Abstract
Osteoporosis treatments need to combine an unequivocally demonstrated reduction of fractures, at various skeletal sites, long-term safety, and a user-friendly profile, optimizing therapeutic adherence. Strontium ranelate is the first compound to simultaneously decrease bone resorption and stimulate bone formation. Its antifracture efficacy, at various skeletal sites, has been established up to 8 years, through studies of the highest methodological standards. Increases in bone mineral density, observed after 1 year of treatment, are predictive of the long-term fracture efficacy, hence suggesting, for the first time in osteoporosis, that bone densitometry can be used as a monitoring tool for both efficacy and compliance. Owing to a positive benefit/risk ratio, strontium ranelate may now be considered as a first-line treatment in the management of osteoporosis.
Keywords: Fractures, hip, osteoporosis, safety, spine, strontium ranelate, treatment
Introduction
Owing to the high personal and societal costs of osteoporosis, the condition remains a challenge to both public health and physicians [Kanis et al. 2008]. Furthermore, the rapid increase in the elderly population means that the prevention of osteoporotic fracture is a socioeconomic priority [Suzuki et al. 2008].
However, the majority of postmenopausal osteoporosis patients remain untreated [Kanis et al. 2008], and even treatment-compliant patients experience new vertebral or nonvertebral fragility fractures during therapy at a rate of 9.5% per year [Adami et al. 2009], leaving a significant proportion of effectively treated patients at risk of fracture. It has also been observed that just 6% of previously untreated patients hospitalized for hip fracture are prescribed antiosteoporotic therapy, with only 41% persisting with treatment at 12 months, at a median duration of 40.3 weeks [Rabenda et al. 2008]. Consequently, it is crucial that effective management strategies are developed.
Strontium ranelate: a dual mode of action
Strontium ranelate is a treatment of postmenopausal osteoporosis that reduces the risk of vertebral and hip fractures. It is the first antiosteoporotic agent that appears to simultaneously increase bone formation and decrease bone resorption, thus resulting in the creation of new bone [Reginster et al. 2007]. Specifically, the dual mode of action of strontium ranelate is due to direct effects on both osteoblasts and osteoclasts, as reflected by the changes in bone markers in clinical trials [Meunier et al. 2004]. Several studies, in various in vitro models, have demonstrated that strontium ranelate increases osteoblast replication, differentiation, and activity [Fromigué et al. 2009; Caverzasio, 2008; Canalis et al. 1996], while in parallel, it downregulates osteoclast differentiation and activity [Hurtel-Lemaire et al. 2009; Takahashi et al. 2003; Baron and Tsouderos, 2002]. A recent in vitro study has shown that strontium ranelate increases the expression of the bone-specific alkaline phosphatase bALP (osteoblast differentiation) and the number of the bone nodules (osteoblast activity) of murine osteoblasts [Bonnelye et al. 2008]. In parallel, strontium ranelate decreases the tartrate resistant acid phosphatase activity (osteoclast differentiation) and the capability of murine osteoclasts to resorb (osteoclast activity), probably by acting on the cytoskeleton of these cells [Bonnelye et al. 2008]. In addition to these direct effects on osteoblasts and osteoclasts, strontium ranelate also modulates the level of osteoprotegerin (OPG) and Receptor Activator for Nuclear Factor kB Ligand (RANKL), two molecules strongly involved in the regulation of osteoclastogenesis by osteoblasts [Atkins et al. 2009; Brennan et al. 2009]. Other in vitro studies have demonstrated the involvement of the calcium-sensing receptor in the effects of strontium ranelate on osteoblasts, osteoclasts and OPG/RANKL regulation [Brennan et al. 2009; Fromigué et al. 2009; Hurtel-Lemaire et al. 2009].
In animal models, strontium ranelate increased bone formation and reduced bone resorption in intact mice, an effect that resulted in increased vertebral bone mass [Delannoy et al. 2002]. In addition, it was found to reduce resorption and long bone loss induced by hind limb immobilization in rats [Hott et al. 2003]. Strontium ranelate prevents bone loss by depressing bone resorption and maintaining bone formation, in estrogendeficient rats [Marie et al. 1993]. Finally, strontium ranelate administration decreased bone resorption and maintained bone formation in adult ovariectomized rats, which resulted in prevention of bone loss, an increase in bone strength and a positive effect on intrinsic bone properties [Bain et al. 2009]. The uncoupling mechanism of action observed in animal models was further supported, in humans, by the variations observed in biochemical markers of bone turnover during treatment of postmenopausal osteoporotic women [Meunier et al. 2002, 2004].
Antifracture efficacy of strontium ranelate
The effects of strontium ranelate in postmenopausal women with vertebral osteoporotic fractures were assessed during a double-blind, placebo-controlled phase II trial [Meunier et al. 2002]. Either strontium ranelate (0.5, 1 or 2g/day) or placebo was given to 353 White women (mean age 66 years; lumbar bone mineral density [BMD] by dual-energy X-ray absorptiometry [DXA] 0.699 ± 0.098 g/cm2 corresponding to mean lumbar T-score of −3.9 ± 1.0; mean number of prevalent vertebral fracture per patient 2.7 ± 2.5; mean menopausal duration 18 ± 8 years; and mean body mass index [BMI] 25 ± 3kg/m2). All patients were also given a daily supplement of calcium (0.5 g) and vitamin D3 (800IU). At the conclusion of this 2-year study, strontium ranelate dose-dependently increased the lumbar BMD values in comparison with baseline (+5.9% for 0.5 g, +8.3% for 1g and +13.6% for 2g). The annual increase in lumbar BMD in the group receiving strontium ranelate 2g/day was +7.3% and significantly different (p < 0.01) when compared with placebo. A significant increase in bone alkaline phosphatase (p < 0.05) was associated with a simultaneous and significant decrease in N-telopeptide crosslinks (p = 0.004) throughout the 2-year period in the group receiving strontium ranelate 2 g/day.
During the second year of treatment, the 2 g/day dose was associated with a 44% reduction in the number of patients experiencing a new vertebral deformity versus placebo (relative risk [RR] 0.56; 95% confidence interval [CI] 0.35–0.89; p < 0.05). Bone histomorphometry showed no mineralization defects. The same percentage of withdrawals following an adverse effect (10%) was observed for patients receiving placebo and for those receiving strontium ranelate 2 g/day [Meunier et al. 2002].
Transiliac-bone biopsies performed in patients included in the SOTI (Spinal Osteoporosis Therapeutic Intervention) and TROPOS (TReatment Of Peripheral OSteoporosis) trials were quantified by X-ray microanalysis for strontium ranelate uptake and the distribution in bone mineral. Changes in the mean and distribution of the degrees of mineralization of bone (MDMB) were measured by quantitative microradiography. In strontium ranelate-treated women, strontium ranelate was dose-dependently deposited into compact and cancellous bone, with significantly higher contents in new bone than in old bone. Measurement of strontium concentration in iliac crest bone biopsies from patients treated up to 60 months in the phase 3 studies with strontium ranelate 2 g/day indicated that the strontium content in total bone (expressed as the ratio Sr/[Sr+Ca] mmol%) reached a plateau at month 36. Strontium ranelate was mainly deposited on new bone. MDMB was not significantly different in strontium ranelate and placebo groups at either compact or cancellous bone levels [Boivin et al. 2009].
Strontium ranelate has been investigated in a large phase 3 program, initiated in 1996, which includes two clinical trials for the treatment of established osteoporosis [Reginster et al. 2005, 2008; Meunier et al. 2004]. The SOTI study was aimed at assessing the effect of strontium ranelate on the risk of vertebral fractures [Meunier et al. 2004]. The TROPOS trial aimed to evaluate the effect of strontium ranelate on peripheral (nonspinal) fractures. The definition of nonvertebral and major nonvertebral fractures was based on a decision of the Study Steering Committee, prior to the lock of the database [Reginster et al. 2005, 2008]. All patients included in these two studies had previously participated in a run-in study: the FIRST (Fracture International Run-in Strontium ranelate Trials) trial, aimed at starting the normalization of calcium and vitamin D. The patients received a calcium/vitamin D supplement throughout the studies, which were individually adapted according to their deficiencies (500 or 1000 mg calcium, and 400 or 800IU vitamin D3). Both studies were multinational, randomized, double-blind and placebo-controlled, with two parallel groups (strontium ranelate 2g/day versus placebo) involving 75 clinical centers in 12 countries in Europe and Australia [Reginster et al. 2005; Meunier et al. 2004]. The study duration was 5 years, with the main statistical analysis planned after 3 years of follow up.
More than 9000 osteoporotic postmenopausal women took part in FIRST. In SOTI, a total of 1649 postmenopausal osteoporotic women were randomized to strontium ranelate or placebo for 4 years, followed by a 1-year treatment-switch period for half of the patients (mean age 70 years), whereas 5091 patients were included in TROPOS (mean age 77 years) for 5 years. In these two studies, the main statistical analysis was performed, after 3 years, in the intent-to-treat population (ITT), defined as patients who took at least one sachet of study treatment and with baseline and postbaseline evaluation of the main criteria.
The primary analysis of SOTI [Meunier et al. 2004] (ITT, n = 1442), evaluating the effect of strontium ranelate 2g/day on vertebral fracture rates, revealed a 41% reduction in RR of experiencing a new vertebral fracture (semiquantitative assessment) with strontium ranelate throughout the 3-year study compared with placebo (139 patients with vertebral fracture versus 222, respectively [RR 0.59; 95% CI 0.48- 0.73; p < 0.001]). The risk of clinical vertebral fractures, which are defined as associated with height loss or back pain and therefore considered as the most severe, was reduced by 38% (RR 0.62; 95% CI 0.47–0.83; p < 0.001). The RR of experiencing a new vertebral fracture was significantly reduced in the strontium ranelate group as compared with the placebo group for the first year. Over the first 12 months, RR reduction was 49% (RR 0.51; 95% CI 0.36–0.74; Cox model p < 0.001). In SOTI, the lumbar BMD increased by 14.4% in the treated group in comparison with the placebo group (p < 0.001). At the third month of therapy, the serum concentration of bone-specific alkaline phosphatase was higher in the strontium ranelate group than in the placebo group (a treatment-related increase of 8.1%, p < 0.001), and this difference persisted at each evaluation during the 3 years. The concentration of serum C-telopeptide crosslinks was lower in the strontium ranelate group than in the placebo group at month 3 (a treatment-related difference of 12.2%, p < 0.001) and at each subsequent evaluation during the 3 years (p < 0.001) [Meunier et al. 2004]. Strontium ranelate was well tolerated without any specific adverse events [Reginster et al. 2005; Meunier et al. 2002, 2004].
The risk of new vertebral fracture over the 4-year treatment period was reduced by 33% with strontium ranelate, relative to placebo (RR 0.67; 95% CI 0.55–0.81; p < 0.001). Similarly, the risk of new clinical vertebral fractures was reduced by 36% (RR 0.64; 95% CI 0.49–0.83; p < 0.01) over 4 years. The number of patients needed to treat for 4 years to prevent one new vertebral fracture was 11 (95% CI 7–24). Among severely affected patients (with two or more prevalent vertebral fractures at baseline), risk reduction with strontium ranelate was 36% (RR, 0.64; 95% CI 0.50–0.81; p < 0.001). The total number of new vertebral fractures was significantly lower in the strontium ranelate group (275) than in the placebo group (421; p < 0.001). The risk of new clinical vertebral fracture was reduced by 36% with strontium ranelate relative to placebo (RR 0.64; 95% CI 0.49–0.83; p < 0.001).
In the patients maintained on strontium ranelate, the progressive increase in lumbar spine seen throughout the 4 years of the trial continued during the fifth year, with an increase of 1.2 ± 5.8% between month 48 and the end of treatment. In the patients switched to placebo, the increase in BMD began to reverse after the switch (-3.2 ± 5.8%) between month 48 and the end of treatment, although BMD was still substantially higher at month 60 (0.819 plusmn 0.147 g/cm2) compared with month 0 (0.734 plusmn 0.123 g/cm2). Both the increase in lumbar BMD in the group maintained on strontium ranelate and the decrease in the group switched to placebo between month 48 and the end of treatment were significant (p < 0.001 and p = 0.002, respectively). BMD in the group switched to placebo increased after subsequent switch back to strontium ranelate; the increase between month 48 and the end of treatment (5.3 ± 7.3%) was similar to the increase seen in strontium ranelate-treated patients during the first year (month 0 to 12) of the trial (6.4 ± 7.7%) [Meunier et al. 2009].
The primary analysis of TROPOS (ITT, n = 4932), evaluating the effect of strontium ranelate 2g/day on nonvertebral fracture, showed a 16% RR reduction in all nonvertebral fractures over a 3-year follow-up period (RR 0.84; 95% CI 0.702–0.995; p = 0.04) [Reginster et al. 2005]. Strontium ranelate treatment was associated with a 19% reduction in risk of major nonvertebral osteoporotic fractures (RR 0.81; 95% CI 0.66–0.98; p = 0.031). In a post-hoc analysis requested by the regulatory authorities, the risk of hip fracture was decreased by 36% (RR 0.64; 95% CI 0.412–0.997; p = 0.046) in a high-risk population (age above 74 years old, femoral-neck BMD T-score of less than or equal to −2.4 according to National Health and Nutrition Examination Survey [NHANES] normative value).
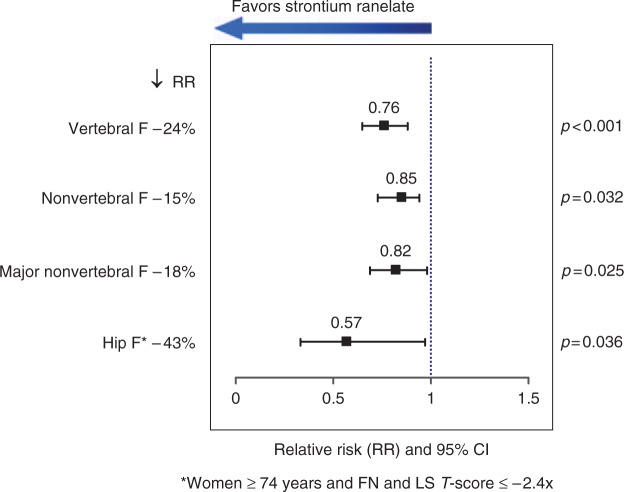
Of the 5091 patients, 2714 (53%) completed the study up to 5 years [Reginster et al. 2008]. The risk of nonvertebral fracture was reduced by 15 % in the strontium ranelate group compared with the placebo group (RR 0.85; 95% CI 0.73–0.99; Figure 1). A post-hoc analysis showed that the risk of hip fracture in a high-risk subset of the population above 74 years old and with a low femoral neck and lumbar BMD was decreased by 43% (RR 0.57; 95% CI 0.33–0.97; p = 0.036), and the risk of vertebral fracture was decreased by 24% (RR 0.76; 95% CI 0.65–0.88; p < 0.001) in the strontium ranelate group. After 5 years, the safety profile of strontium ranelate remained similar to the 3-year findings [Reginster et al. 2008].
Figure 1.
Strontium ranelate reduces vertebral and nonvertebral fracture risks over 5 years. RR, relative risk; CI, confidence interval; F, fracture; FN, femoral neck; LS, lumbar spine.
Postmenopausal osteoporotic women having participated in the 5-year efficacy trials SOTI and TROPOS were invited to enter a 3-year open-label extension study. At the extension baseline, the population treated for 8 years (n = 879; 79.1 ± 5.6 years) had a femoral neck T-score of −2.61 ± 0.71. The cumulative incidences of new vertebral and nonvertebral fractures (13.7% and 12.0%, respectively) over years 6–8 were nonstatistically different from the cumulative incidences in the first 3 years of the original studies (11.5% and 9.6%). Annual relative change in lumbar spine, femoral neck and total hip BMD was significant at every visit, except the 8-year visit for femoral neck and total hip BMD. Strontium ranelate was safe and well tolerated over 8 years. These data indicate that the antifracture efficacy is sustained over 8 years [Reginster et al. 2009].
Efficacy of strontium ranelate according to baseline risk factors
In order to assess the efficacy of strontium ranelate according to the main determinants of vertebral fracture risk (age, baseline BMD, prevalent fractures, family history of osteoporosis, baseline BMI, and addiction to smoking), data from SOTI and TROPOS (n = 5082) were pooled (strontium ranelate 2g/day group [n = 2536]; placebo group [n = 2546]; average age 74 years; 3-year follow up) [Roux et al. 2006].
Strontium ranelate decreased the risk of both vertebral (RR 0.60; 95% CI 0.53–0.69; p < 0.001) and nonvertebral (RR 0.85; 95% CI 0.74–0.99; p = 0.03) fractures. The decrease in risk of vertebral fractures was 37% (p = 0.003) in women aged <70 years, 42% (p < 0.001) for those aged 70–80 years and 32% (p = 0.013) for those aged ≥80 years. The RR of vertebral fracture was 0.28 (95% CI 0.07–0.99; p = 0.045) in osteopenic and 0.61 (95% CI 0.53–0.70; p < 0.001) in osteoporotic women, and baseline BMD was not a determinant of efficacy. The incidence of vertebral fractures in the placebo group increased with the number of prevalent vertebral fractures, but this was not a determinant of the effect of strontium ranelate. In 2605 patients, the risk of experiencing a first vertebral fracture was reduced by 48% (p < 0.001). The risk of experiencing a second vertebral fracture was reduced by 45% (p < 0.001; 1110 patients). Moreover, in patients with more than one prevalent fracture, the risk of experiencing a third or fourth vertebral fracture (or more) was reduced by 33% (p < 0.001; 1365 patients). Family history of osteoporosis, baseline BMI and addiction to smoking were not determinants of efficacy. This study indicates that a 3-year treatment with strontium ranelate is associated with antivertebral fracture efficacy in postmeno-pausal women independently of baseline osteoporotic risk factors [Roux et al. 2006].
Prevalent vertebral fractures increase the risk of subsequent vertebral and nonvertebral fractures [Naves et al. 2003; Van Staa et al. 2002], as well as the risk of hip fracture with at least twofold excess. The risk of further fracture has been shown to be higher among younger people compared with the elderly [Van Staa et al. 2002]. However, few data are available in clinical trials in patients <65 years. The efficacy of strontium ranelate was assessed in osteoporotic patients aged 50–65 years, most of whom had a prevalent vertebral fracture, presenting a subgroup of patients having a very high lifetime risk of fractures [Roux et al. 2008].
Among the patients included in the SOTI study, 385 were aged 50–65 years, of which 353 were eligible for assessment of the efficacy of strontium ranelate on vertebral fractures according to the ITT principle [Roux et al. 2008]. Over 3 years, treatment with strontium ranelate significantly reduced the risk of vertebral fracture by 43% (RR 0.57; 95% CI0.36–0.92; p = 0.019), with a 16.9% incidence of vertebral fractures in the strontium ranelate group versus 29.6% in the placebo group. This efficacy in reducing the risk of vertebral fractures was sustained over 4 years of treatment with strontium ranelate, with a reduction of 35% (RR 0.65; 95% CI 0.42–0.99; p = 0.049) and an incidence of vertebral fractures of 21.6% in the strontium ranelate group versus 32.8% in the placebo group. There was a trend for a reduction in the risk of vertebral fracture over the first year, which was not statistically significant. A significant effect of strontium ranelate compared with placebo was also observed on symptomatic vertebral fractures (defined as radiological fractures plus concomitant back pain or a decrease in body height by at least 1 cm) with a 54% reduction in the risk of symptomatic vertebral fracture over 3 years (RR 0.46; 95% CI 0.22–0.97; p = 0.033), sustained over 4 years with a 52% reduction (RR 0.48; 95% CI 0.24–0.95; p = 0.030). In young patients with severe osteoporosis, clinical, serious and drug-related adverse effects were similar in placebo and treated groups and the overall safety profile was very similar to that already described for the whole SOTI study population over 3 and 4 years. No case of pulmonary embolism or hypersensitivity reaction was observed in this study population [Roux et al. 2008].
Women aged ≥80 years comprise about 8% of the postmenopausal population, but contribute > 30% of all fragility fractures and 60% of hip fractures because of the high prevalence of osteoporosis and high incidence of falls in this group [United Nations, 2003, 2008]. As this group of women constitutes the fastest growing segment of the general population, the number of elderly individuals with osteoporosis will increase markedly in the coming years [Ettinger, et al. 2003]. Despite the important contribution to the public health burden of fractures made by this group, few studies of fracture prevention have focused on the elderly population [McClung et al. 2001].
To determine whether strontium ranelate also reduces fractures in elderly patients, an analysis based on preplanned pooling of data from the SOTI and TROPOS trials included 1488 women between 80 and 100 years of age followed for 3 years [Seeman et al. 2006]. Yearly spinal X-rays were performed in 895 patients. Only radiographically confirmed nonvertebral fractures were included. Baseline characteristics did not differ in placebo and treatment arms. In the ITT analysis, the risk of vertebral, nonvertebral and clinical (symptomatic vertebral and nonvertebral) fractures was reduced within 1 year by 59% (p = 0.002), 41% (p = 0.027) and 37% (p = 0.012), respectively. At the end of 3 years, vertebral, nonvertebral and clinical fracture risks were reduced by 32% (p = 0.013), 31% (p = 0.011) and 22% (p = 0.040), respectively. The medication was well tolerated, and the safety profile was similar to that in younger patients. The investigators concluded that treatment with strontium ranelate safely reduced the risk of vertebral and nonvertebral fractures in women with osteoporosis aged ≥80 years.
Many fractures that occur in women with moderate fracture risk are due to osteopenia. Strontium ranelate was studied in 1431 postmenopausal women with osteopenia [Seeman et al. 2008]. In women with lumbar spine osteopenia, strontium ranelate decreased the risk of vertebral fracture by 41% (RR 0.59; 95% CI 0.43–0.82; p = 0.002; 60 fractures in the strontium group and 90 in the placebo), by 59% in women with no prevalent fractures (RR 0.41; 95% CI 0.17–0.99; p = 0.039; number needed to treat [NNT] = 25) and by 38% in women with prevalent fractures (RR 0.62; 95% CI 0.44–0.88; p = 0.008; NNT = 13). In women with osteopenia both at the lumbar spine and the femoral neck, strontium ranelate reduced the risk of fracture by 52% (RR 0.48; 95% CI 0.24–0.96; p = 0.034; NNT = 11). In women with osteopenia at the lumbar spine or the femoral neck, the RR reduction was 62% (RR 0.38; 95% CI 0.21–0.70; p < 0.001; NNT = 10).
Significant increases in lumbar spine, femoral neck and total hip BMD have been consistently reported in populations exposed to strontium ranelate [Reginster et al. 2005; Reginster and Meunier, 2003; Meunier et al. 2002, 2004]. To analyze the relationship between BMD changes and fracture incidence during 3-year treatment with strontium ranelate, patients from the strontium ranelate arm of the SOTI and TROPOS trials were evaluated [Bruyere et al. 2007a, 2007b]. The outcome measures included BMD at the lumbar spine, femoral neck, total proximal femur assessed at baseline and after a follow up of 1 and 3 years, semiquantitative visual assessment of vertebral fractures and nonvertebral fractures based on written documentation. These assessments were not adjusted for the atomic mass of strontium ranelate since it has been demonstrated that the relationship is independent of this factor [Kendler et al. 2009]. After 3 years of strontium ranelate 2 g/day, each percentage point increase in femoral neck and total proximal femur BMD was associated with a 3% (95% adjusted CI 1–5%) and 2% (1–4%) reduction in risk of new vertebral fracture, respectively. The 3-year changes in femoral neck and total proximal femur BMD explained 76% and 74% of the reduction in vertebral fractures observed during the treatment, respectively. Changes at 3 years in spine BMD were not statistically associated with the incidence of new vertebral fracture (p = 0.10). No significant associations were found between 3-year changes in BMD and incidence of new nonvertebral fractures, but a trend was found for femoral neck BMD (p = 0.09) and total proximal femur BMD (p = 0.07). An increase in femoral neck BMD after 1 year was significantly associated with the reduction in incidence of new vertebral fractures observed after 3 years (p = 0.04). The investigators concluded that, during 3-year strontium ranelate treatment, an increase in femoral neck BMD was associated with a proportional reduction in vertebral fracture incidence. These results are consistent with previous studies that show a reliable prediction of femoral neck BMD for both vertebral and hip fractures. Conversely, the relationship between lumbar spine BMD and the risk of osteoporotic fracture is probably impaired by the artifactual increase in lumbar spine BMD due to osteophytes, which are relatively common in elderly patients.
In a post-hoc analysis of 465 women aged > 74 years with low BMD at the femoral neck (T-score less than or equal to −2.4) selected from the population of the TROPOS trial, BMD was assessed at the femoral neck at baseline and after a follow up of 3 years [Bruyere et al. 2007a, 2007b]. Hip fractures were reported from the hospital database. After adjusting for age, BMI, femoral neck BMD at baseline and number of prevalent vertebral fractures, for each 1% increase in femoral neck BMD observed after 3 years, the risk to experience a hip fracture after 3 years decreased by 7% (95% CI 1–14%; p = 0.04). In patients experiencing a hip fracture over 3 years of treatment with strontium ranelate, femoral neck BMD increased by 3.41% (standard error [SE] 1.02%) compared with 7.235% (SE 0.81%) in patients without hip fracture (p = 0.02). In the 8-year follow up of patients treated with strontium ranelate, there was an association between change in BMD at the total proximal femur and incidence of vertebral fracture (p = 0.02). Each 1% increase in total proximal femur BMD was associated with decreased risk for new vertebral fracture by 5% (95% CI 1–10%) [Reginster et al. 2009].
A recent review noted that the strong correlation between measured BMD increases and fracture risk reduction in patients on strontium ranelate therapy may be of clinical benefit to physicians wishing to evaluate both treatment persistence and fracture risk reduction [Kendler et al. 2009]. Observation of an increase in BMD after 1 year's treatment may also be expected to have a positive impact on compliance by motivating the patients to continue their treatment.
Exploration of bone microarchitecture with strontium ranelate
The safety of strontium ranelate on bone has been investigated through analysis of 141 transiliac bone biopsies performed in a subset of women enrolled in the STRATOS (STRontium Administration for Treatment of OSteoporosis), SOTI or TROPOS trials [Arlot et al. 2008]. Most of the biopsies were unpaired. Histomorphometry provided a two-dimensional (2D) demonstration of the bone safety of strontium ranelate, with higher mineral apposition rate in cancellous bone (+9% versus control, p = 0.019). Osteoblast surfaces were higher (+38% versus control, p = 0.047). Three-dimensional (3D) analysis of 3-year biopsies with strontium ranelate (20 biopsies) and placebo (21 biopsies) using microcomputed tomography showed differences in microarchitecture in the strontium ranelate group versus placebo, higher cortical thickness (+18%, p = 0.008) and trabecular number (+14%, p = 0.05), and lower structure model index (-22%, p = 0.01) and trabecular separation (- 16%, p = 0.04), with no change in cortical porosity. These analyses indicate the bone safety of strontium ranelate in the treatment of postmenopausal osteoporosis and are consistent with a mode of action of strontium ranelate involving dissociation between bone formation and bone resorption. The change in 3D trabecular and cortical microarchitecture may improve bone biomechanical competence and may explain the decreased fracture rate after strontium ranelate treatment.
Quality of life: impact of strontium ranelate
Quality of life was a secondary endpoint in the strontium ranelate phase 3 studies using the Quality of Life Questionnaire in Osteoporosis (QUALIOST), a dedicated questionnaire for vertebral osteoporosis [Marquis et al. 2001]. QUALIOST was used to assess quality of life in 1240 patients from the SOTI trial who completed the questionnaire at baseline and every 6 months [Marquis et al. 2008]. After 3 years of treatment, patients receiving strontium ranelate had a better quality of life compared with placebo (p = 0.016 for global score; p = 0.019 and p = 0.032 for emotional and physical scores, respectively) [Marquis et al. 2008]. The improvement in the emotional score was related to fewer negative feelings and concerns regarding the disease, and the improvement in the physical score was associated with reduced pain and increased mobility.
Safety and tolerability of strontium ranelate
In the SOTI and TROPOS trials, the incidence of adverse events and serious adverse events and withdrawals due to adverse events were similar in the strontium ranelate and placebo groups [Emea, 2007; Shea et al. 2004]. During the first 3 months of treatment, nausea, diarrhea, headache, dermatitis and eczema were more frequently associated with strontium ranelate compared with placebo; but, thereafter, there was no difference in incidence between strontium ranelate and placebo groups concerning nausea and diarrhea.
Whereas no significant increase in venous thromboembolism (VTE) was observed in any of the individual studies, in pooled data from the SOTI and TROPOS trials, there was an apparent increased risk of VTE in the strontium ranelate group (0.6% versus 0.9% per year), although the annual incidence was similar in the strontium ranelate and placebo groups in the individual trials [Reginster et al. 2005; Meunier et al. 2004].
A recently published study used the UK General Practice Research Database (GPRD) to assess the risk of several recently reported adverse events linked to the use of strontium ranelate for osteoporosis in postmenopausal women [Grosso et al. 2008]. Age-adjusted rate ratios for VTE, gastrointestinal disturbance, minor skin complaint and memory loss were 1.1 (95% CI 0.2–5.0), 3.0 (95% CI 2.3–3.8), 2.0 (95% CI 1.3–3.1) and 1.8 (95% CI 0.2–14.1), respectively. No cases of osteonecrosis of the jaw, Stevens—Johnson syndrome or drug rash with eosinophilia and systemic symptoms (DRESS) were found. In addition, a recent analysis of the UK GPRD has shown an absence of increased risk of VTE in osteoporotic patients treated with strontium ranelate, by comparison with untreated patients. Furthermore, the incidence of VTE in strontium ranelate-treated patients was similar with the incidence seen in patients treated with alendronate, an agent that is not especially known to increase this risk [Bréart et al. 2009].
Recently, the postmarketing experience of patients treated with strontium ranelate reported cases of the DRESS syndrome (<20 for 570,000 patient-years of exposure) [Emea, 2007]. This incidence is in the vicinity of what has been previously reported as severe skin reactions with most other currently available antiosteoporosis medications [Musette et al. 2009]. A causative link has not been firmly established, as strontium is a trace element naturally present in the human body and ranelic acid is poorly absorbed. Owing to the possible fatality linked to this syndrome, however, it seems reasonable to discontinue immediately strontium ranelate and other concomitant treatment known to induce such a syndrome in case of suspicious major skin disorders occurring within 2 months of treatment initiation [Tas and Simonart, 2003] and to introduce adapted treatment and follow up to avoid systemic symptoms.
Cost effectiveness of strontium ranelate
It has become increasingly common that recommendations concerning the use of treatments for osteoporosis have been placed in a health economic context, in order to justify resources allocation and inform the development of clinical guidelines. The cost effectiveness of strontium ranelate was compared with nontreatment in UK women using the FRAX® algorithm for fracture assessment. At a willingness-to-pay of £30,000 per quality-adjusted life-year (QALY), strontium ranelate was generally cost effective in women with prior fracture at the threshold of osteoporosis from an age of 65years [Borgstrom et al. 2009]. A validated Markov microsimulation model with a Belgian healthcare cost perspective was used to assess the cost per QALY of strontium ranelate compared with no treatment, on a basis of calcium/vitamin D supplementation if needed. Analyses were performed for women aged 70, 75 and 80 years, either with a BMD T-score ≤ −2.5 SD or with prevalent vertebral fractures. Parameter uncertainty was evaluated using both univariate and probabilistic sensitivity analyses. Strontium ranelate was cost saving at the age of 80 years in both populations. For women with a T-score ≤2.5 SD, the costs per QALY gained of strontium ranelate were respectively € 15,096 and €6913 at 70 and 75 years of age, while these values were €23,426 and €9698 for women with prevalent vertebral fractures. Sensitivity analyses showed that the results were robust over a wide range of assumptions. The authors concluded that, compared with no treatment, long-term strontium ranelate treatment is cost effective for postmenopausal women [Hiligsmann et al. 2010a, 2010b].
The same model was used to compare strontium ranelate with bisphosphonate risedronate. Strontium ranelate appeared to be more effective and less costly than risedronate for women with osteoporosis aged over 75 years and for women with prevalent vertebral fractures aged 80 years. The cost per QALY gained of strontium ranelate compared with risedronate at 75 years of age was € 11,435 for women with prevalent vertebral fractures. When compared with no treatment, the costs per QALY gained of strontium ranelate were € 15,588 and € 7708 at 75 and 80 years of age for women with osteoporosis; the equivalent values were € 16,518 and € 6015 for women with prevalent vertebral fractures. Probabilistic sensitivity analyses showed that strontium ranelate was generally more cost effective than risedronate, in the range of 60% in all cases. The results of this study suggest that strontium ranelate is a cost effective strategy, in a Belgian setting, for the treatment of postmenopausal osteoporotic women aged over 75 years [Hiligsmann et al. 2010a, 2010b].
Conclusions
Strontium ranelate is characterized by a unique mode of action, concomitantly decreasing bone resorption and stimulating bone formation. This treatment appears to reduce fractures at all skeletal sites, including the hip in osteoporotic patients most at risk. Clinical trial evidence suggests that it is effective in a wide scatter of patient profiles, from early postmenopausal women and osteopenic subjects to elderly women over the age of 80 years. The antifracture efficacy of strontium ranelate has been shown to be independent of baseline severity of osteoporosis, bone turnover level or the presence of clinical risk factors. Its safety profile compares favorably with that reported for other antiosteoporosis medications. Strontium ranelate, with its antifracture efficacy demonstrated up to 5 years, may now be considered as a first-line treatment of osteoporosis.
Footnotes
Dr Jean-Yves Reginster has served as a consultant or on a scientific advisory board for Servier, Novartis, Negma, Lilly, Wyeth, Amgen, GlaxoSmithKline, Roche, Merckle, Nycomed, NPS and Theramex. He has received honoraria for speaking from Merck Sharp and Dohme, Lilly, Rottapharm, IBSA, Genevrier, Novartis, Servier, Roche, GlaxoSmithKline, Teijin, Teva, Ebewee Pharma, Zodiac, Analis, Theramex, Nycomed and Novo-Nordisk. He has also received grant support from Bristol Myers Squibb, Merck Sharp & Dohme, Rottapharm, Teva, Lilly, Novartis, Roche, GlaxoSmithKline, Amgen and Servier.
Dr Mickaél Hiligsmann has received grant research funding, consulting fees, lectures fees or reimbursement for attending meetings from Amgen, Novartis and Servier.
Dr Olivier Bruyere has received grant research funding, consulting fees, lectures fees or reimbursement for attending meetings from Servier, GlaxoSmithKline, MSD, Theramex, Galapagos, Rottapharm, IBSA and Wyeth.
References
- Adami S., Giannini S., Bianchi G., Sinigaglia L., Di Munno O., Fiore C.E., et al. (2009) Vitamin D status and response to treatment in post-menopausal osteoporosis. Osteoporos Int 20: 239–244 [DOI] [PubMed] [Google Scholar]
- Arlot M.E., Jiang Y., Genant H.K., Zhao J., Burt-Pichat B., Roux J.P., et al. (2008) Histomorphometric and microCT analysis of bone biopsies from postmenopausal osteoporotic women treated with strontium ranelate. J Bone Miner Res 23: 215–222 [DOI] [PubMed] [Google Scholar]
- Atkins G., Welldon K.J., Halbout P., Findlay D.M. (2009) Strontium ranelate treatment of human primary osteoblasts promotes an osteocyte-like phenotype while eliciting an osteoprotegerin response. Osteoporos Int 20: 653–664 [DOI] [PubMed] [Google Scholar]
- Bain S.D., Jerome C., Shen V., Dupin-Roger I., Ammann P. (2009) Strontium ranelate improves bone strength in ovariectomized rat by positively influencing bone resistance determinants. Osteoporos Int 20: 1417–1428 [DOI] [PubMed] [Google Scholar]
- Baron R., Tsouderos Y. (2002) In vitro effects of S12911–2 on osteoclast function and bone marrow macrophage differentiation. Eur J Pharmacol 450: 11–17 [DOI] [PubMed] [Google Scholar]
- Boivin G., Farlay D., Khebbab M.T., Jaurand X., Delmas P.D., Meunier P.J. (2009) In osteoporotic women treated with strontium ranelate, strontium is located in bone formed during treatment with a maintained degree of mineralization. Osteoporos Int (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- Bonnelye E., Chabadel A., Saltel F., Jurdic P. (2008) Dual effect of strontium ranelate: Stimulation of osteoblast differentiation and inhibition of osteoclast formation and resorption in vitro. Bone 42: 129–138 [DOI] [PubMed] [Google Scholar]
- Borgstrom F., Strom O., Coelho J., Johansson H., Oden A., Mccloskey E. V., et al. (2009) The cost-effectiveness of risedronate in the UK for the management of osteoporosis using the Frax(R). Osteoporos Int 21: 495–505 [DOI] [PubMed] [Google Scholar]
- Bréart G., Cooper C., Meyer O., Speirs C., Deltour N., Reginster JY. (2009) Osteoporosis and venous thromboembolism: a retrospective cohort study in the UK General Practice Research Database (GPRD). Osteoporos Int (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan T., Rybchyn M.S., Green W., Atwa S., Conigrave A.D., Mason R.S. (2009) Osteoblasts play key roles in the mechanisms of action of strontium ranelate. Br J Pharmacol 157: 1291–1300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruyere O., Roux C., Badurski J., Isaia G., De Vernejoul M.C., Cannata J., et al. (2007a) Relationship between change in femoral neck bone mineral density and hip fracture incidence during treatment with strontium ranelate. Curr Med Res Opin 23: 3041–3045 [DOI] [PubMed] [Google Scholar]
- Bruyere O., Roux C., Detilleux J., Slosman D.O., Spector T. D., Fardellone P., et al. (2007b) Relationship between bone mineral density changes and fracture risk reduction in patients treated with strontium ranelate. J Clin Endocrinol Metab 92: 3076–3081 [DOI] [PubMed] [Google Scholar]
- Canalis E., Hott M., Deloffre P., Tsouderos Y, Marie P.J. (1996) The divalent strontium salt S12911 enhances bone cell replication and bone formation in vitro. Bone 18: 517–523 [DOI] [PubMed] [Google Scholar]
- Caverzasio J. (2008) Strontium ranelate promotes osteoblastic cell replication through at least two different mechanisms. Bone 42: 1131–1136 [DOI] [PubMed] [Google Scholar]
- Delannoy P., Bazot D., Marie P.J. (2002) Long-term treatment with strontium ranelate increases vertebral bone mass without deleterious effect in mice. Metabolism 51: 906–911 [DOI] [PubMed] [Google Scholar]
- Emea (2007) Question and answers on the safety of protelos/osseor (strontium ranelate).
- Ettinger M.P. (2003) Aging bone and osteoporosis: strategies for preventing fractures in the elderly. Arch Intern Med 163: 2237–2246 [DOI] [PubMed] [Google Scholar]
- Fromigué O., Haÿ E., Barbara A., Petrel C., Traiffort E., Ruat M., Marie P.J. (2009) Calcium sensing receptor-dependent and -independent activation of osteoblast replication and survival by strontium ranelate. J Cell Mol Med 13: 2189–2199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosso A., Douglas I., Hingorani A., Macallister R., Smeeth L. (2008) Post-marketing assessment of the safety of strontium ranelate; a novel case-only approach to the early detection of adverse drug reactions. Br J Clin Pharmacol 66: 689–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiligsmann M., Bruyere O., Reginster J.Y. (2010a) Cost-effectiveness of strontium ranelate versus risedronate in the treatment of postmenopausal osteoporotic women aged over 75 years. Bone 46: 440–446 [DOI] [PubMed] [Google Scholar]
- Hiligsmann M., Bruyere O., Reginster J.Y. (2010b) Cost-utility of long-term strontium ranelate treatment for postmenopausal osteoporotic women. Osteoporos Int 21: 157–165 [DOI] [PubMed] [Google Scholar]
- Hott M., Deloffre P., Tsouderos Y., Marie P.J. (2003) S12911–2 reduces bone loss induced by short-term immobilization in rats. Bone 33: 115–123 [DOI] [PubMed] [Google Scholar]
- Hurtel-Lemaire A.S., Mentaverri R., Caudrillier A., Cournarie F., Wattel A., Kamel S., et al. (2009) The calcium-sensing receptor is involved in strontium ranelate-induced osteoclast apoptosis. New insights into the associated signaling pathways. J Biol Chem 284: 575–584 [DOI] [PubMed] [Google Scholar]
- Kanis J.A., Burlet N., Cooper C., Delmas P.D., Reginster J.Y., Borgstrom F., et al. (2008) European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int 19: 399–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler D.L., Adachi J.D., Josse R.G., Slosman D.O. (2009) Monitoring strontium ranelate therapy in patients with osteoporosis. Osteoporos Int 20: 1101–1106 [DOI] [PubMed] [Google Scholar]
- Marie P.J., Hott M., Modrowski D., De Pollak C., Guillemain J., Deloffre P., et al. (1993) An uncoupling agent containing strontium prevents bone loss by depressing bone resorption and maintaining bone formation in estrogen-deficient rats. J Bone Miner Res 8: 607–615 [DOI] [PubMed] [Google Scholar]
- Marquis P., Cialdella P., De La Loge C. (2001) Development and validation of a specific quality of life module in post-menopausal women with osteoporosis: the QUALIOST. Qual Life Res 10: 555–566 [DOI] [PubMed] [Google Scholar]
- Marquis P., Roux C, De La Loge C., Diaz-Curiel M., Cormier C, Isaia G., et al. (2008) Strontium ranelate prevents quality of life impairment in post-menopausal women with established vertebral osteoporosis. Osteoporos Int 19: 503–510 [DOI] [PubMed] [Google Scholar]
- Mcclung M.R., Geusens P., Miller P.D., Zippel H., Bensen W. G., Roux C., et al. (2001) Effect of risedronate on the risk of hip fracture in elderly women. Hip intervention program study group. N Engl J Med 344: 333–340 [DOI] [PubMed] [Google Scholar]
- Meunier P.J., Slosman D.O., Delmas P.D., Sebert J.L., Brandi M. L., Albanese C., et al. (2002) Strontium ranelate: dose-dependent effects in established postmenopausal vertebral osteoporosis–a 2-year randomized placebo controlled trial. J Clin Endocrinol Metab 87: 2060–2066 [DOI] [PubMed] [Google Scholar]
- Meunier P.J., Roux C., Seeman E., Ortolani S., Badurski J. E., Spector T. D., et al. (2004) The effects of strontium ranelate on the risk of vertebral fracture in women with postmenopausal osteoporosis. N Engl J Med 350: 459–468 [DOI] [PubMed] [Google Scholar]
- Meunier P.J., Roux C., Ortolani S., Diaz-Curiel M., Compston J., Marquis P., et al. (2009) Effects of long-term strontium ranelate treatment on vertebral fracture risk in postmenopausal women with osteoporosis. Osteoporos Int 20: 1663–1673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musette P., Brandi M.L., Cacoub P., Kaufman J.M., Rizzoli R., Reginster J.Y. (2009) Treatment of osteoporosis: recognizing and managing cutaneous adverse reactions and drug-induced hypersensitivity. Osteoporos Int (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- Naves M., Diaz-Lopez J.B., Gomez C., Rodriguez-Rebollar A., Rodriguez-Garcia M., Cannata-Andia J.B. (2003) The effect of vertebral fracture as a risk factor for osteoporotic fracture and mortality in a Spanish population. Osteoporos Int 14: 520–524 [DOI] [PubMed] [Google Scholar]
- Rabenda V., Vanoverloop J., Fabri V., Mertens R., Sumkay F., Vannecke C., et al. (2008) Low incidence of anti-osteoporosis treatment after hip fracture. J Bone Joint Surg Am 90: 2142–2148 [DOI] [PubMed] [Google Scholar]
- Reginster J.Y., Meunier P.J. (2003) Strontium ranelate phase 2 dose-ranging studies: PREVOS and STRATOS studies. Osteoporos Int 14(Suppl 3): S56-S65 [DOI] [PubMed] [Google Scholar]
- Reginster J.Y., Seeman E., De Vernejoul M.C., Adami S., Compston J., Phenekos C., et al. (2005) Strontium ranelate reduces the risk of nonvertebral fractures in postmenopausal women with osteoporosis: Treatment of Peripheral Osteoporosis (TROPOS) Study. J Clin Endocrinol Metab 90: 2816–2822 [DOI] [PubMed] [Google Scholar]
- Reginster J.Y., Malaise O., Neuprez A., Bruyere O. (2007) Strontium ranelate in the prevention of osteoporotic fractures. Int J Clin Pract 61: 324–328 [DOI] [PubMed] [Google Scholar]
- Reginster J.Y., Felsenberg D., Boonen S., Diez-Perez A., Rizzoli R., Brandi M. L., et al. (2008) Effects of long-term strontium ranelate treatment on the risk of nonvertebral and vertebral fractures in postmenopausal osteoporosis: results of a five-year, randomized, placebo-controlled trial. Arthritis Rheum 58: 1687–1695 [DOI] [PubMed] [Google Scholar]
- Reginster J.Y., Bruyere O., Sawicki A., Roces-Varela A., Fardellone P., Roberts A., et al. (2009) Long-term treatment of postmenopausal osteoporosis with strontium ranelate: results at 8 years. Bone 45: 1059–1064 [DOI] [PubMed] [Google Scholar]
- Roux C., Reginster J.Y., Fechtenbaum J., Kolta S., Sawicki A., Tulassay Z., et al. (2006) Vertebral fracture risk reduction with strontium ranelate in women with postmenopausal osteoporosis is independent of baseline risk factors. J Bone Miner Res 21: 536–542 [DOI] [PubMed] [Google Scholar]
- Roux C., Fechtenbaum J., Kolta S., Isaia G., Andia J.B., Devogelaer J.P. (2008) Strontium ranelate reduces the risk of vertebral fracture in young postmenopausal women with severe osteoporosis. Ann Rheum Dis 67: 1736–1738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman E., Vellas B., Benhamou C., Aquino J.P., Semler J., Kaufman J. M., et al. (2006) Strontium ranelate reduces the risk of vertebral and nonvertebral fractures in women eighty years of age and older. J Bone Miner Res 21: 1113–1120 [DOI] [PubMed] [Google Scholar]
- Seeman E., Devogelaer J.P., Lorenc R., Spector T., Brixen K., Balogh A., et al. (2008) Strontium ranelate reduces the risk of vertebral fractures in patients with osteopenia. J Bone Miner Res 23: 433–438 [DOI] [PubMed] [Google Scholar]
- Shea B., Wells G., Cranney A., Zytaruk N., Robinson V., Griffith L., et al. (2004) Calcium supplementation on bone loss in postmenopausal women. Cochrane Database Syst Rev CD004526. [DOI] [PubMed] [Google Scholar]
- Suzuki A., Sekiguchi S., Asano S., Itoh M. (2008) Pharmacological topics of bone metabolism: recent advances in pharmacological management of osteoporosis. J Pharmacol Sci 106: 530–535 [DOI] [PubMed] [Google Scholar]
- Takahashi N., Sasaki T., Tsouderos Y., Suda T. (2003) S 12911–2 inhibits osteoclastic bone resorption in vitro. J Bone Miner Res 18: 1082–1087 [DOI] [PubMed] [Google Scholar]
- Tas S., Simonart T. (2003) Management of drug rash with eosinophilia and systemic symptoms (DRESS syndrome): an update. Dermatology 206: 353–356 [DOI] [PubMed] [Google Scholar]
- United Nations (2003) World Population Prospects: The 2002 Revision.
- United Nations (2008) World Urbanization Prospects: The 2007 Revision.
- Van Staa T.P., Leufkens H.G., Cooper C. (2002) Does a fracture at one site predict later fractures at other sites? A British cohort study. Osteoporos Int 13: 624–629 [DOI] [PubMed] [Google Scholar]