Abstract
Purpose
To identify potential predictive factors of incidental prostate cancer (IPca) in patients considering tissue-ablation treatment for benign prostatic hyperplasia (BPH).
Materials and Methods
From the 11 centers, 1,613 men who underwent transurethral resection of the prostate (TURP) or open prostatectomy were included. Before surgery, prostate biopsy was performed in all patients with prostate-specific antigen (PSA) ≥4.0 ng/ml or with abnormal digital rectal examination (DRE) findings. The patients with prostate cancer preoperatively or with PSA >20 ng/ml were excluded. As predictive factors of IPca, age, body mass index, PSA, DRE, and transrectal ultrasonography (TRUS) findings, including total prostate volume (TPV), transition zone volume (TZV), and the presence of hypoechoic lesions, were reviewed. PSA density (PSAD) and PSAD in the transition zone (PSAD-TZV) were calculated.
Results
IPca was diagnosed in 78 patients (4.8%). DRE findings, PSA, and TZV were independent predictive factors in the multivariate analysis. In the receiver operating characteristic curve analysis of PSA, PSAD, and PSAD-TZV, the area under the curve (AUC) was the largest for PSAD-TZV (AUC, 0.685).
Conclusions
IPca was detected in 4.8% of the population studied. In addition to DRE findings, the combination of TZV and PSA can be useful predictive factors of IPca in patients considering tissue-ablation treatment as well as TURP.
Keywords: Prostate neoplasms, Prostatic hyperplasia, Transurethral resection of prostate
INTRODUCTION
Transurethral resection of the prostate (TURP) is a standard surgical treatment for symptomatic benign prostatic hyperplasia (BPH). However, it carries risks of potentially fatal complications such as profuse bleeding and transurethral resection syndrome. Recently, photoselective vaporization of the prostate (PVP) with a high-performance potassium titanyl phosphate (KTP) laser was introduced as a minimally invasive surgery for BPH. PVP is associated with a significantly shorter hospital stay and urethral catheter indwelling time and significantly lower blood loss during the operation than is conventional TURP [1,2]. Thus, PVP has become a popular surgical treatment modality for BPH with outcomes comparable to TURP but with fewer complications [1].
In the past, incidental prostate cancers detected after TURP accounted for 1.5 to 15% of all prostate cancers, but the number of cases has been decreasing since the introduction of prostate-specific antigen (PSA) testing in the screening of prostate cancer [3]. Although most of the incidental prostate cancers were considered to be clinically insignificant [4], recent studies have suggested that with increasing tumor volume, especially of T1b cancers, the clinical course becomes more unfavorable and is comparable with that of T2 tumors [5,6].
With the recent increase in the performance of laser ablation of the prostate, undiagnosed prostate cancer may become an important issue. However, the risk of missing prostate cancer during PVP has rarely been investigated. We therefore attempted to extrapolate the incidence of incidental prostate cancer and to analyze its predictive factors during PVP by means of a retrospective review of contemporary TURP.
MATERIALS AND METHODS
The medical records of 1,613 men who underwent TURP or open prostatectomy in 11 medical centers between 2004 and 2008 were reviewed. Before surgery, prostate biopsy was performed in all patients with PSA ≥4.0 ng/ml or with abnormal digital rectal examination (DRE) findings. Patients who were diagnosed as having prostate cancer, high-grade prostate intraepithelial neoplasia, or atypical small acinar proliferation by biopsy were excluded. Also, those with PSA ≥20 ng/ml were excluded irrespective of their biopsy results. Mean resected prostate volume was 18.8±16.5 g. According to the volume of cancer in the resected chips, incidental prostate cancer was staged as clinical stage T1a (cancer volume <5% of total chips) or clinical stage T1b (cancer volume ≥5% of chips). High-risk prostate cancer was defined as such: PSA ≥10 ng/ml or Gleason score ≥7 [7,8]. As potential predictive factors of incidental prostate cancer, age, body mass index, PSA, DRE findings, and transrectal ultrasonography (TRUS) findings, including total prostate volume (TPV), transition zone volume (TZV), and the presence of hypoechoic lesions, were reviewed. PSA density (PSAD, PSA/TPV) and PSAD in the transition zone (PSAD-TZV, PSA/TZV) were calculated to evaluate their clinical usefulness in predicting incidental prostate cancer.
The patients were divided into two groups according to the final pathologic results after surgery: the BPH group and the incidental prostate cancer group. Univariate and multivariate analyses of the clinical factors were performed for comparison between the two groups. Receiver operating characteristic (ROC) curve analysis was used to evaluate the performance of PSA-related values such as PSA, PSAD, and PSAD-TZV as predictors of incidental prostate cancer.
RESULTS
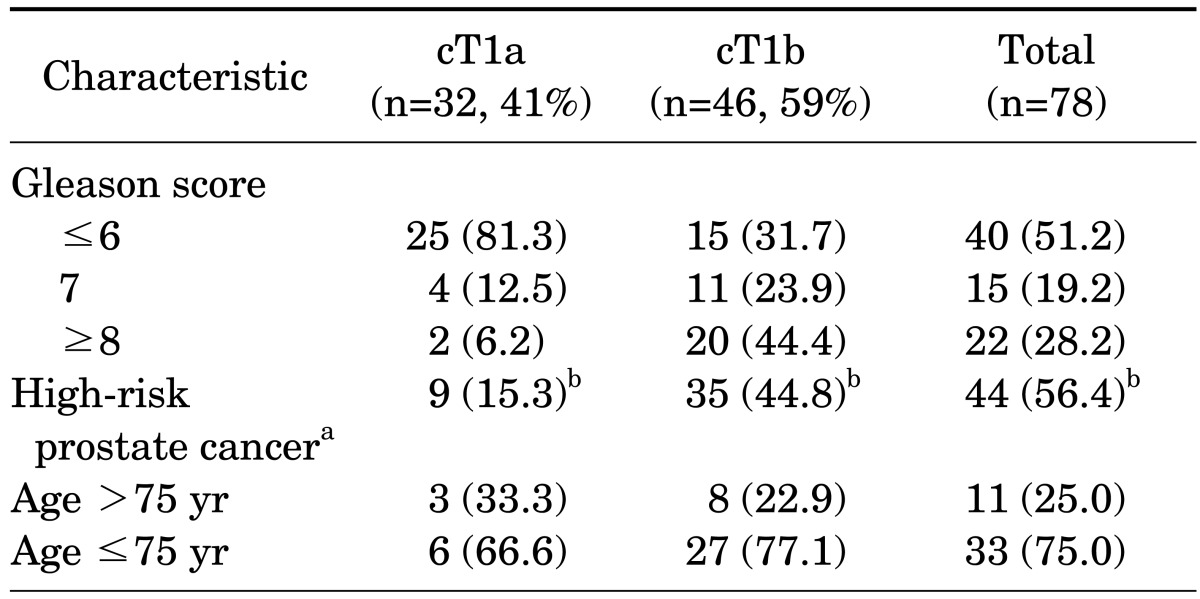
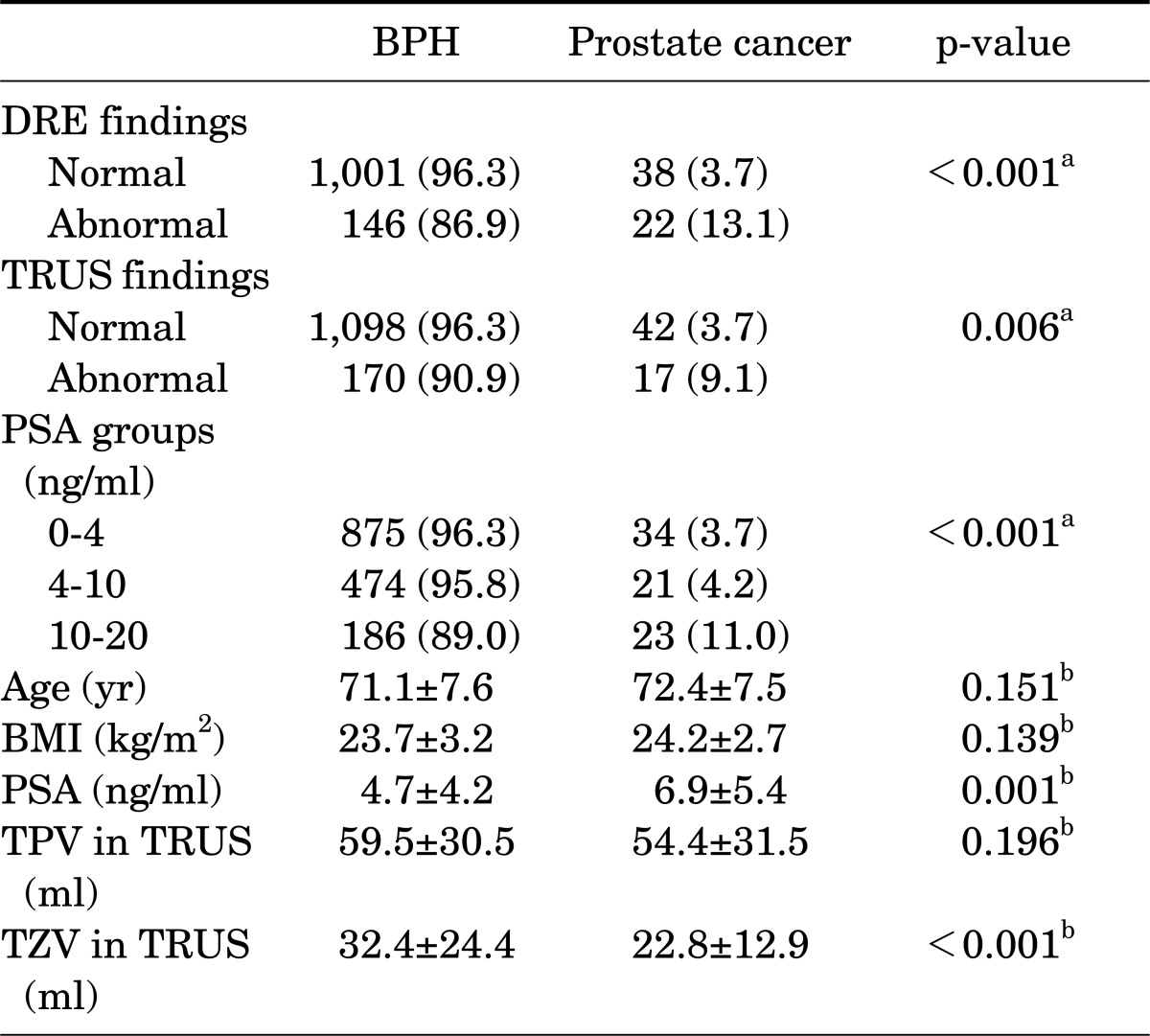
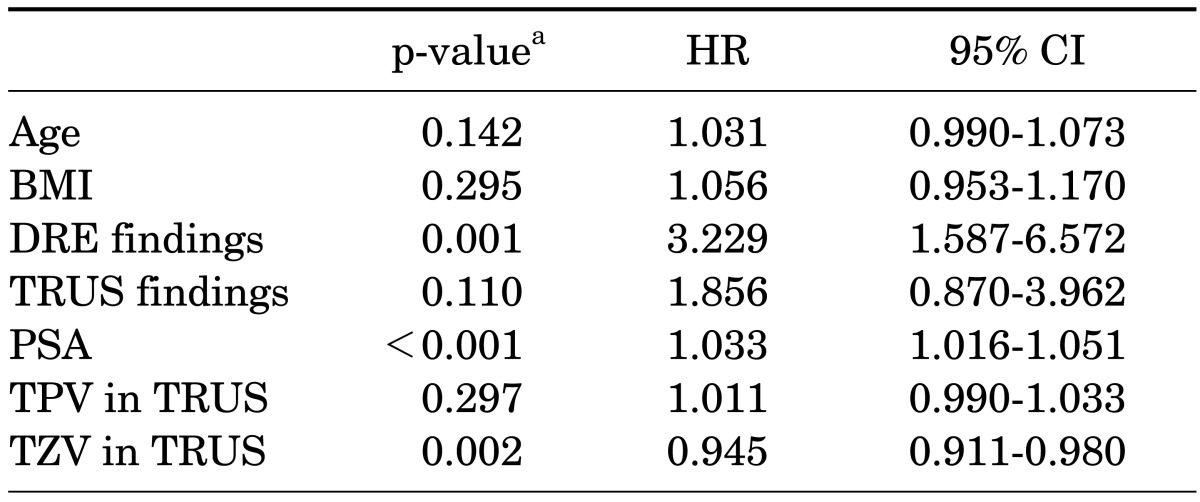
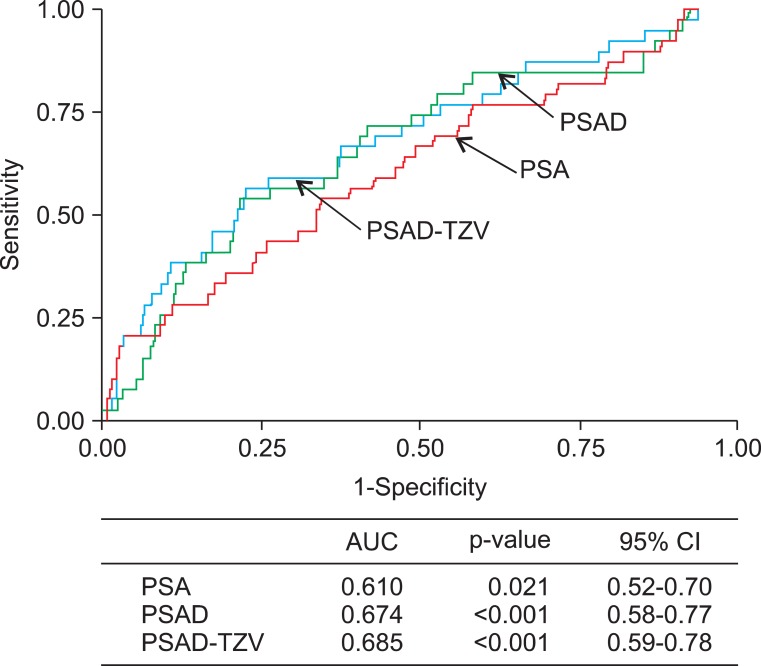
The mean age of the patients was 71.2 years (range, 41 to 80 years) and their mean serum PSA was 4.8±4.3 ng/ml. TURP and open prostatectomy were performed in 1,528 and 85 patients, respectively. Seventy-eight patients (4.8%) had incidental prostate cancer diagnosed after surgery. Among the patients with PSA ≤4.0 ng/ml, prostate cancer was diagnosed in 3.7%. The number of patients with clinical stage T1a and T1b cancer was 32 (41.0%) and 46 (59.0%), respectively, and the proportion of prostate cancer with a Gleason score of 7 or higher was 47.4%. Clinically high-risk cancer accounted for 56.4% of the incidental prostate cancer (Table 1). The treatment received by these patients included androgen deprivation treatment (42.3%), watchful waiting (25.6%), radical prostatectomy (15.4%), and radiation therapy (6.4%), and 6.1% of patients were transferred to another hospital. In the univariate analysis, DRE findings (p<0.001), TRUS findings (p=0.006), PSA (p=0.001), and TZV (p<0.001) were significant predictors of incidental prostate cancer (Table 2). In the multivariate analysis, DRE findings (p=0.001), PSA (p<0.001), and TZV (p=0.002) were independent predictive factors of incidental prostate cancer (Table 3). In the ROC curve analysis of PSA, PSAD, and PSAD-TZV, the area under the curve (AUC) was the largest for PSAD-TZV (AUC, 0.685; Fig. 1) with a cutoff value of 0.17 ng/ml/cc (sensitivity, 66.7%; specificity, 62.5%). Prostate cancer was diagnosed in 8.1% of the patients with PSAD-TZV ≥0.17 ng/ml/ml and in 3.0% of the others (p=0.001).
TABLE 1.
Characteristics of the patients with incidental prostate cancer after surgical treatment for benign prostatic hyperplasia
Values are presented as number (%).
PSA, prostate-specific antigen.
a:PSA ≥10 ng/ml or Gleason score ≥7, b:% from total number of incidental prostate cancer.
TABLE 2.
Univariate analysis of the clinical factors of the patients with BPH for detecting incidental prostate cancer
Values are presented as number (%) or mean±SD.
BPH, benign prostatic hyperplasia; DRE, digital rectal exam; BMI, body mass index; PSA, prostate-specific antigen; TPV, total prostate volume; TRUS, transrectal ultrasonography; TZV, transitional zone volume.
a:Chi-square test, b:Independent student t-test.
TABLE 3.
Multivariate analysis of the clinical factors of patients with BPH for predicting incidental prostate cancer
BPH, benign prostatic hyperplasia; HR, hazard ratio; CI, confidence interval; BMI, body mass index; DRE, digital rectal exam; PSA, prostate-specific antigen; TPV, total prostate volume; TRUS, transrectal ultrasonography; TZV, transitional zone volume.
a:Calculated by logistic regression analysis.
FIG. 1.
Receiver operating characteristic curve analysis of prostate-specific antigen (PSA), PSA density (PSAD), and PSAD-in transition zone volume (PSAD-TZV) on risk of incidental prostate cancer in the patients who underwent surgical treatment for benign prostatic hyperplasia. AUC, area under the curve; CI, confidence interval.
DISCUSSION
The present study raises several important issues that need to be addressed.
First, this report on the contemporary incidence of prostate cancer among patients undergoing TURP is, to our knowledge, the most recent and the second largest of the kind reported recently. Several studies have compared the incidence of incidental prostate cancer among patients undergoing TURP between the pre-PSA era and the PSA era and have shown a decrease in incidental prostate cancer from 15 to 23% to 4 to 6% [9,10]. Most recently, Melchior et al. [11] showed a 5.4% incidence of prostate cancer in 1,931 patients who underwent TURP after a thorough preoperative screening for prostate cancer. In the present study, the incidence of incidental prostate cancer was 4.8%, and among these cases, cT1a and cT1b prostate cancer were detected in 2.0% and 2.8%, respectively. Our result is similar to reports by others and shows that the risk of missing undiagnosed prostate cancer is omnipresent during laser ablation of the prostate [12]. However, the actual risk of missing prostate cancer by laser ablation of the prostate has rarely been investigated. Only two reports have addressed this issue. Ruszat et al. [13] reported the largest series of data on patient follow-up after PVP. By doing thorough preoperative investigation using PSA and DRE in all patients and at least 2 transrectal biopsies in the case of an abnormal PSA or DRE result, they were able to keep the subsequent prostate cancer rate at a very low level of 1.2%. The results reported by Malek et al. [14] are more realistic, with a 5% cancer detection rate at subsequent follow-up after PVP.
The second issue is whether the cancer diagnosed incidentally during TURP is significant. In previous Western studies, the proportion of cancers with a Gleason score of 7 or higher in radical prostatectomy specimens from T1a and T1b cancers was reported to be 0 to 15% and 9.8 to 64%, respectively [15-17]. Also, T1a cancer was more likely to be organ-confined than was T1b cancer, and in terms of pathological stage at radical prostatectomy and progression, T1b was closer to T1c than to T1a [10]. According to Melchior et al. [11], 79% of their incidental cancers discovered during TURP were T1a, and only 21% were T1b. Twenty-five percent of these patients underwent subsequent radical prostatectomy, all of them having either pathologically organ-confined or pT0 cancer. The results of our study look more alarming, with more than half of patients having T1b or high-risk cancer. Also, most of our patients needed an active treatment although the indication for choosing an active treatment could not be clearly explained in all cases. The reason for the relatively high percentage of T1b and high-risk cancers in our patients is unknown but may need to be discussed. First, the patients in this study were drawn from the population in Korea where a PSA screening program is not established. This may have resulted in a higher stage and cancer volume at diagnosis. Another reason might be the generally worse histological findings observed in Eastern patients compared with Western. In a Korean population-based study that reviewed the data of patients with prostate cancer by PSA screening, prostate cancer with a Gleason score of 7 or higher was diagnosed in 46.1%, which was higher than in the Western population [18].
Third, can we predict incidental prostate cancer by use of preoperative parameters? Interestingly, this study demonstrated that PSAD-TZV proved to be more useful than PSA density or PSA alone. The concept of PSAD-TZV has been reported as one of the parameters for improving the diagnostic accuracy of extended-field biopsy or re-biopsy in patients with gray-zone PSA [19-21]. Even though we take into account that the prostate volume measured by TRUS is more accurate in smaller glands [22], the PSAD-TZV might also be regarded as a useful parameter for predicting incidental prostate cancer among patients who are planning to undergo surgical treatment for BPH. Free PSA and complexed PSA, which were not included in the analysis in the present study, can be considered as additional predictive parameters of incidental prostate cancer. Froehner et al. [23] reported that derivatives of prostate-specific antigen, such as percentage free PSA or complexed PSAD, were more useful than total PSA for predicting incidental prostate cancer. However, they did not include the TZV in their analysis. It is unclear whether the clinical stage of incidental prostate cancer can predict progression of prostate cancer, for a hard endpoint such as disease-specific survival required too long a follow-up time to be evaluated. Therefore, it is difficult to select the optimal candidate for aggressive treatment among patients with T1a-T1b prostate cancer [24]. In a limited study, not the stage but PSA before and after surgery and Gleason score were predictive factors of disease progression for incidental prostate cancer [15]. On that basis, we defined high-risk prostate cancer as a Gleason score of 7 or higher or preoperative PSA of 10 ng/ml or higher, and by applying the age of 75 years as the upper limit for definite treatment, the proportion of patients who needed definite treatment for incidental prostate cancer was only 2.0% (33 of 1613; Table 1).
The present study has several limitations to be discussed. We did not observe the emergence of prostate cancer after TURP in a prospective manner and we did not evaluate the disease progression or survival of the patients with incidental prostate cancer. A relatively high proportion of patients underwent imperative TURP owing to recurrent acute urinary retention, infection, or other reasons, so that efforts to screen prostate cancer might have been inadequate. For instance, DRE was not performed in 26% of patients, which may have led to TURP not being performed in a significant number of patients with palpable prostate cancer. We cannot expect our results to apply to all PVP patients, because patients undergoing PVP and TURP may represent different populations. For example, PVP patients may be prescreened to include patients with a relatively younger age, lower prostate volume, and lower PSA than TURP patients. A study comparing the accuracy of PSAD-TZV with that of percentage free PSA or complexed PSAD in detecting incidental prostate cancer is also needed.
In many recent studies, KTP laser ablation has been proved to be a minimally invasive surgical treatment for BPH with comparable efficacy and less morbidity than TURP. Even if the proportion of patients with incidental prostate cancer who need definite treatment is small, an unfavorable pattern was demonstrated in Korean patients. To increase the accuracy of detection of prostate cancer in biopsy, PSA, DRE findings, and transition zone might be used as predictive parameters before surgical treatment for BPH, especially before laser ablation treatment. For patients with a high PSAD-TZV, extended-core or saturated prostate biopsy could be recommended. In addition, even though a prostate biopsy may have a negative result for cancer, patients should be informed of the possibility of emerging prostate cancer in the future. The results of this study may be additional evidence of the usefulness of PSAD-TZV for improving the accuracy of prostate biopsy.
CONCLUSIONS
Incidental prostate cancer was detected in 4.8% of the patients who underwent surgical treatment for BPH and more than half of them showed clinically significant prostate cancer. In addition to DRE findings, a combination of TZV and PSA can be used as useful predictive factors of incidental prostate cancer in patients considering tissue-ablation treatment as well as TURP.
Footnotes
The authors have nothing to disclose.
References
- 1.Bachmann A, Schürch L, Ruszat R, Wyler SF, Seifert HH, Muller A, et al. Photoselective vaporization (PVP) versus transurethral resection of the prostate (TURP): a prospective bi-centre study of perioperative morbidity and early functional outcome. Eur Urol. 2005;48:965–971. doi: 10.1016/j.eururo.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 2.Bouchier-Hayes DM, Anderson P, Van Appledorn S, Bugeja P, Costello AJ. KTP laser versus transurethral resection: early results of a randomized trial. J Endourol. 2006;20:580–585. doi: 10.1089/end.2006.20.580. [DOI] [PubMed] [Google Scholar]
- 3.Andren O, Garmo H, Mucci L, Andersson SO, Johansson JE, Fall K. Incidence and mortality of incidental prostate cancer: a Swedish register-based study. Br J Cancer. 2009;100:170–173. doi: 10.1038/sj.bjc.6604834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cantrell BB, DeKlerk DP, Eggleston JC, Boitnott JK, Walsh PC. Pathological factors that influence prognosis in stage A prostatic cancer: the influence of extent versus grade. J Urol. 1981;125:516–520. doi: 10.1016/s0022-5347(17)55092-2. [DOI] [PubMed] [Google Scholar]
- 5.Andren O, Fall K, Franzen L, Andersson SO, Johansson JE, Rubin MA. How well does the Gleason score predict prostate cancer death? A 20-year followup of a population based cohort in Sweden. J Urol. 2006;175:1337–1340. doi: 10.1016/S0022-5347(05)00734-2. [DOI] [PubMed] [Google Scholar]
- 6.Robinson D, Aus G, Bak J, Gorecki T, Herder A, Rosell J, et al. Long-term follow-up of conservatively managed incidental carcinoma of the prostate: a multivariate analysis of prognostic factors. Scand J Urol Nephrol. 2007;41:103–109. doi: 10.1080/00365590600991268. [DOI] [PubMed] [Google Scholar]
- 7.D'Amico AV, Whittington R, Malkowicz SB, Schultz D, Blank K, Broderick GA, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280:969–974. doi: 10.1001/jama.280.11.969. [DOI] [PubMed] [Google Scholar]
- 8.Klotz L. Active surveillance for prostate cancer: for whom? J Clin Oncol. 2005;23:8165–8169. doi: 10.1200/JCO.2005.03.3134. [DOI] [PubMed] [Google Scholar]
- 9.Jones JS, Follis HW, Johnson JR. Probability of finding T1a and T1b (incidental) prostate cancer during TURP has decreased in the PSA era. Prostate Cancer Prostatic Dis. 2009;12:57–60. doi: 10.1038/pcan.2008.14. [DOI] [PubMed] [Google Scholar]
- 10.Magheli A, Rais-Bahrami S, Carter HB, Peck HJ, Epstein JI, Gonzalgo ML. Subclassification of clinical stage T1 prostate cancer: impact on biochemical recurrence following radical prostatectomy. J Urol. 2007;178(4 Pt 1):1277–1280. doi: 10.1016/j.juro.2007.05.153. [DOI] [PubMed] [Google Scholar]
- 11.Melchior S, Hadaschik B, Thuroff S, Thomas C, Gillitzer R, Thuroff J. Outcome of radical prostatectomy for incidental carcinoma of the prostate. BJU Int. 2009;103:1478–1481. doi: 10.1111/j.1464-410X.2008.08279.x. [DOI] [PubMed] [Google Scholar]
- 12.Jonsson E, Sigbjarnarson HP, Tomasson J, Benediktsdottir KR, Tryggvadottir L, Hrafnkelsson J, et al. Adenocarcinoma of the prostate in Iceland: a population-based study of stage, Gleason grade, treatment and long-term survival in males diagnosed between 1983 and 1987. Scand J Urol Nephrol. 2006;40:265–271. doi: 10.1080/00365590600750110. [DOI] [PubMed] [Google Scholar]
- 13.Ruszat R, Seitz M, Wyler SF, Abe C, Rieken M, Reich O, et al. GreenLight laser vaporization of the prostate: single-center experience and long-term results after 500 procedures. Eur Urol. 2008;54:893–901. doi: 10.1016/j.eururo.2008.04.053. [DOI] [PubMed] [Google Scholar]
- 14.Malek RS, Kuntzman RS, Barrett DM. Photoselective potassium-titanyl-phosphate laser vaporization of the benign obstructive prostate: observations on long-term outcomes. J Urol. 2005;174(4 Pt 1):1344–1348. doi: 10.1097/01.ju.0000173913.41401.67. [DOI] [PubMed] [Google Scholar]
- 15.Capitanio U, Scattoni V, Freschi M, Briganti A, Salonia A, Gallina A, et al. Radical prostatectomy for incidental (stage T1a-T1b) prostate cancer: analysis of predictors for residual disease and biochemical recurrence. Eur Urol. 2008;54:118–125. doi: 10.1016/j.eururo.2008.02.018. [DOI] [PubMed] [Google Scholar]
- 16.Argyropoulos A, Doumas K, Farmakis A, Aristas O, Kontogeorgos G, Lykourinas M. Characteristics of patients with stage T1b incidental prostate cancer. Scand J Urol Nephrol. 2005;39:289–293. doi: 10.1080/00365590510031200. [DOI] [PubMed] [Google Scholar]
- 17.Helfand BT, Mongiu AK, Kan D, Kim DY, Loeb S, Roehl KA, et al. Outcomes of radical prostatectomy for patients with clinical stage T1a and T1b disease. BJU Int. 2009;104:304–309. doi: 10.1111/j.1464-410X.2009.08421.x. [DOI] [PubMed] [Google Scholar]
- 18.Song C, Ahn H, Lee MS, Park J, Kwon TG, Kim HJ, et al. Mass screening for prostate cancer in Korea: a population based study. J Urol. 2008;180:1949–1952. doi: 10.1016/j.juro.2008.07.041. [DOI] [PubMed] [Google Scholar]
- 19.Winkler MH, Kulinskaya E, Gillatt DA. Prediction of prostate cancer in extended-field biopsies of the prostate. BJU Int. 2004;93:516–521. doi: 10.1111/j.1464-410x.2003.04670.x. [DOI] [PubMed] [Google Scholar]
- 20.Djavan B, Remzi M, Zlotta AR, Ravery V, Hammerer P, Reissigl A, et al. Complexed prostate-specific antigen, complexed prostate-specific antigen density of total and transition zone, complexed/total prostate-specific antigen ratio, free-to-total prostate-specific antigen ratio, density of total and transition zone prostate-specific antigen: results of the prospective multicenter European trial. Urology. 2002;60(4 Suppl 1):4–9. doi: 10.1016/s0090-4295(02)01896-4. [DOI] [PubMed] [Google Scholar]
- 21.Ohi M, Ito K, Suzuki K, Yamamoto T, Yamanaka H. Diagnostic significance of PSA density adjusted by transition zone volume in males with PSA levels between 2 and 4ng/ml. Eur Urol. 2004;45:92–96. doi: 10.1016/j.eururo.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 22.Loeb S, Han M, Roehl KA, Antenor JA, Catalona WJ. Accuracy of prostate weight estimation by digital rectal examination versus transrectal ultrasonography. J Urol. 2005;173:63–65. doi: 10.1097/01.ju.0000145883.01068.5f. [DOI] [PubMed] [Google Scholar]
- 23.Froehner M, Buck LM, Koch R, Hakenberg OW, Wirth MP. Derivatives of prostate-specific antigen as predictors of incidental prostate cancer. BJU Int. 2009;104:25–28. doi: 10.1111/j.1464-410X.2009.08349.x. [DOI] [PubMed] [Google Scholar]
- 24.Adolfsson J. The management of category T1a-T1b (incidental) prostate cancer: can we predict who needs treatment? Eur Urol. 2008;54:16–18. doi: 10.1016/j.eururo.2008.03.099. [DOI] [PubMed] [Google Scholar]