Abstract
Oral phosphodiesterase type 5 (PDE5) inhibitors have provided non-invasive, effective, and well-tolerated treatments for patients with erectile dysfunction (ED). However, many patients with ED are unresponsive to 'on-demand' PDE5 inhibitors. In addition, the lack of spontaneity and naturalness of the on-demand regimen could be a reason for decreased compliance with PDE5 inhibitors. Recently, tadalafil and udenafil were approved for low-dose daily administration for the treatment of ED. Since the introduction of the concept of daily administration of PDE5 inhibitors, several reports have supported the potential benefits of this therapy for disease modification, improvement of the treatment response in difficult-to-treat populations, spontaneity, and safety, although further research is needed to better address these hypotheses. In this article, we reviewed the daily administration of PDE5 inhibitors in terms of pharmacokinetics, safety, efficacy, and distinct features.
Keywords: Chronic, Erectile dysfunction, Phosphodiesterase 5 inhibitors
INTRODUCTION
Erectile dysfunction (ED) is a chronic condition defined as the consistent inability to maintain a sufficient erection for satisfactory sexual function. ED is a common disease that affects 10 to 30 million men in the United States [1]. The disease may be associated with underlying cardiovascular, neurological, psychological, and endocrine disorders. At present, oral phosphodiesterase type 5 (PDE5) inhibitors are the first-line therapy for ED patients, providing non-invasive, effective, and well-tolerated treatments for ED [2,3]. Nevertheless, 30 to 40% of patients fail to respond or are not satisfied with on-demand treatment even after correction of the reversible cause of ED [4]. In addition, the success rate is often lower in subpopulations identified as being more difficult to treat, including those with diabetes mellitus (DM), patients with severe vasculogenic ED, and post-radical prostatectomy (RP) patients [5,6]. The lack of spontaneity and naturalness of the on-demand regimen could be reasons for decreased compliance with PDE5 inhibitors [7]. Intermittent administration might not be the optimal treatment for all men and their partners owing to individual patterns of sexual activity [8].
The concept of daily administration of PDE5 inhibitors was introduced in an effort to provide another treatment option that is closer to a natural sexual life [9-12]. Daily tadalafil provides an alternative to on-demand dosing for couples who prefer spontaneous rather than scheduled sexual activity or who have frequent sexual activity. Daily dosing overcomes the requirement for dosing and sexual activity to be temporally linked [2]. In addition, an accumulating body of data supports that daily PDE5 inhibitors could provide potential benefits of improved treatment response in difficult-to-treat populations, disease modification, efficacy, and safety for ED of various etiologies [13-15].
Tadalafil was first approved for low-dose daily administration at a dose of 2.5 mg or 5 mg [2]. The pharmacokinetic characteristics of tadalafil, with its 17.5-hour half-life, make it suitable for daily dosing and achieving a steady-state serum concentration [16]. Recently, udenafil was also approved for low-dose daily administration at a dose of 50 mg [17]. The aim of this article was to provide clinically relevant information on daily PDE5 inhibitors by reviewing their pharmacokinetics, safety, and efficacy.
RATIONALE FOR USING CHRONIC PDE5 INHIBITORS: PHARMACOKINETICS
Tadalafil is a selective and reversible inhibitor of PDE5 [18]. Tadalafil clearance is predominantly by the hepatic metabolism via CYP3A to a catechol that undergoes methylation and is extensively conjugated to form the major circulating metabolite, a methylcatechol glucuronide. (A minor amount of the unconjugated metabolite is also detected in plasma.) Tadalafil is eliminated primarily as a metabolite in feces (61%) and urine (36%) [19]. A significant erectogenic response has been demonstrated within 30 minutes after a single 20-mg tadalafil dose [20] with a duration of up to 36 hours [21]. In particular, the 17.5-hour half-life of tadalafil, which is longer than the half-life of other currently available PDE5 inhibitors, makes it well suited for potential use as daily ED therapy [22]. This longer half-life provides a steady-state serum drug concentration with low-dose, once-daily administration.
Although a direct correlation of plasma concentration with efficacy has not been established, a total tadalafil plasma concentration of 55 ng/ml, which approximates 90% enzyme inhibition in vitro [16], constituted a reasonable pharmacodynamic target for clinical development with the aim of maintaining these concentrations throughout the dosing interval. The support for this target is based on the 90% level of PDE5 inhibition that results in corpus cavernosal relaxation exhibited by sildenafil [23]. Based on this level, a predicted tadalafil plasma concentration of 55 ng/mL provided the pharmacologic rationale for the development of a once-daily, 5-mg dose as potentially efficacious throughout a 24-hour dosing interval in vitro [16].
Examination of tadalafil concentration-time profiles suggested that steady state was essentially attained by day 5 with a plasma concentration 1.6 times that with a single dose [22]. Simulations of various dosing intervals illustrated the similarity and differences between on-demand and daily dosing. In the simulated steady-state, 5 mg of tadalafil taken once daily provided plasma concentrations that were similar to those observed 24 hours after 20 mg of tadalafil taken either two or three times a week. Predicted tadalafil plasma concentrations following the daily 5-mg dose were less than those of the twice-weekly dose and were equivalent to the thrice-weekly dose [16].
When compared with measured tadalafil plasma concentrations following single-dose administration of tadalafil 20 mg, pharmacokinetic simulations indicated that 5 mg of daily tadalafil resulted in plasma concentrations less than half of the 20-mg on-demand dose during the first day. This was approximately equivalent to the second day, but higher for the remainder of the week following 5 mg of daily tadalafil [16]. The simulated cumulative weekly exposure following 5 mg of daily tadalafil was approximately 69% higher than after a single 20-mg dose of tadalafil each week. This simulated result suggested that for a man anticipating a single dose of 20 mg of tadalafil per week as part of his regular sexual activity, a single daily dose of 5 mg of tadalafil would represent a greater weekly exposure to tadalafil [16].
Forgue et al. [22] demonstrated the pharmacokinetics of tadalafil in healthy subjects for peak plasma concentration at steady state (Cmax, ss) and area under the concentration-time curve at steady state (AUCss). The results indicated that the Cmax, ss for 5 mg of daily tadalafil was 26% lower than that for tadalafil 10 mg and 63% lower than that for 20 mg every 2.65 days. In contrast, the AUCss was approximately 33% higher than the exposure following 10 mg of tadalafil every 2.65 days and 33% lower than the exposure following 20 mg of tadalafil every 2.65 days. Therefore, for men who anticipate at least twice-weekly use of tadalafil 20-mg therapy, a decrease in plasma concentrations with a single daily 5-mg tadalafil dose might be advantageous from a tolerability perspective, resulting in less overall tadalafil exposure [16,22].
EFFICACY AND RELIABILITY OF CHRONIC PDE5 INHIBITOR USE
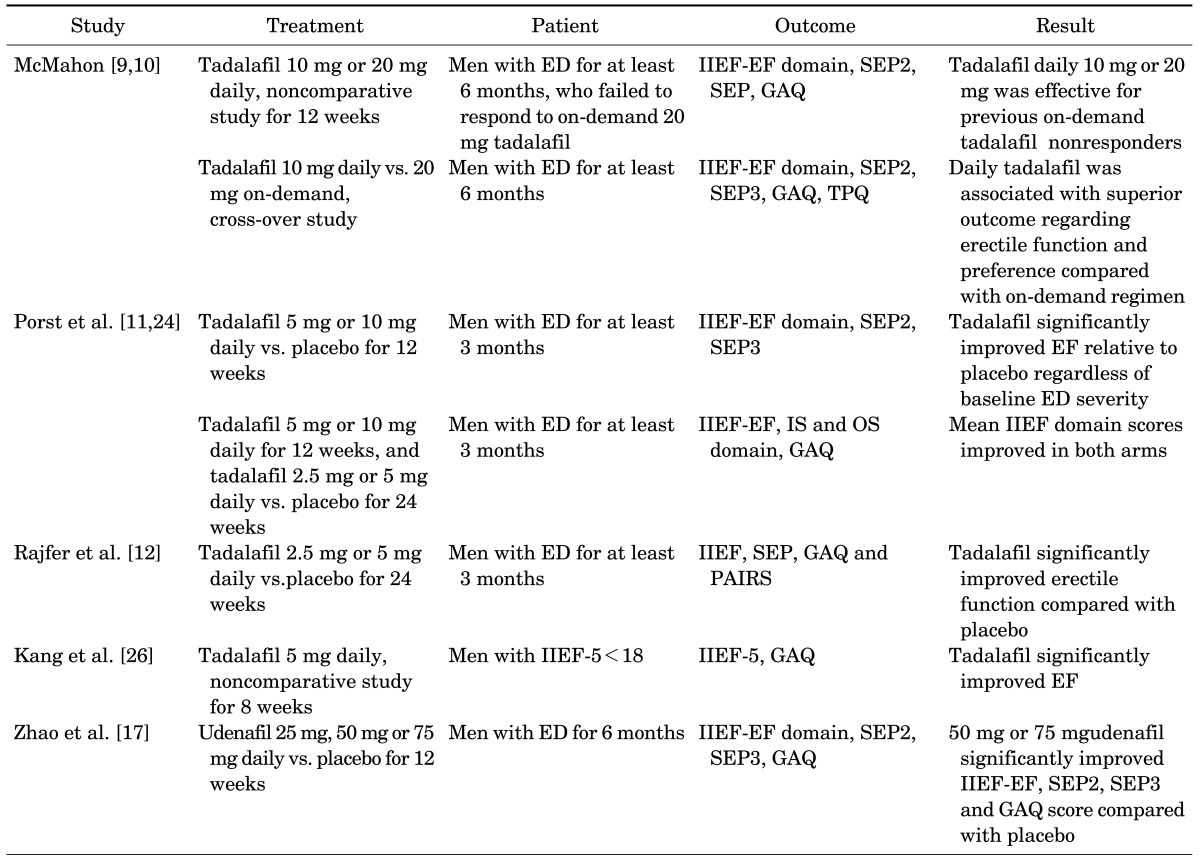
Several randomized, double-blinded, prospective, controlled studies have investigated the improvement in outcomes of chronic PDE5 inhibitor use in ED patients. A summary is in Table 1.
TABLE 1.
Efficacy of daily phosphodiesterase type 5 inhibitors
ED, erectile dysfunction; IIEF-EF, International Index of Erectile Function-Erectile Function; SEP, sexual encounter profile; GAQ, global assessment question; TPQ, treatment preference question; IS, intercourse satisfaction; OS, overall satisfaction; PAIRS, Psychological and Interpersonal Relationship Scales.
In 2004, McMahon [9] published one of the first reports of daily tadalafil in patients with ED. In this open-label, flexible-dose (10 or 20 mg) evaluation, 112 men with ED who failed to respond to 20 mg of tadalafil on at least six occasions and had a sexual encounter profile diary question 3 (SEP3, successful completion of intercourse) affirmative response rate of less than 30% were enrolled. A substantial improvement was seen in mean International Index of Erectile Function-Erectile Function (IIEF-EF) domain scores from 10.3 at baseline to 14.9 after the 4-week on-demand treatment and to 23.1 after 12 weeks of daily dose therapy. Favorable responses to SEP3 were 14% at baseline and increased to 21% after on-demand therapy and were 58% for daily therapy with 10 mg and 52% for daily therapy with 20 mg. IIEF-EF domain scores normalized to 41% and 32% of men treated with daily dose therapy compared with none in the on-demand arm. A follow-up, randomized, open-label crossover trial compared 12 weeks of tadalafil (10 mg daily vs. 20 mg on-demand) in 145 men with ED [10]. The baseline IIEF-EF score was 14.6 and increased to 23.3 in the on-demand arm and 26.4 in the daily dose arm. Positive responses to SEP3 were 30% at baseline and increased to 67% for on-demand therapy and 80% for the daily therapy arm. The daily dose was preferred by 72% of the patients compared with 28% preferring on-demand treatment.
Porst et al. [11] evaluated the efficacy and safety of a single daily dose of 5 mg or 10 mg of tadalafil in a multicenter, randomized, double-blinded, placebo-controlled study of 293 men for 12 to 15 weeks. Both the 5-mg and 10-mg doses significantly improved EF with a mean change in IIEF-EF domain score of 9.7, 9.4, and 0.9 for the 5 mg, 10 mg, and placebo groups, respectively. The authors also reported in 1- to 2-year open-label extension studies that 5 mg of tadalafil once daily for up to 2 years is well tolerated and effective in men with ED [24]. Mean IIEF domain scores improved from baseline to the conclusions of the 1- and 2-year open-label extensions. EF improved by 10.4 and 10.8 at 1 and 2 years, intercourse satisfaction (IS) improved by 4.0 and 3.7, and overall satisfaction (OS) improved to 3.0 and 3.2. At the conclusion of the 2-year open-label extension, positive responses were reported for 95.7% of the global assessment question 1 (GAQ, improved erection) and 92.1% of GAQ2 (improved ability to engage in sexual activity). Interestingly, the mean IIEF-EF score declined to near baseline levels after discontinuation of tadalafil at the end of the 1-year open-label period.
Rajfer et al. [12] reported on 2.5 mg or 5 mg of tadalafil daily vs. placebo in men with ED over 24 weeks. Tadalafil was superior to placebo at both dosages for all primary efficacy endpoints. The mean IIEF-EF score increased by 6.1 in the 2.5-mg tadalafil arm and by 7.0 in the 5-mg arm compared with an increase of 1.2 points for the placebo. Tadalafil also produced significant changes in other IIEF domains including IS, sexual confidence, and OS with sexual life.
Shabsigh et al. [25] examined reliability, defined as successful attempts/total attempts following initial successful intercourse, of tadalafil once daily by using data pooled from two previous studies [11,12]. The first-attempt success rate (SEP3) was significantly higher among men taking 2.5 mg (45.7%) and 5 mg (55.2%) tadalafil compared with placebo (28.5%). Furthermore, following initial success, men taking 5 mg tadalafil had a significantly greater proportion of SEP3 on subsequent attempts (85.9%) compared with men taking placebo (70.2%). Overall, men with ED taking tadalafil once daily experienced a high rate of reliability of efficacy.
In Korea, Kang et al. [26] evaluated the efficacy of 5 mg tadalafil daily in Korean men with ED. A total of 162 men were administered a daily dose of 5 mg of tadalafil and 127 men completed the 8-week clinical trial. IIEF-5 values significantly increased from 11.3 at baseline to 16.9 at 8 weeks. They concluded that 5 mg of tadalafil daily might be effective in improving EF without significant adverse events.
Tadalafil 5 mg was the first ED treatment to be approved for daily administration [2]. Few studies have investigated daily administration of other PDE5 inhibitors, possibly because of pharmacokinetic properties such as a relatively short half-life, which is 3.8 and 3.9 hours for sildenafil and vardenafil, respectively. Of these, sildenafil leads to a significant improvement in the recovery of EF after RP [27] and improved endothelial function and reduces markers of vascular inflammation in men with type 2 diabetes [28]. Zumbe et al. [29] demonstrated that once-daily vardenafil did not produce greater sustained effects on EF than did on-demand vardenafil in men with mild-to-moderate ED, suggesting that daily dosing of PDE5 inhibitors does not produce sustained clinical benefits beyond cessation of treatment above those observed with on-demand administration. In contrast, udenafil has a relatively long half-life (7.3 to 12.1 hours) compared with sildenafil and vardenafil, although this is shorter than tadalafil [30]. A recent study of daily udenafil showed the possibility of daily administration of this PDE5 inhibitor [17]. In this study, udenafil significantly improved EF among ED patients when administered at 50 mg or 75 mg once daily for 12 weeks. The authors concluded that daily udenafil (50 mg) could be another treatment option for ED. In Korea, udenafil 50 mg is also approved for daily administration.
SAFETY OF CHRONIC PDE5 INHIBITOR TREATMENT
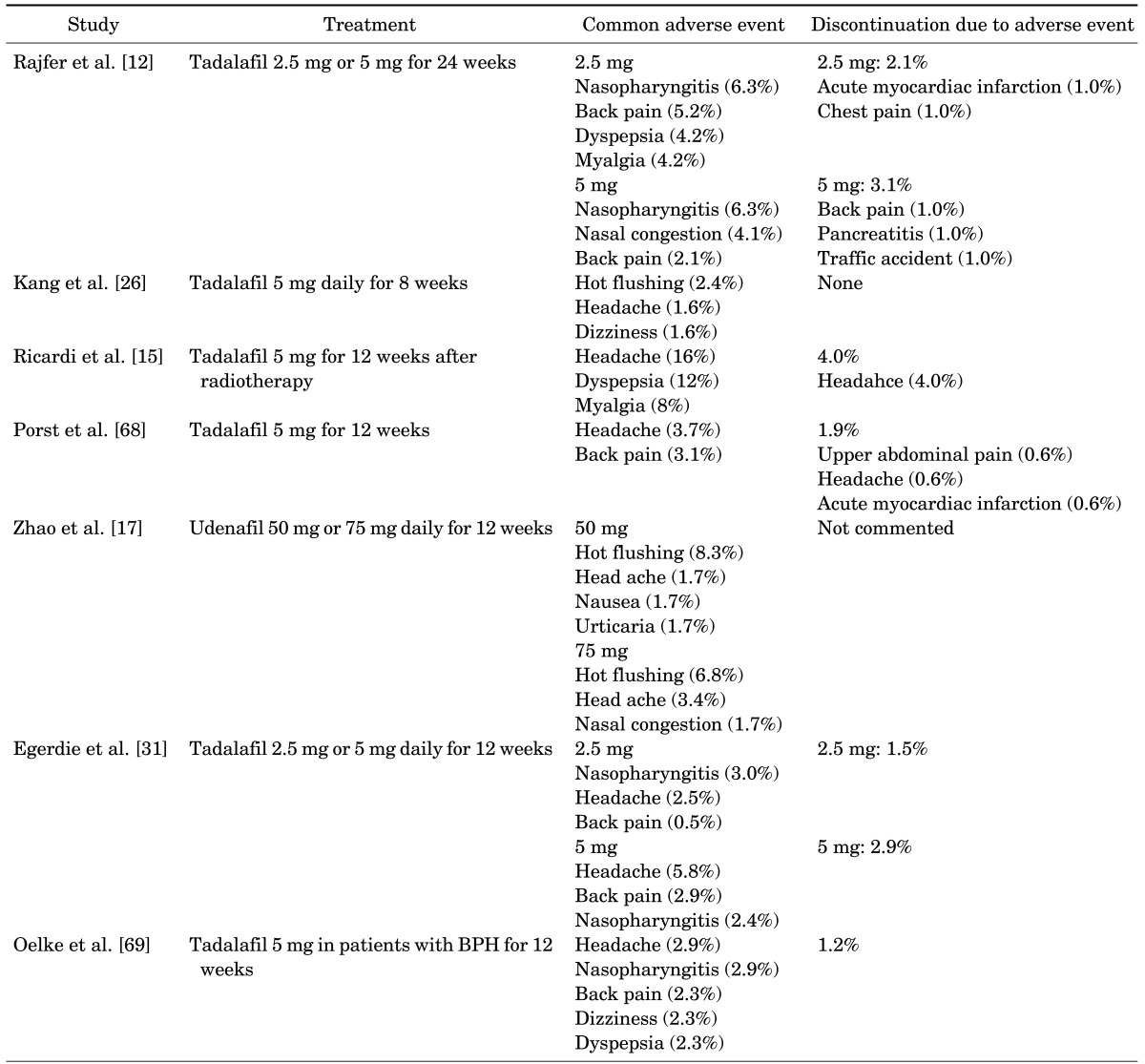
The safety of daily PDE5 inhibitor treatment is established by many studies that indicate minimal, well-tolerated adverse events that are not significantly different from those reported for on-demand regimens (Table 2) [9-12,15,17,24,26,31]. Adverse events are reported as mild or moderate in severity. The most common adverse events reported are headache, dyspepsia, facial flushing, back pain, nasal congestion, and nasopharyngitis [9-12,15,24,26,31-33]. In addition, the rate of discontinuation caused by adverse events is similar to the rate observed with on-demand dosing [24]. The range of reported discontinuation rates from adverse events of daily administration are 1 to 6%. The most common adverse events causing discontinuation are dyspepsia, headache, back pain, and flu-like symptoms.
TABLE 2.
Safety of daily phosphodiesterase type 5 inhibitors
BPH, benign prostatic hyperplasia.
Although PDE5 inhibitors have favorable cardiovascular effects, data on adverse events from daily regimens are lacking [12,24]. Kloner et al. [34] conducted a retrospective analysis of serious cardiovascular adverse events in 36 clinical trials of tadalafil. A serious cardiovascular adverse event was defined as myocardial infarction, cardiovascular death, or cerebrovascular death. Tadalafil 2 to 50 mg was taken as needed, three times/wk, or once daily. The incidence rates of serious cardiovascular adverse events were comparable among men with ED taking tadalafil as needed, three times/wk, or once daily, and these rates were also comparable with placebo-treated patients. In this clinical trial population of men with ED, tadalafil was not associated with an increased risk for serious adverse cardiovascular events.
PDE5 inhibitors cross the blood-retina barrier and can inhibit retina-specific PDE6, which is structurally similar to PDE5 and is involved in regulating the phototransduction cascade. Off-target inhibition of PDE6 is believed to be the mechanism for blurred vision, blue-tinged vision, and altered perception of light with PDE5 inhibitors, symptoms that are typically mild and transient [35,36]. Acute administration of sildenafil at high doses produces only very mild electroretinographic (ERG) changes that are reversible. Some visual complications of long-term use have been suggested, including nonarteritic anterior ischemic optic neuropathy, central serous chorioretinopathy, and vascular events [37-39]. Only a few studies have investigated the retinal effects of chronic PDE5 inhibitors. Cordell et al. [40] assessed ERG changes and changes in retinal function during 6 months of daily use of 5 mg of tadalafil, 20 mg of sildenafil, or placebo. No significant differences were found between the treatment and placebo groups for the outcome of ERG or other parameters such as visual acuity, color discrimination, visual-field testing, and intraocular pressure. The visual safety of daily tadalafil and sildenafil should be assessed over a prolonged period.
Although only two randomized, double-blind, placebo-controlled studies have evaluated semen characteristics after daily PDE5 inhibitors, the results of these studies were consistent. In a noninferiority trial, Hellstrom et al. [41] concluded that there were no deleterious effects of long-term (40 weeks), daily treatment with 20 mg of tadalafil on spermatogenesis or hormones related to testicular function in men older than 45 years. Daily tadalafil was not inferior to placebo for sperm concentration and count, motile sperm, or normal sperm morphology, with a noninferiority margin of 20%. Among the reproductive hormones tested, only total testosterone was significantly different (higher than placebo) between groups at the end-point. Daily vardenafil also has no detrimental effects on semen characteristics or reproductive hormones in men with normal baseline levels [42]. The percentage of patients with a 50% or greater decrease in sperm concentration (baseline to 6 months last observation carried forward) was 0.07%. Vardenafil also had no clinically significant effects on any other semen parameters, or on levels of reproductive hormones, compared with placebo.
ADVANTAGES OF CHRONIC PDE5 INHIBITOR USE IN MEN WITH ED
Accumulating data demonstrate several benefits of daily PDE5 inhibitors as well as efficacy and safety. These advantages include sexual quality of life, efficacy in difficult-to-treat populations, onset of efficacy, and durability and improvement of benign prostatic hyperplasia (BPH) symptoms.
1. Sexual quality of life
Women with partners currently treated with daily PDE5 inhibitors had an improved sexual quality of life [43-45]. Daily 5 mg tadalafil significantly improved EF and sexual quality of life for men with ED but also for their female partners [43]. Tadalafil 5 mg taken once daily as an ED treatment improved OS for men and their female partners [44]. The analysis demonstrated a high concordance among couples in their responses to the man's ED treatment. After 12 weeks of daily 5-mg tadalafil treatment, the participants and their partners were more satisfied [45]. In addition, significant improvements in sexual relationship, confidence, self-esteem, and overall relationship were correlated with EF improvement.
2. Difficult-to-treat populations
Daily PDE5 inhibitor administration has been evaluated in difficult-to-treat populations. Nonresponders to on-demand PDE5 inhibitors range from 30 to 35% [4,46]. Patient education, lifestyle modification, correction of modifiable risk factors, dose adjustment or change in PDE5 inhibitors, counseling, and progression to second-line or third-line treatment are additional salvage methods. Hatzimouratidis et al. [2] reported that nonresponders to on-demand PDE5 inhibitor use were salvaged following daily administration of tadalafil (11.1%) and vardenafil (18.2%). McMahon [9] also showed the ability of daily tadalafil to act as salvage therapy in patients previously unresponsive to on-demand 20 mg tadalafil. The diabetic population has a high prevalence of ED, and ED develops earlier in this population than in men without DM [47]. In addition, ED in men with DM can be more severe and is more likely to be refractory to treatment [48-50]. In several studies of men with diabetes and ED, daily treatment with PDE5 inhibitors was efficacious [13] and improved endothelial function, which suggests that diabetes-induced impairment of endothelial function might be improved by prolonged PDE5 inhibition [28].
Loss of sufficient EF is one of the most common complications following RP. This is mainly due to cavernous nerve damage resulting in penile hypoxia, smooth muscle apoptosis, fibrosis, and veno-occlusive dysfunction [14]. The goal of early penile rehabilitation is to prevent ED after RP by countering pathologic changes using PDE5 inhibitors. Daily low-dose sildenafil leads to a significant improvement in the recovery of EF after RP [27]. Recent animal studies provide further support for daily PDE5 inhibitors in this group of patients [51,52]. In a rat model of bilateral cavernous nerve crush injury, chronic administration of udenafil preserved EF and was beneficial against the pathophysiological consequences of cavernous nerve injury [52]. ED has been suggested as a late effect of radiation therapy (RT) for prostate cancer [53,54]. The underlying mechanism is largely unknown, although some clinical and animal studies suggest that ED after radiotherapy might be caused by radiation damage to the arterial supply of the corpora cavernosa [53,55]. Ricardi et al. [15] compared the efficacy and safety of on-demand 20 mg tadalafil with daily 5 mg tadalafil in ED patients following RT for prostate cancer. In this study, once-a-day 5-mg dosing showed higher compliance and marginally reduced side effects, making it an attractive alternative to on-demand therapy for ED in post-RT prostate cancer patients.
3. Onset of efficacy
Onset of efficacy is an important factor when evaluating PDE5 inhibitors [56]. Several studies have demonstrated the rapid onset of efficacy of on-demand PDE5 inhibitors in men with ED [20,56,57]. Although onset of efficacy is a more important consideration in daily treatment because of the relatively low dose that is used, few studies have investigated this concern. In efficacy and safety trials for daily tadalafil, an average of 80% of men achieved a successful attempt by day 4 with 5 mg of tadalafil, by day 5 with 2.5 mg of tadalafil, and by day 7 with placebo [11,12]. Seftel et al. [58] reported that more men in the tadalafil 5 mg group than in the placebo group achieved successful intercourse by day 2 (48.6% vs 36.6%), although steady-state tadalafil was essentially attained by day 5 [22]. The 2.5-mg tadalafil group was not different from the placebo group by day 2, but did differ by day 3 (35.5% vs. 27.2%). This prospective trial concluded that the onset of efficacy of once-daily 2.5-mg and 5-mg doses of tadalafil was within a few days of initiating therapy.
4. Durability
A sustained erectile effect after cessation of ED treatment is a one of the most interesting and important discussion points when considering daily PDE5 inhibitors for ED treatment. Several previous studies primarily focused on improvement of EF with daily PDE5 inhibitors during treatment, but less is known about what occurs after treatment cessation. Although PDE5 inhibitors are not considered a cure for ED [59], several studies of chronic administration of a PDE5 inhibitor have demonstrated improvement in EF beyond discontinuation of treatment [28,60-62]. Porst et al. [63] reported that in men who demonstrated improved EF while taking 5 mg of daily tadalafil for 1 year, 46.3% continued to show improvement compared with baseline following a 4-week treatment-free period. In this study, more men with diabetes and hypertension had a durable response. This suggests that patients with endothelial damage are more likely to experience sustainable benefit from long-term PDE5 inhibitor treatment for ED. In contrast, some studies had negative findings for durability. Although administration of tadalafil decreased the serum endothelial microparticle concentration, which was selected as a marker of endothelial damage, in patients with arterial ED, this positive effect on endothelial dysfunction disappeared 6 months after tadalafil discontinuation [64]. Following discontinuation of tadalafil after a 1-year open-label extension, the mean IIEF-EF score declined markedly to near baseline levels [24]. Durability of response should be a focus of future research when evaluating daily PDE5 inhibitors.
5. Effect on BPH symptoms
Tadalafil was recently approved in the United States for treatment of the signs and symptoms of BPH and for the treatment of coexisting BPH and ED. Several clinical studies have shown that PDE5 inhibitors can improve BPH-related lower urinary tract symptoms [31,65-69]. Porst et al. [68] assessed the efficacy, onset, and safety of daily 5 mg tadalafil on BPH symptoms in a randomized, double-blind, placebo-controlled study. This study concluded that 5 mg of tadalafil daily for 12 weeks resulted in a clinically meaningful reduction in total International Prostate Symptom Score as early as 1 week and achieved significance at 4 weeks in men with BPH. Egerdie et al. [31] also demonstrated the efficacy of daily 5 mg tadalafil on both ED and BPH in a multinational phase 3 study. Interestingly, 5 mg of tadalafil or 0.5 mg of tamsulosin once daily resulted in significant and similar improvements in BPH symptoms compared with placebo in as little as 1 week and throughout the 12-week treatment period. In addition, tadalafil and tamsulosin similarly improved the maximum urinary flow rate through 12 weeks [69].
LIMITATIONS OF CHRONIC PDE5 INHIBITOR USE IN MEN WITH ED
Several published studies have supported low-dose, chronic PDE5 inhibitors as an efficacious and safe option for treating ED. Nevertheless, some limitations exist to extending their use. The aspect of disease modification and durability after cessation of chronic use should be verified when evaluating daily PDE5 inhibitors. Furthermore, head-to-head trials are needed between on-demand and daily PDE5 inhibitors to remedy the lack of comparative data.
Most clinical trials and research on the chronic use of PDE5 inhibitors has focused on tadalafil because of its pharmacokinetic characteristics. Tadalafil 5 mg is the only drug currently approved for daily administration for ED treatment, but no other choice has been considered for chronic use of a PDE5 inhibitor. One of the most recently developed PDE5 inhibitors, udenafil, has a relatively long half-life that is more similar to that of tadalafil than to sildenafil or vardenafil. Recent data show promising results that udenafil could be another option for chronic use in men with ED.
Treatment cost is an important factor for patient compliance in low-income households, despite the fact that the treatment is highly effective and improves the partner relationship [70]. In addition, cost is the most common reason for rehabilitation neglect in patients who have undergone RP [71]. The influence of costs of daily PDE5 inhibitors is not yet established in men with ED but could negatively influence patient compliance.
CONCLUSIONS
Clinical trials with low-dose, daily PDE5 inhibitors, especially tadalafil, confirm the efficacy and safety of such treatment. Daily administration of a PDE5 inhibitor provides a treatment alternative that could be suitable for difficult-to-treat populations with ED as well as providing spontaneity and naturalness that does not require consideration of the timing of sexual activity. This suggests that the option of daily administration of PDE5 inhibitors could be offered to any ED patients.
Footnotes
The authors have nothing to disclose.
References
- 1.Feldman HA, Goldstein I, Hatzichristou DG, Krane RJ, McKinlay JB. Construction of a surrogate variable for impotence in the Massachusetts Male Aging Study. J Clin Epidemiol. 1994;47:457–467. doi: 10.1016/0895-4356(94)90292-5. [DOI] [PubMed] [Google Scholar]
- 2.Hatzimouratidis K, Amar E, Eardley I, Giuliano F, Hatzichristou D, Montorsi F, et al. Guidelines on male sexual dysfunction: erectile dysfunction and premature ejaculation. Eur Urol. 2010;57:804–814. doi: 10.1016/j.eururo.2010.02.020. [DOI] [PubMed] [Google Scholar]
- 3.Eardley I, Donatucci C, Corbin J, El-Meliegy A, Hatzimouratidis K, McVary K, et al. Pharmacotherapy for erectile dysfunction. J Sex Med. 2010;7(1 Pt 2):524–540. doi: 10.1111/j.1743-6109.2009.01627.x. [DOI] [PubMed] [Google Scholar]
- 4.McMahon CN, Smith CJ, Shabsigh R. Treating erectile dysfunction when PDE5 inhibitors fail. BMJ. 2006;332:589–592. doi: 10.1136/bmj.332.7541.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldstein I, Kim E, Steers WD, Pryor JL, Wilde DW, Natanegara F, et al. Efficacy and safety of tadalafil in men with erectile dysfunction with a high prevalence of comorbid conditions: results from MOMENTUS: multiple observations in men with erectile dysfunction in National Tadalafil Study in the US. J Sex Med. 2007;4:166–175. doi: 10.1111/j.1743-6109.2006.00402.x. [DOI] [PubMed] [Google Scholar]
- 6.McMahon CG. Treatment of erectile dysfunction with chronic dosing of tadalafil. Eur Urol. 2006;50:215–217. doi: 10.1016/j.eururo.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 7.Hackett GI. Patient preferences in treatment of erectile dysfunction: the continuing importance of patient education. Clin Cornerstone. 2005;7:57–65. doi: 10.1016/s1098-3597(05)80049-3. [DOI] [PubMed] [Google Scholar]
- 8.Carson CC, Lue TF. Phosphodiesterase type 5 inhibitors for erectile dysfunction. BJU Int. 2005;96:257–280. doi: 10.1111/j.1464-410X.2005.05614.x. [DOI] [PubMed] [Google Scholar]
- 9.McMahon C. Efficacy and safety of daily tadalafil in men with erectile dysfunction previously unresponsive to on-demand tadalafil. J Sex Med. 2004;1:292–300. doi: 10.1111/j.1743-6109.04042.x. [DOI] [PubMed] [Google Scholar]
- 10.McMahon C. Comparison of efficacy, safety, and tolerability of on-demand tadalafil and daily dosed tadalafil for the treatment of erectile dysfunction. J Sex Med. 2005;2:415–425. doi: 10.1111/j.1743-6109.2005.20360.x. [DOI] [PubMed] [Google Scholar]
- 11.Porst H, Giuliano F, Glina S, Ralph D, Casabe AR, Elion-Mboussa A, et al. Evaluation of the efficacy and safety of once-a-day dosing of tadalafil 5 mg and 10 mg in the treatment of erectile dysfunction: results of a multicenter, randomized, double-blind, placebo-controlled trial. Eur Urol. 2006;50:351–359. doi: 10.1016/j.eururo.2006.02.052. [DOI] [PubMed] [Google Scholar]
- 12.Rajfer J, Aliotta PJ, Steidle CP, Fitch WP, 3rd, Zhao Y, Yu A. Tadalafil dosed once a day in men with erectile dysfunction: a randomized, double-blind, placebo-controlled study in the US. Int J Impot Res. 2007;19:95–103. doi: 10.1038/sj.ijir.3901496. [DOI] [PubMed] [Google Scholar]
- 13.Hatzichristou D, Gambla M, Rubio-Aurioles E, Buvat J, Brock GB, Spera G, et al. Efficacy of tadalafil once daily in men with diabetes mellitus and erectile dysfunction. Diabet Med. 2008;25:138–146. doi: 10.1111/j.1464-5491.2007.02338.x. [DOI] [PubMed] [Google Scholar]
- 14.Magheli A, Burnett AL. Erectile dysfunction following prostatectomy: prevention and treatment. Nat Rev Urol. 2009;6:415–427. doi: 10.1038/nrurol.2009.126. [DOI] [PubMed] [Google Scholar]
- 15.Ricardi U, Gontero P, Ciammella P, Badellino S, Valentino F, Munoz F, et al. Efficacy and safety of tadalafil 20 mg on demand vs. tadalafil 5 mg once-a-day in the treatment of post-radiotherapy erectile dysfunction in prostate cancer men: a randomized phase II trial. J Sex Med. 2010;7:2851–2859. doi: 10.1111/j.1743-6109.2010.01890.x. [DOI] [PubMed] [Google Scholar]
- 16.Wrishko R, Sorsaburu S, Wong D, Strawbridge A, McGill J. Safety, efficacy, and pharmacokinetic overview of low-dose daily administration of tadalafil. J Sex Med. 2009;6:2039–2048. doi: 10.1111/j.1743-6109.2009.01301.x. [DOI] [PubMed] [Google Scholar]
- 17.Zhao C, Kim SW, Yang DY, Kim JJ, Park NC, Lee SW, et al. Efficacy and safety of once-daily dosing of udenafil in the treatment of erectile dysfunction: results of a multicenter, randomized, double-blind, placebo-controlled trial. Eur Urol. 2011;60:380–387. doi: 10.1016/j.eururo.2011.03.025. [DOI] [PubMed] [Google Scholar]
- 18.Corbin JD, Francis SH. Pharmacology of phosphodiesterase-5 inhibitors. Int J Clin Pract. 2002;56:453–459. [PubMed] [Google Scholar]
- 19.Coward RM, Carson CC. Tadalafil in the treatment of erectile dysfunction. Ther Clin Risk Manag. 2008;4:1315–1330. doi: 10.2147/tcrm.s3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosen RC, Padma-Nathan H, Shabsigh R, Saikali K, Watkins V, Pullman W. Determining the earliest time within 30 minutes to erectogenic effect after tadalafil 10 and 20 mg: a multicenter, randomized, double-blind, placebo-controlled, at-home study. J Sex Med. 2004;1:193–200. doi: 10.1111/j.1743-6109.2004.04028.x. [DOI] [PubMed] [Google Scholar]
- 21.Porst H, Padma-Nathan H, Giuliano F, Anglin G, Varanese L, Rosen R. Efficacy of tadalafil for the treatment of erectile dysfunction at 24 and 36 hours after dosing: a randomized controlled trial. Urology. 2003;62:121–125. doi: 10.1016/s0090-4295(03)00359-5. [DOI] [PubMed] [Google Scholar]
- 22.Forgue ST, Patterson BE, Bedding AW, Payne CD, Phillips DL, Wrishko RE, et al. Tadalafil pharmacokinetics in healthy subjects. Br J Clin Pharmacol. 2006;61:280–288. doi: 10.1111/j.1365-2125.2005.02553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ballard SA, Gingell CJ, Tang K, Turner LA, Price ME, Naylor AM. Effects of sildenafil on the relaxation of human corpus cavernosum tissue in vitro and on the activities of cyclic nucleotide phosphodiesterase isozymes. J Urol. 1998;159:2164–2171. doi: 10.1016/S0022-5347(01)63299-3. [DOI] [PubMed] [Google Scholar]
- 24.Porst H, Rajfer J, Casabe A, Feldman R, Ralph D, Vieiralves LF, et al. Long-term safety and efficacy of tadalafil 5 mg dosed once daily in men with erectile dysfunction. J Sex Med. 2008;5:2160–2169. doi: 10.1111/j.1743-6109.2008.00935.x. [DOI] [PubMed] [Google Scholar]
- 25.Shabsigh R, Donatucci C, Costabile R, Perelman MA, Burns P, Zeigler H, et al. Reliability of efficacy in men with erectile dysfunction treated with tadalafil once daily after initial success. Int J Impot Res. 2010;22:1–8. doi: 10.1038/ijir.2009.29. [DOI] [PubMed] [Google Scholar]
- 26.Kang DH, Lee JY, Park SY, Moon HS, Jeong TY, Yoo TK, et al. Efficacy and safety of tadalafil 5 mg administered once daily in Korean men with erectile dysfunction: a prospective, multicenter study. Korean J Urol. 2010;51:647–652. doi: 10.4111/kju.2010.51.9.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bannowsky A, Schulze H, van der Horst C, Hautmann S, Jünemann KP. Recovery of erectile function after nerve-sparing radical prostatectomy: improvement with nightly low-dose sildenafil. BJU Int. 2008;101:1279–1283. doi: 10.1111/j.1464-410X.2008.07515.x. [DOI] [PubMed] [Google Scholar]
- 28.Aversa A, Vitale C, Volterrani M, Fabbri A, Spera G, Fini M, et al. Chronic administration of Sildenafil improves markers of endothelial function in men with Type 2 diabetes. Diabet Med. 2008;25:37–44. doi: 10.1111/j.1464-5491.2007.02298.x. [DOI] [PubMed] [Google Scholar]
- 29.Zumbe J, Porst H, Sommer F, Grohmann W, Beneke M, Ulbrich E. Comparable efficacy of once-daily versus on-demand vardenafil in men with mild-to-moderate erectile dysfunction: findings of the RESTORE study. Eur Urol. 2008;54:204–210. doi: 10.1016/j.eururo.2008.03.056. [DOI] [PubMed] [Google Scholar]
- 30.Kim BH, Lim HS, Chung JY, Kim JR, Lim KS, Sohn DR, et al. Safety, tolerability and pharmacokinetics of udenafil, a novel PDE-5 inhibitor, in healthy young Korean subjects. Br J Clin Pharmacol. 2008;65:848–854. doi: 10.1111/j.1365-2125.2008.03107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Egerdie RB, Auerbach S, Roehrborn CG, Costa P, Garza MS, Esler AL, et al. Tadalafil 2.5 or 5 mg administered once daily for 12 weeks in men with both erectile dysfunction and signs and symptoms of benign prostatic hyperplasia: results of a randomized, placebo-controlled, double-blind study. J Sex Med. 2012;9:271–281. doi: 10.1111/j.1743-6109.2011.02504.x. [DOI] [PubMed] [Google Scholar]
- 32.Montorsi F, Brock G, Lee J, Shapiro J, Van Poppel H, Graefen M, et al. Effect of nightly versus on-demand vardenafil on recovery of erectile function in men following bilateral nerve-sparing radical prostatectomy. Eur Urol. 2008;54:924–931. doi: 10.1016/j.eururo.2008.06.083. [DOI] [PubMed] [Google Scholar]
- 33.Donatucci CF, Wong DG, Giuliano F, Glina S, Dowsett SA, Watts S, et al. Efficacy and safety of tadalafil once daily: considerations for the practical application of a daily dosing option. Curr Med Res Opin. 2008;24:3383–3392. doi: 10.1185/03007990802498440. [DOI] [PubMed] [Google Scholar]
- 34.Kloner RA, Jackson G, Hutter AM, Mittleman MA, Chan M, Warner MR, et al. Cardiovascular safety update of Tadalafil: retrospective analysis of data from placebo-controlled and open-label clinical trials of Tadalafil with as needed, three times-per-week or once-a-day dosing. Am J Cardiol. 2006;97:1778–1784. doi: 10.1016/j.amjcard.2005.12.073. [DOI] [PubMed] [Google Scholar]
- 35.Cote RH. Characteristics of photoreceptor PDE (PDE6): similarities and differences to PDE5. Int J Impot Res. 2004;16(Suppl 1):S28–S33. doi: 10.1038/sj.ijir.3901212. [DOI] [PubMed] [Google Scholar]
- 36.Laties A, Sharlip I. Ocular safety in patients using sildenafil citrate therapy for erectile dysfunction. J Sex Med. 2006;3:12–27. doi: 10.1111/j.1743-6109.2005.00194.x. [DOI] [PubMed] [Google Scholar]
- 37.Gorkin L, Hvidsten K, Sobel RE, Siegel R. Sildenafil citrate use and the incidence of nonarteritic anterior ischemic optic neuropathy. Int J Clin Pract. 2006;60:500–503. doi: 10.1111/j.1368-5031.2006.00904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Escaravage GK, Jr, Wright JD, Jr, Givre SJ. Tadalafil associated with anterior ischemic optic neuropathy. Arch Ophthalmol. 2005;123:399–400. doi: 10.1001/archopht.123.3.399. [DOI] [PubMed] [Google Scholar]
- 39.Fraunfelder FW, Pomeranz HD, Egan RA. Nonarteritic anterior ischemic optic neuropathy and sildenafil. Arch Ophthalmol. 2006;124:733–734. doi: 10.1001/archopht.124.5.733. [DOI] [PubMed] [Google Scholar]
- 40.Cordell WH, Maturi RK, Costigan TM, Marmor MF, Weleber RG, Coupland SG, et al. Retinal effects of 6 months of daily use of tadalafil or sildenafil. Arch Ophthalmol. 2009;127:367–373. doi: 10.1001/archophthalmol.2009.36. [DOI] [PubMed] [Google Scholar]
- 41.Hellstrom WJ, Gittelman M, Jarow J, Steidle C, McMurray J, Talley D, et al. An evaluation of semen characteristics in men 45 years of age or older after daily dosing with tadalafil 20 mg: results of a multicenter, randomized, double-blind, placebo-controlled, 9-month study. Eur Urol. 2008;53:1058–1065. doi: 10.1016/j.eururo.2007.09.046. [DOI] [PubMed] [Google Scholar]
- 42.Jarvi K, Dula E, Drehobl M, Pryor J, Shapiro J, Seger M. Daily vardenafil for 6 months has no detrimental effects on semen characteristics or reproductive hormones in men with normal baseline levels. J Urol. 2008;179:1060–1065. doi: 10.1016/j.juro.2007.10.077. [DOI] [PubMed] [Google Scholar]
- 43.Rubio-Aurioles E, Kim ED, Rosen RC, Porst H, Burns P, Zeigler H, et al. Impact on erectile function and sexual quality of life of couples: a double-blind, randomized, placebo-controlled trial of tadalafil taken once daily. J Sex Med. 2009;6:1314–1323. doi: 10.1111/j.1743-6109.2009.01222.x. [DOI] [PubMed] [Google Scholar]
- 44.Althof SE, Rubio-Aurioles E, Kingsberg S, Zeigler H, Wong DG, Burns P. Impact of tadalafil once daily in men with erectile dysfunction--including a report of the partners' evaluation. Urology. 2010;75:1358–1363. doi: 10.1016/j.urology.2009.11.066. [DOI] [PubMed] [Google Scholar]
- 45.Seftel AD, Buvat J, Althof SE, McMurray JG, Zeigler HL, Burns PR, et al. Improvements in confidence, sexual relationship and satisfaction measures: results of a randomized trial of tadalafil 5 mg taken once daily. Int J Impot Res. 2009;21:240–248. doi: 10.1038/ijir.2009.22. [DOI] [PubMed] [Google Scholar]
- 46.Hatzichristou D, Rosen RC, Broderick G, Clayton A, Cuzin B, Derogatis L, et al. Clinical evaluation and management strategy for sexual dysfunction in men and women. J Sex Med. 2004;1:49–57. doi: 10.1111/j.1743-6109.2004.10108.x. [DOI] [PubMed] [Google Scholar]
- 47.Feldman HA, Goldstein I, Hatzichristou DG, Krane RJ, McKinlay JB. Impotence and its medical and psychosocial correlates: results of the Massachusetts Male Aging Study. J Urol. 1994;151:54–61. doi: 10.1016/s0022-5347(17)34871-1. [DOI] [PubMed] [Google Scholar]
- 48.Bacon CG, Hu FB, Giovannucci E, Glasser DB, Mittleman MA, Rimm EB. Association of type and duration of diabetes with erectile dysfunction in a large cohort of men. Diabetes Care. 2002;25:1458–1463. doi: 10.2337/diacare.25.8.1458. [DOI] [PubMed] [Google Scholar]
- 49.Ziegler D, Merfort F, van Ahlen H, Yassin A, Reblin T, Neureither M. Efficacy and safety of flexible-dose vardenafil in men with type 1 diabetes and erectile dysfunction. J Sex Med. 2006;3:883–891. doi: 10.1111/j.1743-6109.2006.00295.x. [DOI] [PubMed] [Google Scholar]
- 50.Price DE, Gingell JC, Gepi-Attee S, Wareham K, Yates P, Boolell M. Sildenafil: study of a novel oral treatment for erectile dysfunction in diabetic men. Diabet Med. 1998;15:821–825. doi: 10.1002/(SICI)1096-9136(199810)15:10<821::AID-DIA697>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 51.Hotta Y, Hattori M, Kataoka T, Ohno R, Mikumo M, Maeda Y, et al. Chronic vardenafil treatment improves erectile function via structural maintenance of penile corpora cavernosa in rats with acute arteriogenic erectile dysfunction. J Sex Med. 2011;8:705–711. doi: 10.1111/j.1743-6109.2010.02153.x. [DOI] [PubMed] [Google Scholar]
- 52.Lee CH, Kim HS, Goo MJ, Kang KK, Ahn BO, Kim SH, et al. Chronic administration of udenafil, a selective phosphodiesterase type 5 inhibitor, promotes erectile function recovery in an animal model of bilateral cavernous nerve crush injury. J Sex Med. 2011;8:1330–1340. doi: 10.1111/j.1743-6109.2011.02228.x. [DOI] [PubMed] [Google Scholar]
- 53.van der Wielen GJ, van Putten WL, Incrocci L. Sexual function after three-dimensional conformal radiotherapy for prostate cancer: results from a dose-escalation trial. Int J Radiat Oncol Biol Phys. 2007;68:479–484. doi: 10.1016/j.ijrobp.2006.12.015. [DOI] [PubMed] [Google Scholar]
- 54.Turner SL, Adams K, Bull CA, Berry MP. Sexual dysfunction after radical radiation therapy for prostate cancer: a prospective evaluation. Urology. 1999;54:124–129. doi: 10.1016/s0090-4295(99)00032-1. [DOI] [PubMed] [Google Scholar]
- 55.van der Wielen GJ, Mulhall JP, Incrocci L. Erectile dysfunction after radiotherapy for prostate cancer and radiation dose to the penile structures: a critical review. Radiother Oncol. 2007;84:107–113. doi: 10.1016/j.radonc.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 56.Shabsigh R, Seftel AD, Rosen RC, Porst H, Ahuja S, Deeley MC, et al. Review of time of onset and duration of clinical efficacy of phosphodiesterase type 5 inhibitors in treatment of erectile dysfunction. Urology. 2006;68:689–696. doi: 10.1016/j.urology.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 57.Nagao K, Kobayashi H, Fujikawa K, Tachibana T, Iwamoto Y, Ishii N, et al. Vardenafil allows successful intercourse initiated rapidly after dosing in Japanese patients with diabetes mellitus and erectile dysfunction. J Sex Med. 2009;6:2851–2857. doi: 10.1111/j.1743-6109.2009.01439.x. [DOI] [PubMed] [Google Scholar]
- 58.Seftel A, Goldfischer E, Kim ED, Dula E, Zeigler H, Burns P. Onset of efficacy of tadalafil once daily in men with erectile dysfunction: a randomized, double-blind, placebo controlled trial. J Urol. 2011;185:243–248. doi: 10.1016/j.juro.2010.09.035. [DOI] [PubMed] [Google Scholar]
- 59.Jannini EA, Isidori AM, Gravina GL, Aversa A, Balercia G, Bocchio M, et al. The ENDOTRIAL study: a spontaneous, open-label, randomized, multicenter, crossover study on the efficacy of sildenafil, tadalafil, and vardenafil in the treatment of erectile dysfunction. J Sex Med. 2009;6:2547–2560. doi: 10.1111/j.1743-6109.2009.01375.x. [DOI] [PubMed] [Google Scholar]
- 60.Aversa A, Caprio M, Rosano GM, Spera G. Endothelial effects of drugs designed to treat erectile dysfunction. Curr Pharm Des. 2008;14:3768–3778. doi: 10.2174/138161208786898725. [DOI] [PubMed] [Google Scholar]
- 61.Rosano GM, Aversa A, Vitale C, Fabbri A, Fini M, Spera G. Chronic treatment with tadalafil improves endothelial function in men with increased cardiovascular risk. Eur Urol. 2005;47:214–220. doi: 10.1016/j.eururo.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 62.Konstantinopoulos A, Giannitsas K, Athanasopoulos A, Spathas D, Perimenis P. The impact of daily sildenafil on levels of soluble molecular markers of endothelial function in plasma in patients with erectile dysfunction. Expert Opin Pharmacother. 2009;10:155–160. doi: 10.1517/14656560802678211. [DOI] [PubMed] [Google Scholar]
- 63.Porst H, Glina S, Ralph D, Zeigler H, Wong DG, Woodward B. Durability of response following cessation of tadalafil taken once daily as treatment for erectile dysfunction. J Sex Med. 2010;7:3487–3494. doi: 10.1111/j.1743-6109.2010.01908.x. [DOI] [PubMed] [Google Scholar]
- 64.La Vignera S, Condorelli RA, Vicari E, D'Agata R, Calogero AE. Endothelial apoptosis decrease following tadalafil administration in patients with arterial ED does not last after its discontinuation. Int J Impot Res. 2011;23:200–205. doi: 10.1038/ijir.2011.28. [DOI] [PubMed] [Google Scholar]
- 65.McVary KT, Monnig W, Camps JL, Jr, Young JM, Tseng LJ, van den Ende G. Sildenafil citrate improves erectile function and urinary symptoms in men with erectile dysfunction and lower urinary tract symptoms associated with benign prostatic hyperplasia: a randomized, double-blind trial. J Urol. 2007;177:1071–1077. doi: 10.1016/j.juro.2006.10.055. [DOI] [PubMed] [Google Scholar]
- 66.Roehrborn CG, McVary KT, Elion-Mboussa A, Viktrup L. Tadalafil administered once daily for lower urinary tract symptoms secondary to benign prostatic hyperplasia: a dose finding study. J Urol. 2008;180:1228–1234. doi: 10.1016/j.juro.2008.06.079. [DOI] [PubMed] [Google Scholar]
- 67.Stief CG, Porst H, Neuser D, Beneke M, Ulbrich E. A randomised, placebo-controlled study to assess the efficacy of twice-daily vardenafil in the treatment of lower urinary tract symptoms secondary to benign prostatic hyperplasia. Eur Urol. 2008;53:1236–1244. doi: 10.1016/j.eururo.2008.01.075. [DOI] [PubMed] [Google Scholar]
- 68.Porst H, Kim ED, Casabe AR, Mirone V, Secrest RJ, Xu L, et al. Efficacy and safety of tadalafil once daily in the treatment of men with lower urinary tract symptoms suggestive of benign prostatic hyperplasia: results of an international randomized, double-blind, placebo-controlled trial. Eur Urol. 2011;60:1105–1113. doi: 10.1016/j.eururo.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 69.Oelke M, Giuliano F, Mirone V, Xu L, Cox D, Viktrup L. Monotherapy with tadalafil or tamsulosin similarly improved lower urinary tract symptoms suggestive of benign prostatic hyperplasia in an international, randomised, parallel, placebo-controlled clinical trial. Eur Urol. 2012;61:917–925. doi: 10.1016/j.eururo.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 70.Stroberg P, Hedelin H, Bergström A. Is sex only for the healthy and wealthy? J Sex Med. 2007;4:176–182. doi: 10.1111/j.1743-6109.2006.00233.x. [DOI] [PubMed] [Google Scholar]
- 71.Teloken P, Mesquita G, Montorsi F, Mulhall J. Post-radical prostatectomy pharmacological penile rehabilitation: practice patterns among the international society for sexual medicine practitioners. J Sex Med. 2009;6:2032–2038. doi: 10.1111/j.1743-6109.2009.01269.x. [DOI] [PubMed] [Google Scholar]