Abstract
AIM: To analyze the expression of the tumor-related proteins in differentiated-type early gastric carcinoma (DEGC) samples.
METHODS: Tumor specimens were obtained from 102 patients (75 males and 27 females) who had received an endoscopic tumor resection at Tottori University Hospital between 2007 and 2009. Ninety-one cancer samples corresponded to noninvasive or intramucosal carcinoma according to the Vienna classification system, and 11 samples were submucosal invasive carcinomas. All of the EGCs were histologically differentiated carcinomas. All patients were classified as having Helicobacter pylori (H. pylori) infections by endoscopic atrophic changes or by testing seropositive for H. pylori IgG. All of the samples were histopathologically classified as either tubular or papillary adenocarcinoma according to their structure. The immunohistochemical staining was performed in a blinded manner with respect to the clinical information. Two independent observers evaluated protein expression. All data were statistically analyzed then.
RESULTS: The rates of aberrant activation-induced cytidine deaminase (AID) expression and P53 overexpression were both 34.3% in DEGCs. The expression of Mlh1 was lost in 18.6% of DEGCs. Aberrant AID expression was not significantly associated with P53 overexpression in DEGCs. However, AID expression was associated with the severity of mononuclear cell activity in the non-cancerous mucosa adjacent to the tumor (P = 0.064). The rate of P53 expression was significantly greater in flat or depressed tumors than in elevated tumors. The frequency of Mlh1 loss was significantly increased in distal tumors, elevated gross-type tumors, papillary histological-type tumors, and tumors with a severe degree of endoscopic atrophic gastritis (P < 0.05).
CONCLUSION: Aberrant AID expression, P53 overexpression, and the loss of Mlh1 were all associated with clinicopathological features and gastric mucosal alterations in DEGCs. The aberrant expression of AID protein may partly contribute to the induction of nuclear P53 expression.
Keywords: Gastric cancer, Activation-induced cytidine deaminase, P53, Mlh1, Endoscopic resection
INTRODUCTION
Gastric cancer (GC) is the second leading cause of cancer death and the fourth most common malignant tumor in the world[1]. The mortality rate associated with the disease is high, with a 5-year survival rate of approximately 20% being observed worldwide[2]. The 5-year survival rate for GC is over 50% in Japan[3]. One of the main factors limiting the survival rate is late tumor detection. Therefore, a better understanding of the clinicopathological characteristics in early GC (EGC) is critical. Infection with Helicobacter pylori (H. pylori), especially when “cag” pathogenicity island (cag PAI) positive, increases the risk of developing GC by more than 6-fold. Therefore, cag PAI is considered an important carcinogenic trigger[4]. Almost all H. pylori strains in Japan are cag PAI-positive[5]. Infection with H. pylori causes chronic inflammation of the gastric mucosa, which slowly progresses through the premalignant stages of atrophic gastritis, intestinal metaplasia and adenoma/dysplasia to GC[6]. The Japanese Research Society for Gastric Cancer has proposed that GC is divided into differentiated and undifferentiated types according to the degree of glandular formation by the tumor cells[7]. Additionally, each type of cancer might follow different genetic pathways during carcinogenesis[8]. The frequency of differentiated-type carcinomas among total EGC is approximately 60%. Therefore, differentiated-type early gastric carcinoma (DEGC) is considered to represent the initial phase of GC[9].
Gastric carcinoma results from the accumulation of genetic and epigenetic alterations[8]. The frequency of MLH1 DNA methylation is 20%-30%[8,10] and the frequency of P53 gene mutations is 25%-50%[8,11] in sporadic GC. MLH1 is a DNA mismatch repair gene. Hypermethylation of the MLH1 promoter region is the main cause of microsatellite instability (MSI) in primary GCs[12]. Activation-induced cytidine deaminase (AID) is a DNA- and RNA-editing enzyme that was originally identified as an inducer of somatic hypermutation and class-switch recombination in the immunoglobulin genes[13]. Previous reports indicate that AID transgenic mice develop malignant T-cell lymphomas and lung adenomas. This finding suggests that aberrant AID expression results in tumor-related gene mutations and might be a cause of human malignancy[14]. It has been reported that cag PAI-positive H. pylori infection causes the aberrant expression of AID in the gastric epithelium. Aberrant AID expression leads to the accumulation of nucleotide alterations in the P53 gene[15]. Although the relationship between AID and Mlh1 is currently unclear, the expression of P53 has been reported to be inversely associated with Mlh1 loss in GC[8]. Elucidation of the relationship between the clinicopathological characteristics and the molecular events in EGC might improve the early detection, treatment, and surveillance of GC.
In this study, we evaluated AID, P53, and Mlh1 expression in endoscopically resected DEGCs and investigated their relationships with clinicopathological characteristics and background mucosa.
MATERIALS AND METHODS
Patient and tissue samples
Tumor specimens were obtained from 102 patients (75 males and 27 females) who had received an endoscopic tumor resection at Tottori University Hospital between 2007 and 2009 (Table 1). The mean age (± SD) was 70.6 ± 7.8 years (range: 55-92 years). The male patients were statistically younger than the female patients (69.4 ± 7.9 vs 74.2 ± 6.6, P = 0.006). We classified the DEGCs based on the Japanese classification of GC, 13th edition (7) according to location, macroscopic, and morphological types. The tumor location was defined as the upper third, middle third, or lower third of the tissue. The macroscopic type of DEGC was determined as elevated, depressed, or flat. All of the samples were histopathologically classified as either tubular or papillary adenocarcinoma according to their structure.
Table 1.
Patients and tissue samples n (%)
| Total (n = 102) | Male (n = 75) | Female (n = 27) | Gender difference | |
| Age (yr, mean ± SD) | 70.6 ± 7.8 | 69.4 ± 7.9 | 74.2 ± 6.6 | P = 0.006 |
| Tumor size (cm) | ||||
| < 2.0 | 76 (74.5) | 54 (72.0) | 22 (81.5) | P = 0.332 |
| ≥ 2.0 | 26 (25.5) | 21 (28.0) | 5 (18.5) | |
| Tumor location | ||||
| Upper third | 23 (22.5) | 19 (25.3) | 4 (14.8) | P = 0.095 |
| Middle third | 40 (39.2) | 32 (41.6) | 8 (29.6) | |
| Lower third | 39 (38.2) | 24 (32.0) | 15 (55.6) | |
| Gross tumor appearance | ||||
| Flat/depressed | 63 (61.8) | 47 (62.7) | 16 (59.3) | P = 0.755 |
| Elevated | 39 (38.2) | 28 (37.3) | 11 (40.7) | |
| Histological type | ||||
| Tubular | 88 (86.3) | 66 (88.0) | 22 (81.5) | P = 0.605 |
| Papillary | 14 (13.7) | 9 (12.0) | 5 (18.5) | |
| Depth of invasion | ||||
| Mucosa | 91 (89.2) | 66 (88.0) | 25 (92.6) | P = 0.509 |
| Submucosa | 11 (10.8) | 9 (12.0) | 2 (7.4) |
Ninety-one cancer samples corresponded to noninvasive or intramucosal carcinoma according to the Vienna classification system[16], and 11 samples were submucosal invasive carcinomas. All of the EGCs were histologically differentiated carcinomas. All patients were classified as having H. pylori infections by endoscopic atrophic changes or by testing seropositive for H. pylori IgG. Two experienced pathologists (Yashima K and Ito H) verified the pathological diagnoses. Moreover, we confirmed that these patients had no H. pylori eradication history. All specimens were assigned a new number without personal information to maintain anonymity. This study was approved by the institutional ethics committee of Tottori University (No. 314).
Evaluation of endoscopic gastric atrophy
All endoscopic examinations were performed using video scopes (model GIF-Q260; Olympus, Tokyo, Japan) and two endoscopists (Takeda Y and Yashima K) evaluated gastric atrophy according to the location of the atrophic border as described by Kimura et al[17]. A difference in the color and height of the gastric mucosa defines the border between the pyloric and fundic gland regions. We scored endoscopic gastric atrophy as marked (O2-O3), moderate (C3-O1) or mild (C1-C2). Previously, Takao et al[18] reported a significant correlation between endoscopic gastric atrophy (Kimura-Takemoto classification[17]) and the histological gastritis (updated Sydney system[19]). This suggests that the degree of endoscopic gastric atrophy can be considered as the grade of atrophic gastritis.
Evaluation of surrounding mucosal inflammation
We evaluated mononuclear cell activity in the non-cancerous mucosa adjacent to a tumor and scored it as mild, moderate or marked according to the updated Sydney system[19].
Immunohistochemical staining
Paraffin-embedded sections (4 μm) were immunohistochemically stained with an anti-AID rat monoclonal antibody (EK2 5G9, Cell Signaling TECHNOLOGY, Danvers, CA, USA; dilution 1:400), an anti-P53 mouse monoclonal antibody (DO-7, Dakopatts, Copenhagen, Denmark; dilution 1:50), and an anti-Mlh1 mouse monoclonal antibody (G168-15, PharMingen, San Diego, CA, USA; dilution 1:50) using the avidin-biotin-peroxidase complex technique.
The immunohistochemical staining was performed in a blinded manner with respect to the clinical information. The sections were deparaffinized in xylene and rehydrated in ethanol. The sections were then immersed in a citrate buffer (0.01 mol/L, pH 6.0) and heated in a microwave oven for 20-30 min to retrieve antigens. The endogenous tissue peroxidase activity was blocked by incubation with 3% H2O2. The sections were subsequently incubated with primary antibody overnight at 4 °C. As a negative control, the primary antibody was replaced with normal serum IgG at a similar dilution. The detection reaction followed the Vectastain Elite ABC kit protocol (Vector Laboratories, Burlingame, CA, USA) with diaminobenzidine as the chromogen. The sections were counterstained with hematoxylin. The sections were incubated with biotinylated anti-rat or anti-mouse IgG and avidin-biotin-peroxidase. The sections were subsequently visualized using diaminobenzidine tetrahydrochloride. Two independent observers (Takeda Y and Yashima K) evaluated protein expression.
Assessment of AID immunostaining
The internal positive controls were lymphocytes of germinal centers in lymphoid follicles (Figure 1A). The follicles contain activated B cells and intensely stained positive for AID in all specimens. The cytoplasm was scored as positive when > 30% of tumor cells were stained as strongly as the germinal centers.
Figure 1.
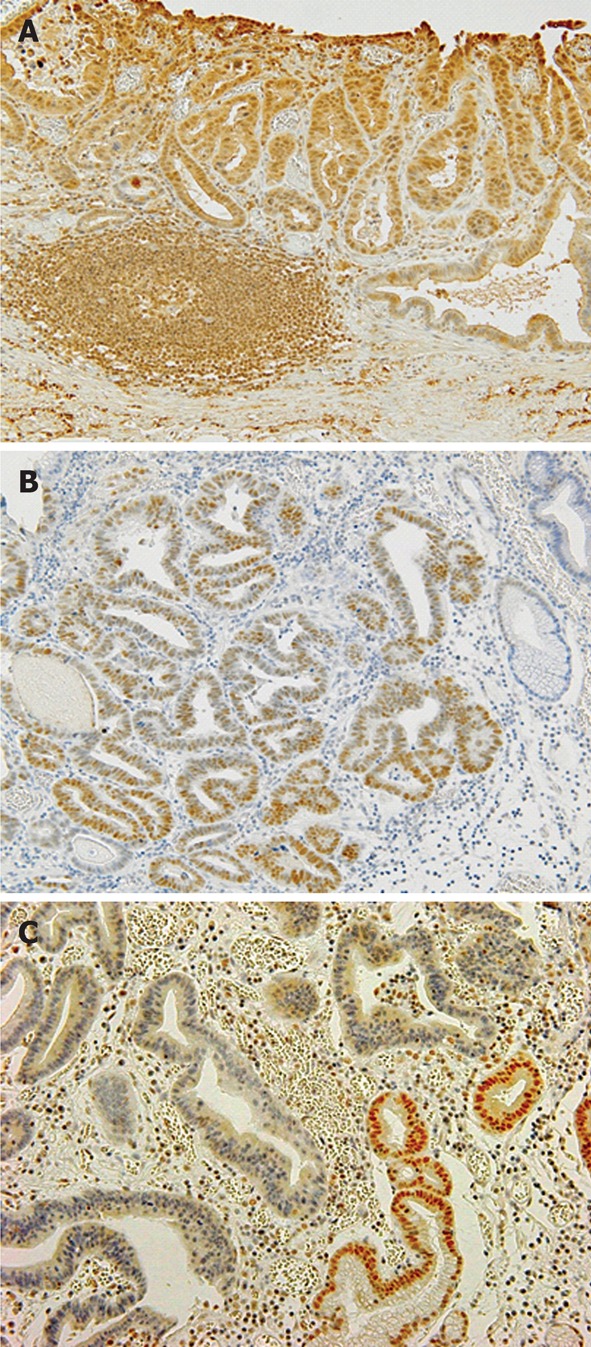
Representative findings of activation-induced cytidine deaminase, P53 and Mlh1 immunohistological stain in differentiated-type early gastric carcinoma. A: Positive activation-induced cytidine deaminase immunostaining in cytoplasm of differentiated-type early gastric carcinoma (DEGC); B: Overexpression of P53 in DEGC; C: No nuclear immunoreactivity for Mlh1 protein in DEGC.
Assessment of P53 immunostaining
The tumors were scored as positive for P53 when a distinct nuclear immunoreaction occurred in > 25% of tumor cells[20] as shown in Figure 1B.
Assessment of Mlh1 immunostaining
The evaluation of Mlh1 expression was classified as being either normal or decreased (Figure 1C). Tissue specimens with definite nuclear staining in < 30% of the tumor cells were categorized as having decreased staining[21].
Statistical analysis
All data were statistically analyzed by the χ2 test with Yates’ correction, Fisher’s test and the Mann-Whitney test (U-test) using Stat View 5.0 software (SAS Institute, Cary, NC, USA). Statistical significance was established at P < 0.05.
RESULTS
Frequency of aberrant AID, P53, and Mlh1 expression
Aberrant AID expression and P53 overexpression in DEGCs were detected in 35 (34.3%) cases. The loss of Mlh1 expression was observed in 19 (18.6%) cases. Among elderly patients (≥ 65 years old), the loss of Mlh1 expression in DEGCs was significantly higher in female patients than in male patients [10/26 (38.5%) vs 6/54 (11.1%), P = 0.004] (Table 2).
Table 2.
Frequency of aberrant activation-induced cytidine deaminase, p53 and Mlh1 expression
| Total |
Age < 65 yr (n = 22) |
Age ≥ 65 yr (n = 80) |
|||||
| Male | Female | Male | Female | ||||
| AID | |||||||
| + | 35 | 6 | 1 | P = 0.689 | 16 | 12 | P = 0.147 |
| - | 67 | 15 | 0 | 38 | 14 | ||
| P53 | |||||||
| + | 35 | 11 | 0 | P = 1.000 | 20 | 4 | P = 0.086 |
| - | 67 | 10 | 1 | 34 | 22 | ||
| Mlh1 | |||||||
| + | 83 | 18 | 1 | P = 0.278 | 48 | 16 | P = 0.004 |
| - | 19 | 3 | 0 | 6 | 10 | - | |
AID: Activation-induced cytidine deaminase.
Relationships between AID, P53 and Mlh1 expression
The overexpression of P53 was significantly more frequent in patients with Mlh1-positive tumors than Mlh1-negative tumors [33/83(39.7%) vs 2/19(10.5%), P = 0.015] (Table 3). The overexpression of P53 was not associated with aberrant AID expression (P = 0.657).
Table 3.
Relationships among activation-induced cytidine deaminase, P53 and Mlh1 expression
|
AID |
Mlh1 |
|||||
| + | - | + | - | |||
| P53 | ||||||
| + | 11 | 24 | P = 0.657 | 33 | 2 | P = 0.015 |
| - | 24 | 43 | 50 | 17 | ||
AID: Activation-induced cytidine deaminase.
Relationship of AID, P53, and Mlh1 expression with tumor features
The aberrant AID expression frequency was correlated with the location of DEGCs. However, there was no correlation between AID expression and tumor growth or histological type. The incidence of P53 overexpression in DEGCs was significantly more frequent in flat or depressed tumors than in elevated type tumors [28/64 (43.8%) vs 7/38 (18.4%), P = 0.009] (Table 4). The overexpression of P53 was found more often in tubular tumors than in papillary adenocarcinoma [34/88 (38.6%) vs. 1/14 (7.1%), P = 0.045]. A loss of Mlh1 expression was closely associated with distal location (P = 0.027), elevated gross type (P = 0.039) and papillary histological type (P = 0.033).
Table 4.
Relationships of activation-induced cytidine deaminase, P53 and Mlh1 expression with tumor features
|
AID |
P53 |
Mlh1 |
|||||||
| + | - | + | - | + | - | ||||
| Tumor location | |||||||||
| Upper third | 4 | 19 | P = 0.067 | 8 | 15 | P = 0.543 | 22 | 1 | P = 0.027 |
| Middle third | 13 | 27 | 16 | 24 | 34 | 6 | |||
| Lower third | 18 | 21 | 11 | 28 | 27 | 12 | |||
| Tumor growth | |||||||||
| Flat/depressed | 19 | 45 | P = 0.202 | 28 | 36 | P = 0.009 | 56 | 8 | P = 0.039 |
| Elevated | 16 | 22 | 7 | 31 | 27 | 11 | |||
| Histological type | |||||||||
| Tubular | 29 | 59 | P = 0.673 | 34 | 54 | P = 0.045 | 75 | 13 | P = 0.033 |
| Papillary | 6 | 8 | 1 | 13 | 8 | 6 | |||
AID: Activation-induced cytidine deaminase.
Relationships of AID, P53, and Mlh1 expression with background mucosa
Although aberrant AID expression was not related to gastric atrophy, mononuclear cell activity tended to be marked in the surrounding mucosa adjacent to DEGCs with aberrant AID expression (P = 0.064). The P53 expression in DEGCs was not associated with gastric atrophy and mononuclear cell activity in the surrounding mucosa. The loss of Mlh1 expression in DEGCs was associated with marked endoscopic gastric atrophy (P = 0.020) and mild mononuclear cell activity (P = 0.053) (Table 5).
Table 5.
Relationships of activation-induced cytidine deaminase, P53 and Mlh1 expression with background mucosa
|
AID |
P53 |
Mlh1 |
|||||||
| + | - | + | - | + | - | ||||
| Endoscopic gastric atrophy | |||||||||
| Mild | 8 | 17 | P = 0.540 | 9 | 16 | P = 0.376 | 20 | 5 | P = 0.020 |
| Moderate | 19 | 29 | 19 | 29 | 44 | 4 | |||
| Marked | 8 | 21 | 7 | 22 | 19 | 10 | |||
| Mononuclear cell activity | |||||||||
| Mild | 5 | 17 | P = 0.064 | 4 | 17 | P = 0.232 | 14 | 8 | P = 0.053 |
| Moderate | 24 | 47 | 28 | 44 | 61 | 10 | |||
| Marked | 6 | 3 | 3 | 6 | 8 | 1 | |||
AID: Activation-induced cytidine deaminase.
DISCUSSION
The present study examined AID, P53 and Mlh1 expression in endoscopically resected DEGCs, and these results were compared with the clinicopathological characteristics and the surrounding mucosa. Aberrant AID expression in endoscopically resected DEGCs significantly correlated with marked mononuclear cell activity in tumor background mucosa but not with P53 overexpression. In addition, P53 expression significantly correlated with flat or depressed types of gross tumor appearance. The loss of Mlh1 expression correlated with elevated type, papillary type histology, distal location and severe endoscopic atrophic gastritis.
Infection with H. pylori triggers aberrant AID expression in the gastric epithelium, which leads to the accumulation of altered nucleotides in the P53 gene[15,22,23]. The rate of aberrant AID expression in DEGC (34.3%) was slightly higher than the 26.9% and 22.5% described in two previous reports[23,24]. The variability in the findings may be caused by differences in the stage of carcinoma progression and the degree of tumor differentiation. All of our data were obtained from endoscopically resected, well-differentiated early carcinomas.
Previously, Kim et al[23] found a significant association between aberrant AID expression and the nuclear overexpression of P53 in various types of GCs. However, we did not find a relationship between aberrant AID expression and P53 overexpression in DEGCs. Similarly, Goto et al[24] found no correlation between AID and P53 in early differentiated and poorly differentiated GCs. There are several possible explanations for these different findings. One explanation is that nonsense mutations were considered to be false-negative. Additionally, P53 protein could accumulate to repair damaged DNA in false-positive cells without P53 mutations[25]. The rate of P53 expression might also increase with tumor progression[26]. Moreover, P53 protein might become altered through cigarette smoking, as in lung and esophageal carcinogenesis[27,28]. Further investigation is needed to clarify the correlation between the expression of P53 and aberrant AID expression.
The expression of AID in gastric epithelial cells could be altered by the direct action of H. pylori macromolecules through the type IV secretion system encoded by cag PAI[29]. Additionally, H. pylori infection is associated with inflammatory cytokines, such as tumor necrosis factor α, that are produced during gastric inflammation[15]. Furthermore, AID expression in tumors such as hepatocellular carcinoma, cholangiocarcinoma and colon cancer is also mediated by proinflammatory cytokine stimulation via nuclear factor κB[30-32]. Aberrant AID expression correlates with chronic active inflammation, glandular atrophy and intestinal metaplasia in the non-neoplastic gastric mucosa[24]. The present study found that aberrant AID expression in tumors correlated with mononuclear cell activity in the mucosa surrounding the tumor, which would support the mechanisms of AID expression.
The 33.7% frequency of P53 overexpression in the DEGC was consistent with previous findings[8]. The expression of P53 was associated with flat or depressed macroscopic tumor features but not with the other clinicopathological features of age, gender, or location and tumor size. In agreement with our results, Sasaki et al[9] also demonstrated that P53 overexpression is more frequent in depressed-type differentiated GCs.
Several epigenetic alterations in GC have been described[10,16]. DNA methylation of MLH1 promoter region CpG islands is closely associated with a loss of Mlh1 expression in GCs that exhibit MSI[16]. MLH1 hypermethylation is evident in 20%-28% of differentiated carcinomas[10,33]. The reported frequency of negative Mlh1 expression in both early and sporadic GC ranges from 13%-20%[34,35]. In the present study, the frequency of lost Mlh1 expression in DEGCs was 18.6%. Our study and previous studies have shown that GCs with reduced Mlh1 expression are statistically more prevalent among elderly women. Previous reports have suggested that[36], high-frequency MSI (MSI-H) GCs are characterized by an antral location and proliferation. A loss of Mlh1 expression was associated with the lower third of the stomach and elevated gross type in our study. Additionally, our findings were consistent with the Guos report[37], which showed a higher prevalence of MSI-H in papillary type GC than in early well-differentiated carcinoma.
Chronic gastritis induced by H. pylori infection usually progresses to atrophic gastritis, which is an established risk factor for GC. The risk increases with the degree and the extent of atrophic gastritis. However, no clinicopathological studies regarding the relationship between molecular events and the degree of endoscopic atrophy in patients with GC have been published. Factors such as aging, dietary habits, alcohol consumption, cigarette smoking and autoimmunity promote atrophic gastritis[38,39]. The frequency of Mlh1 loss increases in tumors that cause a severe degree of endoscopic atrophic gastritis. Our results suggest that several factors are involved in the gastric atrophic changes found in patients with DEGC accompanied by aberrant Mlh1 expression. However, more studies are required to identify the mechanism of this association. Moreover, significantly less mononuclear cell infiltration was evident in patients with DEGC that had lost Mlh1. These results might be a consequence of a reduction in H. pylori density accompanied with severe glandular atrophy, which might contribute to reduced inflammatory infiltration.
In conclusion, we investigated the relationships between AID, P53 and Mlh1 expression, clinicopathological characteristics, and mucosal alterations. Our results suggest that aberrant AID expression may partly contributes to P53 overexpression.
COMMENTS
Background
Helicobacter pylori infection causes aberrant activation-induced cytidine deaminase (AID) expression in gastric epithelial tissues, which results in alterations in various tumor-related genes. However, the precise molecular mechanisms underlying gastric carcinogenesis are not fully understood, particularly in endoscopically resected early gastric tumors.
Research frontiers
In this study, the authors analyzed the expression of the tumor-related proteins: AID, P53, and Mlh1 in 102 differentiated-type early gastric carcinoma (DEGC) samples obtained by endoscopic resection.
Innovations and breakthroughs
The authors found that the rates of aberrant AID expression and P53 overexpression were both 34.3% in DEGCs. The expression of Mlh1 was lost in 18.6% of DEGCs. Aberrant AID expression was not significantly associated with P53 overexpression in DEGCs. However, AID expression was associated with the severity of mononuclear cell activity in the non-cancerous mucosa adjacent to the tumor. The rate of P53 expression was significantly greater in flat or depressed tumors than in elevated tumors. The frequency of Mlh1 loss was significantly increased in distal tumors, elevated gross type tumors, papillary histological type tumors, and tumors with a severe degree of endoscopic atrophic gastritis.
Applications
Collectively, the data of this study suggested that aberrant AID expression, P53 overexpression, and the loss of Mlh1 were all associated with clinicopathological features and gastric mucosal alterations in DEGCs. Furthermore, the aberrant expression of AID protein may partly contribute to the induction of nuclear P53 expression.
Peer review
Takeda et al analyzed expression of the tumor-related proteins: AID, P53, and Mlh1 in 102 DEGC samples obtained by endoscopic resection and found aberrant AID expression, P53 overexpression and the loss of Mlh1 expression were associated with some of the clinicopathological features and gastric mucosal alterations in DEGCs, and that the aberrant expression of AID protein might partly contribute to the induction of nuclear P53 expression.
Footnotes
Peer reviewer: Jian-Kun Hu, MD, PhD, Associate Professor, Department of Gastrointestinal Surgery, West China Hospital, Sichuan University, Chengdu 610041, Sichuan Province, China
S- Editor Wang JL L- Editor Hughes D E- Editor Zheng XM
References
- 1.Parkin DM. International variation. Oncogene. 2004;23:6329–6340. doi: 10.1038/sj.onc.1207726. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Clegg LX, Ward E, Ries LA, Wu X, Jamison PM, Wingo PA, Howe HL, Anderson RN, Edwards BK. Annual report to the nation on the status of cancer, 1975-2001, with a special feature regarding survival. Cancer. 2004;101:3–27. doi: 10.1002/cncr.20288. [DOI] [PubMed] [Google Scholar]
- 3.Arai T, Takubo K. Clinicopathological and molecular characteristics of gastric and colorectal carcinomas in the elderly. Pathol Int. 2007;57:303–314. doi: 10.1111/j.1440-1827.2007.02101.x. [DOI] [PubMed] [Google Scholar]
- 4.Helicobacter and Cancer Collaborative Group. Gastric cancer and Helicobacter pylori: a combined analysis of 12 case control studies nested within prospective cohorts. Gut. 2001;49:347–353. doi: 10.1136/gut.49.3.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ito Y, Azuma T, Ito S, Miyaji H, Hirai M, Yamazaki Y, Sato F, Kato T, Kohli Y, Kuriyama M. Analysis and typing of the vacA gene from cagA-positive strains of Helicobacter pylori isolated in Japan. J Clin Microbiol. 1997;35:1710–1714. doi: 10.1128/jcm.35.7.1710-1714.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Correa P. Human gastric carcinogenesis: a multistep and multifactorial process--First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res. 1992;52:6735–6740. [PubMed] [Google Scholar]
- 7.Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma, 13th ed. Tokyo: Kanehara & Co., Ltd; 1998. [DOI] [PubMed] [Google Scholar]
- 8.Tamura G. Alterations of tumor suppressor and tumor-related genes in the development and progression of gastric cancer. World J Gastroenterol. 2006;12:192–198. doi: 10.3748/wjg.v12.i2.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sasaki I, Yao T, Nawata H, Tsuneyoshi M. Minute gastric carcinoma of differentiated type with special reference to the significance of intestinal metaplasia, proliferative zone, and p53 protein during tumor development. Cancer. 1999;85:1719–1729. [PubMed] [Google Scholar]
- 10.Hong SH, Kim HG, Chung WB, Kim EY, Lee JY, Yoon SM, Kwon JG, Sohn YK, Kwak EK, Kim JW. DNA hypermethylation of tumor-related genes in gastric carcinoma. J Korean Med Sci. 2005;20:236–241. doi: 10.3346/jkms.2005.20.2.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamazaki K, Tajima Y, Makino R, Nishino N, Aoki S, Kato M, Sakamoto M, Morohara K, Kaetsu T, Kusano M. Tumor differentiation phenotype in gastric differentiated-type tumors and its relation to tumor invasion and genetic alterations. World J Gastroenterol. 2006;12:3803–3809. doi: 10.3748/wjg.v12.i24.3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fleisher AS, Esteller M, Wang S, Tamura G, Suzuki H, Yin J, Zou TT, Abraham JM, Kong D, Smolinski KN, et al. Hypermethylation of the hMLH1 gene promoter in human gastric cancers with microsatellite instability. Cancer Res. 1999;59:1090–1095. [PubMed] [Google Scholar]
- 13.Muramatsu M, Kinoshita K, Fagarasan S, Yamada S, Shinkai Y, Honjo T. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell. 2000;102:553–563. doi: 10.1016/s0092-8674(00)00078-7. [DOI] [PubMed] [Google Scholar]
- 14.Okazaki IM, Hiai H, Kakazu N, Yamada S, Muramatsu M, Kinoshita K, Honjo T. Constitutive expression of AID leads to tumorigenesis. J Exp Med. 2003;197:1173–1181. doi: 10.1084/jem.20030275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matsumoto Y, Marusawa H, Kinoshita K, Endo Y, Kou T, Morisawa T, Azuma T, Okazaki IM, Honjo T, Chiba T. Helicobacter pylori infection triggers aberrant expression of activation-induced cytidine deaminase in gastric epithelium. Nat Med. 2007;13:470–476. doi: 10.1038/nm1566. [DOI] [PubMed] [Google Scholar]
- 16.Schlemper RJ, Riddell RH, Kato Y, Borchard F, Cooper HS, Dawsey SM, Dixon MF, Fenoglio-Preiser CM, Fléjou JF, Geboes K, et al. The Vienna classification of gastrointestinal epithelial neoplasia. Gut. 2000;47:251–255. doi: 10.1136/gut.47.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kimura K, Takemoto T. An endoscopic recognition of the atrophic border and its significance in chronic gastritis. Endoscopy. 1969;1:87–97. [Google Scholar]
- 18.Takao T, Ishikawa T, Ando T, Takao M, Matsumoto T, Isozaki Y, Okita M, Nagao Y, Oyamada H, Yokoyama K, et al. Multifaceted Assessment of Chronic Gastritis: A Study of Correlations between Serological, Endoscopic, and Histological Diagnostics. Gastroenterol Res Pract. 2011;2011:631461. doi: 10.1155/2011/631461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996;20:1161–1181. doi: 10.1097/00000478-199610000-00001. [DOI] [PubMed] [Google Scholar]
- 20.Andachi H, Yashima K, Koda M, Kawaguchi K, Kitamura A, Hosoda A, Kishimoto Y, Shiota G, Ito H, Makino M, et al. Reduced Fhit expression is associated with mismatch repair deficiency in human advanced colorectal carcinoma. Br J Cancer. 2002;87:441–445. doi: 10.1038/sj.bjc.6600501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baek MJ, Kang H, Kim SE, Park JH, Lee JS, Paik YK, Kim H. Expression of hMLH1 is inactivated in the gastric adenomas with enhanced microsatellite instability. Br J Cancer. 2001;85:1147–1152. doi: 10.1054/bjoc.2001.2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morisawa T, Marusawa H, Ueda Y, Iwai A, Okazaki IM, Honjo T, Chiba T. Organ-specific profiles of genetic changes in cancers caused by activation-induced cytidine deaminase expression. Int J Cancer. 2008;123:2735–2740. doi: 10.1002/ijc.23853. [DOI] [PubMed] [Google Scholar]
- 23.Kim CJ, Song JH, Cho YG, Cao Z, Kim SY, Nam SW, Lee JY, Park WS. Activation-induced cytidine deaminase expression in gastric cancer. Tumour Biol. 2007;28:333–339. doi: 10.1159/000124239. [DOI] [PubMed] [Google Scholar]
- 24.Goto A, Hirahashi M, Osada M, Nakamura K, Yao T, Tsuneyoshi M, Takayanagi R, Oda Y. Aberrant activation-induced cytidine deaminase expression is associated with mucosal intestinalization in the early stage of gastric cancer. Virchows Arch. 2011;458:717–724. doi: 10.1007/s00428-011-1086-x. [DOI] [PubMed] [Google Scholar]
- 25.Ashcroft M, Kubbutat MH, Vousden KH. Regulation of p53 function and stability by phosphorylation. Mol Cell Biol. 1999;19:1751–1758. doi: 10.1128/mcb.19.3.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hurlimann J, Saraga EP. Expression of p53 protein in gastric carcinomas. Association with histologic type and prognosis. Am J Surg Pathol. 1994;18:1247–1253. doi: 10.1097/00000478-199412000-00008. [DOI] [PubMed] [Google Scholar]
- 27.Pfeifer GP, Denissenko MF, Olivier M, Tretyakova N, Hecht SS, Hainaut P. Tobacco smoke carcinogens, DNA damage and p53 mutations in smoking-associated cancers. Oncogene. 2002;21:7435–7451. doi: 10.1038/sj.onc.1205803. [DOI] [PubMed] [Google Scholar]
- 28.Saeki H, Ohno S, Miyazaki M, Araki K, Egashira A, Kawaguchi H, Watanabe M, Morita M, Sugimachi K. p53 protein accumulation in multiple oesophageal squamous cell carcinoma: relationship to risk factors. Oncology. 2002;62:175–179. doi: 10.1159/000048264. [DOI] [PubMed] [Google Scholar]
- 29.Viala J, Chaput C, Boneca IG, Cardona A, Girardin SE, Moran AP, Athman R, Mémet S, Huerre MR, Coyle AJ, et al. Nod1 responds to peptidoglycan delivered by the Helicobacter pylori cag pathogenicity island. Nat Immunol. 2004;5:1166–1174. doi: 10.1038/ni1131. [DOI] [PubMed] [Google Scholar]
- 30.Endo Y, Marusawa H, Kou T, Nakase H, Fujii S, Fujimori T, Kinoshita K, Honjo T, Chiba T. Activation-induced cytidine deaminase links between inflammation and the development of colitis-associated colorectal cancers. Gastroenterology. 2008;135:889–898, 898.e1-898.e3. doi: 10.1053/j.gastro.2008.06.091. [DOI] [PubMed] [Google Scholar]
- 31.Endo Y, Marusawa H, Kinoshita K, Morisawa T, Sakurai T, Okazaki IM, Watashi K, Shimotohno K, Honjo T, Chiba T. Expression of activation-induced cytidine deaminase in human hepatocytes via NF-kappaB signaling. Oncogene. 2007;26:5587–5595. doi: 10.1038/sj.onc.1210344. [DOI] [PubMed] [Google Scholar]
- 32.Komori J, Marusawa H, Machimoto T, Endo Y, Kinoshita K, Kou T, Haga H, Ikai I, Uemoto S, Chiba T. Activation-induced cytidine deaminase links bile duct inflammation to human cholangiocarcinoma. Hepatology. 2008;47:888–896. doi: 10.1002/hep.22125. [DOI] [PubMed] [Google Scholar]
- 33.Ohmura K, Tamura G, Endoh Y, Sakata K, Takahashi T, Motoyama T. Microsatellite alterations in differentiated-type adenocarcinomas and precancerous lesions of the stomach with special reference to cellular phenotype. Hum Pathol. 2000;31:1031–1035. doi: 10.1053/hupa.2000.16669. [DOI] [PubMed] [Google Scholar]
- 34.Kim HG, Lee S, Kim DY, Ryu SY, Joo JK, Kim JC, Lee KH, Lee JH. Aberrant methylation of DNA mismatch repair genes in elderly patients with sporadic gastric carcinoma: A comparison with younger patients. J Surg Oncol. 2010;101:28–35. doi: 10.1002/jso.21432. [DOI] [PubMed] [Google Scholar]
- 35.Nakajima T, Akiyama Y, Shiraishi J, Arai T, Yanagisawa Y, Ara M, Fukuda Y, Sawabe M, Saitoh K, Kamiyama R, et al. Age-related hypermethylation of the hMLH1 promoter in gastric cancers. Int J Cancer. 2001;94:208–211. doi: 10.1002/ijc.1454. [DOI] [PubMed] [Google Scholar]
- 36.Falchetti M, Saieva C, Lupi R, Masala G, Rizzolo P, Zanna I, Ceccarelli K, Sera F, Mariani-Costantini R, Nesi G, et al. Gastric cancer with high-level microsatellite instability: target gene mutations, clinicopathologic features, and long-term survival. Hum Pathol. 2008;39:925–932. doi: 10.1016/j.humpath.2007.10.024. [DOI] [PubMed] [Google Scholar]
- 37.Guo RJ, Arai H, Kitayama Y, Igarashi H, Hemmi H, Arai T, Hanai H, Sugimura H. Microsatellite instability of papillary subtype of human gastric adenocarcinoma and hMLH1 promoter hypermethylation in the surrounding mucosa. Pathol Int. 2001;51:240–247. doi: 10.1046/j.1440-1827.2001.01197.x. [DOI] [PubMed] [Google Scholar]
- 38.Nakamura M, Haruma K, Kamada T, Mihara M, Yoshihara M, Sumioka M, Fukuhara T, Chayama K. Cigarette smoking promotes atrophic gastritis in Helicobacter pylori-positive subjects. Dig Dis Sci. 2002;47:675–681. doi: 10.1023/a:1017901110580. [DOI] [PubMed] [Google Scholar]
- 39.Dai YC, Tang ZP, Zhang YL. How to assess the severity of atrophic gastritis. World J Gastroenterol. 2011;17:1690–1693. doi: 10.3748/wjg.v17.i13.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]


