Abstract
AIM: To explore the value of serum M2-pyruvate kinase (M2-PK) in colorectal cancer (CRC) mass screening.
METHODS: We conducted a molecular epidemiology study in Hangzhou, China, from year 2006 to year 2008. Serum samples were collected from 93 CRC, 41 advanced adenomas, 137 adenomas, 47 non-adenomatous polyps, and 158 normal participants in a community setting. Serum M2-PK and carcinoembryonic antigen (CEA) were measured using Enzyme-linked immunosorbent assay. SPSS 16.0 software was used to perform data analysis. Area under the receiver operating characteristic curve (AUC), sensitivity, and specificities were estimated for serum M2-PK in diagnosis of colorectal lesions and compared with CEA.
RESULTS: Average serum M2-PK value among 158 normal people was 2.96 U/mL and not affected by gender (P = 0.47) or age (P = 0.59). Average serum M2-PK (U/mL) was 14.75 among stage III and 13.10 among stage I and II CRC patients, about 4 times higher than that among normal people. Average serum M2-PK was 8.58, 6.70, 5.13 and 2.51 U/mL among advanced adenoma, adenomas, non-adenomatous polyps, and inflammatory bowel disease patients, respectively. AUC for serum M2-PK was greater than that for CEA among all colorectal lesions. AUC for serum M2-PK was 0.89 (0.84, 0.94) (95% confidence interval), higher than that for CEA [0.70 (0.62-0.79)] in CRC stage I and II, 0.89 (0.84-0.94) vs 0.73 (0.63-0.83) in CRC stage III, 0.81 (0.74-0.86) vs 0.63 (0.53 - 0.73) in advanced adenomas, 0.69 (0.64-0.76) vs 0.54 (0.47-0.60) in adenomas, and 0.69 (0.62-0.78) vs 0.58 (0.48-0.68) in non-adenomatous polyps. The diagnostic sensitivity for all colorectal lesions increased with decrease in the cut-off value of serum M2-PK. The diagnostic sensitivity (%) of serum M2-PK was 100.00 for CRC, 95.12 advanced adenoma, 82.48 adenoma, and 82.98 non-adenomatous polyp. There were no CRC cases missed and 40.51% of unnecessary colonoscopies were avoided when the cut-off value was 2.00 U/mL.
CONCLUSION: Serum M2-PK can be used as a primary screening test in CRC mass screening. It may be a promising non-invasive biomarker for CRC early detection.
Keywords: Serum M2-pyruvate kinase, Colorectal cancer screening, Serum biomarker, Carcinoembryonic antigen
INTRODUCTION
Colorectal cancer (CRC) is the third most common cancer in men and the second most common in women worldwide[1]. Data from China indicate that CRC incidence is rapidly rising, making it the 2nd-5th most common cancers across different cities[2-4] in the past decades. One of the most important ways to reduce CRC mortality and morbidity is to conduct CRC screening in the population. However, the compliance rate for the immunochemical fecal occult blood test (iFOBT) in a CRC mass screening is not high and is even lower for colonoscopy[5,6].
In order to increase the compliance rate, a screening protocol combining iFOBT with a high risk factors questionnaire (HRFQ) approach as the primary test to screen high risk populations, followed by colonoscopy as a follow-up test to detect CRC and other colorectal diseases, has been recommended by the Department of Disease Prevention and Control, the Ministry of Health of China, for CRC mass screening in China, based on the work of Zheng and her team[7]. This protocol has been used in the China national screening program in the general population in recent years[5,6,8]. The combined HRFQ has improved compliance rate and screening net sensitivity due to the simultaneous screening design and the overall effectiveness of our screening program. However, the overall false positive rate is high, as is the case in all CRC mass screening programs worldwide[8-11].
In our CRC mass screening program, 73.3% (false positive rate from iFOBT or HRFQ) of high risk populations previously underwent unnecessary colonoscopy examinations[6,8]. It is therefore worth further exploring a new simple noninvasive method with high compliance and high sensitivity to identify high risk populations, reduce unnecessary demand for colonoscopies from community residents, and save colonoscopy resources for populations genuinely in need. A serum biomarker with high sensitivity is usually regarded as an ideal primary mass screening test as this is simple, fast, convenient to both participants and clinicians, acceptable to participants, and noninvasive. To date, no effective serum biomarkers can be recommended for CRC mass screening.
We believe that serum tumor M2-pyruvate kinase (M2-PK) can be developed as an effective serum biomarker for CRC mass screening. There are four pyruvate kinase isoforms existing in mammals. The M1 isoform is predominantly expressed in most adult and differentiated tissues; L and R isoforms are expressed in liver and red blood cells; the dimeric form of the M2 isoform is a splice variant of M1 expressed in cancer cells and undifferentiated tissues[12]. Notably it has been reported that tumor tissues exclusively express the embryonic M2 isoform of pyruvate kinase[13,14]. Tumor M2-PK can be detected in blood and fecal samples, probably due to high expression in tumor cells and release into the body fluids[15].
Some studies have reported that fecal M2-PK is a promising biomarker for CRC screening and have recommended fecal M2-PK as a CRC screening marker[16-18]. However, several further studies do not support this view[19-21]. Blood tests are more convenient than fecal tests and can achieve a higher compliance rate in the general population. Clinical studies indicate that serum M2-PK has a higher sensitivity than serum carcinoembryonic antigen (CEA), is a valuable tumor marker in detection of gastrointestinal cancer[22,23] and has advantages in finding localized CRC[24]. No study has investigated the value of serum M2-PK in CRC mass screening in a community setting.
We investigated the potential value of serum M2-PK as a primary test for CRC screening in a community setting and compared its value with serum CEA which is currently one of the most commonly used diagnostic serum biomarkers and still regarded as the best single diagnostic marker for CRC[25,26] due to high specificity. However, serum CEA is not recommended as a screening test for CRC due to low sensitivity[27].
MATERIALS AND METHODS
Study design and population
We conducted a molecular epidemiology study to explore the value of serum M2-PK in CRC mass screening. The study protocol was reviewed and approved by the Institutional Review Board at Zhejiang University Cancer Institute. From July 2006 to December 2008, CRC screening was conducted among community residents aged 40-74 years in Hangzhou City[6,8] following the CRC screening protocol recommended by the China Ministry of Health. All participants gave written informed consent. When participants turned in the signed consent, we collected serum samples from 93 CRC, 41 advanced adenomas, 137 adenomas, 47 non-adenomatous polyps, 7 inflammatory bowel diseases (IBD), and 158 normal people in the community. According to CRC TNM protocol updated by UICC and AJCC in 2009, among the 93 cases (84 cases from consecutive community patients, 9 cases from our CRC screening site) of CRC, 55 cases were diagnosed as stage 0, I and II, and 38 cases were diagnosed as stage III.
Validation of colorectal lesions
All participants in this study were examined by colonoscopy. If colonoscopy showed a positive result, a biopsy and histopathological diagnosis were carried out after receipt of a signed consent form. Based on the International Classification and guidelines for Colonoscopy Surveillance after Polypectomy[28], CRC was defined as the invasion of malignant cells beyond the muscular mucosa. Patients with intramucosal carcinoma or carcinoma in situ were classified as having high-grade dysplasia. Histological classification of total polyps included adenoma (tubular, tubulovillous, or villous) and non-adenomatous polyps. Pathology slides of positive lesions were re-examined and diagnosed by consensus of at least two independent pathologists.
Serum M2-PK determined by enzyme-linked immunosorbent assay
Serum M2-PK was detected strictly in accordance with enzyme-linked immunosorbent assay kit instructions. The test kit measures the dimeric form of tumor M2-PK. The microtiter plates provided in the test kits were pre-coated with antibody specific to tumor M2-PK. Standards or samples are then added to the appropriate microtiter plate wells with a biotin-conjugated polyclonal antibody preparation specific for tumor M2-PK. Our assay carefully followed the instructions of the test kit. Serum CEA was detected automatically by an Abbott i2000SR automatic light meter. Standard serum M2-PK kits (Product ID E0588h) were purchased from Uscn life Science and Technology Company, USA). Serum samples were processed using the following steps: (1) 4 mL elbow vein blood was collected in CRC patients or high risk population under fasting state; (2) the vein blood was kept at under 4 °C for 1.5 h, until its natural coagulation; (3) the blood was centrifuged at 3000 r/min centrifugation at 4 °C for 5 min; and (4) serum obtained from blood supernatants was again centrifuged for 5 min. Supernatants of serum were removed and placed in Eppendorf tubes and packed immediately in a freezer at -80 °C.
Statistical analysis
SPSS 16.0 for Windows was used to perform data analysis. Mean ± SD were estimated for serum M2-PK, CEA, and age by colorectal lesion. Linear regressions and t tests were used to compare the serum M2-PK value between gender and age groups. Area under the receiver operating characteristic curve (AUC) and 95% confidence intervals (CI) were estimated for the value of serum M2-PK in diagnosis of CRC, advanced adenomas, adenomas, non-adenomatous polyps, and IBD and compared with serum CEA value. The meaning of AUC is defined as: no diagnostic value if AUC < 0.5; Low diagnostic value if AUC between 0.5-0.7; moderate diagnostic value if AUC between 0.7-0.9; high diagnostic value if AUC > 0.9[29]. The various diagnostic sensitivities and specificities, positive predictive values (PPV) and negative predictive values (NPV), and their 95% CIs were estimated by setting different M2-PK cut-off values for the various colorectal lesions compared with the normal people. The different M2-PK cut-off values were chosen according to the research purposes and scheduled sensitivity and specificity[30].
RESULTS
Table 1 shows the basic characteristics of the study population. The average age was 59.17 ± 10.71 for 93 CRC cases and 57.15 ± 7.96 for 158 normal participants. Among the normal group, there was no significant difference in serum M2-PK between men and women (P = 0.47) or between different age groups (P = 0.59). The average serum M2-PK value in U/mL was 14.75 ± 13.39 among the stage III and 13.10 ± 12.07 among the stage I and II of CRC patients, about 4 fold higher than that (2.96 ± 2.17) among the normal group. The average serum M2-PK value in U/mL was 8.58, 6.70, 5.13, and 2.51 among advanced adenoma, adenomas, nonadenomatous polyps, and IBD, respectively. The average serum CEA value in ng/mL was 5.68 ± 5.43 among stage III CRC patients and 5.74 ± 7.49 among stage I and II, about 2 fold higher than that (1.98 ± 1.02) among the normal group.
Table 1.
Basic characteristics of study population for the value of serum pyruvate kinase Isoenzyme M2 and carcinoembryonic antigen in colorectal cancer mass screening in Hangzhou, China, 2006-2008 (mean ± SD)
| Colorectal lesion | n |
Gender |
Age (yr) | M2-PK (U/mL) | CEA (ng/mL) | |
| Male | Female | |||||
| Colorectal cancer | ||||||
| Stage I and II | 55 | 53 | 40 | 59.17 ± 10.71 | 13.10 ± 12.07 | 5.74 ± 7.49 |
| Stage III | 38 | 14.75 ± 13.39 | 5.68 ± 5.43 | |||
| Advanced adenoma | 41 | 25 | 16 | 60.17 ± 7.78 | 8.58 ± 7.65 | 2.68 ± 1.43 |
| Adenoma | 137 | 68 | 69 | 60.34 ± 8.16 | 6.70 ± 6.97 | 2.58 ± 3.74 |
| Nonadenomatous polyp | 47 | 25 | 22 | 59.04 ± 8.08 | 5.13 ± 3.73 | 2.55 ± 2.09 |
| IBD | 7 | 1 | 6 | 57.43 ± 7.16 | 2.51 ± 1.94 | 1.71 ± 0.91 |
| Normal | 158 | 56 | 102 | 57.15 ± 7.96 | 2.96 ± 2.17 | 1.98 ± 1.02 |
IBD: Inflammatory bowel disease; M2-PK: M2-pyruvate kinase; CEA: Carcinoembryonic antigen.
The average AUC of serum M2-PK was significantly (P ≤ 0.01) greater than that of CEA among all kinds of colorectal lesions except non-adenomatous polyps (marginal significance, P = 0.09) and IBD (no significance, P = 0.40), as shown in Table 2. The AUC of serum M2-PK was 0.89 with 95% CI: 0.84-0.94, significantly higher than that of CEA (0.70: 0.62-0.79) for stage I and II CRC patients, 0.89 (0.84-0.94) vs 0.73 (0.63-0.83) for stage III CRC, 0.81 (0.74-0.86) vs 0.63 (0.53 - 0.73) for advanced adenomas, 0.69 (0.64-0.76) vs 0.54 (0.47-0.60) for adenomas, 0.69 (0.62-0.78) vs 0.58 (0.48-0.68) for non-adenomatous polyps, and 0.42 (0.21-0.63) vs 0.41 (0.21-0.61) for IBD.
Table 2.
The area under the receiver operating characteristic curve and 95% confidence interval of serum pyruvate kinase isoenzyme M2 in U/mL and carcinoembryonic antigen in ng/mL in diagnosing colorectal lesions in colorectal cancer mass screening in Hangzhou, China, 2006-2008
| Colorectal lesion | Test | AUC | SE | P-value | 95% CI |
| CRC stage I and II | CEA | 0.70 | 0.04 | < 0.0001 | 0.62-0.79 |
| M2-PK | 0.89 | 0.03 | < 0.0001 | 0.84-0.94 | |
| CRC stage III | CEA | 0.73 | 0.05 | < 0.0001 | 0.63-0.83 |
| M2-PK | 0.89 | 0.03 | < 0.0001 | 0.84-0.94 | |
| Advanced adenoma | CEA | 0.63 | 0.05 | 0.01 | 0.53-0.73 |
| M2-PK | 0.81 | 0.04 | < 0.0001 | 0.74-0.86 | |
| Adenoma | CEA | 0.54 | 0.03 | 0.28 | 0.47-0.60 |
| M2-PK | 0.69 | 0.03 | < 0.0001 | 0.64-0.76 | |
| Nonadenomatous polyp | CEA | 0.58 | 0.05 | 0.09 | 0.48-0.68 |
| M2-PK | 0.69 | 0.04 | < 0.0001 | 0.62-0.78 | |
| Inflammatory bowel disease | CEA | 0.41 | 0.10 | 0.40 | 0.21-0.61 |
| M2-PK | 0.42 | 0.10 | 0.40 | 0.21-0.63 |
AUC: Area under the receiver operating characteristic curve; CRC: Colorectal cancer; CI: Confidence interval; M2-PK: M2-pyruvate kinase; CEA: Carcinoembryonic antigen.
The diagnostic sensitivity and specificity with 95% CI of serum M2-PK at different cut-off values are shown in Table 3. When the cut-off value of M2-PK was
Table 3.
Diagnostic sensitivity and specificity in percentage at 95% confidence interval of serum M2-pyruvate kinase using various cut-off value settings for different colorectal lesions compared with 158 normal people in colorectal cancer mass screening in Hangzhou, 2006-2008 (95% CI)
| M2-PK (U/mL) |
Colorectal cancer |
Advanced adenoma |
Adenoma |
Non-adenomatous polyp |
||||
| Sensitivity | Specificity | Sensitivity | Specificity | Sensitivity | Specificity | Sensitivity | Specificity | |
| 2.00 | 100.00 (100.00-100.00) | 40.51 (32.85-48.16) | 95.12 (88.53-100.00) | 40.51 (32.85-48.16) | 82.48 (76.12-88.85) | 40.51 (32.85-48.16) | 82.98 (72.23-93.72) | 40.51 (32.85-48.16) |
| 2.50 | 94.62 (90.04-99.21) | 55.06 (47.31-62.82) | 85.37 (74.55-96.18) | 55.06 (47.31-62.82) | 71.53 (63.98-79.09) | 55.06 (47.31-62.82) | 76.60 (64.49-88.70) | 55.06 (47.31-62.82) |
| 3.00 | 91.40 (85.70-97.10) | 65.19 (57.76-72.62) | 75.61 (62.46-88.75) | 65.19 (57.76-72.62) | 61.31 (53.16-69.47) | 65.19 (57.76-72.62) | 65.96 (52.41-79.50) | 65.18 (57.76-72.62) |
| 3.50 | 87.10 (80.28-93.91) | 68.99 (61.78-76.20) | 73.17 (59.61-86.73) | 68.99 (61.78-76.20) | 56.93 (48.64-65.23) | 68.99 (61.78-76.20) | 55.32 (41.11-69.53) | 68.99 (61.78-76.20) |
| 4.00 | 81.72 (73.87-89.58) | 74.05 (67.22-80.89) | 65.85 (51.34-80.37) | 74.05 (67.22-80.89) | 49.64 (41.26-58.01) | 74.05 (67.22-80.89) | 48.94 (34.64-63.23) | 74.05 (67.22-80.89) |
M2-PK: M2-pyruvate kinase.
2.00 U/mL the sensitivity was 100.00% for CRC, i.e., there were no CRC cases missed. The sensitivity was 95.12%, 81.75%, and 82.98% for advanced adenomas, adenomas, and non-adenomatous polyps (missing rate was 4.88%, 18.25% and 17.02%), respectively. The specificity was 40.51% at the cut-off value of 2.00 U/mL, i.e., a total of 40.51% of unnecessary colonoscopies could be avoided. When the cut-off value increased from 2.00 to 4.00 U/mL, sensitivities of CRC decreased from 100.00% to 81.72% and specificities of CRC increased from 40.51% to 74.05%.
For the comparison of sensitivity and specificity between serum M2-PK and serum CEA in diagnosing positive colorectal lesions, the cut-off value of serum M2-PK was set at 2.00 U/mL and of CEA 5.00 ng/mL. The sensitivity of serum M2-PK was higher but the specificity was lower than that of CEA (Figure 1).
Figure 1.
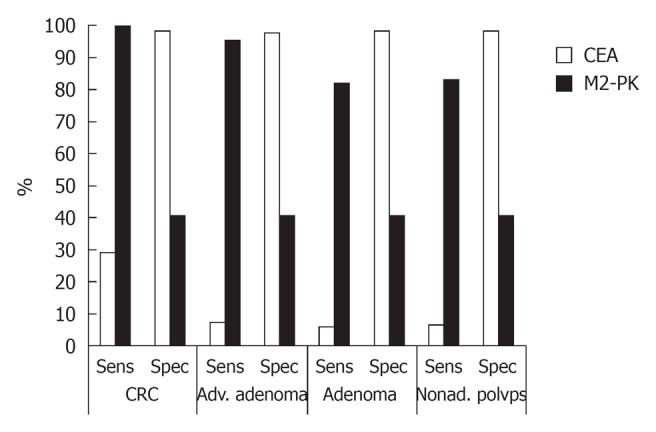
Comparison of diagnostic values between serum M2-pyruvate kinase and carcinoembryonic antigen for positive colorectal lesions based on sensitivity and specificity in Hangzhou, 2006-2008. M2-PK: M2-pyruvate kinase; CEA: Carcinoembryonic antigen; CRC: Colorectal cancer; Adv.: Advanced; Nonad.: Nonadenomatous; Sens: Sensitivity; Spec: Specificity.
The PPV and NPV with 95% CIs of serum M2-PK with various cut-off value settings for different colorectal lesions compared with 158 normal people in this CRC primary screening are shown in Table 4. The PPV varied from 49.73% to 64.96% and NPV from 100.00% to 87.31% when the cut-off value settings of serum M2-PK were changed from 2.00 to 4.00 U/mL.
Table 4.
Positive predictive value and negative predictive value in percentage at 95% confidence interval of serum M2-pyruvate kinase using various cut-off value settings for different colorectal lesions compared with 158 normal people in colorectal cancer mass screening in Hangzhou, China, 2006-2008 (95% CI)
| M2-PK (U/mL) |
Colorectal cancer |
Advanced adenoma |
Adenoma |
Nonadenomatous polyps |
||||
| PPV | NPV | PPV | NPV | PPV | NPV | PPV | NPV | |
| 2.00 | 49.73 (42.57-56.90) | 100.00 (100.00-100.00) | 29.32 (21.59-37.06) | 96.97 (92.83-100.00) | 54.59 (47.81-61.37) | 72.73 (63.42-82.03) | 29.32 (21.59-37.06) | 88.89 (81.63-96.15) |
| 2.50 | 55.35 (47.62-63.07) | 94.57 (89.93-99.20) | 33.02 (24.07-41.97) | 93.55 (88.56-98.54) | 57.99 (50.55-65.43) | 69.05 (60.98-77.12) | 33.64 (24.69-42.60) | 88.78 (82.23-95.03) |
| 3.00 | 60.71 (52.62-68.80) | 92.79 (87.98-97.60) | 36.05 (25.90-46.19) | 91.15 (85.91-96.39) | 60.43 (52.30-68.56) | 66.03 (58.59-73.46) | 36.05 (25.90-46.19) | 86.55 (80.43-92.68) |
| 3.50 | 62.31 (53.98-70.64) | 90.08 (84.76-95.41) | 37.97 (27.27-48.68) | 90.83 (85.67-96.00) | 61.42 (52.95-69.88) | 64.88 (57.66-72.10) | 34.67 (23.90-45.44) | 83.85 (77.52-90.17) |
| 4.00 | 64.96 (56.31-73.60) | 87.31 (67.22-80.89) | 39.71 (28.08-51.34) | 89.31 (84.02-94.60) | 62.39 (53.29-71.48) | 62.90 (55.96-69.85) | 35.94 (24.18-47.69) | 82.98 (76.78-89.18) |
M2-PK: M2-pyruvate kinase; PPV: Positive predictive value; NPV: Negative predictive value.
DISCUSSION
This study explored the potential value of serum M2-PK in screening CRC and other colorectal lesions in the population and compared its value to that of serum CEA. Overall, the serum M2-PK has a higher diagnostic value than CEA for all types of colorectal lesions except IBD. The serum M2-PK has a moderate to high diagnostic value for early and advanced stages of CRC but CEA has a low to moderate diagnostic value for all stages of CRC. For advanced adenoma the serum M2-PK has a moderate diagnostic value while CEA has a low to moderate value. For both adenoma and non-adenomatous polyps the serum M2-PK has a low to moderate diagnostic value while CEA has a zero to low value. According to this study, both serum M2-PK and CEA have no diagnostic value to IBD. The sensitivity of serum M2-PK is much higher than that of serum CEA in diagnosing all positive colorectal lesions except IBD. The post-hoc statistical power in this study was 100% for all positive colorectal lesions except IBD. Serum M2-PK has the capacity to find more CRC and precancerous lesions than CEA.
We used community patients’ samples to test the value of serum M2-PK and found that serum M2-PK has the advantage of detecting earlier stages of CRC. The sensitivity of serum M2-PK for CRC was 100% in this study when the cut-off value was set up at 2.00 U/mL, much higher than that of colonoscopy, iFOBT, and fecal M2-PK[17,31,32]. One of the major goals of CRC mass screening is to reduce mortality through the detection of early-stage CRC, adenocarcinoma and adenoma[31]. A CRC mass screening should avoid missing any CRC cases at the primary stage and confirm the diagnosis at the secondary or later stage of screening, making it possible to achieve the goal of fewer or no deaths from CRC. At this point, a higher-sensitivity screening test is to be preferred to a test with higher specificity in a primary screening. In addition, a serum test avoids the inconvenience of a fecal test and it is simpler, faster, and safer than colonoscopy. Thus, the compliance rate for serum M2-PK in a CRC mass screening is predicted to be higher than that for fecal M2-PK, iFOBT, and colonoscopy. Using serum M2-PK as a primary screening test, the effectiveness of a CRC mass screening should be increased due to high compliance and high sensitivity.
This study showed serum M2-PK is more useful than serum CEA in CRC mass screening because of higher sensitivity and diagnostic value in finding early CRC. The sensitivities of serum CEA were 29.03%, 7.31%, 5.84% and 6.38%, respectively, in diagnosing CRC, advanced adenomas, adenomas, and non-adenomatous polyps, when the serum CEA cut-off value was 5.00 ng/mL. The low sensitivity of serum CEA in detecting early CRC and precancerous lesions limits its application in CRC mass screening.
Adenoma is regarded as a precancerous lesion of CRC. Advanced adenoma is a severe type and defined as adenoma with a diameter of ≥ 10 mm, a villous adenoma, and an adenoma with high grade dysplasia[4,31]. The detection rates of early CRC and advanced adenoma have been used as important indicators in evaluating the effectiveness of a CRC mass screening programs[31]. The projected annual transition rates from advanced adenoma to CRC range from 2.6% to 5.6% among people ≥ 55 years old[33]. Studies show that fecal M2-PK is not a good marker for the detection of colorectal adenomas[34]. Until now, there have been no effective serum biomarkers for finding early CRC and advanced adenomas. Our study indicates that serum M2-PK can obtain a moderate diagnostic value in detecting advanced adenomas, better than that of serum CEA.
Fecal M2-PK can be an indicator of IBD[35,36] and some studies showed plasma M2-PK to have elevated levels in acute and serious inflammation disease[37,38]. However, our study did not find that serum M2-PK is a good index for IBD, for three possible reasons. One is that there were only seven cases of IBD in our study. The second is that the inflammatory process in these seven female patients may be in the early stage, not as severe as those in the other studies. The third possible reason is that there may actually be little difference between IBD cases and normal people. Since there are a considerable number of IBD patients among high risk CRC populations, future research should test the value of serum M2-PK for diagnosing IBD in a large study population.
The findings that serum M2-PK among normal people is low and not influenced by age and gender in this study are expected. Tumor M2-PK is an enzyme within tumor metabolism. The serum level of tumor M2-PK among normal people should be low compared to that among colorectal lesion patients and should not vary by gender and age. The average level of serum M2-PK among 158 normal people was 2.96 U/mL which is much lower than those in clinical patients or volunteers[22,23]. Our community-based results for serum levels of M2-PK associated with the TNM Classification of Malignant Tumors and Duke’s staging in CRC are supported by these patient-based clinical studies[22,23]. Because our result for normal serum level of M2-PK was based on a large sample size (158) from communities in a CRC mass screening program, it is reliable and can be generalizable.
Serum M2-PK with high sensitivity can achieve moderate to high diagnostic value in detecting early CRC and advanced adenomas and is superior to serum CEA. It also plays an important role in reducing costs, inconvenience, and colonoscopy-related complications during CRC screening. In addition, the compliance rate for serum M2-PK should be improved compared to other tests in a mass screening program. Thus the effectiveness of CRC mass screening programs should be improved greatly. In the long run, the healthcare burden from CRC should be minimized due to low CRC incidence and mortality in the community, the desired outcome of a successful CRC screening program.
Overall, we conclude that serum M2-PK can be used as an efficient primary screening test for CRC mass screening. It is simpler and faster than a fecal test and cheaper, more convenient, and safer than colonoscopy. It is a promising non-invasive biomarker for CRC early detection. We will test its value in other community settings and or in a large study population in the future.
ACKNOWLEDGMENTS
The authors appreciate the previous screening work contributed by researchers, physicians, nurses and staff in our research team. Also the authors thank endoscopy physicians, nurses, and staff in local hospitals for performing the colonoscopies on participants in this study.
COMMENTS
Background
Colorectal cancer (CRC) has become a big burden to global health over the past decades. Mass screening is an effective way to reduce CRC mortality and incidence in the population. However, the low compliance for current screening tests affects the effectiveness of CRC mass screening programs. A serum test avoids the inconvenience of a fecal test and it is simpler, faster, and safer than colonoscopy. Therefore, a serum test can obtain a higher compliance for CRC screening in the general population than a fecal test or colonoscopy. A serum biomarker test with high sensitivity is intuitively an ideal test for CRC mass screening. To date, no effective serum biomarkers can be recommended for CRC mass screening.
Research frontiers
M2 isoform of pyruvate kinase (M2-PK) is a splice variant of M1 and expressed in cancer cells and undifferentiated tissues. The dimeric form of M2-PK is termed tumor M2-PK. Clinical research shows tumor M2-PK is associated with the TNM Classification of Malignant Tumors and Duke’s staging in CRC. The authors hypothesized that serum tumor M2-PK can be developed as an efficient primary screening test in CRC screening in the population. No previous study has investigated the value of serum tumor M2-PK in CRC mass screening in a community setting.
Innovations and breakthroughs
The level of serum M2-PK among normal people was low and not affected by gender and age. Serum M2-PK among CRC patients was about 4 fold higher than that among the normal. Serum M2-PK has moderate value in diagnosing CRC and advanced adenoma. The diagnostic sensitivity of serum M2-PK was 100.0% for CRC, i.e., there were no CRC cases missed, and 40.5% of unnecessary colonoscopies avoided when the cut-off value was 2.00 U/mL. This is the first study that has investigated the value of serum M2-PK in CRC mass screening in a community setting.
Applications
Results from this study suggest that serum M2-PK can be used as a primary screening test in CRC mass screening due to its high sensitivity and high compliance. It is a promising non-invasive biomarker for CRC early detection.
Terminology
Advanced adenoma is a severe type of adenoma and defined as adenoma with a diameter of ≥ 10 mm, a villous adenoma, and an adenoma with high grade dysplasia.
Peer review
The author investigated the potential value of serum M2-PK as a promising non-invasive biomarker for CRC mass screening, due to lower sensitivity of serum CEA for CRC screening. Positive results had been achieved in this study.
Footnotes
Supported by The National 11th 5-Year Key Programs for Department of Science and Technology of China, No. 2006BAI02A08
Peer reviewers: Joseph T Tseng, Assistant Professor, Institute of Bioinformatics, College of Bioscience and Biotechnology, National Cheng-Kung University, No.1 University Rd, Tainan, 701, Taiwan, China; Yu-Min Li, PhD, Professor, Second Hospital of Lanzhou University, Lanzhou 730030, Gansu Province, China
S- Editor Wang JL L- Editor Hughes D E- Editor Zheng XM
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Yang L, Parkin DM, Ferlay J, Li L, Chen Y. Estimates of cancer incidence in China for 2000 and projections for 2005. Cancer Epidemiol Biomarkers Prev. 2005;14:243–250. [PubMed] [Google Scholar]
- 3.Zhang SW, Chen WQ, Kong LZ, Li LD, Lu FZ, Li GL, Meng J, Zhao P. [Malignant tumor incidence and mortality among some cities and counties in China, 1998-2002] Zhongguo Zhongliu. 2006;15:430–448. [Google Scholar]
- 4.Sung JJ, Lau JY, Young GP, Sano Y, Chiu HM, Byeon JS, Yeoh KG, Goh KL, Sollano J, Rerknimitr R, et al. Asia Pacific consensus recommendations for colorectal cancer screening. Gut. 2008;57:1166–1176. doi: 10.1136/gut.2007.146316. [DOI] [PubMed] [Google Scholar]
- 5.Cai SR, Zhang SZ, Zhu HH, Zheng S. Barriers to colorectal cancer screening: a case-control study. World J Gastroenterol. 2009;15:2531–2536. doi: 10.3748/wjg.15.2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meng W, Bi XW, Bai XY, Pan HF, Cai SR, Zhao Q, Zhang SZ. Barrier-focused intervention to increase colonoscopy attendance among nonadherent high-risk populations. World J Gastroenterol. 2009;15:3920–3925. doi: 10.3748/wjg.15.3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dong ZW. Guidelines of cancer screening, early detection and early treatment of China. 1st ed. Beijing: Peking University Medical Press; 2005. pp. 34–46. [Google Scholar]
- 8.Meng W, Cai SR, Zhou L, Dong Q, Zheng S, Zhang SZ. Performance value of high risk factors in colorectal cancer screening in China. World J Gastroenterol. 2009;15:6111–6116. doi: 10.3748/wjg.15.6111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cai SR, Zhang SZ, Zhu HH, Huang YQ, Li QR, Ma XY, Yao KY, Zheng S. Performance of a colorectal cancer screening protocol in an economically and medically underserved population. Cancer Prev Res (Phila) 2011;4:1572–1579. doi: 10.1158/1940-6207.CAPR-10-0377. [DOI] [PubMed] [Google Scholar]
- 10.Kronborg O, Regula J. Population screening for colorectal cancer: advantages and drawbacks. Dig Dis. 2007;25:270–273. doi: 10.1159/000103899. [DOI] [PubMed] [Google Scholar]
- 11.Zhu MM, Xu XT, Nie F, Tong JL, Xiao SD, Ran ZH. Comparison of immunochemical and guaiac-based fecal occult blood test in screening and surveillance for advanced colorectal neoplasms: a meta-analysis. J Dig Dis. 2010;11:148–160. doi: 10.1111/j.1751-2980.2010.00430.x. [DOI] [PubMed] [Google Scholar]
- 12.Clower CV, Chatterjee D, Wang Z, Cantley LC, Vander Heiden MG, Krainer AR. The alternative splicing repressors hnRNP A1/A2 and PTB influence pyruvate kinase isoform expression and cell metabolism. Proc Natl Acad Sci USA. 2010;107:1894–1899. doi: 10.1073/pnas.0914845107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Christofk HR, Vander Heiden MG, Harris MH, Ramanathan A, Gerszten RE, Wei R, Fleming MD, Schreiber SL, Cantley LC. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature. 2008;452:230–233. doi: 10.1038/nature06734. [DOI] [PubMed] [Google Scholar]
- 14.Mazurek S, Boschek CB, Hugo F, Eigenbrodt E. Pyruvate kinase type M2 and its role in tumor growth and spreading. Semin Cancer Biol. 2005;15:300–308. doi: 10.1016/j.semcancer.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 15.Kaura B, Bagga R, Patel FD. Evaluation of the Pyruvate Kinase isoenzyme tumor (Tu M2-PK) as a tumor marker for cervical carcinoma. J Obstet Gynaecol Res. 2004;30:193–196. doi: 10.1111/j.1447-0756.2004.00187.x. [DOI] [PubMed] [Google Scholar]
- 16.Ewald N, Schaller M, Bayer M, Akinci A, Bretzel RG, Kloer HU, Hardt PD. Fecal pyruvate kinase-M2 (tumor M2-PK) measurement: a new screening concept for colorectal cancer. Anticancer Res. 2007;27:1949–1952. [PubMed] [Google Scholar]
- 17.Hardt PD, Mazurek S, Toepler M, Schlierbach P, Bretzel RG, Eigenbrodt E, Kloer HU. Faecal tumour M2 pyruvate kinase: a new, sensitive screening tool for colorectal cancer. Br J Cancer. 2004;91:980–984. doi: 10.1038/sj.bjc.6602033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tonus C, Neupert G, Sellinger M. Colorectal cancer screening by non-invasive metabolic biomarker fecal tumor M2-PK. World J Gastroenterol. 2006;12:7007–7011. doi: 10.3748/wjg.v12.i43.7007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haug U, Hundt S, Brenner H. Sensitivity and specificity of faecal tumour M2 pyruvate kinase for detection of colorectal adenomas in a large screening study. Br J Cancer. 2008;99:133–135. doi: 10.1038/sj.bjc.6604427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shastri YM, Naumann M, Oremek GM, Hanisch E, Rösch W, Mössner J, Caspary WF, Stein JM. Prospective multicenter evaluation of fecal tumor pyruvate kinase type M2 (M2-PK) as a screening biomarker for colorectal neoplasia. Int J Cancer. 2006;119:2651–2656. doi: 10.1002/ijc.22243. [DOI] [PubMed] [Google Scholar]
- 21.Shastri YM, Loitsch S, Hoepffner N, Povse N, Hanisch E, Rösch W, Mössner J, Stein JM. Comparison of an established simple office-based immunological FOBT with fecal tumor pyruvate kinase type M2 (M2-PK) for colorectal cancer screening: prospective multicenter study. Am J Gastroenterol. 2008;103:1496–1504. doi: 10.1111/j.1572-0241.2008.01824.x. [DOI] [PubMed] [Google Scholar]
- 22.Schulze G. The tumor marker tumor M2-PK: an application in the diagnosis of gastrointestinal cancer. Anticancer Res. 2000;20:4961–4964. [PubMed] [Google Scholar]
- 23.Zhang B, Chen JY, Chen DD, Wang GB, Shen P. Tumor type M2 pyruvate kinase expression in gastric cancer, colorectal cancer and controls. World J Gastroenterol. 2004;10:1643–1646. doi: 10.3748/wjg.v10.i11.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schneider J, Schulze G. Comparison of tumor M2-pyruvate kinase (tumor M2-PK), carcinoembryonic antigen (CEA), carbohydrate antigens CA 19-9 and CA 72-4 in the diagnosis of gastrointestinal cancer. Anticancer Res. 2003;23:5089–5093. [PubMed] [Google Scholar]
- 25.Wild N, Andres H, Rollinger W, Krause F, Dilba P, Tacke M, Karl J. A combination of serum markers for the early detection of colorectal cancer. Clin Cancer Res. 2010;16:6111–6121. doi: 10.1158/1078-0432.CCR-10-0119. [DOI] [PubMed] [Google Scholar]
- 26.El-Awady S, Lithy R, Morshed M, Khafagy W, Abd Monem H, Waleed O, Badr S, Fekry A, El Nakeeb A, Ghazy H, et al. Utility of serum preoperative carcinoemberyonic antigen in colorectal cancer patients. Hepatogastroenterology. 2009;56:361–366. [PubMed] [Google Scholar]
- 27.Locker GY, Hamilton S, Harris J, Jessup JM, Kemeny N, Macdonald JS, Somerfield MR, Hayes DF, Bast RC. ASCO 2006 update of recommendations for the use of tumor markers in gastrointestinal cancer. J Clin Oncol. 2006;24:5313–5327. doi: 10.1200/JCO.2006.08.2644. [DOI] [PubMed] [Google Scholar]
- 28.Winawer SJ, Zauber AG, Fletcher RH, Stillman JS, O’brien MJ, Levin B, Smith RA, Lieberman DA, Burt RW, Levin TR, et al. Guidelines for colonoscopy surveillance after polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer and the American Cancer Society. CA Cancer J Clin. 2006;56:143–159; quiz 184-185. doi: 10.3322/canjclin.56.3.143. [DOI] [PubMed] [Google Scholar]
- 29.Song HL, He J, Huang PX, Li SY. Application of parametric and nonparametric methods in estmiating AUC ROC. Shanghai: China 2nd Military Medical University Press; 2006. pp. 726–728. [Google Scholar]
- 30.Chen WZ, Pan XP, Song XB, Ni ZZ. [Selection of the best cut-off value in ROC curve] Zhongguo Weisheng Tongji. 2006;23:157–158. [Google Scholar]
- 31.Roessler M, Rollinger W, Palme S, Hagmann ML, Berndt P, Engel AM, Schneidinger B, Pfeffer M, Andres H, Karl J, et al. Identification of nicotinamide N-methyltransferase as a novel serum tumor marker for colorectal cancer. Clin Cancer Res. 2005;11:6550–6557. doi: 10.1158/1078-0432.CCR-05-0983. [DOI] [PubMed] [Google Scholar]
- 32.Helm J, Choi J, Sutphen R, Barthel JS, Albrecht TL, Chirikos TN. Current and evolving strategies for colorectal cancer screening. Cancer Control. 2003;10:193–204. doi: 10.1177/107327480301000302. [DOI] [PubMed] [Google Scholar]
- 33.Brenner H, Hoffmeister M, Stegmaier C, Brenner G, Altenhofen L, Haug U. Risk of progression of advanced adenomas to colorectal cancer by age and sex: estimates based on 840,149 screening colonoscopies. Gut. 2007;56:1585–1589. doi: 10.1136/gut.2007.122739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shastri YM, Stein JM. Faecal tumour pyruvate kinase M2: not a good marker for the detection of colorectal adenomas. Br J Cancer. 2008;99:1366; author reply 1367. doi: 10.1038/sj.bjc.6604656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chung-Faye G, Hayee B, Maestranzi S, Donaldson N, Forgacs I, Sherwood R. Fecal M2-pyruvate kinase (M2-PK): a novel marker of intestinal inflammation. Inflamm Bowel Dis. 2007;13:1374–1378. doi: 10.1002/ibd.20214. [DOI] [PubMed] [Google Scholar]
- 36.Walkowiak J, Banasiewicz T, Krokowicz P, Hansdorfer-Korzon R, Drews M, Herzig KH. Fecal pyruvate kinase (M2-PK): a new predictor for inflammation and severity of pouchitis. Scand J Gastroenterol. 2005;40:1493–1494. doi: 10.1080/00365520500319112. [DOI] [PubMed] [Google Scholar]
- 37.Staib P, Hoffmann M, Schinköthe T. Plasma levels of tumor M2-pyruvate kinase should not be used as a tumor marker for hematological malignancies and solid tumors. Clin Chem Lab Med. 2006;44:28–31. doi: 10.1515/CCLM.2006.006. [DOI] [PubMed] [Google Scholar]
- 38.Schneider J, Morr H, Velcovsky HG, Weisse G, Eigenbrodt E. Quantitative detection of tumor M2-pyruvate kinase in plasma of patients with lung cancer in comparison to other lung diseases. Cancer Detect Prev. 2000;24:531–535. [PubMed] [Google Scholar]


