune system, by regulating the degree of local costimulation through MEK/ERK, can modify the effect of glucocorticoids on T cells. Moreover, these findings suggest that MAPK inhibitors may offer a therapeutic solution for glucocorticoid resistance.
Introduction
Glucocorticoids are pleiotropic hormones with a wide spectrum of immunomodulatory and anti-inflammatory properties. Endogenous glucocorticoids, the end-effectors of the hypothalamus-pituitary-adrenal axis, play an important role in regulating the homeostasis of the immune system (1). Synthetic steroids have become the mainstay of modern therapies for multiple chronic inflammatory diseases, including autoimmune and allergic disorders. Glucocorticoids exert their action primarily by suppressing innate immunity and inhibiting the production of various proinflammatory mediators, such as cytokines and chemokines (2). Their effect on adaptive immunity is more complex and usually reflects their capacity to modulate the functions of CD4+ T lymphocytes (2, 3). It is generally recognized that glucocorticoids suppress peripheral Th cell responses (3). However, recent investigations have raised questions about the absolute validity of this notion and showed that the effect of glucocorticoids on T cell functions may depend on the activation and differentiation state of T cells, as well as on the conditions or agents used for stimulation (2, 3). Thus, resting T cells appear to differ from activated T cells in their response to glucocorticoids, while infections, cytokines, and proinflammatory factors can alter the sensitivity of T cells (4, 5). Furthermore, glucocorticoids often selectively affect various Th cell functions (3, 6).
In clinical practice, reduced T cell sensitivity to the suppressive action of glucocorticoids is a common problem that complicates the management of chronic inflammatory diseases. Patients with glucocorticoid resistance usually suffer from severe inflammation associated with significantly high levels of cytokines, growth factors, and costimulatory factors in the inflamed tissues (7, 8). Interestingly, in these individuals, glucocorticoids fail to inhibit the proliferation and cytokine production of T cells located at the site of inflammation, whereas they appropriately suppress the function of T cells in other peripheral tissues (5, 9, 10). It is possible that signals from the local inflammatory milieu that promote the development of effector T cell responses interfere with the inhibitory signals delivered by glucocorticoids and diminish their suppressive effect.
Glucocorticoids mediate their biological functions by binding to their intracellular receptor, which then translocates to the nucleus and positively or negatively regulates the expression of several genes. Over the last two decades several research groups have attempted to delineate the regulatory effects of glucocorticoids on T cell activation and the mechanisms involved. The repressive action of glucocorticoids on T cell functions has been mainly attributed to their inhibitory effect on the secretion of growth factors and other cytokines, including IL-2, GM-CSF, IL-4, IL-6, and IFN-γ (11, 12). The inhibition has been found to be largely due to the ability of the activated glucocorticoid receptor to hinder the activity of transcription factors, such as activator protein-1 (AP-1), nuclear factor of activated T cells (NF-AT), NF-κB, and STATs, that mediate the upregulation of cytokine gene expression following T cell activation (13). Disruption of the transcriptional activity of various nuclear factors by glucocorticoids is believed to be involved in the development of glucocorticoid resistance (13).
The aim of this study was to determine whether the sensitivity of T cells to the suppressive effect of glucocorticoids could be modulated by exogenous factors that alter the strength of signaling during T cell stimulation. For this purpose, purified naive CD4+ T cells treated with the synthetic glucocorticoid analogue dexamethasone (Dex) were stimulated via the T cell receptor (TCR) in the presence and absence of anti-CD28 or IL-2. The experiments demonstrate that treatment with Dex led to suppression of the ability of T cells to respond to TCR stimulation, an action associated with inhibition of c-Fos expression and NF-κB, NF-AT, and AP-1 activity. However, enhancement of stimulatory T cell signaling by addition of anti-CD28 or IL-2 reversed the inhibitory effect of Dex on c-Fos and AP-1 induction and restored T cell proliferation. Signal transduction via the MAPK pathway and especially activation of the extracellular signal–regulated kinase (ERK) cascade was crucial for the development of costimulation-mediated T cell resistance to glucocorticoids.
Methods
Mice, donors, and reagents.
BALB/c mice 5–6 weeks old were purchased from Charles River Laboratories (Raleigh, North Carolina, USA) and housed in the animal facilities of Columbia University’s College of Physicians and Surgeons. For each experiment, eight to ten mice were used. For the studies with human cells, peripheral blood was obtained from four healthy adult individuals for each experiment.
The anti-mouse and anti-human CD3 and CD28 mAb’s used for T cell stimulation were purchased from BD Pharmingen (San Diego, California, USA). Murine recombinant IL-2 was obtained from R&D Systems Inc. (Minneapolis, Minnesota, USA). Human recombinant IL-2 was purchased from BD Pharmingen. The MAPK kinase (MEK) inhibitor U0126 and the JNK inhibitor SP600125 were purchased from Calbiochem-Novabiochem Corp. (San Diego, California, USA).
For the murine cell cultures, complete RPMI containing 10 μg/ml penicillin-streptomycin, 2 mM L-glutamine, 0.1 mM nonessential amino acids, 0.1 mM β-mercaptoethanol, 1 mM sodium pyruvate, 10 mM HEPES, and 10% FBS was used. For the human cell cultures, RPMI containing 10 μg/ml penicillin-streptomycin, 2 mM L-glutamine, and 10% FBS was used.
Cell culture and proliferation.
Murine naive CD4+ T cells were purified from mouse spleen and lymph node cell suspensions using the MACS separation column system with anti–MHC class II, anti-CD8, and anti-CD62L mAb’s (Miltenyi Biotec, Auburn, California, USA). The purity of the obtained CD4+CD62Lhigh cell population was around 98%. Human lymphocytes were obtained from whole blood following Ficoll (Sigma-Aldrich, St. Louis, Missouri, USA) separation. The naive CD4+ T cells were subsequently isolated using Ab-conjugated Dynabeads (Miltenyi Biotec) and then negatively depleted of CD45RO+ cells to give a greater than 98% CD4+CD45RA+ population. Purified murine or human CD4+ T cells were incubated overnight with 10–7 M Dex (Sigma-Aldrich) or control (PBS). The next day the T cells were washed extensively and set up in culture with plate-bound anti-CD3 (5 μg/ml) alone, or with anti-CD3 plus soluble anti-CD28 (3 μg/ml) or IL-2 (50–80 U/ml). In some experiments, 10 μM (maximum concentration used) or 5 μM (half of the maximum) JNK or ERK inhibitor was also added to the cultures. The proliferative capacity of the cells was assessed after 72 hours. [3H]thymidine (1 μCi/well) was added for the last 12 hours of culture, and the incorporated radioactivity was measured by liquid scintillation spectroscopy.
Cell division cycle profile using CFSE.
Dex- and control-treated purified naive CD4+ T cells were resuspended in serum-free RPMI and incubated with 5 μg CFSE (Molecular Probes Inc., Eugene, Oregon, USA) for 10 minutes at 37°C. CFSE was subsequently neutralized with complete RPMI. CFSE-labeled T cells were cultured with the appropriate mAb’s as described above. CFSE labeling efficiency and fluorescein intensities were measured before culture and after 24, 48, and 72 hours using a FACScan flow cytometer (Becton, Dickinson and Co., Franklin Lakes, New Jersey, USA). Histogram overlays and peak distribution analyses were performed using CellQuest software (Becton, Dickinson and Co.). All plots represent live CD4+ T cells.
Flow cytometry.
Before and 24, 48, and 72 hours after the initiation of in vitro culture, CD4+ T cells were harvested, washed with PBS, resuspended in binding buffer containing 2.5 mM CaCl2, and stained with propidium iodide and annexin V (BD Pharmingen) for 15 minutes. After a final wash the cells were analyzed on a FACScan flow cytometer. Analysis was performed on 10,000 collected events.
Western blot.
Whole cell extracts were obtained from T cells as previously described (14). One hundred micrograms of total cell protein was resolved by SDS-PAGE on 10% polyacrylamide gels and electroblotted onto PVDF membranes (Amersham Pharmacia Biotech, Arlington Heights, Illinois, USA). After saturation of nonspecific binding sites with BSA in TBS–Tween 20 (5%), the membranes were probed overnight with the appropriate Ab against phosphorylated ERK (Cell Signaling Technology Inc., Beverly, Massachusetts, USA), phosphorylated JNK (Cell Signaling Technology Inc.), c-Jun (Santa Cruz Biotechnology Inc., Santa Cruz, California, USA), c-Fos (Santa Cruz Biotechnology Inc.), or β-actin (Santa Cruz Biotechnology Inc.) and subsequently incubated with a secondary HRP-labeled anti-IgG Ab. Finally the membranes were treated with chemiluminescent detection reagents (Amersham Pharmacia Biotech) and processed for autoradiography.
Electrophoretic mobility shift assay.
Nuclear extracts were obtained from T cells as previously described (14). Ten micrograms of nuclear extracts was incubated at room temperature for 30 minutes with the appropriate 32P-labeled double-stranded oligonucleotide probe and poly(dI-dC) in a total volume of 20 μl of binding buffer. The electrophoretic analysis of the binding reaction mixture was performed on a 4.5% polyacrylamide gel in 0.25× Tris-borate-EDTA (TBE) buffer. The gels were dried and visualized by autoradiography. The consensus oligonucleotide probes (AP-1, 5′-CGCTTGATGACTCAGCCGGAA-3′; NF-κB, 5′-AGTTGAGGGGACTTTCCCAGGG-3; NF-AT, 5′-GGAGGAAAAACTGTTTCATACAGAAGGCGT-3′; and STAT5, 5′-GATTCCCCGAAAT-3′) were purchased from Gene Link Inc. (Hawthorne, New York, USA). The sequence specificity of the binding was tested by competition with a molar excess of homologous and heterologous oligonucleotides.
Statistical analysis.
Pairs of groups were compared by using the Student’s t test. Values of P < 0.05 were considered significant.
Results
Costimulation promotes the development of T cell resistance to glucocorticoids.
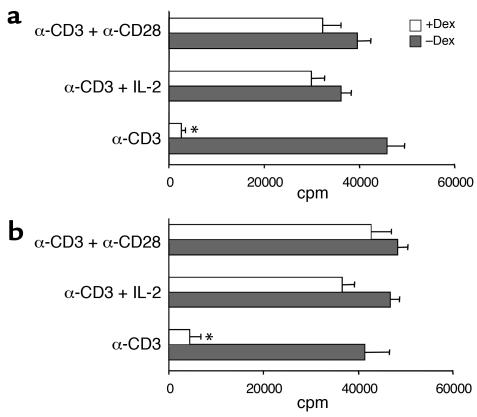
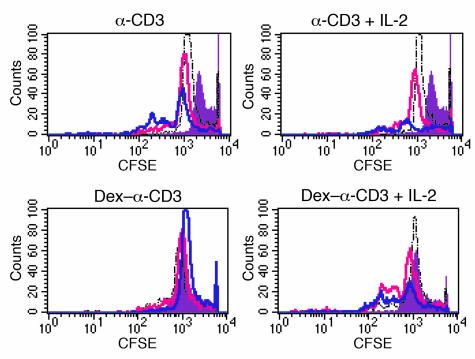
To examine the effect of glucocorticoids on T cell activation, murine purified naive CD4+ T cells were cultured overnight with Dex and subsequently stimulated via the TCR with anti-CD3 cross-linking. Treatment with Dex suppressed the ability of T cells to proliferate in response to anti-CD3 (Figure 1a). However, enhancement of the strength of stimulatory signaling during TCR ligation by addition of costimulation abrogated the inhibitory action of Dex and restored proliferation. Dex-treated T cells stimulated with anti-CD3 plus anti-CD28 or anti-CD3 plus exogenous IL-2 proliferated as well as control T cells not treated with Dex (Figure 1a). Results from similar experiments performed using control and Dex-treated human naive CD4+ T cells also indicated that the presence of strong costimulatory signals during TCR triggering favors the development of glucocorticoid resistance (Figure 1b). To further confirm the differences in the ability of control and Dex-treated T cells to divide following activation, analysis with CFSE was performed. Staining with CFSE demonstrated that Dex-treated T cells failed to undergo cell division in response to anti-CD3 stimulation (Figure 2). In contrast, Dex-treated T cells stimulated with anti-CD3 and IL-2 (Figure 2) or anti-CD28 (data not shown) showed a division pattern analogous to that of control T cells, although a slower initiation of cell proliferation with fewer cells dividing during the first 24 hours was observed. Nevertheless, by 72 hours the proliferation pattern of the two groups of T cells (Dex-treated and control) activated with anti-CD3 plus IL-2 was similar.
Figure 1.
Costimulation abrogates the inhibitory effect of Dex on TCR-induced proliferation. (a) Murine or (b) human Dex-treated and control naive CD4+ T cells were stimulated with anti-CD3, anti-CD3 plus IL-2, or anti-CD3 plus anti-CD28 for 3 days, and T cell proliferation was assessed by measuring 3H incorporation. Results are expressed as mean counts per minute (± SD) of triplicate cultures. *Reduction in proliferation is statistically significant.
Figure 2.
Costimulation promotes T cell resistance to Dex. Dex-treated and control T cells were stained with CFSE and stimulated with anti-CD3 or anti-CD3 plus IL-2 for 3 days. Cells were harvested before stimulation and on days 1, 2, and 3 and analyzed by flow cytometry. Results from a representative experiment are shown. Filled purple: before stimulation; dashed black line: after 24 hours; pink line: after 48 hours; blue line: after 72 hours.
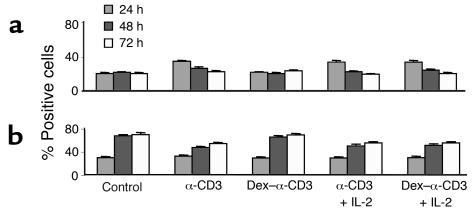
Previous studies have suggested that peripheral CD4+ T cells may undergo apoptosis following Dex treatment (3). We also found that a number of T cells died during the overnight treatment with Dex, but these cells were eliminated prior to in vitro stimulation. To address the possibility that increased susceptibility to apoptosis was responsible for the alterations in the proliferative potential of Dex-treated T cells, the cells were labeled with annexin V and propidium iodide and analyzed by flow cytometry. The number of apoptotic (annexin-positive) and dead (propidium iodide–positive) cells was assessed after 24, 48, and 72 hours of in vitro stimulation. The degree of apoptosis among the Dex-treated T cells that failed to proliferate after stimulation with anti-CD3 was similar to that of unstimulated T cells cultured in medium alone and remained rather stable throughout the culture period (Figure 3). In contrast, control and Dex-treated T cells proliferating in response to anti-CD3 plus IL-2 had higher levels of apoptosis during the first 24 hours; these levels were subsequently reduced and remained lower than the levels of the nonproliferating T cells. These results confirm that the failure of the Dex-sensitive T cells to proliferate and the delayed onset of the Dex-resistant T cell expansion were not due to excessive apoptosis.
Figure 3.
Stimulation of Dex-treated T cells is not associated with enhanced apoptosis. Dex-treated and control naive CD4+ T cells were stimulated with anti-CD3 or anti-CD3 plus IL-2 and stained with (a) annexin V and (b) propidium iodide 24, 48, and 72 hours later. The number of positively stained cells was assessed by flow cytometry. Results are shown as mean ± SD of duplicate samples. Control, medium alone.
Costimulation prevents the inhibitory effect of glucocorticoids on AP-1 activity.
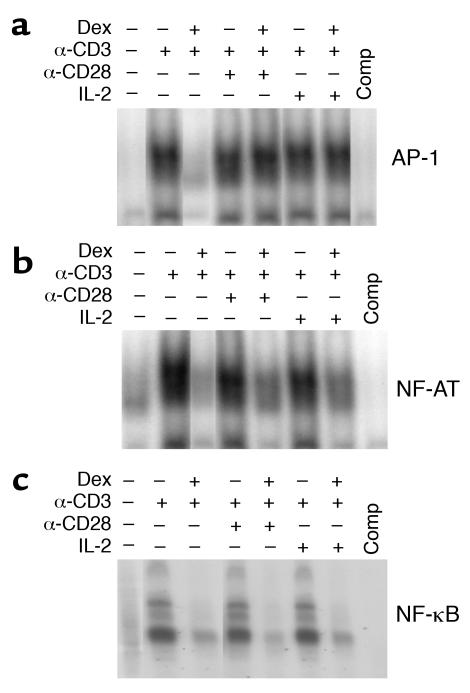
TCR triggering rapidly induces a broad spectrum of signaling events that lead to activation of NF-AT, AP-1, and NF-κB, the main transcription factors responsible for the upregulation of IL-2 expression and the progress of the cell cycle (15). It has been shown that glucocorticoids exert their immunosuppressive action by interfering with the function of several transcription factors involved in the positive regulation of activation-induced genes (11–13, 16). This prompted us to determine whether the inhibitory effect of Dex on TCR-induced proliferation and its abrogation by costimulation were associated with alterations in the function of the three major transcription factors. For this purpose the activation of NF-AT, AP-1, and NF-κB was determined, based on the ability of each factor to bind to a radiolabeled nucleotide probe corresponding to its DNA binding site. Cell extracts were generated from Dex-treated and untreated T cells stimulated with anti-CD3 alone and anti-CD3 plus IL-2 or anti-CD28. Unstimulated T cells contained little transcriptional activity, while stimulation with anti-CD3 or anti-CD3 plus IL-2 or anti-CD28 induced AP-1, NF-AT, and NF-κB activation. Dex treatment prevented the induction of AP-1 activity in response anti-CD3, as indicated by the lack of AP-1 binding to its DNA target (Figure 4a). However, enhancement of the strength of TCR signaling by anti-CD28 or IL-2 signals restored the function of AP-1, and the binding to its cognate DNA was analogous to that observed in control T cells (Figure 4a). In addition to its effect on AP-1, Dex also interfered with the transcriptional activity of NF-AT and NF-κB. As shown in Figure 4b, Dex prevented the TCR-induced DNA binding of NF-AT. This effect was partially reversed by the addition of anti-CD28 or IL-2 (Figure 4b). In contrast, the inhibitory action of Dex on NF-κB function was persistent and was not influenced by the level of stimulatory signaling, as determined by the presence or absence of costimulatory signals (Figure 4c). These data suggest that the costimulation-mediated abrogation of the inhibitory effect of Dex on AP-1 and NF-AT activation may be responsible for the development of glucocorticoid resistance and the induction of proliferation in the Dex-treated T cells that were stimulated with anti-CD3 plus anti-CD28 or IL-2.
Figure 4.
Costimulation restores the inhibition of AP-1 activation by Dex. Nuclear extracts were prepared from Dex-treated and control naive CD4+ T cells stimulated with anti-CD3, anti-CD3 plus IL-2, or anti-CD3 plus anti-CD28 and analyzed by electrophoretic mobility shift assay (EMSA) using 32P-labeled oligonucleotide probes corresponding to (a) AP-1, (b) NF-AT, or (c) NF-κB DNA binding sites. As a control, the competition (Comp) for DNA binding with excess oligonucleotide was assessed. Results from a representative experiment are shown.
MEK/ERK signaling is critical for the development of glucocorticoid resistance.
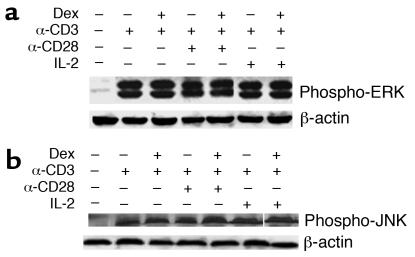
CD28 signals, as well as IL-2 signals, are transduced via the MAPK family members (17, 18). The MAPK system, and in particular ERK and JNK, have been shown to play a deterministic role in T cell activation and differentiation (19). The effect of glucocorticoids on MAPKs has not been clearly established, but interference with the signaling chain that leads to activation of AP-1 has been postulated. To examine this possibility, the effect of Dex on ERK and JNK activation was investigated. Prior to T cell stimulation, activation of ERK or JNK was not observed in either the control or the Dex-treated group (data not shown). In contrast, stimulation with anti-CD3 and anti-CD3 plus anti-CD28 or IL-2 was associated with ERK and JNK phosphorylation (Figure 5). The phosphorylation of ERK and JNK was assessed respectively at 5–10 minutes and 2–4 hours after cross-linking with anti-CD3. These time points were chosen because they correspond to the peak activity of these kinases in response to TCR triggering. Treatment with Dex did not affect the phosphorylation of ERK and JNK, irrespective of the presence or absence of costimulatory signals (Figure 5).
Figure 5.
Dex treatment does not affect ERK and JNK activation. Cell extracts were prepared from Dex-treated and control naive CD4+ T cells stimulated with anti-CD3, anti-CD3 plus IL-2, or anti-CD3 plus anti-CD28. The phosphorylation of (a) ERK and (b) JNK was assessed by Western blotting using specific Ab’s. Results from a representative experiment are shown.
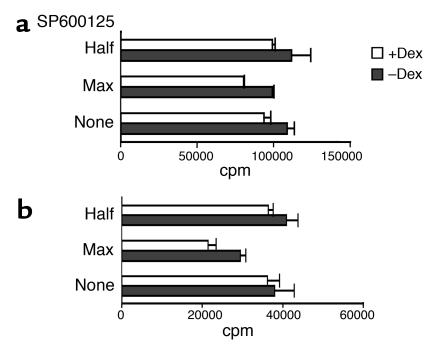
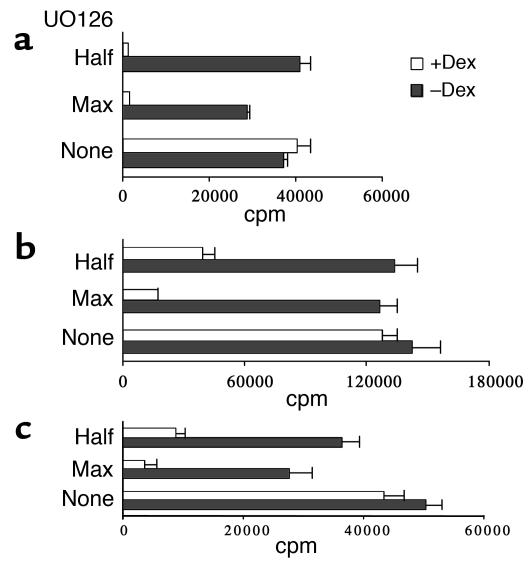
Because signaling through ERK and JNK was not altered by Dex, we wanted to investigate its potential involvement in the development of glucocorticoid resistance. For this purpose, control and Dex-treated T cells were stimulated in the presence of increasing concentrations of specific MAPK inhibitors, and the effect on proliferation was assessed. Addition of SP600125, a JNK inhibitor, did not affect the ability of either control or Dex-treated T cells to proliferate in response to anti-CD3 plus IL-2 (Figure 6a). Limited reduction was observed in the proliferation of Dex-treated T cells stimulated with anti-CD3 plus anti-CD28 (Figure 6b). Signaling through ERK was blocked by using the compound U0126, a selective inhibitor of MEK catalytic activity that results in direct prevention of ERK phosphorylation (20). Presence of U0126 in the cultures completely suppressed the proliferation of both murine (Figure 7, a and b) and human (Figure 7c) Dex-treated T cells in response to anti-CD3 and IL-2 or anti-CD28. At only the highest concentration did U0126 have a minor effect on the proliferation of control T cells (Figure 7). These findings indicate that the signaling pathways that operate in normal T cells and are sufficient to sustain induction of proliferation in the absence of ERK activation are inhibited by Dex. This suggests that enhanced delivery of signals transduced through the MEK/ERK pathway is essential for the development of resistance to glucocorticoids and priming for proliferation.
Figure 6.
Inhibition of JNK activation does not affect T cell resistance to Dex. Dex-treated and control murine naive CD4+ T cells were stimulated for 3 days with (a) anti-CD3 plus IL-2 or (b) anti-CD3 plus anti-CD28 in the presence of 10 μM (max) or 5 μM (half) of SP600125. T cell proliferation was assessed by measuring 3H incorporation. Results are expressed as mean counts per minute (± SD) of triplicate cultures.
Figure 7.
Inhibition of MEK/ERK activity abrogates T cell resistance to Dex. Dex-treated and control murine naive CD4+ T cells were stimulated for 3 days with (a) anti-CD3 plus IL-2 or (b) anti-CD3 plus anti-CD28 in the presence of 10 μM (max) or 5 μM (half) of U0126. (c) Purified human naive CD4+ T cells were also stimulated with anti-CD3 plus anti-CD28 in the presence and absence of U0126. T cell proliferation was assessed by measuring 3H incorporation. Results are expressed as mean counts per minute (± SD) of triplicate cultures.
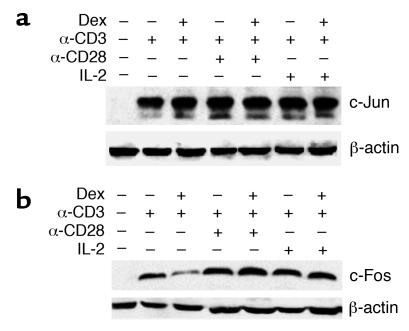
JNK and ERK signaling leads to AP-1 activation through the induction of Jun and Fos proteins (19). In particular, sufficient synthesis of c-Fos and c-Jun in response to T cell stimulation is a prerequisite for the formation and activation of the heterodimeric AP-1 complex. Thus, in order to further analyze the basis on which costimulatory signaling through the MAPKs influences T cell responsiveness to glucocorticoids, we examined the levels of c-Jun and c-Fos expression in Dex-sensitive and Dex-resistant T cells. Stimulation of T cells with anti-CD3, as well as with anti-CD3 plus anti-CD28 or IL-2, resulted in upregulation of c-Jun expression, which was not influenced by treatment with Dex (Figure 8a). In contrast, Dex suppressed the induction of c-Fos following anti-CD3 triggering, while addition of anti-CD28 or IL-2 led to an increase in the levels of c-Fos expression (Figure 8b). These results imply that although Dex does not entirely block signaling through the MEK/ERK pathway, it diminishes the induction of c-Fos under conditions of limited stimulation. Enhancement of stimulatory signaling increases the synthesis of c-Fos, promoting the formation of functional AP-1 and the development of glucocorticoid resistance. The MEK/ERK inhibitor U0126 is known to downregulate the expression of Fos and Jun (20), and this may explain its role in preventing the induction of costimulation-mediated glucocorticoid resistance.
Figure 8.
Costimulation restores the Dex-mediated inhibition of c-Fos induction. Cell extracts were prepared from Dex-treated and control human naive CD4+ T cells stimulated with anti-CD3, anti-CD3 plus IL-2, or anti-CD3 plus anti-CD28. The induction of (a) c-Jun and (b) c-Fos expression was assessed by Western blotting using specific Ab’s. Results from a representative experiment are shown.
STAT5 signaling does not play a primary role in IL-2–induced glucocorticoid resistance.
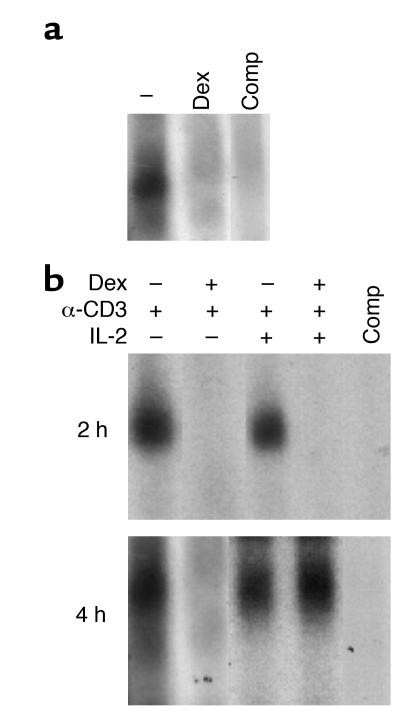
Although IL-2 signals are transduced via ERK, under normal circumstances activation of STAT5 by IL-2 is also an important signaling event (17, 21). The fact that STAT5-deficient T cells show defective proliferation following IL-2 or TCR stimulation highlights the significance of STAT5 signaling in T cell activation (22). It was therefore important to examine the effect of glucocorticoids on the activation of STAT5. For this purpose the function of STAT5 was assessed by analyzing the IL-2–induced DNA binding of activated STAT5. Preliminary experiments showed that there was a good correlation between the DNA-binding capacity of STAT5 and the levels of STAT5 expression and phosphorylation in resting and activated control and Dex-treated T cells (data not shown). Stimulation of T cells with IL-2 for 20 minutes resulted in rapid induction of a DNA-protein complex that bound specifically to the oligonucleotide element that corresponds to STAT5 (Figure 9a). However, treatment with Dex inhibited the binding of STAT5 to its DNA site, indicating that IL-2 signaling through STAT5 was blocked (Figure 9a). Although Dex inhibited STAT5 function in resting T cells, it was possible that the development of Dex resistance following stimulation with anti-CD3 plus costimulation modulated this effect; TCR signaling has been shown to modulate STAT5 activity (23). To address this question, we examined the IL-2–induced STAT5 activation in Dex-treated and control T cells that were cultured with anti-CD3 or anti-CD3 plus IL-2 for 4 or 24 hours. After harvest, the cells were washed extensively, starved for 3–4 hours, and stimulated with IL-2 for 20 minutes. Dex persistently blocked the activation of STAT5 in T cells that were cultured with anti-CD3 alone (Figure 9b). Inhibition of STAT5 signaling was also noticed in Dex-treated T cells cultured with anti-CD3 and IL-2 for 4 hours. However, after 24 hours of stimulation, the inhibitory effect of Dex on STAT5 activation in these cells was abolished (Figure 9b). Activation of STAT5 was also observed in these cells before the secondary stimulation with IL-2 for 20 minutes (data not shown). This finding indicates that Dex adequately blocked the IL-2–induced activation of STAT5 in weakly stimulated T cells, but addition of strong stimulation through the MAPKs was capable of reversing this effect over time. This may explain the almost normal proliferation of Dex-resistant T cells after 72 hours of stimulation with anti-CD3 plus costimulation.
Figure 9.
Dex alters IL-2–induced activation of STAT5. (a) Dex-treated and control naive CD4+ T cells were stimulated with IL-2 for 20 minutes, and cell extracts were prepared and assessed for STAT5 DNA binding using EMSA. (b) Dex-treated and control naive CD4+ T cells stimulated with anti-CD3 or anti-CD3 plus IL-2 for 4 or 24 hours were harvested, washed, rested, and stimulated with IL-2 for 20 minutes. STAT5 DNA binding was assessed in the cell extracts by EMSA.
Discussion
In this study we used a simple in vitro system to determine whether and how immunoregulatory factors that influence the outcome of T cell activation affect the responsiveness of T cells to glucocorticoids. Glucocorticoids are potent immunosuppressive agents, but their mode of action is not an on-or-off phenomenon. Indeed, on an individual basis, the response to glucocorticoids can differ greatly, varying from profound inhibition of immune functions to complete resistance. The factors that control the sensitivity of immune cells to glucocorticoids are not well understood. Our results demonstrate that the quantity and quality of stimulatory signaling during T cell activation can differentially modulate the sensitivity of naive CD4+ T cells to glucocorticoids. Specifically, we found that costimulation by enhancing the degree of MAPK signaling attenuates glucocorticoid-mediated transrepression and promotes the development of resistance.
We found that costimulation through IL-2 and CD28 was adequate to abrogate the suppressive action of Dex on T cell proliferation. The abrogation of Dex-induced suppression was also associated with restoration of T cell cytokine production (D. Tsitoura, unpublished observations). CD28, as well as IL-2, support T cell activation by substantially increasing the production of endogenous IL-2 that, in turn, enhances the competence of T cells to sustain an autocrine-driven clonal expansion (24, 25). This effect is mediated by enhancement of IL-2 gene transcription and stabilization of mRNA (25, 26). Downregulation of IL-2 expression is considered one of the main mechanisms of glucocorticoid suppressive action, due to disruption of the function of IL-2’s transcriptional regulators (11, 12, 16). Our data agree with this evidence and show that prevention of TCR-induced proliferation by Dex is associated with inhibition of the activity of AP-1, NF-AT, and NF-κB, all of which positively control the transcription of IL-2 and other growth factors. It seems that when both glucocorticoid and stimulatory signals are present, the responsiveness of T cells is determined by their competitive effect on the transcriptional regulation of IL-2 and the predominance of the strongest signal. The finding that IL-2 and anti-CD28 promoted the development of resistance to Dex by abrogating its suppressive effect on AP-1 function supports this notion. Interestingly, it has been shown that stimulation with IL-2 or anti-CD28 can also rescue T cells from glucocorticoid-induced cell death (27, 28). In vivo, the degree of costimulation in the local environment is modulated according to the need for immune defense. Therefore, cross-talk between glucocorticoids and costimulatory signals may represent a physiological safety step for the optimum control of immune reactivity and the support of the most beneficial immune functions. An analogous phenomenon where glucocorticoids influence the threshold of T cell activation by allowing or dampening the transduction of TCR-generated signals has been proposed to play a deterministic role in thymic selection, as well as in Th subset differentiation in the periphery (3, 29, 30). In addition to costimulatory signals, other proinflammatory mediators or cytokines may also be able to modulate quantitatively and qualitatively the sensitivity of T cells to glucocorticoids (D. Tsitoura, unpublished observations). In these cases, the molecular mechanisms underlying the alteration of the effect of glucocorticoids may vary from the ones that are responsible for the development of costimulation-mediated glucocorticoid resistance.
Our data indicate that the pathways involved in the transduction of costimulatory signals to the nucleus are differentially affected by Dex. Dex exerted a profound suppressive effect on NF-κB transactivation that was not influenced by the intensity of stimulatory signaling. Nonreversible and independent on the presence of costimulation induction by Dex of the inhibitory protein I-κB, which sequesters NF-κB in the cytoplasm and prevents its translocation to the nucleus, could be responsible for this outcome (31, 32). Dex also inhibited the activation of STAT5 in primary resting T cells. In contrast, Dex did not interfere with ERK and JNK activation, thus allowing the transmission of TCR, CD28, or IL-2 signals to their physiological targets. Induction of AP-1 is a direct consequence of ERK and JNK signaling. Although Dex did not interrupt the upstream signaling leading to AP-1 generation, it did modulate its function in a way that was dependent on the strength of incoming signals. We found that under conditions of limited stimulation, Dex effectively suppressed the expression of the c-Fos element of AP-1. Enhancement of stimulatory signals increased the synthesis of c-Fos, supporting the formation of the AP-1 complex. The interaction of the AP-1 complex with glucocorticoids appears to be dynamic and reciprocal (33). Several reports have described that glucocorticoids antagonize and repress the activation-induced transcriptional activity of AP-1 (34–36). However, the inverse outcome whereby overexpression of AP-1 leads to restriction of the suppressive properties of glucocorticoids has also been demonstrated (37, 38). Based on these observations, a plausible explanation for our results is that the presence of costimulation during TCR stimulation enhances the MAPK signaling and leads to a higher degree of AP-1 formation and activation, which in turn antagonizes the effects of glucocorticoids on IL-2 transcription, reverses the repression, and promotes T cell resistance. AP-1, in addition to its separate function, participates in the formation of NF-AT (39). Thus, enhancement of MAPK signaling may also indirectly support the activation of NF-AT that further sustains the upregulation of IL-2 and other activation-induced genes. This probably explains the increase in NF-AT DNA-binding activity observed in the Dex-resistant T cells. Interestingly, lack of inhibition of AP-1 activity by glucocorticoids has been documented in monocytes from steroid-resistant asthmatics compared with monocytes from steroid-responsive patients (40).
Among the MAPKs, signaling via MEK/ERK was found to be essential for the development of costimulation-mediated resistance to glucocorticoids. JNK signaling did not appear to play an equally important role. The different contribution of the two signaling pathways in the development of glucocorticoid resistance probably reflects their distinctive significance in T cell responses (19). ERK signaling is critically involved in IL-2 gene upregulation, cyclin activation, and cell division, while JNK participates mostly in Th subset differentiation (41, 42). Lack of the JNK gene does not affect T cell proliferation or IL-2 production (43). Under normal circumstances, engagement of TCR or other costimulatory receptors leads to parallel activation of several secondary messenger cascades, including ERK, that act in a complementary way in order to deliver the signal to the nucleus and elicit the appropriate immune response. In our study this was highlighted by the fact that addition of U0126, the MEK/ERK inhibitor, was not sufficient to alter the proliferation of control T cells. However, because the function of most of these effector signaling routes is disrupted in Dex-treated T cells, MEK/ERK signaling becomes critical in determining the outcome of T cell stimulation. A vital role for ERK in T cell activation has also been reported in other conditions of limited signal transduction, such as in activation of primary T cells that do not express high levels of various surface receptors and therefore do not respond adequately to the stimulatory effects of cytokines (44). Furthermore, the duration and strength of ERK signaling has been found to be critically involved in the regulation of positive and negative selection in the thymus (45). ERK, in addition to its importance in signal transduction, may also influence glucocorticoid sensitivity through its effect on glucocorticoid receptor phosphorylation. It has been shown that ERK2 activation contributes to the phosphorylation of serine/threonine residues of glucocorticoid receptor (46). Glucocorticoid receptor phosphorylation has been associated with reduction of ERK activity in T cell responses (47, 48).
Previous studies have documented that glucocorticoids interfere with IL-2–induced signaling via the JAK3-STAT5 pathway (49, 50). In particular, it has been shown that Dex blocks JAK3 phosphorylation, as well as STAT5 phosphorylation, nuclear translocation, and DNA binding (49, 50). Our data support the observation that Dex inhibits STAT5 activation by IL-2 in resting T cells. However, we found that activation of Dex-treated T cells under conditions that promote the development of Dex resistance led over time to abrogation of the suppressive effect on STAT5. The late restoration of the STAT5 signaling cascade in Dex-resistant T cells may explain the delay these cells experience in the onset of efficient proliferation. How restoration was achieved is not clear. It is possible that T cell activation alters the conformation of the glucocorticoid receptor complex that directly interacts with STAT5 (51, 52). Synergistic interaction between the glucocorticoid receptor and STAT5 leading to enhancement of STAT5 activity has been demonstrated by several groups (51–54). Alternatively, activation of T cells in the presence of costimulation may induce the synthesis of proteins that limit the inhibitory effect of glucocorticoids on STAT5 signaling.
In summary, the results of this study indicate that costimulation-induced enhancement of ERK signaling promotes the resistance of T cells to glucocorticoids. Steroid resistance is an important problem for the treatment of chronic inflammatory diseases. Most patients with steroid insensitivity do not present a complete lack of T cell responsiveness to the suppressive effect of glucocorticoids but require excessively high doses to show a therapeutic response. In these cases, therapeutic inhibition of the signaling pathways that are not affected by glucocorticoids may act synergistically and support the suppressive effect of glucocorticoids. In this context, development of inhibitory agents that target the MAPKs and particularly the MEK/ERK cascade may have significant therapeutic potential.
Acknowledgments
This work was supported by NIH Public Health Service grant PO1 AI-50514, the Arthritis Foundation, and the American Lung Association.
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Nonstandard abbreviations used: dexamethasone (Dex); activator protein-1 (AP-1); nuclear factor of activated T cells (NF-AT); T cell receptor (TCR); extracellular signal–regulated kinase (ERK); MAPK kinase (MEK); electrophoretic mobility shift assay (EMSA).
References
- 1.Webster JI, Tonelli L, Sternberg EM. Neuroendocrine regulation of immunity. Annu. Rev. Immunol. 2002;20:125–163. doi: 10.1146/annurev.immunol.20.082401.104914. [DOI] [PubMed] [Google Scholar]
- 2.Rook GA. Glucocorticoids and immune function. Baillieres Best Pract. Res. Clin. Endocrinol. Metab. 1999;13:567–581. doi: 10.1053/beem.1999.0044. [DOI] [PubMed] [Google Scholar]
- 3.Ashwell JD, Lu FW, Vacchio MS. Glucocorticoids in T cell development and function. Annu. Rev. Immunol. 2000;18:309–345. doi: 10.1146/annurev.immunol.18.1.309. [DOI] [PubMed] [Google Scholar]
- 4.Brinkmann V, Kristofic C. Regulation by corticosteroids of Th1 and Th2 cytokine production in human CD4+ effector T cells generated from CD45RO- and CD45RO+ subsets. J. Immunol. 1995;155:3322–3328. [PubMed] [Google Scholar]
- 5.Kino T, Chrousos GP. Tissue-specific glucocorticoid resistance-hypersensitivity syndromes: multifactorial states of clinical importance. J. Allergy Clin. Immunol. 2002;109:609–613. doi: 10.1067/mai.2002.123708. [DOI] [PubMed] [Google Scholar]
- 6.Almawi WY, Melemedjian OK, Rieder MJ. An alternate mechanism of glucocorticoid anti-proliferative effect: promotion of a Th2 cytokine-secreting profile. Clin. Transplant. 1999;13:365–374. doi: 10.1034/j.1399-0012.1999.130501.x. [DOI] [PubMed] [Google Scholar]
- 7.Leung DY, de Castro M, Szefler SJ, Chrousos GP. Mechanisms of glucocorticoid-resistant asthma. Ann. N. Y. Acad. Sci. 1998;840:735–746. doi: 10.1111/j.1749-6632.1998.tb09612.x. [DOI] [PubMed] [Google Scholar]
- 8.Andrews RC, Walker BR. Glucocorticoids and insulin resistance: old hormones, new targets. Clin. Sci. (Lond.) 1999;96:513–523. doi: 10.1042/cs0960513. [DOI] [PubMed] [Google Scholar]
- 9.Leung DY, et al. Dysregulation of interleukin 4, interleukin 5, and interferon gamma gene expression in steroid-resistant asthma. J. Exp. Med. 1995;181:33–40. doi: 10.1084/jem.181.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lane SJ, Lee TH. Mononuclear cells in corticosteroid-resistant asthma. Am. J. Respir. Crit. Care Med. 1996;154:S49–S51. doi: 10.1164/ajrccm/154.2_Pt_2.S49. [DOI] [PubMed] [Google Scholar]
- 11.Crabtree GR, Gillis S, Smith KA, Munck A. Mechanisms of glucocorticoid-induced immunosuppression: inhibitory effects on expression of Fc receptors and production of T-cell growth factor. J. Steroid Biochem. 1980;12:445–449. doi: 10.1016/0022-4731(80)90305-2. [DOI] [PubMed] [Google Scholar]
- 12.Kunicka JE, et al. Immunosuppression by glucocorticoids: inhibition of production of multiple lymphokines by in vivo administration of dexamethasone. Cell. Immunol. 1993;149:39–49. doi: 10.1006/cimm.1993.1134. [DOI] [PubMed] [Google Scholar]
- 13.Adcock IM. Glucocorticoid-regulated transcription factors. Pulm. Pharmacol. Ther. 2001;14:211–219. doi: 10.1006/pupt.2001.0283. [DOI] [PubMed] [Google Scholar]
- 14.Mui AL, Wakao H, O’Farrell AM, Harada N, Miyajima A. Interleukin-3, granulocyte-macrophage colony stimulating factor and interleukin-5 transduce signals through two STAT5 homologs. EMBO J. 1995;14:1166–1175. doi: 10.1002/j.1460-2075.1995.tb07100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cantrell D. T cell antigen receptor signal transduction pathways. Annu. Rev. Immunol. 1996;14:259–274. doi: 10.1146/annurev.immunol.14.1.259. [DOI] [PubMed] [Google Scholar]
- 16.Paliogianni F, Raptis A, Ahuja SS, Najjar SM, Boumpas DT. Negative transcriptional regulation of human interleukin 2 (IL-2) gene by glucocorticoids through interference with nuclear transcription factors AP-1 and NF-AT. J. Clin. Invest. 1993;91:1481–1489. doi: 10.1172/JCI116353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gaffen SL. Signaling domains of the interleukin 2 receptor. Cytokine. 2001;14:63–77. doi: 10.1006/cyto.2001.0862. [DOI] [PubMed] [Google Scholar]
- 18.Isakov N, Altman A. Protein kinase C(theta) in T cell activation. Annu. Rev. Immunol. 2002;20:761–794. doi: 10.1146/annurev.immunol.20.100301.064807. [DOI] [PubMed] [Google Scholar]
- 19.Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002;298:1911–1912. doi: 10.1126/science.1072682. [DOI] [PubMed] [Google Scholar]
- 20.Favata MF, et al. Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J. Biol. Chem. 1998;273:18623–18632. doi: 10.1074/jbc.273.29.18623. [DOI] [PubMed] [Google Scholar]
- 21.Lin JX, Leonard WJ. The role of Stat5a and Stat5b in signaling by IL-2 family cytokines. Oncogene. 2000;19:2566–2576. doi: 10.1038/sj.onc.1203523. [DOI] [PubMed] [Google Scholar]
- 22.Nakajima H, et al. An indirect effect of Stat5a in IL-2-induced proliferation: a critical role for Stat5a in IL-2-mediated IL-2 receptor alpha chain induction. Immunity. 1997;7:691–701. doi: 10.1016/s1074-7613(00)80389-1. [DOI] [PubMed] [Google Scholar]
- 23.Lee IH, Li WP, Hisert KB, Ivashkiv LB. Inhibition of interleukin 2 signaling and signal transducer and activator of transcription (STAT)5 activation during T cell receptor-mediated feedback inhibition of T cell expansion. J. Exp. Med. 1999;190:1263–1274. doi: 10.1084/jem.190.9.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwartz RH. Costimulation of T lymphocytes: the role of CD28, CTLA-4, and B7/BB1 in interleukin-2 production and immunotherapy. Cell. 1992;71:1065–1068. doi: 10.1016/s0092-8674(05)80055-8. [DOI] [PubMed] [Google Scholar]
- 25.Gomez J, Gonzalez A, Martinez AC, Rebollo A. IL-2-induced cellular events. Crit. Rev. Immunol. 1998;18:185–220. [PubMed] [Google Scholar]
- 26.June CH, Ledbetter JA, Lindsten T, Thompson CB. Evidence for the involvement of three distinct signals in the induction of IL-2 gene expression in human T lymphocytes. J. Immunol. 1989;143:153–161. [PubMed] [Google Scholar]
- 27.Zubiaga AM, Munoz E, Huber BT. IL-4 and IL-2 selectively rescue Th cell subsets from glucocorticoid-induced apoptosis. J. Immunol. 1992;149:107–112. [PubMed] [Google Scholar]
- 28.Wagner DH, Jr, et al. Rescue of thymocytes from glucocorticoid-induced cell death mediated by CD28/CTLA-4 costimulatory interactions with B7-1/B7-2. J. Exp. Med. 1996;184:1631–1638. doi: 10.1084/jem.184.5.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jamieson CA, Yamamoto KR. Crosstalk pathway for inhibition of glucocorticoid-induced apoptosis by T cell receptor signaling. Proc. Natl. Acad. Sci. U. S. A. 2000;97:7319–7324. doi: 10.1073/pnas.97.13.7319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van Laethem F, et al. Glucocorticoids attenuate T cell receptor signaling. J. Exp. Med. 2001;193:803–814. doi: 10.1084/jem.193.7.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Auphan N, DiDonato JA, Rosette C, Helmberg A, Karin M. Immunosuppression by glucocorticoids: inhibition of NF-kappa B activity through induction of I kappa B synthesis. Science. 1995;270:286–290. doi: 10.1126/science.270.5234.286. [DOI] [PubMed] [Google Scholar]
- 32.Scheinman RI, Cogswell PC, Lofquist AK, Baldwin AS., Jr Role of transcriptional activation of I kappa B alpha in mediation of immunosuppression by glucocorticoids. Science. 1995;270:283–286. doi: 10.1126/science.270.5234.283. [DOI] [PubMed] [Google Scholar]
- 33.Herrlich P. Cross-talk between glucocorticoid receptor and AP-1. Oncogene. 2001;20:2465–2475. doi: 10.1038/sj.onc.1204388. [DOI] [PubMed] [Google Scholar]
- 34.Jonat C, et al. Antitumor promotion and antiinflammation: down-modulation of AP-1 (Fos/Jun) activity by glucocorticoid hormone. Cell. 1990;62:1189–1204. doi: 10.1016/0092-8674(90)90395-u. [DOI] [PubMed] [Google Scholar]
- 35.Schule R, et al. Functional antagonism between oncoprotein c-Jun and the glucocorticoid receptor. Cell. 1990;62:1217–1226. doi: 10.1016/0092-8674(90)90397-w. [DOI] [PubMed] [Google Scholar]
- 36.Adcock IM, et al. The effects of glucocorticoids on phorbol ester and cytokine stimulated transcription factor activation in human lung. Life Sci. 1994;55:1147–1153. doi: 10.1016/0024-3205(94)00243-6. [DOI] [PubMed] [Google Scholar]
- 37.Yang-Yen HF, et al. Transcriptional interference between c-Jun and the glucocorticoid receptor: mutual inhibition of DNA binding due to direct protein-protein interaction. Cell. 1990;62:1205–1215. doi: 10.1016/0092-8674(90)90396-v. [DOI] [PubMed] [Google Scholar]
- 38.Touray M, Ryan F, Jaggi R, Martin F. Characterisation of functional inhibition of the glucocorticoid receptor by Fos/Jun. Oncogene. 1991;6:1227–1234. [PubMed] [Google Scholar]
- 39.Macian F, Lopez-Rodriguez C, Rao A. Partners in transcription: NFAT and AP-1. Oncogene. 2001;20:2476–2489. doi: 10.1038/sj.onc.1204386. [DOI] [PubMed] [Google Scholar]
- 40.Adcock IM, Lane SJ, Brown CR, Lee TH, Barnes PJ. Abnormal glucocorticoid receptor-activator protein 1 interaction in steroid-resistant asthma. J. Exp. Med. 1995;182:1951–1958. doi: 10.1084/jem.182.6.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Su B, Karin M. Mitogen-activated protein kinase cascades and regulation of gene expression. Curr. Opin. Immunol. 1996;8:402–411. doi: 10.1016/s0952-7915(96)80131-2. [DOI] [PubMed] [Google Scholar]
- 42.Dong C, Davis RJ, Flavell RA. Signaling by the JNK group of MAP kinases. c-Jun N-terminal kinase. J. Clin. Immunol. 2001;21:253–257. doi: 10.1023/a:1010975124110. [DOI] [PubMed] [Google Scholar]
- 43.Yang DD, et al. Differentiation of CD4+ T cells to Th1 cells requires MAP kinase JNK2. Immunity. 1998;9:575–585. doi: 10.1016/s1074-7613(00)80640-8. [DOI] [PubMed] [Google Scholar]
- 44.Qian D, Weiss A. T cell antigen receptor signal transduction. Curr. Opin. Cell Biol. 1997;9:205–212. doi: 10.1016/s0955-0674(97)80064-6. [DOI] [PubMed] [Google Scholar]
- 45.Mariathasan S, et al. Duration and strength of extracellular signal-regulated kinase signals are altered during positive versus negative thymocyte selection. J. Immunol. 2001;167:4966–4973. doi: 10.4049/jimmunol.167.9.4966. [DOI] [PubMed] [Google Scholar]
- 46.Krstic MD, Rogatsky I, Yamamoto KR, Garabedian MJ. Mitogen-activated and cyclin-dependent protein kinases selectively and differentially modulate transcriptional enhancement by the glucocorticoid receptor. Mol. Cell. Biol. 1997;17:3947–3954. doi: 10.1128/mcb.17.7.3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Webster JC, et al. Mouse glucocorticoid receptor phosphorylation status influences multiple functions of the receptor protein. J. Biol. Chem. 1997;272:9287–9293. doi: 10.1074/jbc.272.14.9287. [DOI] [PubMed] [Google Scholar]
- 48.Irusen E, et al. p38 Mitogen-activated protein kinase-induced glucocorticoid receptor phosphorylation reduces its activity: role in steroid-insensitive asthma. J. Allergy Clin. Immunol. 2002;109:649–657. doi: 10.1067/mai.2002.122465. [DOI] [PubMed] [Google Scholar]
- 49.Paliogianni F, Ahuja SS, Balow JP, Balow JE, Boumpas DT. Novel mechanism for inhibition of human T cells by glucocorticoids. Glucocorticoids inhibit signal transduction through IL-2 receptor. J. Immunol. 1993;151:4081–4089. [PubMed] [Google Scholar]
- 50.Bianchi M, Meng C, Ivashkiv LB. Inhibition of IL-2-induced Jak-STAT signaling by glucocorticoids. Proc. Natl. Acad. Sci. U. S. A. 2000;97:9573–9578. doi: 10.1073/pnas.160099797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lechner J, et al. Promoter-dependent synergy between glucocorticoid receptor and Stat5 in the activation of beta-casein gene transcription. J. Biol. Chem. 1997;272:20954–20960. doi: 10.1074/jbc.272.33.20954. [DOI] [PubMed] [Google Scholar]
- 52.Stocklin E, Wissler M, Gouilleux F, Groner B. Functional interactions between Stat5 and the glucocorticoid receptor. Nature. 1996;383:726–728. doi: 10.1038/383726a0. [DOI] [PubMed] [Google Scholar]
- 53.Wyszomierski SL, Yeh J, Rosen JM. Glucocorticoid receptor/signal transducer and activator of transcription 5 (STAT5) interactions enhance STAT5 activation by prolonging STAT5 DNA binding and tyrosine phosphorylation. Mol. Endocrinol. 1999;13:330–343. doi: 10.1210/mend.13.2.0232. [DOI] [PubMed] [Google Scholar]
- 54.Goleva E, Kisich KO, Leung DY. A role for STAT5 in the pathogenesis of IL-2-induced glucocorticoid resistance. J. Immunol. 2002;169:5934–5940. doi: 10.4049/jimmunol.169.10.5934. [DOI] [PubMed] [Google Scholar]