Abstract
The pathogenesis of osteoarthritis (OA) appears to be the result of a complex interplay between mechanical, cellular, and biochemical forces. Obesity is the strongest risk factor for disease onset and mechanical factors dominate the risk for disease progression. This narrative review focuses on the influence of biomechanics and obesity on the etiology of OA and its symptomatic presentation. We need to revisit the way we currently manage the disease and focus on the modifiable, primarily through nonpharmacologic intervention. Greater therapeutic attention to the important role of mechanical factors and obesity in OA etiopathogenesis is required if we are to find ways of reducing the public health impact of this condition.
Keywords: osteoarthritis, management, nonpharmacologic
Introduction
Osteoarthritis (OA) affects an estimated 21 million Americans [1998; Lawrence et al. 1998], and recent estimates suggest that symptomatic knee-OA occurs in 13% of people aged 60 and over [Dunlop et al. 2001; 1998]. The risk of mobility disability (defined as needing help walking or climbing stairs) attributable to knee OA alone is greater than that due to any other medical condition in people aged 65 and over [Guccione et al. 1994; 2001]. Although this prevalence is high, it is expected to increase even further with the increasing prevalence of obesity and the aging population [1994]. Despite its frequency and impact from disability, OA is a condition that is often poorly managed in clinical practice.
Sir William Osler, considered to be the ‘Father of Modern Medicine’, once said ‘osteoarthritis is an easy disease to take care of when the patient walks in the front door, I walk out the back door’ [Balint et al. 1987]. No-one denies that the management of OA is a challenge, however, modern clinicians are armed with a plethora of effective treatment options many of which are not used effectively and the importance of modifiable risk factors is not recognized.
The two modifiable risk factors that account for the majority of disease development and its progression are obesity and altered joint mechanics. Numerous studies have highlighted the importance of mechanical factors on the etiopathogenesis of this disease [Andriacchi et al. 2004; Carter et al. 2004; Jackson et al. 2004; Shakoor and Moisio, 2004; Teichtahl et al. 2003; Kerin et al. 2002]. Obesity is the single most important risk factor for development of severe OA of the knee, more so than other potentially damaging factors including heredity [Coggon et al. 2001; Felson and Zhang, 1998]. Despite the recognition that both joint mechanics and obesity are critically important in disease genesis and symptoms, little is done to effectively intervene on these important risk factors. Instead, the primary focus of current therapeutic interventions is palliative—primarily limited to analgesia, and, when this fails, surgical intervention.
This narrative review focuses on the influence of biomechanics and obesity on the etiology of OA and its symptomatic presentation. We need to revisit the way we currently manage the disease and focus on what is modifiable, primarily through nonpharmacologic intervention. In order for this change in clinical management of OA to occur we need to recognize the missed opportunity to intervene on these modifiable risk factors. Pursuant to that recognition, a coordinated process to address OA management and outcome-oriented efforts to address current shortcomings in clinical practice should be undertaken. The paper is a narrative review of methods focused on redressing limitations in the current standards of clinical management of OA.
Problems with current clinical management of OA
Major efforts are underway internationally to reform health care to optimize outcomes and to reduce costs. One of the most expensive aspects of health-care delivery is the management of OA. According to the US Centers for Disease Control (CDC), arthritis and other rheumatic conditions (AORC) cost the United States US $128 billion in 2003, a 24% increase since 1997 and an amount equal to 1.2% of the gross domestic product (GDP) [Centers for Disease Control and Prevention (CDC), 2007]. The most prevalent and disabling form of arthritis is OA. The burden of disability for our patients and the increasing societal cost of treating this illness should encourage reflection and redress our current methods of managing this prevalent disease, both in the public-health and clinical-care settings.
In the absence of a cure, current therapeutic modalities are primarily aimed at reducing pain and improving joint function by agents targeted toward symptoms that do not facilitate any improvement in joint structure or long-term disease amelioration [Zhang et al. 2008; Hunter, 2007; Hunter and Felson, 2006; Zhang et al. 2005; Jordan et al. 2003; Anonymous, 2000]. Despite a number of evidence-based guidelines for OA management, [Zhang et al. 2008; Hunter, 2007; Hunter and Felson, 2006; Zhang et al. 2005; Jordan et al. 2003; Anonymous, 2000] a number of reports have outlined suboptimal care for patients with OA [DeHaan et al. 2007; Jawad, 2005; Pencharz et al. 2002 Glazier et al. 1998]. These studies demonstrate inadequate uptake of conservative, nonpharmacologic treatment options such as weight loss and exercise, inappropriate surgical interventions such as arthroscopic debridement and lavage and the inappropriate use of imaging. These are just a few of the examples which are discussed further here, but inevitably the limitations of current management methods need to be recognized and endorsed by the interested stakeholders (including clinicians engaged in OA management) and processes (such as quality and outcome indicators) put in place to address them.
The typical management of OA in clinical practice is palliative—analgesia followed by surgical intervention. While both analgesia and surgery have a critical role to play, at present this is all many clinicians do. The most widely used analgesic agents, nonsteroidal anti-inflammatory drugs (NSAIDs) and cyclooxygenase (COX)-2 inhibitors are associated with high rates of adverse events (AE) [Ortiz, 2004; Felson et al. 2000]. NSAIDs alone cause over 16,500 deaths and over 103,000 hospitalizations per year in the US [Wolfe et al. 1999]. In addition, these drugs rarely relieve symptoms completely. In clinical trials of OA, different NSAIDs performed similarly, with people reporting an approximate 30% reduction in pain and 15% improvement in function [Todd and Clissold, 1990].
Many individuals with OA ultimately require total joint replacement, a procedure that is also not without inherent morbidity and cost. Although OA can be treated surgically, there are many patients for whom this is inappropriate, because of medical comorbidity, old age or other circumstances [Zhang et al. 2008]. In the younger and more active individual, it is desirable to delay arthroplasty due to the limited lifespan of the prosthesis and the necessary lifestyle changes that accompany it. Surgery should be resisted when symptoms can be managed by other treatment modalities. At present there is no metric whereby the use of conservative management prior to surgery is monitored.
Another major challenge with current clinical practice is the overuse of imaging for a diagnosis that can be made clinically. In light of the current lack of therapy that can modify the disease course, and the imprecision of imaging measurement, there is currently no rationale for obtaining serial radiographs if the clinical state remains unchanged. Magnetic resonance imaging (MRI) should be used infrequently to facilitate the diagnosis of other causes of knee pain that can be confused with OA (e.g. osteochondritis dissecans, avascular necrosis). Various imaging procedures such as CT, MRI, and PET scans have grown to a $100 billion industry in the United States. According to Dr Vijay Rao, chairwoman of the radiology department of the Thomas Jefferson University Hospital, of the over 95 million high-tech scans done in the US annually, 20–50% were unnecessary because their results failed to help treat or diagnose the patient's ailment [Rao, 2009]. The penchant to obtain serial radiographs and MRIs in persons with OA typically does not alter management.
One important consequence of the penchant for imaging is to drive up the rates of unnecessary surgical intervention. One frequent and unfortunate consequence of this in knee OA is meniscal resection. The menisci have many functions in the knee, including the equal distribution of stress between the relatively incongruous tibiofemoral joint surfaces, stability enhancement and lubrication [Seedhom et al. 1974]. The absence of a functioning meniscus increases peak and average contact stresses in the medial compartment in a range of 40 700% [Baratz et al. 1986; Fukubayashi and Kurosawa, 1980; Kurosawa et al. 1980]. Degenerative meniscal lesions such as horizontal cleavages, oblique or complex tears are associated with older age [Englund et al. 2008]. By the time radiographic disease develops, the overwhelming majority of patients have meniscal lesions [Englund et al. 2008; Bhattacharyya et al. 2003]. In asymptomatic patients with a mean age of 65 years, a tear was found in 67% using MRI, whereas in patients with symptomatic knee OA, a meniscal tear was found in 91% [Bhattacharyya et al. 2003]. Resection of a non-obstructive degenerate meniscus may only remove the earliest evidence of OA while disease progression proceeds [Moseley et al. 2002]. Arthroscopic debridement and meniscal resection remains the most frequently performed procedure by orthopedic surgeons in the US [Brinker et al. 2002; Hall and Lawrence, 1998]. Up to 1 million knee arthroscopies are performed annually in the US. In a well-designed placebo surgery trial, improvement in symptoms could be attributed to a placebo effect [Moseley et al. 2002]. For a small subgroup of knees with loose bodies or flaps of meniscus or cartilage that are causing mechanical symptoms, especially locking or catching of the joint, arthroscopic removal of these unstable tissues may improve joint function and alleviate these mechanical symptoms. The majority of people with knee OA do not complain of these symptoms. Recent guidelines (including the most recent from OARSI [Zhang et al. 2008] and AAOS [2009]) recommend against the use of routine arthroscopy for knee OA management. To date, these recommendations have not been reflected in changes in clinical practice.
Our inability as medical professionals to adequately satisfy the demands of our patient's complaints of pain and functional limitation is leading to the pursuit of other treatments with limited efficacy. With few conservative options offered by their doctors, increasing numbers of patients are turning to untested alternative remedies and aggressively marketed dietary supplements with little substantive evidence to support their efficacy [Gardiner et al. 2007]. These are just some of the reasons for us to reflect on OA management, to recognize these limitations, and to redress them accordingly.
The genesis of joint symptoms
A full understanding of the risk factors for pain and other symptoms in knee OA requires consideration of a range of biopsychosocial factors [Dieppe and Lohmander, 2005]. The symptoms of OA are typically described as mechanical—i.e. they occur with physical activity. However, subjects with the same degree of structural damage experience widely differing levels of pain, a phenomenon that is poorly understood [Hunter et al. 2008b]. Differences in joint forces and joint stress during functional activities may assist in explaining the dissociation between radiographic structural findings and pain. Malalignment has been previously shown to be a predictor for functional decline in knee OA and plays a role in the ‘mechanical’ nature of OA-related pain [Sharma et al. 2001].
Lower-limb OA may lead to adaptive strategies in gait to reduce the loading in an otherwise vulnerable joint [Mundermann et al. 2004; Hurwitz et al. 2002]. These strategies include adopting a toe-out gait and/or slower walking speed. They can be reinforced or learned by appropriate rehabilitation and offer a potential for another conservative approach to knee OA therapy.
In addition to strategies adopted by patients, prescribed medication may alter joint loading. Commensurate with reductions in pain, NSAIDs also offer improvements in a person's walking speed [Blin et al. 1990]. Some of the increase in joint loading may be a direct consequence of faster walking speeds following the use of the NSAIDs, but previous analyses have also demonstrated increases in both the adduction and quadriceps moment in people who experienced pain relief with piroxicam [Schnitzer et al. 1993]. Of particular concern is the fact that anti-inflammatory or analgesic therapy may actually be associated with an increase in joint forces. Whether this is the mechanism that explains the potential for increased structural damage associated with NSAID use remains unclear [Huskisson et al. 1995].
The impact of modifiable factors on disease onset and progression
OA occurs in joints when the dynamic equilibrium between the breakdown and repair of joint tissues becomes unbalanced [Eyre, 2004]. This progressive joint failure may cause pain and disability [Guccione et al. 1994], although many people with structural changes consistent with OA are asymptomatic [Hannan et al. 2000]. OA can occur in any synovial joint in the body but is most common in the knees, hips and hands.
OA is perhaps best understood as resulting from excessive mechanical stress applied in the context of systemic susceptibility. Susceptibility to OA may be increased in part by genetic inheritance (a positive family history increases risk), age, ethnicity, nutritional factors and female gender [Felson, 2004a]. The susceptibility to OA can also be influenced by the mechanical environment. For example, the predilection for OA being more prevalent in women than men may partly be explained on the basis of the female knee being more mechanically vulnerable to OA. Quadricep strength in men is greater than in women and this difference may play an important role in improving joint stability [Hassan et al. 2002; Slemenda et al. 1998]. The higher fat-mass and lower muscle-mass in women may explain some of the gender difference in OA susceptibility although this is conjectural and needs to be formally tested [Madsen et al. 1997]. Other gender differences that impact upon joint loading include pelvic dimensions, knee morphology, Q-angle, and neuromuscular strength [Huston et al. 2000]. For instance, disproportionate loading of the lateral compartment in women likely arises from differences in knee stability/stiffness that is reduced in women as a result of decreased neuromuscular strength and increased ligamentous laxity [Hewett, 2000; Huston et al. 2000; Shelbourne et al. 1998].
Local mechanical factors such as the adduction moment, malalignment, and muscle strength make the knee joint vulnerable to the progression of OA [Felson, 2004b]. The human knee is a complex joint with considerable forces on the articular surfaces during weight bearing. In theory, any shift from a neutral or collinear alignment of the hip, knee, and ankle affects load distribution at the knee [Tetsworth and Paley, 1994]. The medial compartment bears a resultant 60–70% of the force across the neutrally aligned knee during weight bearing [Andriacchi, 1994] and, because it is subjected to more load than the lateral compartment, this may play a role in the predisposition to medial tibiofemoral compartment progression in OA [Ledingham et al. 1993]. In a varus knee, this axis passes medial to the knee and a moment arm is created, which further increases force across the medial compartment. In contrast, in a valgus knee, the load-bearing axis passes lateral to the knee, and the resulting moment arm increases force across the lateral compartment [Tetsworth and Paley, 1994]. Varus and valgus malalignment, respectively, have been shown to increase the risk of subsequent medial and lateral knee OA radio-graphic progression [Sharma et al. 2001]. Malalignment provides only a static impression of the mechanical forces being imparted on the joint in one plane. The dynamic moment that provides a major contribution to the total loading across the knee joint is usually labeled the adduction (or external varus) moment at the knee [Andriacchi, 1994]. Higher maximum adduction moments at the knee are related to OA disease severity and to a higher rate of progression of knee OA [Miyazaki et al. 2002; Sharma et al. 1998]. Of the risk factors known to be associated with disease progression, the adduction moment is the most potent that has been identified [Miyazaki et al. 2002]. Understanding the role alignment plays in OA etiopathogenesis is important because it modulates the effect of standard risk-factors for knee OA progression including obesity [Sharma et al. 2000], quadricep strength [Sharma et al. 2003], laxity [Sharma et al. 2003] and stage of disease [Cerejo et al. 2002; Sharma et al. 2001].
Local mechanical factors mediate the impact of more systemic factors such as obesity on the knee [Felson et al. 2004]. Obesity is the single most important risk factor for development of severe OA of the knee and more so than other potentially damaging factors including heredity [Coggon et al. 2001; Felson and Zhang, 1998]. Because obesity is both a risk factor for OA and has been increasing in prevalence over the past four decades [Ogden et al. 2006; Flegal et al. 2002], it is likely that more individuals will be affected by knee OA in the future.
Relation of joint mechanics to patellofemoral osteoarthritis
The patellofemoral (PF) joint transmits relatively high forces through relatively small contact areas. The PF joint-reaction force (JRF) increases with increasing knee flexion. The JRF during walking (10–15° of flexion) is approximately 50% of bodyweight. Walking upstairs (60°), the JRF is 3.3 times bodyweight. During squats (130°), the JRF is 7.8 times bodyweight [Grelsamer and Weinstein, 2001]. It is therefore not surprising that the patella is involved in over half of cases of symptomatic knee OA, with combined tibiofemoral and PF OA found in 41% of subjects and isolated PF disease found in 11% of subjects [McAlindon et al. 1996]. The dominant pattern of PF OA is lateral PF involvement in approximately 80% of cases [Hunter et al. 2007; Elahi et al. 2000; Harrison et al. 1994; Iwano et al. 1990]. Consistent with this, the pain and disease progression of PF OA occur when abnormal kinematics (lateral patellar tilt) produce excessive pressure on the lateral patellar facet [Ficat, 1978; Ficat and Hungerford, 1977]. A number of studies have demonstrated that the relation of the patella with reference to the femur plays a critical role in determining the rate of disease progression and predisposition to symptoms in persons with PF OA [Hunter et al. 2007; Kalichman et al. 2007a; 2007b; Niu et al. 2005].
Relation of joint mechanics to hip osteoarthritis
There is strong evidence that mechanical and structural changes around the hip are major etiological factors in the development of hip OA [Harris, 1986; Tanzer and Noiseux, 2004]. Childhood diseases such as Legg-Perthe's disease and slipped capital femoral epiphysis predispose the hip to OA at a young age [Rab, 1999; Goodman et al. 1997; Leunig et al. 1997; Snow et al. 1993]. Acetabular dysplasia is well known to be a major precursor of OA [Cooperman et al. 1983; Harris, 1986]. In these patients, overload of the acetabular rim is the pathomechanism that ultimately leads to OA [Klaue et al. 1991]. It was originally assumed that 50% of hip OA was idiopathic (not associated with any obvious deformity) [Lloyd-Roberts, 1955]. More recent studies have found that 90% or more of hip OA cases can be attributed to anatomical abnormalities [Harris, 1986; Solomon, 1976; Tanzer and Noiseux, 2004].
The need to refocus treatment strategies on modifiable risk factors
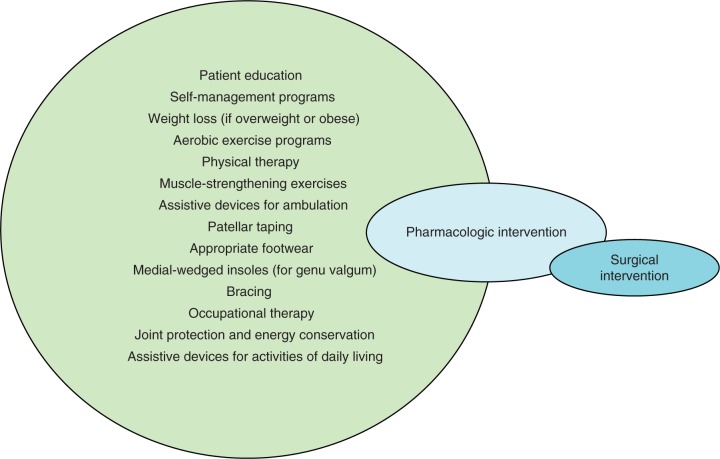
At present, therapeutic interventions are palliative—they are primarily limited to analgesia and when this fails, surgical intervention. We need to change this paradigm to intervene when structural changes may be reversible. In the absence of pharmacologic agents that can modify disease, we need to reappraise our current treatment strategies to focus on modifiable risk factors for symptom genesis and disease progression (Figure 1). The overwhelming preponderance of treatments available for OA is drugs and/or surgery. For example, in a meta-analysis of trials in OA, 60% of them were assessing the effect of drug treatment and 26% were evaluating surgical procedures [Tallon et al 2000]. The remarkable lack of studies evaluating rehabilitation techniques, nonpharmacologic interventions, and other self-management techniques, has been labeled ‘research agenda bias’ by Tallon and colleagues [Tallon et al 2000] and is, in part, a consequence of lucrative drug development opportunities and reimbursement to procedural specialists. The toxicity/ adverse event profile of the most commonly used existing therapies (such as NSAIDs, COX-2 inhibitors and total joint replacement) is very unfavorable when compared to conservative interventions such as weight loss, braces and orthotics [Jordan et al 2003].
Figure 1.
The tadpole of revised osteoarthritis management. We have a vast array of nonpharmacologic tools that are currently grossly underutilized. When these are ineffective, pharmacologic options should be introduced. When both nonpharmacologic and pharmacologic interventions fail then surgery is an option.
In an era where the prevalence of OA is exploding due to the combined effects of aging and obesity we are at a critical nexus where many of the current therapies are either ineffective or even harmful. The withdrawal of COX-2 inhibitors due to safety concerns [Ortiz, 2004], the inability to demonstrate the efficacy of many currently widely-used therapies (including viscosupple-ments and glucosamine [Clegg et al. 2006; Lo et al 2003]) has created an urgent need to address the overwhelming burden of symptoms related to this condition in our community with safe and effective therapies.
As a clinician managing OA, efforts should be made where possible to influence modifiable risk factors. In the first instance, the clinical encounter should target identification of individual risk factors (including altered alignment, obesity and muscle weakness) and the therapeutic intervention should be tailored to target the individual. The majority of people with OA are overweight or obese and there is good evidence for the efficacy of weight management for OA [Messier et al 2004] and this is advocated by most OA guidelines. For each kilogram of weight lost, the knee will experience a fourfold reduction in load during daily activities [Messier et al. 2005]. In practice however, weight management is not frequently implemented [DeHaan et al 2007; Hutchings et al 2007; Jordan et al 2004]. If someone is overweight or obese they should be engaged in a combination diet and exercise program aimed at a weight reduction of greater than 5% of body-weight [Messier et al 2004].
Another pivotal and frequently ignored [DeHaan et al 2007; Hutchings et al 2007; Jordan et al 2004] aspect of conservative treatment of OA is exercise. Exercise increases aerobic capacity, muscle strength and endurance, and also facilitates weight loss [Messier et al 2004; Ettinger et al 1997]. All patients capable of exercise should be encouraged to partake in a low-impact aerobic exercise program (walking, biking, swimming or other aquatic exercise) designed not to aggravate their underlying symptoms. Quadricep weakness is common among patients with knee OA, in whom it had been believed to be a manifestation of disuse atrophy, which develops because of unloading of the painful extremity [Hurley, 1999]. Quadricep strengthening exercises have been demonstrated to lead to improvements in pain and function [2005; Roddy et al 2005a; 2005b]. Quadriceps weakness is also a risk factor for the development of knee OA, presumably by decreasing stability of the knee joint and reducing the shock-attenuating capacity of the muscle [Hurley, 1999; Slemenda et al 1998]. Recently, a clinical trial evaluated the effects of lower-extremity strength training on knee OA progression [Mikesky et al 2006]. The strength training group experienced less frequent progression than the range of motion comparator. Guidelines routinely advocate exercise, [Zhang et al 2008; Hunter, 2007; Hunter and Felson, 2006; Zhang et al 2005; Jordan et al 2003; Anonymous, 2000] however, clinical practice does not reflect this recommendation [DeHaan et al 2007; Hutchings et al 2007; Jordan et al 2004].
Despite their current underemphasis in clinical trials and practice, therapies targeting the pathomechanics of OA are efficacious [Andriacchi et al 2004; Hinman et al 2003; Pollo et al 2002; Draper et al 2000; Kirkley et al 1999]. The etiopathogenesis of OA is in large part the result of local mechanical factors acting within the context of systemic or local susceptibility. Recent studies suggest that mechanical forces play an important role in predisposition to both symptoms and structural change [Andriacchi et al 2004; Carter et al 2004; Jackson et al 2004; Shakoor and Moisio, 2004; Teichtahl et al 2003; Kerin et al 2002]. At present, there are a number of therapeutic options that can modify joint forces, including patella taping, braces, orthotics, shoes and osteotomies for the knee and surgical correction of hip deformity associated with femoro-acetabular impingement syndrome [Pollo et al 2002; Draper et al 2000; Kirkley et al 1999]. Observational study data would suggest that through altering joint mechanics we may also alter the rate of disease progression however this remains to be demonstrated in a well-designed clinical trial. Further reading about devices that target pathomechanics is available in a recent review [Gross and Hillstrom, 2008].
Evidence-based guidelines have the potential to improve the quality of health care by promoting interventions of proven benefit and discouraging unnecessary, ineffective or harmful interventions [Woolf et al 1999]. Recent years have seen a number of evidence-based guidelines developed for OA management, [Zhang et al 2008; Hunter, 2007; Hunter and Felson, 2006; Zhang et al 2005; Jordan et al 2003; Anonymous, 2000] including the most recent additions from OARSI and AAOS. There is some consistency [Misso et al 2008; Poitras et al 2007; Pencharz et al 2002] in the numerous guidelines that are available for OA management, [Zhang et al 2008; Hunter, 2007; Hunter and Felson, 2006; Zhang et al 2005; Jordan et al. 2003; Anonymous, 2000] yet, despite some dissemination attempts, clinical practice does not reflect these recommendations [DeHaan et al 2007; Jawad, 2005; Pencharz et al 2002; Glazier et al 1998]. There are delays in uptake, particularly of nonpharmacological recommendations, and variance in the application of recommendations by clinicians in different contexts [DeHaan et al 2007; Denoeud et al 2005; Jawad, 2005; Mazieres et al 2005; Sarzi-Puttini et al 2005].
In addition, qualitative information suggests that the needs of patients are not being met with regard to the quantity and quality of information provided about OA and its treatment, the emotional needs of patients, and patient—clinician communication [Rosemann et al 2006; Chard et al 2002].
Given the number of guidelines available relevant to OA, and the consistency of recommendations within them, and considering the time and resources required for guideline development, future efforts to guide management of OA of the hip and/or knee are better directed towards implementing practices known to be effective [Misso et al 2008]. There is worldwide interest in developing and implementing patient-centered models of care to support the integration of evidence into practice and improve patient health outcomes for people with chronic conditions, including OA [Chassany et al 2006; Ouwens et al 2005].
Another burgeoning issue that will likely drive this change is that the continued growth of health care spending is not sustainable and has been responsible in part for recent calls for health-care reform. The major platforms of health-care reform are cost containment through optimizing effectiveness and quality. Management of musculoskeletal disease is expensive and a large portion of that relates to OA. If clinicians do not become involved in quality metrics, outcomes reporting and monitoring standards, it will be forced upon them by payers/insurers. A recent study demonstrates that rheumatologists in clinical practice do not follow up OA patients and don't use health status measures and, if they were to, would not know how to interpret them [Bellamy et al 2009]. This recent study clearly demonstrates that rheumatologists are poorly prepared for the brave new world of monitoring quality of care in OA. If patients are not followed up and metrics of health status are not measured, and even if they were are uninterpretable, we have a lot of education and innovation ahead of us. The brave new world of electronic health records, benchmarking, using data to inform shared decision-making and therapeutic goal-setting is upon us. Current rheumatologists are ill-prepared for these aspects of OA management. Rheumatology fellowship educational reforms and clear efforts in continuing medical education are desperately needed to ready us for these changes. While these data do not speak to other medical or surgical specialties that care for persons with OA, I have no reason to believe they are better prepared than rheumatologists.
Future therapeutic prospects
There is no OA equivalent to measuring high lipid levels, atherosclerosis, hypertension, or high glucose and glucose tolerance, for example, as we have for cardiovascular disease and diabetes, where one can detect and treat the disease precursors pre-emptively before the associated processes lead to end-organ failure. Instead, the ‘watchful waiting’ of steady decline to end-stage joint disease is a major cause of disablement and loss of quality of life. A number of obstacles exist to revising the status of OA care, but foremost amongst these is our penchant to utilize radiography to diagnose and study OA. Radiography is the mainstay of imaging of OA for diagnostics and clinical investigations, and is the only structural outcome metric accepted by the FDA for efficacy of a pharmaceutical intervention.
Recent advances in other prevalent rheumatic diseases has resulted in diseases that were associated with inexorable decline, being treated proactively with associated preservation of structure and function. The advance of biologic therapy in rheumatoid arthritis has seen dramatic shifts in preservation of structure and discussion of a new classification of disease remission. Recent evolution in medical care for osteoporosis has seen a marked reduction in fracture rates with their associated morbidity, with the appropriate institution of antiresorptive drug therapy. Unfortunately, we don't have this proactive stance available in OA, and with current structural definitions and measurement strategies that is unlikely to change. We desperately need to focus on earlier disease where changes may be reversible, if we are not to continue current therapeutic approaches that are largely palliative.
If we are to develop interventions for OA that target the joint before it is irreversibly damaged [Brandt et al 2006] we need to identify disease earlier and target the tissue that leads to the cascade of events we describe as joint failure. The denudation of cartilage (subchondral bone exposed) is irreversible and by the time people develop radiographic OA, the overwhelming majority have areas of denuded cartilage [Hunter et al 2008a]. MRI studies provide strong evidence that ascertainment of disease on radiographs only provides insights into late stage disease [Reichenbach et al 2008; Amin et al 2005; Hunter et al 2004; Karachalios et al 2004].
One large and as yet unmet treatment goal is modification of the underlying joint structure. Some studies, with varying levels of evidence, suggest that glucosamine sulfate, chondroitin sulfate, sodium hyaluronan, doxycycline, MMP inhibitors, bisphosphonates, calcitonin, diacerein and avocado-soybean unsaponifiables may modify disease progression [Abramson et al 2006]. It may be a while before a disease-modifying drug is available as current trial strategies remain neglectful of some simple fundamentals. Cartilage is not a direct source of symptoms and yet this remains the major focus of drug development opportunities. As important as the appropriate focus on tissues likely to be generating symptoms, therapeutic development needs to be cognizant of the aberrant mechanical forces at play in people with OA [Brandt et al 2006]. There are promising therapies being developed for new OA targets for both symptoms and structure, but we need to pay heed to the lessons we have learned and consider the obstacles to development if they are to be effective [Hunter and Hellio Le Graverand-Gastineau, 2008].
Conclusion
The pathogenesis of OA appears to be the result of a complex interplay between mechanical, cellular, and biochemical forces. Obesity is the strongest risk factor for disease onset and mechanical factors dominate the risk for disease progression. Greater therapeutic attention to the important role of mechanical factors and obesity in OA etiopathogenesis is required if we are to find ways of reducing the public health impact of this condition. Therapies directed at reducing joint loads such as weight loss and mechanical interventions are not used as frequently as they should be.
Footnotes
This is a narrative review and the comments and editorial expressed herein represent those of the author and do not reflect those of any official scientific role or institution that the author may hold or be affiliated with. The corresponding author had final responsibility for the decision to submit for publication. Dr Hunter receives research support from AstraZeneca, DonJoy, Lilly, Merck, NIH, Pfizer, Stryker and Wyeth.
References
- (1994) Arthritis prevalence and activity limitations—United States, 1990. MMWR Morb Mortal Wkly Rep 43: 433–438 [PubMed] [Google Scholar]
- (1998) Prevalence and impact of chronic joint symptoms—seven states, 1996. MMWR Morb Mortal Wkly Rep 47: 345–351 [PubMed] [Google Scholar]
- (2001) Prevalence of disabilities and associated health conditions among adults—United States, 1999. MMWR Morb Mortal Wkly Rep 50: 120–125 [PubMed] [Google Scholar]
- (2005) Review: Both aerobic and home-based quadriceps strengthening exercises reduce pain and disability in knee osteoarthritis. ACP Journal Club 143: 71. [PubMed] [Google Scholar]
- (2009) Guideline on the Treatment of Osteoarthritis (OA) of the Knee. Internet Communication.
- Abramson S.B., Attur M., Yazici Y. (2006) Prospects for disease modification in osteoarthritis. Nat Clin Pract Rheumatol 2: 304–312 [DOI] [PubMed] [Google Scholar]
- American Academy of Orthopaedic Surgeons (AAOS) (2008) Treatment of osteoarthritis of the knee (nonarthroplasty). Rosemont (IL): American Academy of Orthopaedic Surgeons; p. 263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin S., LaValley M.P., Guermazi A., Grigoryan M., Hunter D.J., Clancy M., et al. (2005) The relationship between cartilage loss on magnetic resonance imaging and radiographic progression in men and women with knee osteoarthritis. Arthritis Rheum 52: 3152–3159 [DOI] [PubMed] [Google Scholar]
- Andriacchi T.P. (1994) Dynamics of knee malalignment. Orthop Clin North Am 25: 395–403 [PubMed] [Google Scholar]
- Andriacchi T.P., Mundermann A., Smith R.L., Alexander E.J., Dyrby C.O., Koo S. (2004) A framework for the in vivo pathomechanics of osteoarthritis at the knee. Ann Biomed Eng 32: 447–57 [DOI] [PubMed] [Google Scholar]
- Anonymous (2000) Recommendations for the medical management of osteoarthritis of the hip and knee: 2000 update. American College of Rheumatology Subcommittee on Osteoarthritis Guidelines. Arthritis Rheum 43: 1905–1915 [DOI] [PubMed] [Google Scholar]
- Balint G., Rooney P.J., Buchanan W.W. (1987) A legacy for rheumatology from Sir William Osler. Clin Rheumatol 6: 423–435 [DOI] [PubMed] [Google Scholar]
- Baratz M.E., Fu F.H., Mengato R. (1986) Meniscal tears: The effect of meniscectomy and of repair on intraarticular contact areas and stress in the human knee. A preliminary report. Am J Sports Med 14: 270–275 [DOI] [PubMed] [Google Scholar]
- Bellamy E., Wilson C., Bellamy N. (2009) Osteoarthritis measurement in routine rheumatology outpatient practice (OMIRROP) in Australia: A survey of practice style, instrument use, responder criteria, and state-attainment criteria. J Rheumatol 36(5): 1049–1055 [DOI] [PubMed] [Google Scholar]
- Bhattacharyya T, Gale D., Dewire P., Totterman S., Gale M.E., McLaughlin S., et al. (2003) The clinical importance of meniscal tears demonstrated by magnetic resonance imaging in osteoarthritis of the knee. J Bone Joint Surg Am 85-A: 4–9 [DOI] [PubMed] [Google Scholar]
- Blin O., Pailhous J., Lafforgue P., Serratrice G. (1990) Quantitative analysis of walking in patients with knee osteoarthritis: A method of assessing the effectiveness of non-steroidal anti-inflammatory treatment. Ann Rheum Dis 49: 990–993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt K.D., Radin E.L., Dieppe P.A., Van De P.L. (2006) Yet more evidence that osteoarthritis is not a cartilage disease. Ann Rheum Dis 65: 1261–1264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinker M.R., O'Connor D.P., Pierce P., Woods G.W., Elliott M.N. (2002) Utilization of orthopaedic services in a capitated population. J Bone Joint Surg Am 84-A: 1926–1932 [DOI] [PubMed] [Google Scholar]
- Carter D.R., Beaupre G.S., Wong M., Smith R.L., Andriacchi T.P., Schurman D.J. (2004) The mechanobiology of articular cartilage development and degeneration. Clin Orthop Relat Res S69–S77 [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC). (2007) National and state medical expenditures and lost earnings attributable to arthritis and other rheumatic conditions—United States, 2003. MMWR Morb Mortal Wkly Rep 56: 4–7 [PubMed] [Google Scholar]
- Cerejo R., Dunlop D.D., Cahue S., Channin D., Song J., Sharma L. (2002) The influence of alignment on risk of knee osteoarthritis progression according to baseline stage of disease. Arthritis Rheum 46: 2632–2636 [DOI] [PubMed] [Google Scholar]
- Chard J., Dickson J., Tallon D., Dieppe P. (2002) A comparison of the views of rheumatologists, general practitioners and patients on the treatment of osteoarthritis. Rheumatology 41: 1208–1210 [PubMed] [Google Scholar]
- Chassany O., Boureau F., Liard F., Bertin P., Serrie A., Ferran P., et al. (2006) Effects of training on general practitioners' management of pain in osteoarthritis: A randomized multicenter study. J Rheumatol 33: 1827–1834 [PubMed] [Google Scholar]
- Clegg D.O., Reda D.J., Harris C.L., Klein M.A., O'Dell J.R., Hooper M.M., et al. (2006) Glucosamine, chondroitin sulfate, and the two in combination for painful knee osteoarthritis. N Eng J Med 354: 795–808 [DOI] [PubMed] [Google Scholar]
- Coggon D., Reading I., Croft P., McLaren M., Barrett D., Cooper C., et al. (2001) Knee osteoarthritis and obesity. Int J Obes Relat Metab Disord 25: 622–627 [DOI] [PubMed] [Google Scholar]
- Cooperman D.R., Wallensten R., Stulberg S.D. (1983) Acetabular dysplasia in the adult. Clin Orthop Relat Res 175: 79–85 [PubMed] [Google Scholar]
- DeHaan M.N., Guzman J., Bayley M.T., Bell M.J. (2007) Knee osteoarthritis clinical practice guidelines — how are we doing? J Rheumatol 34: 2099–2105 [PubMed] [Google Scholar]
- Denoeud L., Mazieres B., Payen-Champenois C., Ravaud P. (2005) First line treatment of knee osteoarthritis in outpatients in France: Adherence to the EULAR 2000 recommendations and factors influencing adherence. Ann Rheum Dis 64: 70–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieppe P.A., Lohmander L.S. (2005) Pathogenesis and management of pain in osteoarthritis. Lancet 365: 965–973 [DOI] [PubMed] [Google Scholar]
- Draper E.R., Cable J.M., Sanchez-Ballester J., Hunt N., Robinson J.R., Strachan R.K. (2000) Improvement in function after valgus bracing of the knee. An analysis of gait symmetry. f Bone foint Surg Br 82: 1001–1005 [DOI] [PubMed] [Google Scholar]
- Dunlop D.D., Manheim L.M., Song J., Chang R.W. (2001) Arthritis prevalence and activity limitations in older adults. Arthritis Rheum 44: 212–221 [DOI] [PubMed] [Google Scholar]
- Elahi S., Cahue S., Felson D.T., Engelman L., Sharma L. (2000) The association between varus-valgus alignment and patellofemoral osteoarthritis. Arthritis Rheum 43: 1874–1880 [DOI] [PubMed] [Google Scholar]
- Englund M., Guermazi A., Gale D., Hunter D.J., Aliabadi P., Clancy M., et al. (2008) Incidental meniscal findings on knee MRI in middle-aged and elderly persons. N Eng f Med 359: 1108–1115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettinger W.H.J., Burns R., Messier S.P., Applegate W., Rejeski W.J., Morgan T., et al. (1997) A randomized trial comparing aerobic exercise and resistance exercise with a health education program in older adults with knee osteoarthritis. The Fitness Arthritis and Seniors Trial (FAST). JAMA 277: 25–31 [PubMed] [Google Scholar]
- Eyre D.R. (2004) Collagens and cartilage matrix homeostasis. Clin Orthop Relat Res S118–122 [DOI] [PubMed] [Google Scholar]
- Felson D.T. (2004a) An update on the pathogenesis and epidemiology of osteoarthritis. Radial Clin North Am 42: 1–9 [DOI] [PubMed] [Google Scholar]
- Felson D.T. (2004b) Risk factors for osteoarthritis: Understanding joint vulnerability. Clin Orthop Relat Res S16–21 [DOI] [PubMed] [Google Scholar]
- Felson D.T., Goggins J., Niu J., Zhang Y., Hunter D.J. (2004) The effect of body weight on progression of knee osteoarthritis is dependent on alignment. Arthritis Rheum 50: 3904–3909 [DOI] [PubMed] [Google Scholar]
- Felson D.T., Lawrence R.C., Hochberg M.C., McAlindon T, Dieppe P.A., Minor M.A., et al. (2000) Osteoarthritis: New insights. Part 2: treatment approaches. Ann Intern Med 133: 726—-737 [DOI] [PubMed] [Google Scholar]
- Felson D.T., Zhang Y. (1998) An update on the epidemiology of knee and hip osteoarthritis with a view to prevention. Arthritis Rheum 41: 1343–1355 [DOI] [PubMed] [Google Scholar]
- Ficat P. (1978) [The syndrome of lateral hyperpressure of the patella]. [French]. Acta Orthop Belg 44: 65–76 [PubMed] [Google Scholar]
- Ficat R.P., Hungerford D.S. (1977) Disorders of the Patello-femoral Joint, The Williams and Wilkins Co.: Baltimore [Google Scholar]
- Flegal K.M., Carroll M.D., Ogden C.L., Johnson C.L., Flegal K.M., Carroll M.D., et al. (2002) Prevalence and trends in obesity among US adults, 1999–2000. JAMA 288: 1723–1727 [DOI] [PubMed] [Google Scholar]
- Fukubayashi T., Kurosawa H. (1980) The contact area and pressure distribution pattern of the knee. A study of normal and osteoarthrotic knee joints. Acta Orthop Scand 51: 871–879 [DOI] [PubMed] [Google Scholar]
- Gardiner P., Graham R., Legedza A.T., Ahn A.C., Eisenberg D.M., Phillips R.S. (2007) Factors associated with herbal therapy use by adults in the United States. Altern Ther Health Med 13: 22–29 [PubMed] [Google Scholar]
- Glazier R.H., Dalby D.M., Badley E.M., Hawker G.A., Bell M.J., Buchbinder R., et al. (1998) Management of common musculoskeletal problems: A survey of Ontario primary care physicians. CMAJ 158: 1037–1040 [PMC free article] [PubMed] [Google Scholar]
- Goodman D.A., Feighan J.E., Smith A.D., Latimer B., Buly R.L., Cooperman D.R. (1997) Subclinical slipped capital femoral epiphysis. Relationship to osteoarthrosis of the hip. J Bone Joint Surg Am 79: 1489–1497 [DOI] [PubMed] [Google Scholar]
- Grelsamer R.P., Weinstein C.H. (2001) Applied biomechanics of the patella. Clin Orthop Relat Res 389: 9–14 [DOI] [PubMed] [Google Scholar]
- Gross K.D., Hillstrom H.J. (2008) Noninvasive devices targeting the mechanics of osteoarthritis. Rheum Dis Clin North Am 34: 755–776 [DOI] [PubMed] [Google Scholar]
- Guccione A.A., Felson D.T., Anderson J.J., Anthony J.M., Zhang Y, Wilson P.W., et al. (1994) The effects of specific medical conditions on the functional limitations of elders in the Framingham Study. Am J Public Health 84: 351–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall M.J., Lawrence L. (1998) Ambulatory surgery in the United States, 1996. Adv Data 300: 1–16 [PubMed] [Google Scholar]
- Hannan M.T., Felson D.T., Pincus T. (2000) Analysis of the discordance between radiographic changes and knee pain in osteoarthritis of the knee. J Rheumatol 27: 1513–1517 [PubMed] [Google Scholar]
- Harris W.H. (1986) Etiology of osteoarthritis of the hip. Clin Orthop Relat Res 213: 20–33 [PubMed] [Google Scholar]
- Harrison M.M., Cooke T.D., Fisher S.B., Griffin M.P. (1994) Patterns of knee arthrosis and patellar subluxation. Clin Orthop Relat Res 309: 56–63 [PubMed] [Google Scholar]
- Hassan B.S., Doherty S.A., Mockett S., Doherty M. (2002) Effect of pain reduction on postural sway, proprioception, and quadriceps strength in subjects with knee osteoarthritis. Ann Rheum Dis 61: 422–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewett T.E. (2000) Neuromuscular and hormonal factors associated with knee injuries in female athletes. Strategies for intervention. Sports Med 29: 313–327 [DOI] [PubMed] [Google Scholar]
- Hinman R.S., Crossley K.M., McConnell J., Bennell K.L. (2003) Efficacy of knee tape in the management of osteoarthritis of the knee: Blinded randomised controlled trial. BMJ 327: 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter D.J., Niu J., Zhang Y, Totterman S., Tamez-Pena J., Dabrowski C., et al. (2008a) Change in Cartilage Morphometry: A sample of the progression cohort of the Osteoarthritis Initiative. Ann Rheum Dis 68: 349–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter D.J., Le Graverand-Gastineau M.P. Hellio. (2008) How close are we to having structure-modifying drugs available? Rheum Dis Clin North Am 34: 789–802 [DOI] [PubMed] [Google Scholar]
- Hunter D.J., McDougall J.J., Keefe F.J. (2008b) The symptoms of osteoarthritis and the genesis of pain. Rheum Dis Clin North Am 34: 623–643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter D.J., Patil V., Niu J.B., McLennan C, LaValley M., Genant H., et al. (2004) The etiology of knee pain in the community. Arthritis Rheum 50: 1885 [Google Scholar]
- Hunter D.J., Zhang Y.Q., Niu J.B., Felson D.T., Kwoh K., Newman A., et al. (2007) Patella malalignment, pain and patellofemoral progression: The Health ABC Study. Osteoarthritis Cartilage 15: 1120–1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter D. (2007) In the clinic. Osteoarthritis. Ann Intern Med 147: ITC8–16 [DOI] [PubMed] [Google Scholar]
- Hunter D., Felson D. (2006) Osteoarthritis. BMJ 332: 639–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley M.V. (1999) The role of muscle weakness in the pathogenesis of osteoarthritis. Rheum Dis Clin North Am 25: 283–298 [DOI] [PubMed] [Google Scholar]
- Hurwitz D.E., Ryals A.B., Case J.P., Block J.A., Andriacchi T.P. (2002) The knee adduction moment during gait in subjects with knee osteoarthritis is more closely correlated with static alignment than radio-graphic disease severity, toe out angle and pain. J Orthop Res 20: 101–107 [DOI] [PubMed] [Google Scholar]
- Huskisson E.C., Berry H., Gishen P., Jubb R.W., Whitehead J. (1995) Effects of antiinflammatory drugs on the progression of osteoarthritis of the knee. LINK Study Group. Longitudinal Investigation of Nonsteroidal Antiinflammatory Drugs in Knee Osteoarthritis. J Rheumatol 22: 1941–1946 [PubMed] [Google Scholar]
- Huston L.J., Greenfield M.L., Wojtys E.M. (2000) Anterior cruciate ligament injuries in the female athlete. Potential risk factors. Clin Orthop Relat Res 372: 50–63 [DOI] [PubMed] [Google Scholar]
- Hutchings A., Calloway M., Choy E., Hooper M., Hunter D.J., Jordan J.M., et al. (2007) The Longitudinal Examination of Arthritis Pain (LEAP) study: Relationships between weekly fluctuations in patient-rated joint pain and other health outcomes. J Rheumatol 34: 2291–2300 [PubMed] [Google Scholar]
- Iwano T, Kurosawa H., Tokuyama H., Hoshikawa Y. (1990) Roentgenographic and clinical findings of patellofemoral osteoarthrosis. With special reference to its relationship to femorotibial osteoarthrosis and etiologic factors. Clin Orthop Relat Res 252: 190–197 [PubMed] [Google Scholar]
- Jackson B.D., Wluka A.E., Teichtahl A.J., Morris M.E., Cicuttini F.M. (2004) Reviewing knee osteoarthritis—a biomechanical perspective. J Sci Med Sport 7: 347–357 [DOI] [PubMed] [Google Scholar]
- Jawad A.S. (2005) Analgesics and osteoarthritis: Are treatment guidelines reflected in clinical practice? Am J Ther 12: 98–103 [DOI] [PubMed] [Google Scholar]
- Jordan K.M., Arden N.K., Doherty M., Bannwarth B., Bijlsma J.W., Dieppe P., et al. (2003) EULAR Recommendations 2003: An evidence based approach to the management of knee osteoarthritis: Report of a Task Force of the Standing Committee for International Clinical Studies Including Therapeutic Trials (ESCISIT). Ann Rheum Dis 62: 1145–1155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan K.M., Sawyer S., Coakley P., Smith H.E., Cooper C., Arden N.K. (2004) The use of conventional and complementary treatments for knee osteoarthritis in the community. Rheumatology (Oxford) 43: 381–384 [DOI] [PubMed] [Google Scholar]
- Kalichman L., Zhang Y.Q., Niu J.B., Goggins J., Gale D., Zhu YY, et al. (2007a) The association between patellar alignment on magnetic resonance imaging and radiographic manifestations of knee osteoarthritis. Arthritis Res Ther 9: R26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalichman L., Zhu Y, Zhang Y, Niu J., Gale D., Felson D.T., et al. (2007b) The association between patella alignment and knee pain and function: An MRI study in persons with symptomatic knee osteoarthritis. Osteoarthritis and Cartilage 15: 1235–1240 [DOI] [PubMed] [Google Scholar]
- Karachalios T, Zibis A., Papanagiotou P., Karantanas A.H., Malizos K.N., Roidis N., et al. (2004) MR imaging findings in early osteoarthritis of the knee. Eur J Radiol 50: 225–230 [DOI] [PubMed] [Google Scholar]
- Kerin A., Patwari P., Kuettner K, Cole A., Grodzinsky A. (2002) Molecular basis of osteoarthritis: Biomechanical aspects. Cell Mol Life Sci 59: 27–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkley A., Webster-Bogaert S., Litchfield R., Amendola A., MacDonald S., McCalden R., et al. (1999) The effect of bracing on varus gonarthrosis. J Bone Joint Surg Am 81: 539–548 [DOI] [PubMed] [Google Scholar]
- Klaue K, Durnin C.W., Ganz R. (1991) The acetabular rim syndrome. A clinical presentation of dysplasia of the hip. J Bone Joint Surg Br 73: 423–429 [DOI] [PubMed] [Google Scholar]
- Kurosawa H., Fukubayashi T., Nakajima H. (1980) Load-bearing mode of the knee joint: Physical behavior of the knee joint with or without menisci. Clin Orthop Relat Res 149: 283–290 [PubMed] [Google Scholar]
- Lawrence R.C., Helmick C.G., Arnett F.C., Deyo R.A., Felson D.T., Giannini E.H., et al. (1998) Estimates of the prevalence of arthritis and selected musculoskeletal disorders in the United States. Arthritis Rheum 41: 778–799 [DOI] [PubMed] [Google Scholar]
- Ledingham J., Regan M., Jones A., Doherty M. (1993) Radiographic patterns and associations of osteoarthritis of the knee in patients referred to hospital. Ann Rheum Dis 52: 520–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leunig M., Werlen S., Ungersbock A., Ito K., Ganz R. (1997) Evaluation of the acetabular labrum by MR arthrography. J Bone Joint Surg Br 79: 230–234 [DOI] [PubMed] [Google Scholar]
- Lloyd-Roberts G.C. (1955) Osteoarthritis. J Bone Joint Surg Br 37-B: 8–47 [DOI] [PubMed] [Google Scholar]
- Lo G.H., LaValley M., McAlindon T., Felson D.T. (2003) Intra-articular hyaluronic acid in treatment of knee osteoarthritis: A meta-analysis. JAMA 290: 3115–3121 [DOI] [PubMed] [Google Scholar]
- Madsen O.R., Brot C, Petersen M.M., Sorensen O.H. (1997) Body composition and muscle strength in women scheduled for a knee or hip -replacement. A comparative study of two groups of osteoarthritic women. Clin Rheumatol 16: 39–44 [DOI] [PubMed] [Google Scholar]
- Mazieres B., Scmidely N., Hauselmann H.J., Martin-Mola E., Serni U., Verbruggen A.A., et al. (2005) Level of acceptability of EULAR recommendations for the management of knee osteoarthritis by practitioners in different European countries. Ann Rheum Dis 64: 1158–1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAlindon T., Zhang Y., Hannan M., Naimark A., Weissman B., Castelli W., et al. (1996) Are risk factors for patellofemoral and tibiofemoral knee osteoarthritis different? J Rheumatol 23: 332–337 [PubMed] [Google Scholar]
- Messier S.P., Gutekunst D.J., Davis C, DeVita P., Messier S.P., Gutekunst D.J., et al. (2005) Weight loss reduces knee-joint loads in overweight and obese older adults with knee osteoarthritis. Arthritis Rheumat 52: 2026–2032 [DOI] [PubMed] [Google Scholar]
- Messier S.P., Loeser R.F., Miller G.D., Morgan T.M., Rejeski W.J., Sevick M.A., et al. (2004) Exercise and dietary weight loss in overweight and obese older adults with knee osteoarthritis: The Arthritis, Diet, and Activity Promotion Trial. Arthritis Rheum 50: 1501–1510 [DOI] [PubMed] [Google Scholar]
- Mikesky A.E., Mazzuca S.A., Brandt K.D., Perkins S.M., Damush T., Lane K.A. (2006) Effects of strength training on the incidence and progression of knee osteoarthritis. Arthritis Rheum 55: 690–699 [DOI] [PubMed] [Google Scholar]
- Misso M.L., Pitt V.J., Jones K.M., Barnes H.N., Piterman L., Green S.E. (2008) Quality and consistency of clinical practice guidelines for diagnosis and management of osteoarthritis of the hip and knee: A descriptive overview of published guidelines. Med J Aust 189: 394–399 [DOI] [PubMed] [Google Scholar]
- Miyazaki T., Wada M., Kawahara H., Sato M., Baba H., Shimada S. (2002) Dynamic load at baseline can predict radiographic disease progression in medial compartment knee osteoarthritis. Ann Rheum Dis 61: 617–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moseley J.B., O'Malley K., Petersen N.J., Menke T.J., Brody B.A., Kuykendall D.H., et al. (2002) A controlled trial of arthroscopic surgery for osteoarthritis of the knee. N Eng J Med 347: 81–88 [DOI] [PubMed] [Google Scholar]
- Mundermann A., Dyrby C.O., Hurwitz D.E., Sharma L., Andriacchi T.P. (2004) Potential strategies to reduce medial compartment loading in patients with knee osteoarthritis of varying severity: Reduced walking speed. Arthritis Rheum 50: 1172–1178 [DOI] [PubMed] [Google Scholar]
- Niu J., Zhang Y.Q., Nevitt M., Xu L., Felson D.T., Zhu Y.Y., et al. (2005) Patellar malalignment and knee pain among subjects with no radiographic knee osteoarthritis: The Beijing Osteoarthritis Study. Arthritis Rheum 52: 1191. [DOI] [PubMed] [Google Scholar]
- Ogden C.L., Carroll M.D., Curtin L.R., McDowell M.A., Tabak C.J., Flegal K.M., et al. (2006) Prevalence of overweight and obesity in the United States, 1999–2004. JAMA 295: 1549–1555 [DOI] [PubMed] [Google Scholar]
- Ortiz E. (2004) Market withdrawal of Vioxx: Is it time to rethink the use of COX-2 inhibitors? J Manag Care Pharm 10: 551–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouwens M., Wollersheim H., Hermens R., Hulscher M., Grol R. (2005) Integrated care programmes for chronically ill patients: A review of systematic reviews. Int J Qual Health Care 17: 141–146 [DOI] [PubMed] [Google Scholar]
- Pencharz J.N., Grigoriadis E., Jansz G.F., Bombardier C. (2002) A critical appraisal of clinical practice guidelines for the treatment of lower-limb osteoarthritis. Arthritis Res 4: 36–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poitras S., Avouac J., Rossignol M., Avouac B., Cedraschi C, Nordin M., et al. (2007) A critical appraisal of guidelines for the management of knee osteoarthritis using Appraisal of Guidelines Research and Evaluation criteria. Arthritis Res Ther 9: R126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollo F.E., Otis J.C., Backus S.I., Warren R.F., Wickiewicz T.L. (2002) Reduction of medial compartment loads with valgus bracing of the osteoarthritic knee. Am J Sports Med 30: 414–421 [DOI] [PubMed] [Google Scholar]
- Rab G.T. (1999) The geometry of slipped capital femoral epiphysis: Implications for movement, impingement, and corrective osteotomy. J Pediatr Orthop 19: 419–424 [DOI] [PubMed] [Google Scholar]
- Rao V. (2009) Good or Useless, Medical Scans Cost the Same, http://www.nytimes.com/2009/03/02/health/02scans.html (accessed July 03, 2009).
- Reichenbach S., Guermazi A., Niu J., Neogi T, Hunter D.J., Roemer F.W., et al. (2008) Prevalence of bone attrition on knee radiographs and MRI in a community-based cohort. Osteoarthritis Cartilage 16: 1005–1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roddy E., Zhang W, Doherty M., Arden N.K., Barlow J., Birrell F., et al. (2005a) Evidence-based recommendations for the role of exercise in the management of osteoarthritis of the hip or knee—the MOVE consensus. Rheumatology (Oxford) 44: 67–73 [DOI] [PubMed] [Google Scholar]
- Roddy E., Zhang W, Doherty M., Roddy E., Zhang W, Doherty M. (2005b) Aerobic walking or strengthening exercise for osteoarthritis of the knee? A systematic review. Ann Rheum Dis 64: 544–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosemann T, Wensing M., Joest K., Backenstrass M., Mahler C., Szecsenyi J. (2006) Problems and needs for improving primary care of osteoarthritis patients: The views of patients, general practitioners and practice nurses. BMC Musculoskelet Disord 7: 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarzi-Puttini P., Cimmino M.A., Scarpa R., Caporali R., Parazzini F., Zaninelli A., et al. (2005) Do physicians treat symptomatic osteoarthritis patients properly? Results of the AMICA experience. Semin Arthritis Rheum 35: 38–42 [DOI] [PubMed] [Google Scholar]
- Schnitzer T.J., Popovich J.M., Andersson G.B., Andriacchi T.P. (1993) Effect of piroxicam on gait in patients with osteoarthritis of the knee. Arthritis Rheum 36: 1207–1213 [DOI] [PubMed] [Google Scholar]
- Seedhom B.B., Dowson D., Wright V. (1974) Proceedings: Functions of the menisci. A preliminary study. Ann Rheum Dis 33: 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakoor N., Moisio K. (2004) A biomechanical approach to musculoskeletal disease. Best Pract Res Clin Rheumatol 18: 173–186 [DOI] [PubMed] [Google Scholar]
- Sharma L., Dunlop D.D., Cahue S., Song J., Hayes K.W. (2003) Quadriceps strength and osteoarthritis progression in malaligned and lax knees. Ann Intern Med 138: 613–619 [DOI] [PubMed] [Google Scholar]
- Sharma L., Hurwitz D.E., Thonar E.J., Sum J.A., Lenz M.E., Dunlop D.D., et al. (1998) Knee adduction moment, serum hyaluronan level, and disease severity in medial tibiofemoral osteoarthritis. Arthritis Rheum 41: 1233–1240 [DOI] [PubMed] [Google Scholar]
- Sharma L., Lou C, Cahue S., Dunlop D.D. (2000) The mechanism of the effect of obesity in knee osteoarthritis: The mediating role of malalignment. Arthritis Rheum 43: 568–575 [DOI] [PubMed] [Google Scholar]
- Sharma L., Song J., Felson D.T., Cahue S., Shamiyeh E., Dunlop D.D. (2001) The role of knee alignment in disease progression and functional decline in knee osteoarthritis. JAMA 286: 188–195 [DOI] [PubMed] [Google Scholar]
- Shelbourne K.D., Davis T.J., Klootwyk T.E. (1998) The relationship between intercondylar notch width of the femur and the incidence of anterior cruciate ligament tears. A prospective study. Am J Sports Med 26: 402–08 [DOI] [PubMed] [Google Scholar]
- Slemenda C, Heilman D.K., Brandt K.D., Katz B.P., Mazzuca S.A., Braunstein E.M., et al. (1998) Reduced quadriceps strength relative to body weight: A risk factor for knee osteoarthritis in women? Arthritis Rheum 41: 1951–1959 [DOI] [PubMed] [Google Scholar]
- Snow S.W., Keret D., Scarangella S., Bowen J.R. (1993) Anterior impingement of the femoral head: A late phenomenon of Legg-Calve-Perthes' disease. J Pediatr Orthop 13: 286–289 [DOI] [PubMed] [Google Scholar]
- Solomon L. (1976) Patterns of osteoarthritis of the hip. J Bone Joint Surg Br 58: 176–183 [DOI] [PubMed] [Google Scholar]
- Tallon D., Chard J., Dieppe P. (2000) Relation between agendas of the research community and the research consumer. Lancet 355: 2037–2040 [DOI] [PubMed] [Google Scholar]
- Tanzer M., Noiseux N. (2004) Osseous abnormalities and early osteoarthritis: The role of hip impingement. Clin Orthop Relat Res 429: 170–177 [PubMed] [Google Scholar]
- Teichtahl A., Wluka A., Cicuttini F.M. (2003) Abnormal biomechanics: A precursor or result of knee osteoarthritis? Br J Sports Med 37: 289–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetsworth K., Paley D. (1994) Malalignment and degenerative arthropathy. Orthop Clin North Am 25: 367–377 [PubMed] [Google Scholar]
- Todd P.A., Clissold S.P. (1990) Naproxen. A reappraisal of its pharmacology, and therapeutic use in rheumatic diseases and pain states. Drugs 40: 91–137 [DOI] [PubMed] [Google Scholar]
- Wolfe M.M., Lichtenstein D.R., Singh G. (1999) Gastrointestinal toxicity of nonsteroidal antiinflammatory drugs. N Eng J Med 340: 1888–1899 [DOI] [PubMed] [Google Scholar]
- Woolf S.H., Grol R., Hutchinson A., Eccles M., Grimshaw J. (1999) Clinical guidelines: Potential benefits, limitations, and harms of clinical guidelines. BMJ 318: 527–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., Moskovitz R., Nuki G., Abramson S., Altman R., Arden N., et al. (2008) OARSI recommendations for the management of hip and knee osteoarthritis, Part II: OARSI evidence-based, expert consensus guidelines. Osteoarthritis Cartilage 16: 137–162 [DOI] [PubMed] [Google Scholar]
- Zhang W., Doherty M., Arden N., Bannwarth B., Bijlsma J., Gunther K.P., et al. (2005) EULAR evidence based recommendations for the management of hip osteoarthritis: Report of a task force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Ann Rheum Dis 64: 669–681 [DOI] [PMC free article] [PubMed] [Google Scholar]