Abstract
Denosumab is a subcutaneously (SC) administered investigational fully human monoclonal antibody to receptor activator of nuclear factor-kB ligand (RANKL), a cytokine member of the tumor necrosis factor family that is the principal mediator of osteoclastic bone resorption. RANKL stimulates the formation, activity, and survival of osteoclasts, and is implicated in the pathogenesis of postmenopausal osteoporosis and other skeletal disorders associated with increased bone remodeling. Denosumab binds RANKL, preventing it from binding to RANK, thereby reducing the formation, activity, and survival of osteoclasts and slowing the rate of bone resorption. Postmenopausal women with low bone mineral density (BMD) treated with denosumab have a reduction of bone turnover markers and an increase in BMD that is rapid, sustained, and reversible. In postmenopausal women with osteoporosis, denosumab reduces the risk of vertebral, hip, and nonvertebral fractures. In postmenopausal women with low BMD randomized to receive denosumab or alendronate, denosumab is associated with a significantly greater increase in BMD and further reduction in bone turnover markers compared with alendronate. In postmenopausal women with low BMD who were previously treated with alendronate, those who switched to denosumab have a significantly greater BMD increase and further reduction in bone turnover markers compared with those continuing alendronate. Denosumab is well tolerated with a favorable safety profile. It is a promising emerging drug for the prevention and treatment of osteoporosis, offering a long dosing interval of every 6 months and convenient SC dosing, with the potential of improving long-term adherence to therapy compared with current oral treatments.
Keywords: osteoporosis, treatment, prevention, denosumab, FRAX, safety
Introduction
Osteoporosis is a common skeletal disease characterized by low bone mineral density (BMD) and poor bone quality, with a reduction in bone strength and increase in fracture risk [Klibanski et al. 2001]. It has been estimated that over 200 million people worldwide have osteoporosis [Cooper et al. 1992], including about 75 million in the United States (US), Europe, and Japan [European Foundation for Osteoporosis and Bone Disease and National Osteoporosis Foundation, 1997]. Postmenopausal women are at particularly high risk for osteoporosis due to declining estrogen levels, with low BMD being a strong risk factor for fracture. Approximately 30% of all postmenopausal women in the US and Europe have osteoporosis, with at least 40% of these women having one or more fragility fractures in their remaining lifetime [Melton et al. 1992]. Vertebral fractures are the most common type of fragility fracture [Riggs and Melton, 1995], with 5% of 50 year-old Caucasian women and 25% of 80 year-old women having at least one vertebral fracture [Melton et al. 1989]. Hip fractures in white women are more common than breast cancer, with a lifetime risk of 1 in 6 [Cummings and Melton, 2002]. Fractures of the hip and spine are associated with increased morbidity and mortality [Cooper, 1997]. Any type of fracture is a sentinel event that greatly increases the risk of future fractures [Kanis et al. 2004]. Despite the high prevalence of osteoporosis and the availability of cost-effective drugs that are proven to reduce fracture risk, it is both underdiagnosed and under-treated. Bone density testing to identify patients at risk for fracture is commonly not done [Curtis et al. 2008], and even patients with previous fractures are typically not evaluated or treated for osteoporosis [Feldstein et al. 2003]. When treatment is started, many patients do not take medication correctly or for a sufficient length of time to benefit from reduction in fracture risk [McCombs et al. 2004]. Patients with good compliance to therapy have larger BMD increases [Yood et al. 2003], greater reduction in fracture risk [Caro et al. 2004], and lower healthcare costs than patients who are less compliant [McCombs et al. 2004]. Strategies that have been suggested to improve long-term compliance to therapy include reducing the frequency of drug dosing and simplifying drug administration [US Department of Health and Human Services, 2004].
The World Health Organization fracture risk assessment tool (FRAX) provides an estimate of 10-year fracture probability in untreated women and men aged 40–90, based on validated clinical risk factors for fracture and BMD at the femoral neck, when available [Kanis and on behalf of the World Health Organization Scientific Group (2007), 2007]. When economic modeling is conducted with FRAX and country-specific assumptions (e.g. societal willingness to pay, consequences of fractures, costs of fracture care, and treatment to prevent fractures), cost-effective thresholds for intervention with pharmacological therapy can be determined. Since many fractures occur in patients who do not have BMD in the osteoporosis range (T-score ≤ −2.5) [Wainwright et al. 2005], FRAX may be most useful in identifying those with low bone mass (osteopenia, T-score between −1.0 and −2.5) who are at sufficiently high risk for fracture to benefit from therapy. Ultimately, however, the decision to treat and the selection of an individual drug must be customized according to each individual patient, with consideration of other factors that include the patient's co-morbidities, previous treatment experiences, preferences, availability of treatment, and insurance coverage.
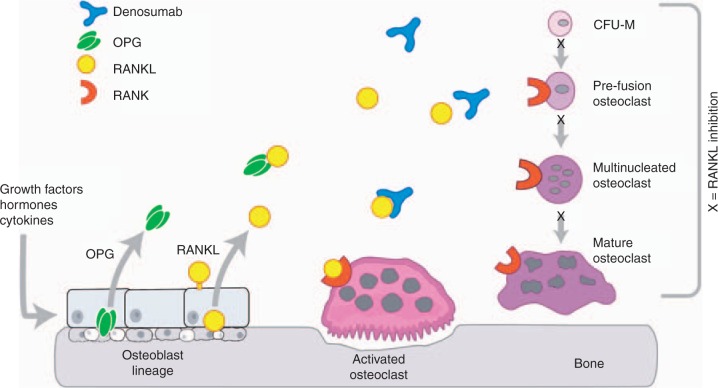
Medication for the prevention and treatment of osteoporosis stabilizes or increases BMD and reduces fracture risk through its effects on bone remodeling. This is the dynamic process by which the adult skeleton is continually broken down and reformed in discrete bone remodeling units located on the surface of trabecular bone and in Haversian systems of cortical bone through the coordinated activity of osteoclasts and osteoblasts. When bone resorption is greater than bone formation, as commonly occurs in postmenopausal women, bone loss is the result, with an increase in skeletal fragility and risk of fractures [Felsenberg and Boonen, 2005]. The receptor activator of nuclear factor-KB ligand (RANKL)/ receptor activator of nuclear factor-KB (RANK)/ osteoprotegerin (OPG) signaling pathway (Figure 1) is the principal regulator of osteoclastic bone resorption [Bekker et al. 2004]. When RANKL, expressed on the surface of osteoblasts and other cells, binds to RANK on the cell surface of osteoclasts and pre-osteoclasts, it increases osteoclast formation, activity, and survival, resulting in increased bone resorption. OPG is a naturally occurring soluble nonsignaling ‘decoy receptor’ that binds to RANKL, reducing the formation, activity, and survival of osteoclasts, with inhibition of bone resorption [Simonet et al. 1997]. An increase in the RANKL:OPG ratio has been implicated in the pathogenesis of postmenopausal osteoporosis and other skeletal diseases associated with elevated bone resorption [Hofbauer and Schoppet, 2004].
Figure 1.
The receptor activator of nuclear factor-kB ligand (RANKL)/receptor activator of nuclear factor-kB (RANK)/osteoprotegerin (OPG) signaling pathway. Denosumab is a fully human monoclonal antibody to RANKL that prevents the binding of RANKL to RANK and thereby inhibits bone resorption in a manner similar to native OPG. Adapted from [Kostenuik, 2005] with permission from Elsevier and [Lewiecki, 2006] with permission from Future Medicine Ltd.
Drugs for the prevention and treatment of osteoporosis are classified as antiresorptive (anti-catabolic) or anabolic (bone-forming) depending on their effect on bone remodeling [McClung et al. 2005; Riggs and Parfitt, 2005; Riggs et al. 1996]. Antiresorptive drugs include estrogens (with or without progesterone), bisphosphonates (alendronate, risedronate, ibandronate, and zoledronic acid), an estrogen agonist/antagonist (raloxifene), and salmon calcitonin. Teriparatide and recombinant human parathyroid hormone [1–84] are the only approved anabolic agents. Strontium ranelate appears to have both antiresorptive and anabolic properties [Meunier et al. 2002].
Denosumab (previously AMG 162; Amgen Inc., Thousand Oaks, CA, USA) is an investigational fully human monoclonal antibody (IgG2 immunoglobulin isotype) antiresorptive drug, administered by subcutaneous (SC) injection, with a high affinity and specificity for human RANKL. By binding to RANKL, it prevents the interaction of RANKL to RANK and inhibits bone resorption by suppressing osteoclast formation, activity, and survival (Figure 1). This is a review of the data from completed clinical trials on the use of denosumab for the prevention and treatment of postmenopausal osteoporosis.
Pharmacokinetics
The pharmacokinetics of denosumab are nonlinear with dose, and similar to other fully human monoclonal antibodies. A phase 1 dose-escalation trial in healthy postmenopausal women given a single dose of SC denosumab (0.01 to 3.0 mg/kg) showed three phases: a prolonged absorption phase with maximum serum concentration (Cmax) observed at 5 to 21 days post-dose, increasing as dose increased; a prolonged β-phase, with serum half-life as long as 32 days with the maximum dose; and a rapid terminal phase when serum concentration dropped below 1000 ng/ml [Bekker et al. 2004]. The effect on reduction of bone resorption, as measured by urinary N-telopeptide (NTX), is dose-dependent, rapid (within 12 hours, the earliest time point measured), profound (up to 84% decrease from baseline at the 3 month time point), sustained (up to 6 months), and reversible (rise in NTX at the end of the monitoring period). Reduction of serum bone-specific alkaline phosphatase (BSAP) occurs later and is less pronounced than was observed for NTX.
The duration of denosumab's antiresorptive effect appears to be a consequence of its long half-life and its effects on osteoclast recruitment, function, and survival. A 6-month dosing interval, when administered for the treatment of osteoporosis, offers the potential of improving adherence to therapy compared with other osteoporosis treatments that require more frequent dosing. SC administration alleviates concerns regarding possible malabsorption of oral agents, particularly the oral bisphosphonates, and may have greater acceptance by primary care physicians than intravenous bisphosphonates.
Efficacy
Denosumab compared with placebo in postmenopausal women with low BMD
The efficacy and safety of denosumab were evaluated in a phase 2 randomized, placebo-controlled, dose-ranging study in 412 post-menopausal women with low BMD (lumbar spine T-score −1.8 to −4.0, and total hip or femoral neck T-score −1.8 to −3.5) [McClung et al. 2006]. The subjects were randomized to 9 groups (41–54 subjects per group) receiving SC denosumab 6, 14, or 30 mg every 3 months; SC denosumab 14, 60, 100, or 210 mg every 6 months; open-label alendronate 70 mg weekly; or placebo. The primary endpoint was percentage change in lumbar spine BMD at 12 months compared to baseline. Bone turnover was assessed by measurement of serum and urine telopeptides and serum bone-specific alkaline phosphatase (BSAP). Denosumab treatment for 12 months resulted in a BMD increase of 3.0–6.7% at the lumbar spine compared to baseline, while there was a 0.8% loss with placebo (p<0.001). At the total hip, there was a BMD increase of 1.9–3.6% compared to baseline, with a 0.6% loss in the placebo group (p<0.001). At the distal one-third radius, there was a BMD increase of 0.4–1.3% with denosumab, compared to a 2.0% loss with placebo (p<0.001). In exploratory comparisons (i.e. preliminary assessments to determine whether additional investigation was warranted) with alendronate, the BMD changes were at least as large with denosumab, with an apparently greater BMD increase at the total hip and distal one-third with denosumab 30 mg every 3 months and 60 mg every 6 months. While the significance of this finding was uncertain due to the small number of patients and the study not being designed to test equivalence, it raised the possibility that the novel mechanism of action of denosumab might result in an effect on skeletal sites high in cortical bone that is different than alendronate. Denosumab groups showed a statistically significant decrease (82% mean decrease with the 60 mg dose) in serum C-telopeptide (CTX) compared to placebo and compared to alendronate (p<0.001 for both comparisons) as early as 3 days, the first scheduled time of CTX measurement. Serum CTX reached a maximum mean decrease of 88% compared to 5% with placebo. The effects on urinary NTX were similar to those of serum CTX. The reduction of BSAP with denosumab was significant compared to placebo (p<0.001), but was delayed by about 1 month compared to CTX. The effect on bone turnover was rapid, sustained, and reversible. The dose of 60 mg every 6 months was selected for further study in phase 3 clinical trials due to the finding that higher doses were not associated with additional increases in BMD and the greater convenience of dosing every 6 months compared with every 3 months.
A pre-specified exploratory analysis evaluated the efficacy and safety of denosumab after 24 months of treatment [Lewiecki et al. 2007]. Outcome measures included BMD at the lumbar spine, total hip, distal one-third radius, and total body; bone turnover markers; and safety. Of the original 412 women randomized, 337 (81.8%) completed the 24-month study. BMD increases at the lumbar spine at 24 months were in the range 4.13–8.89% with denosumab compared to a 1.18% decrease with placebo (p <0.001 for all doses denosumab versus placebo). Compared to open label alendronate, BMD increases with denosumab at all four skeletal sites were similar or greater, except with the denosumab dose of 14mg every 6 months, for which the BMD change at the lumbar spine was less than with alendronate.
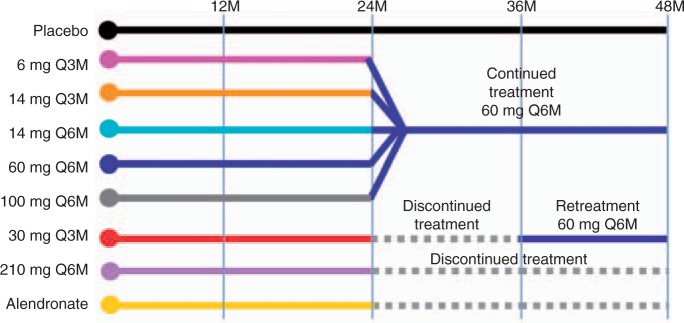
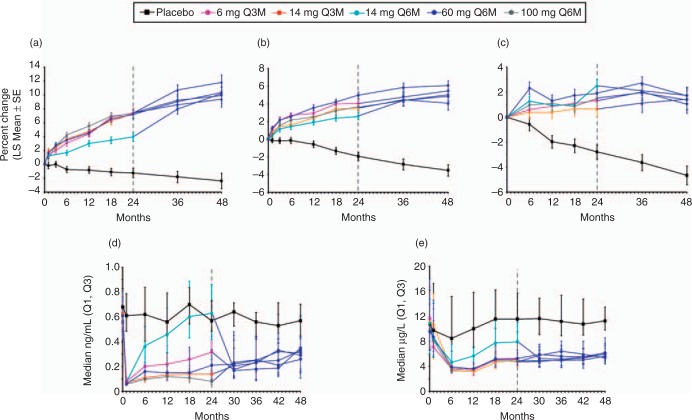
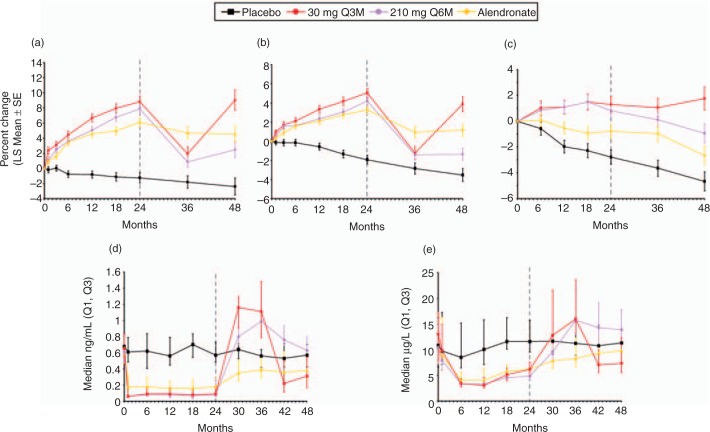
This study was extended for an additional 24 months for a total of 48 months, with blinded doses of SC denosumab or placebo administered every 6 months [Miller et al. 2008]. Denosumab-treated patients who continued the study were reassigned based on their randomization group at enrolment (Figure 2). Patients randomized to denosumab 6 and 14mg respectively every 3 months, and those receiving 14, 60, and 100mg every 6 months received denosumab 60 mg every 6 months. Patients randomized to the 6-monthly 210 mg dosage received placebo for the remainder of the study. Patients randomized to the 3-monthly 30 mg dosage received placebo for 12 months then were subsequently retreated with denosumab 60 mg, every 6 months, for 12 months. Open-label alendronate patients discontinued alendronate therapy after 24 months and received no additional drug therapy. The placebo group was maintained for the entire 48 months. Of patients initially randomized in the study, 64% (262/412) completed 48 months. Continuous, long-term denosumab treatment (Figure 3) increased BMD at the lumbar spine (9.4–11.8%) and total hip (4.0–6.1%). Bone turnover markers were reduced over 48 months. Discontinuation of denosumab (Figure 4) was associated with a BMD decrease of 6.6% at the lumbar spine and 5.3% at the total hip within the first 12 months of treatment discontinuation, followed by a plateau of BMD over the fourth year of the study at a level that was approximately the same as baseline. This level of BMD was nevertheless greater than that of patients on placebo, who lost BMD at all skeletal sites. Retreatment with denosumab increased lumbar spine BMD by 9.0% from original baseline values. Bone turnover markers increased to levels transiently greater than baseline after discontinuation of denosumab, peaking 6–12 months after discontinuation and returning to levels near baseline that were not significantly different than placebo 24 months after discontinuation. Retreatment with denosumab 12 months after discontinuation rapidly reduced bone turnover markers to levels that were similar to continuously treated patients at 42 and 48 months. The clinical consequences of the increase in bone turnover markers and loss of BMD after denosumab discontinuation are not known. While no increase in fracture risk was observed after denosumab was discontinued, additional analyses may be helpful in determining whether there are any adverse clinical consequences associated with the transient period of increased bone remodeling. Similar patterns in BMD and bone turnover marker changes have been observed in the first year after withdrawal of estrogen therapy, with a return of bone turnover markers to near baseline within 2 years after estrogen discontinuation [Wasnich et al. 2004; Sornay-Rendu et al. 2003; Gallagher et al. 2002; Greenspan et al. 2002]. It is reassuring to note that the transient increase in bone remodeling after discontinuation of estrogen therapy has not resulted in clear evidence of increased fracture risk in several large studies [Banks et al. 2004; Yates et al. 2004; Barrett-Connor et al. 2003; Cauley et al. 1995].
Figure 2.
Study design of the denosumab phase 2 study in postmenopausal women with low bone mineral density showing reallocation of groups. Reprinted from [Miller et al. 2008] with permission from Elsevier. Q3M, every 3 months; Q6M, every 6 months.
Figure 3.
Continuation of denosumab for 48 months of continuous therapy. Percentage change in bone mineral density (BMD) and bone turnover marker (BTM) values in patients who continued denosumab treatment for 48 months: (a) lumbar spine; (b) total hip; (c) distal 1/3 radius; (d) serum C-telopeptide; (e) bone-specific alkaline phosphatase. BMD values are shown as percentage change from baseline (least square mean ± standard error), while BTM levels are shown as absolute values (median with interquartile range) at the end of each dosing cycle. The dashed line at month 24 indicates the time at which patients were reallocated to the 60 mg Q6M dose. Reprinted from [Miller et al. 2008] with permission from Elsevier.
Figure 4.
Discontinuation and retreatment with denosumab over 48 months. Percentage change in bone mineral density (BMD) and bone turnover markers (BTM) values in patients who discontinued denosumab treatment for the last 24 months (210 mg, 6 monthly[Q6M]), were re-treated with denosumab 60 mg Q6M at month 36 (30 mg, 3 monthly [Q3M]), or discontinued alendronate treatment: (a) lumbar spine; (b) total hip; (c) distal 1/3 radius; (d) serum C-telopeptide; (e) bone-specific alkaline phosphatase. BMD values are shown as percentage change from baseline (least square mean ± standard error), while BTM levels are shown as absolute values (median with interquartile range) at the end of each dosing cycle. The dashed line at month 24 indicates the time at which dosing was reallocated. Reprinted from [Miller et al. 2008] with permission from Elsevier.
An additional extension of the denosumab phase 2 study to a total of 72 months, evaluating patients treated with denosumab for the entire duration, has been completed. The results have not yet been reported.
A phase 3 trial evaluated the efficacy and safety of denosumab in postmenopausal women with low bone mass (osteopenia). DEFEND (DEnosumab FortifiEs boNe Density) enrolled 332 postmenopausal women with lumbar spine T-scores between −1.0 and −2.5 who were randomized to receive SC denosumab 60 mg every 6 months (n = 166) or placebo (n = 166) [Bone et al. 2008] (Table 1). All subjects were instructed to take supplements of 1000mg calcium per day, with variable vitamin D supplementation determined according to the baseline serum level of 25-hydroxyvitamin D. The primary efficacy end-point was the percentage change from baseline in lumbar spine BMD measured by dual-energy X-ray absorptiometry (DXA) at 24 months compared to placebo. Secondary endpoints included percentage change from baseline in BMD at the total hip, femoral neck, one-third radius, and total body at 24 months; per cent change from baseline in trabecular, cortical, and per cent change from baseline in bone turnover markers. The mean baseline age of study subjects was 59.4 years, with a mean baseline lumbar spine T-score of −1.61. The study was completed by 86% of randomized subjects. Denosumab significantly increased BMD at the lumbar spine compared with placebo at 24 months (denosumab 6.5% versus placebo −0.6%, p < 0.0001), with significant BMD increases also reported at the total hip, one-third radius, and total body (p < 0.0001 for each compared with placebo). There was also a significant decrease in markers of bone resorption and formation compared with placebo. It was concluded that 6-monthly SC denosumab 60 mg increased BMD and reduced bone turnover markers in postmenopausal women with osteopenia, with an overall incidence of adverse events that was similar to placebo.
Table 1.
Key phase 3 trials of denosumab in women with postmenopausal osteoporosis (PMO) or low bone mineral density (BMD)
| Study acronym | DEFEND* | DECIDE$ | STAND‡ | FREEDOM§ |
|---|---|---|---|---|
| [Bone et al. 2008] | [Brown et al. 2009] | [Kendler et al. 2008] | [Cummings et al. 2008; Amgen Inc. 2008] | |
| Description | Prevention of PMO∥ | Denosumab compared with alendronate in patients starting treatment | Switching to denosumab compared with continuing alendronate in patients previously treated with alendronate | Treatment of PMO |
| Primary endpoint | Percentage change in LS¶ BMD# at 24 months with denosumab compared with placebo | Percentage change in TH** BMD at 12 months with denosumab compared with compared with alendronate | Percentage change in TH BMD at 12 months with denosumab compared with alendronate | New vertebral fractures at 36 months with denosumab placebo |
| Number randomized | 332 | 1,189 | 504 | 7,868 |
| Mean age (years) | 59.4 | 64.4 | 68 | 72.3 |
| Baseline T-score | Between −1.0 and −2.5 at LS | ≤−2.0 at LS or TH | ≤−2.0 to ≥ −4.0 at LS or TH | <−2.5 to ≥ − 4.0 at LS or TH |
| Efficacy results | Increased BMD with denosumab compared with placebo | Increased BMD with denosumab compared with alendronate | Increased BMD with denosumab compared with alendronate | Decreased risk of fracture with denosumab compared with placebo |
| Safety results | Similar AEs‡‡ and SAEs§§ with denosumab comopared with placebo, except for greater number of SAE infections with denosumab | Similar AEs and SAEs with denosumab compared with alendronate | Similar AEs and SAEs with denosumab compared with alendronate | Similar AEs and SAEs with denosumab compared with placebo |
DEnosumab FortifiEs boNe Density;
Determining Efficacy: Comparison of Initiating Denosumab versus alEndronate;
Study of Transitioning from AleNdronate to Denosumab;
Fracture REduction Evaluation of Denosumab in Osteoporosis every 6 Months;
PMO, postmenopausal osteoporosis;
LS, lumbar spine;
BMD, bone mineral density;
TH, total hip;
AE, adverse event;
SAE, serious adverse event.
Denosumab compared with alendronate in women with low BMD initiating treatment
DECIDE (Determining Efficacy: Comparison of Initiating Denosumab versus alEndronate) was a 1-year, phase 3 double-blind, double-dummy non-inferiority trial in 1,189 postmenopausal women with lumbar spine or total hip T-score of −2.0 or less who were randomized to receive SC denosumab 60 mg every 6 months plus weekly oral placebo (n = 594) or oral alendronate 70 mg weekly plus SC placebo injections every 6 months (n = 595) [Brown et al. 2009] (Table 1).
All subjects were instructed to take at least 500 mg supplemental calcium per day, with the dose of vitamin D supplementation adjusted according to baseline serum 25-hydroxyvitamin D. The primary endpoint was per cent change from baseline of the total hip BMD at month 12 in subjects treated with denosumab compared with alendronate. Key secondary endpoints included the per cent change from baseline in BMD at the femoral neck, trochanter, lumbar spine, and one-third distal radius at month 12; and per cent change in serum CTX-I and P1NP from baseline at months 1, 3, 6, 9, and 12. The mean baseline lumbar spine T-score was −2.6 and mean baseline age was 64 years, with 94% of subjects completing 12 months of study. At 12 months, there was a significantly greater BMD increase with denosumab compared with alendronate at the total hip (denosumab 3.5 versus alendronate 2.6%, p < 0.0001) and all other measured skeletal sites, with the treatment difference 0.6% at the femoral neck, 1.0% at the trochanter, 1.1% at the lumbar spine, and 0.6% at the distal one-third radius (p ≤ 0.0002 for all sites). There was a statistically significant greater reduction in bone turnover markers (CTX-I and P1NP) with denosumab compared with placebo. This head-to-head blinded clinical trial of denosumab versus alendronate showed a significantly greater increase in BMD and greater reduction of bone turnover markers with denosumab, with a similar pattern and frequency of adverse events (AEs).
Switching to denosumab compared with continuing alendronate in women with low BMD previously treated with alendronate
STAND (Study of Transitioning from AleNdronate to Denosumab) was a 1-year phase 3 double-blind, active-controlled, double-dummy study in 504 postmenopausal women aged 55-years and older being treated with alendronate, with lumbar spine or total hip T-score in the range from −2.0 to −4.0 [Roux et al. 2009; Kendler et al. 2008] (Table 1). Subjects were randomized to receive SC denosumab 60 mg every 6 months or continuing oral alendronate 70 mg weekly. All subjects were given daily supplements of calcium 1000 mg and vitamin D at least 400 IU. The primary endpoint was percentage change in BMD at the total hip at 12 months for denosumab compared to alendronate. The study design allowed testing of the primary endpoint for superiority if noninferiority was demonstrated. The mean age of subjects at the time of randomization was 68 ± 8 years and mean duration of alendronate therapy was 44 ± 33 months. At 12 months, there was a statistically significant greater increase in BMD with denosumab compared to continuing alendronate at the total hip (denosumab 1.90%, alendronate 1.05%, p < 0.0001), lumbar spine, and distal one-third radius. It was concluded that in postmenopausal women taking alendronate, those switched to denosumab had increased BMD more than those continuing alendronate, with a similar incidence of AEs.
Denosumab compared with placebo for the treatment of postmenopausal osteoporosis
FREEDOM (Fracture REduction Evaluation of Denosumab in Osteoporosis every 6 Months) was a three-year, phase 3 clinical trial in 7,868 postmenopausal women with osteoporosis who were randomized to receive either SC denosumab 60 mg (n = 3902) or placebo (n = 3906) every 6 months [Cummings et al. 2008] (Table 1). The primary efficacy endpoint was new vertebral fractures at 36 months, with secondary endpoints that included time to first hip and nonvertebral fractures. Study subjects were between ages 60 and 90 years (mean 72.3 years) with a baseline T-score at the lumbar spine or total hip <−2.5 to ≥−4.0 (mean baseline T-score = −2.8 at the lumbar spine), approximately 23% 111of whom had at least one prevalent vertebral fracture at the time of entry into the study. All patients received elemental calcium 1000 mg and vitamin D 400–800 IU daily. About 83% of subjects (denosumab n = 3,272, placebo n = 3,206) completed the 36-month study. Treatment with denosumab was associated with a significant 68% reduction in the risk of new vertebral fractures compared with placebo (2.3% denosumab versus 7.2% placebo, p<0.0001), 40% reduction in the risk of hip fractures (0.7% denosumab versus 1.2% placebo, p = 0.036), and 20% reduction in the risk of nonvertebral fractures (6.5% denosumab versus 8.0% placebo, p = 0.011) [Cummings et al. 2009].
Safety
Potential safety issues with RANKL inhibition
RANKL is expressed by numerous cell types, including endothelial cells, bone marrow stromal cells, primitive mesenchymal cells surrounding cartilage, chondrocytes, activated T lymphocytes, and immature CD4/CD8 thymocytes as well as osteoblasts and pre-osteoblasts.
RANK is expressed on the surface of cells that include chondrocytes, mammary gland epithelial cells, trophoblast cells, dendritic cells, and mature T-cells as well as osteoclasts. OPG is released by cells that include endothelial cells, smooth muscle cells, dendritic cells, and mature B-lymphocytes as well as osteoblasts, and expressed in organs that include the heart, lung, spleen, thymus, kidney and intestine [Kearns et al. 2008; Neumann et al. 2005]. While the role, if any, of the RANKL/RANK/OPG signaling pathway in the regulation and function of many of these nonskeletal cells and organ systems is not well understood, its presence raises the possibility that RANKL inhibition could have clinically significant nonskeletal effects. This suggests the potential for an impact of denosumab on immune function, atherosclerosis, vascular calcification, and mammary cell activity. Preclinical data regarding these safety issues and others are reviewed, with the safety findings from key phase 2 and 3 clinical trials of denosumab presented in the following section.
Immune function
Bone remodeling is modulated through a complex interaction of osteoclasts and osteoblasts with immune cells (e.g. T- and B-lymphocytes, dendritic cells), cytokines (e.g. interleukin-1, interleukin-6, tumor necrosis factor-a), and circulating hormones (e.g. vitamin D, parathyroid hormone, testosterone, and leptin) [Clowes et al. 2005]. A local or systemic imbalance in these factors may result in bone loss, as commonly occurs in many chronic inflammatory diseases. It has been shown that activated T-cells directly stimulate osteoclastogenesis through RANKL [Kong et al. 1999a]. It has also been demonstrated that RANKL produced by activated T- and B-lymphocytes increases bone resorption by interacting with osteoclast precursors and that RANKL prolongs the survival of dendritic cells, thereby increasing T-cell activity [Xing et al. 2005; Josien et al. 1999; Wong et al. 1997]. The intimate relationship of bone remodeling with RANKL/RANK/OPG signaling and regulators of immune function suggests that inhibition of RANKL could impact the immune response to infection or malignancy [Whyte, 2006]. Preclinical studies have shown that the development of an intact immune system is dependent on the RANKL/RANK/OPG pathway, with RANK and RANKL knockout mice having a deficiency of splenic B-cells and failure to develop peripheral lymph nodes [Dougall et al. 1999; Kong et al. 1999b]. The RANKL/ RANK/OPG system appears to have a non-essential role in immune function for adults who already have a fully developed immune system. Continuous lifelong overexpression of OPG in transgenic rats and mice, which are perhaps better animal models for the effects of prolonged human exposure to denosumab than the RANK knockout mouse, is not associated with abnormalities of peripheral lymph node development or impaired innate or humoral immune responses [Stolina et al. 2007; Stolina et al. 2005]. Humans with osteoclast-poor osteo-petrosis due to mutations of the gene encoding RANKL (TNFSF11), the human equivalent of the RANKL knockout mouse, do not have apparent defects in immune parameters [Sobacchi et al. 2007]. However, in a study of individuals with osteoclast-poor osteopetrosis due to mutations of the gene encoding RANK (TNFRSF11A), the human equivalent of the RANK knockout mouse, hypogammaglobuline-mia associated with impairment of function of immunoglobulin-secreting B-cells was reported [Guerrini et al. 2008]. The significance of this finding with regard to exogenous RANKL inhibition in adults with a fully developed immune system is not known.
Atherosclerosis and vascular calcification
High-serum OPG levels in humans have been associated with the presence and severity of coronary artery disease [Jono et al. 2002], stroke [Browner et al. 2001], cardiovascular morbidity [Rasmussen et al. 2006] and mortality [Morena et al. 2006], progression of atherosclerosis [Kiechl et al. 2004], and vascular calcification [Nitta et al. 2004]. Possible explanations for these observations include: an active role of OPG in the pathogenesis of vascular disease; OPG being a compensatory counter-regulatory response to mitigate the progression of vascular disease; OPG being a non-compensatory (neutral) response to vascular disease; or a common etiology for vascular disease and OPG production. Animal studies have helped to define the relationship between OPG and vascular calcification. OPG knockout mice develop medial calcification of the aorta and renal arteries that is prevented by transgenic over-expression of OPG [Min et al. 2000] without affecting atherosclerosis [Morony et al. 2008], and OPG prevents vascular calcification in a rat model of rapidly progressive arterial calcification [Price et al. 2001]. In mice that are deficient in apolipoprotein E, OPG inhibits the progression and calcification of advanced atherosclerotic lesions [Bennett et al. 2006]. The animal models support the concept that OPG protects against vascular calcification rather than causing it.
Effects on mammary cells
RANK knockout mice have a defect in mammary gland development during pregnancy and lactation due to the inability of sprouted alveolar buds to differentiate and mature [Fata et al. 2000]. However, OPG transgenic mice and rats with a 100-fold over expression of OPG do not have a failure of lactation and have normal suckling behavior [Simonet et al. 1997], suggesting that a small permissive level of RANKL is sufficient for mammary gland development and function.
Magnitude of reduction of bone turnover
Bone remodeling serves to maintain mineral homeostasis, strengthen bone in the areas of greatest mechanical stress, and repair bone microcracks. Excessive bone remodeling (turnover) is implicated in the pathogenesis of postmenopausal osteoporosis, with most current treatments directed toward reducing bone turnover. While there is no clear demarcation between desirable and undesirable levels of bone turnover, oversuppression of bone turnover has been proposed as a potential factor leading to increased risk of unusual fractures [Odvina et al. 2005] or osteonecrosis of the jaw (ONJ) [Marx et al. 2007] with bisphosphonate therapy. However, unusual fractures and ONJ may occur in patients who have not been treated with bisphosphonates, and the role, if any, of bone turnover levels in the pathogenesis of these conditions is unclear. Some osteoporosis patients (3–5%) have very low bone turnover in the absence of antiresorptive therapy [Kimmel et al. 1990], and may be at risk for further reduction in bone turnover if treated with antiresorptive drugs. The definition and consequences of excessive bone turnover in individual patients remains to be determined. There is no evidence that the magnitude of reduction in the bone remodeling rate achieved with denosumab has undesirable skeletal effects; continued surveillance in clinical trials and after marketing, if approved, is appropriate.
Fracture healing
In rats with fractures, administration of OPG does not influence early callus formation or fracture strength, although it does impair callus remodeling and consolidation [Ulrich-Vinther and Andre-assen, 2005]. In mice, severe osteoclast depletion with high dose RANK-Fc treatment has no significant effect on fracture healing [Flick et al. 2003]. In a recent study of male RANKL knock-in mice that expressed a chimeric (human/murine) form of RANKL, denosumab delayed the removal of cartilage and the remodeling of fracture callus but did not diminish the mechanical integrity of the healing fractures [Gerstenfeld et al. 2009]. To date there are no reports of impaired fracture healing in humans treated with denosumab.
Observed adverse events and serious adverse events in clinical trials of denosumab in women with low BMD and postmenopausal osteoporosis
In the phase 2 trial of denosumab in postmenopausal women with low BMD, AEs were generally similar in the placebo, denosumab, and alendro-nate groups respectively during the first 24 months of treatment [Lewiecki et al. 2007]. However, there were 6 cases (1.9%) of serious adverse events (SAEs) of infections in the denosumab group (2 cases of diverticulitis, 3 cases of pneumonia, and 1 case of labyrinthitis) compared to none in the placebo group or open label alendronate group. There was 1 death, caused by gastric cancer, in the denosumab group, and none in the placebo or alendronate groups. Discontinuation rates over the first 24 months were 2.2% for placebo, 2.9% for denosumab, and 6.4% for alendronate. No neutralizing antibodies to denosu-mab were observed in the first 24 months of treatment. At 48 months, reported SAEs were 10.9% (5/46) in the placebo group, 17.8% (56/314) in the denosumab group, and 17.4% (8/46) in the alendronate group. The incidence of malignant neoplasms was similar in all treatment groups. The overall incidence of infections was also similar in all treatment groups, although infections requiring hospitalization (SAEs) occurred in 3.2% (10/314) of denosumab-treated patients and none of those who received placebo or alendronate. All infections were common community-acquired infections that responded appropriately to standard antibiotic therapy, with no reports of opportunistic infections.
In DEFEND, AEs were similar in both groups. SAEs occurred numerically more often with denosumab than placebo, with the difference not statistically significant (denosumab 11% versus placebo 5.5%, p = 0.074), primarily due to the greater number of subjects with infections treated as hospital inpatients (denosumab 8 versus placebo 1). The overall incidence of infections was balanced (denosumab 60% versus placebo 61%). In DECIDE, no significant difference was observed in the overall incidence of AEs between denosumab and alendronate-treated subjects (80.9% versus 82.3%; p =0.60), including gastrointestinal disorders, infections, and neoplasms. SAEs were similar between denosumab-treated subjects (34 [5.7%]) and alendro-nate-treated subjects (37 [6.3%]). The incidence and types of infections were similar between the treatment groups (221 [37.3%] denosumab; 207 [35.3%] alendronate). Infection SAEs were similar between treatment groups, with nine (1.5%) for denosumab- and six (1.0%) alendronate-treated subjects. In STAND, AEs and SAEs were balanced in both treatment groups. Reported selected SAEs of interest included infections (alendronate 3 [1.2%], denosumab 1 [0.4%]) and neoplasms (alendronate 3[1.2%], denosumab 3 [1.2%]).
In FREEDOM, denosumab did not increase the overall risk of malignancies, infections, cardiovascular events, hypocalcemia, or delayed fracture healing [Cummings et al. 2009]. There were no cases of ONJ. Ninety (2.3%) deaths occurred in the placebo and 70 (1.8%) in the denosumab group (p = 0.08).
Considering the collective data in the reported clinical trials of denosumab for the prevention and treatment of postmenopausal osteoporosis, the risk-benefit ratio appears favorable. Continuing observation of patients in clinical trials for possible adverse immune effects, including infections and malignancy, is appropriate.
Conclusion
Denosumab is an investigational fully human monoclonal antibody to RANKL that reduces bone resorption in a rapid, sustained, and reversible manner. Treatment with SC denosumab 60mg, every 6 months, increases BMD and reduces bone turnover markers in postmenopausal women with low BMD. It increases BMD and decreases bone turnover markers more than alendronate, and in postmenopausal women previously treated with alendronate, those switching to denosumab increase BMD and reduce bone turnover markers more than those continuing alendronate. In women with postmenopausal osteoporosis, SC denosumab 60mg, every 6 months, significantly reduces the risk of vertebral, hip, and nonvertebral fractures compared with placebo. The safety and tolerability of denosumab are generally similar to placebo and alendronate. The infrequent dosing interval may improve compliance compared to oral medications that require more frequent dosing. SC administration may be more acceptable for use by physicians who are uncomfortable with intravenous administration of bisphosphonates, as well as being more convenient for patients. Denosumab enhances the choices of therapeutic agents for the management of postmenopausal osteoporosis, and may be particularly useful for patients who are unable to benefit from oral bisphosphonates due to a contraindication, intolerance, malabsorption, or poor compliance.
Footnotes
The author declares the following potential conflict of interest. He has received grant or research support from Merck, Eli Lilly, Novartis, sanofiaventis, Amgen, Pfizer, Wyeth, Roche, GlaxoSmithKline and Procter & Gamble, and has been a consultant, advisory board member, or sponsored speaker for Merck, Eli Lilly, Novartis, Procter & Gamble, sanofiaventis, Roche, GlaxoSmithKline, Wyeth, Amgen and Upsher-Smith.
References
- Amgen Inc (2008) Form 8-K. Available at http://investors.amgen.com/phoenix.zhtml?c=61656&p=irol-sec [Accessed 2008 September 16].
- Banks E., Beral V., Reeves G., Balkwill A., Barnes I. (2004) Fracture incidence in relation to the pattern of use of hormone therapy in postmenopausal women. JAMA 291: 2212–2220 [DOI] [PubMed] [Google Scholar]
- Barrett-Connor E., Wehren L.E., Siris E.S., Miller P., Chen Y.T., Abbott T.A., III, et al. (2003) Recency and duration of postmenopausal hormone therapy: Effects on bone mineral density and fracture risk in the National Osteoporosis Risk Assessment (NORA) study. Menopause 10: 412–419 [DOI] [PubMed] [Google Scholar]
- Bekker P.J., Holloway D.L., Rasmussen A.S., Murphy R., Martin S.W., Leese P.T., et al. (2004) A single-dose placebo-controlled study of AMG 162, a fully human monoclonal antibody to RANKL, in postmenopausal women. J Bone Miner Res 19: 1059–1066 [DOI] [PubMed] [Google Scholar]
- Bennett B.J., Scatena M., Kirk E.A., Rattazzi M., Varon R.M., Averill M., et al. (2006) Osteoprotegerin inactivation accelerates advanced atherosclerotic lesion progression and calcification in older ApoE-/- mice. Arterioscler Thromb Vasc Biol 26: 2117–2124 [DOI] [PubMed] [Google Scholar]
- Bone H.G., Bolognese M.A., Yuen C.K., Kendler D.L., Wang H., Liu Y., et al. (2008) Effects of denosumab on bone mineral density and bone turnover in postmenopausal women. J Clin Endocrinol Metab 93: 2149–2157 [DOI] [PubMed] [Google Scholar]
- Brown J.P., Prince R.L., Deal C, Recker R.R., Kiel D.P., de Gregorio L.H., et al. (2009) Comparison of the effect of denosumab and alendronate on BMD and biochemical markers of bone turnover in postmenopausal women with low bone mass: A randomized, blinded, phase 3 trial. J Bone Miner Res 24: 153–161 [DOI] [PubMed] [Google Scholar]
- Browner W.S., Lui L.Y., Cummings S.R. (2001) Associations of serum osteoprotegerin levels with diabetes, stroke, bone density, fractures, and mortality in elderly women. J Clin Endocrinol Metab 86: 631–637 [DOI] [PubMed] [Google Scholar]
- Caro J.J., Ishak K.J., Huybrechts K.F., Raggio G., Naujoks C. (2004) The impact of compliance with osteoporosis therapy on fracture rates in actual practice. Osteoporos Int 15: 1003–1008 [DOI] [PubMed] [Google Scholar]
- Cauley J.A., Seeley D.G., Ensrud K., Ettinger B., Black D., Cummings S.R. (1995) Estrogen replacement therapy and fractures in older women. Ann Intern Med 122: 9–16 [DOI] [PubMed] [Google Scholar]
- Clowes J.A., Riggs B.L., Khosla S. (2005) The role of the immune system in the pathophysiology of osteoporosis. Immunol Rev 208: 207–227 [DOI] [PubMed] [Google Scholar]
- Cooper C. (1997) The crippling consequences of fractures and their impact on quality of life. Am J Med 103: 12S—19S [DOI] [PubMed] [Google Scholar]
- Cooper C., Campion G., Melton L.J., III (1992) Hip fractures in the elderly: A world-wide projection. Osteoporos Int 2: 285–289 [DOI] [PubMed] [Google Scholar]
- Cummings S., Zanchetta J., McClung M., Christiansen C, Siris E., Delmas P.D., et al. (2009) The effects of twice-yearly denosumab on fracture risk in women with osteoporosis. Osteoporos Int 20: S16 [Google Scholar]
- Cummings S.R., McClung M.R., Christiansen C., Siris E., Adami S., Kutilek S., et al. (2008) A phase III study of the effects of denosumab on vertebral, nonvertebral, and hip fracture in women with osteoporosis: Results from the FREEDOM trial. J Bone Miner Res 23: S80 [Google Scholar]
- Cummings S.R., Melton L.J. (2002) Epidemiology and outcomes of osteoporotic fractures. Lancet 359: 1761–1767 [DOI] [PubMed] [Google Scholar]
- Curtis J.R., Carbone L., Cheng H., Hayes B., Laster A., Matthews R., et al. (2008) Longitudinal trends in use of bone mass measurement among older Americans, 1999–2005. J Bone Miner Res 23: 1061–1067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougall W.C., Glaccum M., Charrier K., Rohrbach K., Brasel K., De Smedt T., et al. (1999) RANK is essential for osteoclast and lymph node development. Genes Dev 13: 2412–2424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Foundation for Osteoporosis and Bone Disease, National Osteoporosis Foundation (1997) Who are candidates for prevention and treatment for osteoporosis? Osteoporos Int 7: 1–6 [DOI] [PubMed] [Google Scholar]
- Fata J.E., Kong YY, Li J., Sasaki T, Irie-Sasaki J., Moorehead R.A., et al. (2000) The osteoclast differentiation factor osteoprotegerin-ligand is essential for mammary gland development. Cell 103: 41–50 [DOI] [PubMed] [Google Scholar]
- Feldstein A., Elmer P.J., Orwoll E., Herson M., Hillier T.(2003) Bone mineral density measurement and treatment for osteoporosis in older individuals with fractures — A gap in evidence-based practice guideline implementation. Arch Intern Med 163: 2165–2172 [DOI] [PubMed] [Google Scholar]
- Felsenberg D., Boonen S. (2005) The bone quality framework: Determinants of bone strength and their interrelationships, and implications for osteoporosis management. Clin Ther 27: 1–11 [DOI] [PubMed] [Google Scholar]
- Flick L.M., Weaver J.M., Ulrich-Vinther M., Abuzzahab F., Zhang X., Dougall W.C., et al. (2003) Effects of receptor activator of NFkappaB (RANK) signaling blockade on fracture healing. J Orthop Res 21: 676–684 [DOI] [PubMed] [Google Scholar]
- Gallagher J.C., Rapuri P.B., Haynatzki G., Detter J.R. (2002) Effect of discontinuation of estrogen, calcitriol, and the combination of both on bone density and bone markers. J Clin Endocrinol Metab 87: 4914–4923 [DOI] [PubMed] [Google Scholar]
- Gerstenfeld L.C., Sacks D.J., Pelis M., Mason Z.D., Graves D.T., Barrero M., et al. (2009) Comparison of effects of the bisphosphonate alendronate versus the RANKL inhibitor denosumab on murine fracture healing. J Bone Miner Res 24: 196–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenspan S.L., Emkey R.D., III, Bone H.G., Weiss S.R., Bell N.H., Downs R.W., Jr, et al. (2002) Significant differential effects of alendronate, estrogen, or combination therapy on the rate of bone loss after discontinuation of treatment of postmenopausal osteoporosis–A randomized, double-blind, placebo-controlled trial. Ann Intern Med 137: 875–883 [DOI] [PubMed] [Google Scholar]
- Guerrini M.M., Sobacchi C, Cassani B., Abinun M., Kilic S.S., Pangrazio A., et al. (2008) Human osteoclast-poor osteopetrosis with hypogammaglobu-linemia due to TNFRSF11A (RANK) mutations. Am J Hum Genet 83: 64–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofbauer L.C., Schoppet M. (2004) Clinical implications of the osteoprotegerin/RANKL/ RANK system for bone and vascular diseases. JAMA 292: 490–495 [DOI] [PubMed] [Google Scholar]
- Jono S., Ikari Y, Shioi A., Mori K., Miki T, Hara K., et al. (2002) Serum osteoprotegerin levels are associated with the presence and severity of coronary artery disease. Circulation 106: 1192–1194 [DOI] [PubMed] [Google Scholar]
- Josien R., Wong B.R., Li H.L., Steinman R.M., Choi Y. (1999) TRANCE, a TNF family member, is differentially expressed on T cell subsets and induces cytokine production in dendritic cells. J Immunol 162: 2562–2568 [PubMed] [Google Scholar]
- Kanis J.A., Johnell O., De Laet C, Johansson H., Oden A., Delmas P., et al. (2004) A meta-analysis of previous fracture and subsequent fracture risk. Bone 35: 375–382 [DOI] [PubMed] [Google Scholar]
- Kanis J.A. on behalf of the World Health Organization Scientific Group. (2007) Assessment of osteoporosis at the primary health-care level. Technical Report. World Health Organization Collaborating Centre for Metabolic Bone Diseases, Printed by the University of Sheffield: University of Sheffield, UK [Google Scholar]
- Kearns A.E., Khosla S., Kostenuik P.J. (2008) Receptor activator of nuclear factor kappa B ligand and osteoprotegerin regulation of bone remodeling in health and disease. Endocr Rev 29: 155–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler D.L., Benhamou C.L., Brown J.P., Lillestol M., Roux C., Man H.S., et al. (2008) Effects of denosumab vs alendronate on bone mineral density (BMD), bone turnover markers (BTM), and safety in women previously treated with alendronate. J Bone Miner Res 23: S473. [DOI] [PubMed] [Google Scholar]
- Kiechl S., Schett G., Wenning G., Redlich K, Oberhollenzer M., Mayr A., et al. (2004) Osteoprotegerin is a risk factor for progressive atherosclerosis and cardiovascular disease. Circulation 109: 2175–2180 [DOI] [PubMed] [Google Scholar]
- Kimmel D.B., Recker R.R., Gallagher J.C., Vaswani A.S., Aloia J.F. (1990) A comparison of iliac bone histomorphometric data in post-menopausal osteoporotic and normal subjects. Bone Miner 11: 217–235 [DOI] [PubMed] [Google Scholar]
- Klibanski A., Adams-Campbell L., Bassford T., Blair S.N., Boden S.D., Dickersin K., et al. (2001) Osteoporosis prevention, diagnosis, and therapy. JAMA 285: 785–795 [DOI] [PubMed] [Google Scholar]
- Kong Y.Y., Feige U, Sarosi I., Bolon B., Tafuri A., Morony S., et al. (1999) Activated T cells regulate bone loss and joint destruction in adjuvant arthritis through osteoprotegerin ligand. Nature 402: 304–309 [DOI] [PubMed] [Google Scholar]
- Kong Y.Y., Yoshida H., Sarosi I., Tan H.L., Timms E., Capparelli C., et al. (1999b) OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature 397: 315–323 [DOI] [PubMed] [Google Scholar]
- Kostenuik P.J. (2005) Osteoprotegerin and RANKL regulate bone resorption, density, geometry and strength. Curr Opin Pharmacol 5: 618–625 [DOI] [PubMed] [Google Scholar]
- Lewiecki E.M. (2006) Denosumab: A promising drug for the prevention and treatment of osteoporosis. Women's Health 2: 517–525 [DOI] [PubMed] [Google Scholar]
- Lewiecki E.M., Miller P.D., McClung M.R., Cohen S.B., Bolognese M.A., Liu Y, et al. (2007) Two-year treatment with denosumab (AMG 162) in a randomized phase 2 study of postmenopausal women with low bone mineral density. J Bone Miner Res 22: 1832–1841 [DOI] [PubMed] [Google Scholar]
- Marx R.E., Cillo J.E., Jr., Ulloa J.J. (2007) Oral bisphosphonate-induced osteonecrosis: Risk factors, prediction of risk using serum CTX testing, prevention, and treatment. J Oral Maxillofac Surg 65: 2397–2410 [DOI] [PubMed] [Google Scholar]
- McClung M.R., Lewiecki E.M., Cohen S.B., Bolognese M.A., Woodson G.C., Moffett A.H., et al. (2006) Denosumab in postmenopausal women with low bone mineral density. N Engl J Med 354: 821–31 [DOI] [PubMed] [Google Scholar]
- McClung M.R., San M.J., Miller P.D., Civitelli R., Bandeira F., Omizo M., et al. (2005) Opposite bone remodeling effects of teriparatide and alendronate in increasing bone mass. Arch Intern Med 165: 1762–1768 [DOI] [PubMed] [Google Scholar]
- McCombs J.S., Thiebaud P., Laughlin-Miley C., Shi J. (2004) Compliance with drug therapies for the treatment and prevention of osteoporosis. Maturitas 48: 271–287 [DOI] [PubMed] [Google Scholar]
- Melton L.J., III, Chrischilles E.A., Cooper C, Lane A.W., Riggs B.L. (1992) Perspective How many women have osteoporosis? J Bone Miner Res 7: 1005–1010 [DOI] [PubMed] [Google Scholar]
- Melton L.J., III, Kan S.H., Frye M.A., Wahner H.W., O'Fallon W.M., Riggs B.L. (1989) Epidemiology of vertebral fractures in women. Am J Epidemiol 129: 1000–1011 [DOI] [PubMed] [Google Scholar]
- Meunier P.J., Slosman D.O., Delmas P.D., Sebert J.L., Brandi M.L., Albanese C., et al. (2002) Strontium ranelate: Dose-dependent effects in established postmenopausal vertebral osteoporosis — A 2-year randomized placebo controlled trial. J Clin Endocrinol Metab 87: 2060–2066 [DOI] [PubMed] [Google Scholar]
- Miller P.D., Bolognese M.A., Lewiecki E.M., McClung M.R., Ding B., Austin M., et al. (2008) Effect of denosumab on bone density and turnover in postmenopausal women with low bone mass after long-term continued, discontinued, and restarting of therapy: A randomized blinded phase 2 clinical trial. Bone 43: 222–229 [DOI] [PubMed] [Google Scholar]
- Min H., Morony S., Sarosi I., Dunstan C.R., Capparelli C, Scully S., et al. (2000) Osteoprotegerin reverses osteoporosis by inhibiting endosteal osteoclasts and prevents vascular calcification by blocking a process resembling osteoclastogenesis. J Exp Med 192: 463–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morena M., Terrier N., Jaussent I., Leray-Moragues H, Chalabi L., Rivory J.P., et al. (2006) Plasma osteoprotegerin is associated with mortality in hernodialysis patients. J Am Soc Nephrol 17: 262–270 [DOI] [PubMed] [Google Scholar]
- Morony S., Tintut Y, Zhang Z., Cattley R.C., Van G., Dwyer D., et al. (2008) Osteoprotegerin inhibits vascular calcification without affecting atherosclerosis in ldlr(-/-) mice. Circulation 117: 411–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann E., Gay S., Muller-Ladner U. (2005) The RANK/RANKL/osteoprotegerin system in rheumatoid arthritis: New insights from animal models. Arthritis Rheum 52: 2960–2967 [DOI] [PubMed] [Google Scholar]
- Nitta K, Akiba T, Uchida K, Otsubo S., Takei T, Yumura W, et al. (2004) Serum osteoprotegerin levels and the extent of vascular calcification in haemodialy-sis patients. Nephrol Dial Transplant 19: 1886–1889 [DOI] [PubMed] [Google Scholar]
- Odvina C.V., Zerwekh J.E., Rao D.S., Maalouf N, Gottschalk F.A., Pak C.Y. (2005) Severely suppressed bone turnover: A potential complication of alendronate therapy. J Clin Endocrinol Metab 90: 1294–1301 [DOI] [PubMed] [Google Scholar]
- Price P.A., June H.H., Buckley J.R., Williamson M.K. (2001) Osteoprotegerin inhibits artery calcification induced by warfarin and by vitamin D. Arterioscler Thromb Vasc Biol 21: 1610–1616 [DOI] [PubMed] [Google Scholar]
- Rasmussen L.M., Tarnow L., Hansen T.K., Parving H.H., Flyvbjerg A. (2006) Plasma osteoprotegerin levels are associated with glycaemic status, systolic blood pressure, kidney function and cardiovascular morbidity in type 1 diabetic patients. Eur J Endocrinol 154: 75–81 [DOI] [PubMed] [Google Scholar]
- Riggs B.L., Melton L.J., III (1995) The worldwide problem of osteoporosis: Insights afforded by epidemiology. Bone 17: 505S—11S [DOI] [PubMed] [Google Scholar]
- Riggs B.L., Melton L.J., III, O'Fallon W.M. (1996) Drug therapy for vertebral fractures in osteoporosis: Evidence that decreases in bone turnover and increases in bone mass both determine antifracture efficacy. Bone 18: 197S—201S [DOI] [PubMed] [Google Scholar]
- Riggs B.L., Parfitt A.M. (2005) Drugs used to treat osteoporosis: The critical need for a uniform nomenclature based on their action on bone remodeling. J Bone Miner Res 20: 177–184 [DOI] [PubMed] [Google Scholar]
- Roux C., Brown J.P., Kendler D.L., Lillestol M., Siddhanti S., Man H.S., et al. (2009) Assessment of bone mineral density and bone turnover markers in postmenopausal women transitioned from alendronate to denosumab. Osteoporos Int 20: S10 [Google Scholar]
- Simonet W.S., Lacey D.L., Dunstan C.R., Kelley M., Chang M.S., Luthy R., et al. (1997) Osteoprotegerin: A novel secreted protein involved in the regulation of bone density. Cell 89: 309–319 [DOI] [PubMed] [Google Scholar]
- Sobacchi C, Frattini A., Guerrini M.M., Abinun M., Pangrazio A., Susani L., et al. (2007) Osteoclast-poor human osteopetrosis due to mutations in the gene encoding RANKL. Nat Genet 39: 960–962 [DOI] [PubMed] [Google Scholar]
- Sornay-Rendu E., Garnero P., Munoz F., Duboeuf F., Delmas P.D. (2003) Effect of withdrawal of hormone replacement therapy on bone mass and bone turnover: The OFELY study. Bone 33: 159–166 [DOI] [PubMed] [Google Scholar]
- Stolina M., Dwyer D., Morony S., Corbin T., McCabe J., Zack D., et al. (2005) Rats and mice overexpressing soluble OPG have high bone mass but no alteration in immunological parameters or lymphocyte function. Arthritis Rheum 20: S708 [Google Scholar]
- Stolina M., Dwyer D., Ominsky M.S., Corbin T., Van G., Bolon B., et al. (2007) Continuous RANKL Inhibition in Osteoprotegerin Transgenic Mice and Rats Suppresses Bone Resorption without Impairing Lymphorganogenesis or Functional Immune Responses. J Immunol 179: 7497–7505 [DOI] [PubMed] [Google Scholar]
- Ulrich-Vinther M., Andreassen T.T. (2005) Osteoprotegerin treatment impairs remodeling and apparent material properties of callus tissue without influencing structural fracture strength. Calcif Tissue Int 76: 280–286 [DOI] [PubMed] [Google Scholar]
- US Department of Health and Human Services (2004) Bone Health and Osteoporosis: A Report of the Surgeon General US Department of Health and Human Services, Office of the Surgeon General: Rockville, MD [Google Scholar]
- Wainwright S.A., Marshall L.M., Ensrud K.E., Cauley J.A., Black D.M., Hillier T.A., et al. (2005) Hip fracture in women without osteoporosis. J Clin Endocrinol Metab 90: 2787–2793 [DOI] [PubMed] [Google Scholar]
- Wasnich R.D., Bagger Y.Z., Hosking D.J., McClung M.R., Wu M., Mantz A.M., et al. (2004) Changes in bone density and turnover after alendronate or estrogen withdrawal. Menopause 11: 622–630 [DOI] [PubMed] [Google Scholar]
- Whyte M.P. (2006) The long and the short of bone therapy. N Engl J Med 354: 860–863 [DOI] [PubMed] [Google Scholar]
- Wong B.R., Josien R., Lee S.Y., Sauter B., Li H.L., Steinman R.M., et al. (1997) TRANCE (tumor necrosis factor [TNF]-related activation-induced cytokine), a new TNF family member predominantly expressed in T cells, is a dendritic cell-specific survival factor. J Exp Med 186: 2075–2080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing L., Schwarz E.M., Boyce B.F. (2005) Osteoclast precursors, RANKL/RANK, and immunology. Immunol Rev 208: 19–29 [DOI] [PubMed] [Google Scholar]
- Yates J., Barrett-Connor E., Barlas S., Chen Y.T., Miller P.D., Siris E.S. (2004) Rapid loss of hip fracture protection after estrogen cessation: Evidence from the National Osteoporosis Risk Assessment. Obstet Gynecol 103: 440–446 [DOI] [PubMed] [Google Scholar]
- Yood R.A., Emani S., Reed J.I., Lewis B.E., Charpentier M., Lydick E. (2003) Compliance with pharmacologic therapy for osteoporosis. Osteoporos Int 14: 965–968 [DOI] [PubMed] [Google Scholar]