Abstract
Human African trypanosomiasis (HAT), also known as sleeping sickness, is a major cause of mortality and morbidity in sub-Saharan Africa. Current therapy with melarsoprol for CNS HAT has unacceptable side-effects with an overall mortality of 5%. This review discusses the issues of diagnosis and staging of CNS disease, its neuropathogenesis, and the possibility of new therapies for treating late-stage disease.
Historical perspective
Human African trypanosomiasis (HAT), also known as sleeping sickness, comes in two variants: East African and West African. Caused by protozoan parasites of the genus Trypanosoma, it has emerged over the last few decades as a major threat to human health in Africa. While for centuries there was an awareness of the disease and of its propensity to induce a fatal sleep disorder, it was not until the period 1894–1910 that the cause of sleeping sickness in humans and cattle was discovered. Preeminent in this discovery was David Bruce, who, while working in Zululand on a wasting disease of cattle known as nagana, identified trypanosomes in the blood of affected cattle (1, 2). He then established experimentally that healthy game animals were host reservoirs of the disease, which was transmitted by the bite of the tsetse fly to domestic animals, which then became ill (2). In 1899 the causative parasite was identified as Trypanosoma brucei, and in 1902 Everett Dutton first identified, in a European patient, a subspecies of trypanosomes called Trypanosoma brucei gambiense (2) that is now recognized as the cause of West African sleeping sickness. In 1903, Aldo Castellani, working with Bruce, identified trypanosomes in the blood and cerebrospinal fluid (CSF) of a patient with sleeping sickness (2, 3), and in 1910 J.W.W. Stephens and H.B. Fantham first described Trypanosoma brucei rhodesiense (2), which is now recognized as causing East African sleeping sickness. There are characteristic differences between the biology and clinical features of T.b. gambiense disease and T.b. rhodesiense disease, probably due to a greater adaptation of the gambiense parasite to humans.
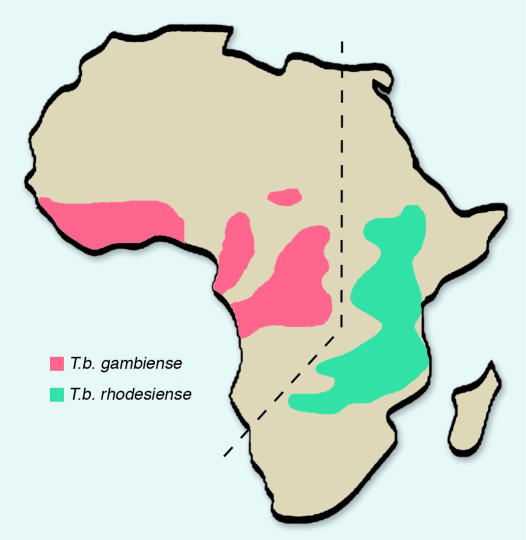
Currently, HAT occurs in 36 countries in sub-Saharan Africa; about 60 million people worldwide are at risk from developing the disease (4). The annual incidence of the disease is approximately 300,000 cases, and the area of Africa that is infested by the tsetse fly encompasses approximately ten million square kilometers (2, 5) — a third of the land mass of Africa (Figure 1). HAT was almost brought under control during the early 1950s (6), with a significant decrease in the number of newly registered cases from 1949 to 1965, but a variety of factors led to its recent reemergence. These include socio-economic unrest — especially war — causing disruption of disease surveillance and control, inadequate financial allocation of critical resources to the disease during peacetime, increasing parasite drug resistance, changes in climate and vegetation, the emergence of new virulent parasite strains, unpredicted population movements of animal reservoirs, and changes in host disease susceptibility (5, 6). Many of these factors may operate simultaneously, and there have been several significant epidemics and focal resurgences of the disease in various regions of Africa in recent years.
Figure 1.
Diagrammatic representation of the distribution of the two types of human African trypanosomiasis in Africa. Figure modified with permission from Butterworth-Heinemann (5).
Trypanosome biology
In both types of HAT the disease is transmitted by the blood-sucking tsetse fly of the genus Glossina. Infected wild animals and domestic animals, such as cattle, are the reservoirs of parasites causing human disease. The flies become infective approximately 21 days after feeding on an infected animal host (5). The fly ingests the trypanosomes during a blood meal from the infected animal, following which the parasites undergo a series of morphological and biochemical changes in the fly’s anterior midgut where the infection is initially established (7). Long slender parasitic forms produced in the midgut then move to the salivary glands to become epimastigotes, which then change into short stumpy infective metacyclic trypanosomes which enter via the wound of a bitten individual. A primary lesion known as a trypanosomal chancre usually develops 5–15 days later at the site of the bite, soon after which the trypanosomes invade the bloodstream, lymph nodes, and other tissues. HAT is invariably fatal if untreated. A fly remains infective for life, and human/fly contact is therefore a crucial component of the disease. The life cycle of the organism in the human and the tsetse fly is summarized in Figure 2.
Figure 2.
Diagrammatic representation of the life cycle of Trypanosoma brucei in the human and the tsetse fly. Image credit: Alexander J. da Silva and Melanie Moser, Centers for Disease Control Public Health Image Library.
Much work has been carried out on the sequencing and mapping of the trypanosome genome (8). Trypanosomes causing HAT are diploid and have a haploid nuclear DNA content of approximately 35 Mb (8, 9). Three classes of chromosome in T. brucei have been identified based on size, namely megabase (1–6 Mb), of which there are at least 11, intermediate (200–900 kb), and at least 100 minichromosomes (50–150 kb) (8, 9). The entire genome contains about 10,000 genes, 10% of which are thought to be variant surface glycoprotein (VSG) genes encoding variable surface glycoproteins (9). The VSG genes are of great pathogenic importance as they provide the molecular basis for the antigenic variation seen in trypanosome infection, and only one of them is expressed at any one time, the rest being transcriptionally silent (9). The VSGs are distributed over the surface of the trypanosome and are anchored to the outer membrane by a glycosylphosphatidylinositol (GPI) anchor (9). Ten million copies of a single species of VSG cover the trypanosome surface at any one time. During infection of the host, a constant low frequency gene conversion process switches transcriptionally inactive basic copy VSG genes in and out of the expression site, and this antigenic variation allows the parasite to continuously evade the host’s immune response. As a result, the parasite undergoes rapid multiplication in the blood of the host, producing waves of parasitaemia that characterize this disease (10).
Clinical features
There are two recognized stages in the clinical presentation of HAT, namely the early hemolymphatic stage, and the late encephalitic stage when the CNS is involved. However, the transition from the early to the late stage is not always distinct in rhodesiense infection (5). The tempo of the disease is usually acute in rhodesiense disease — CNS invasion by the parasite occurs early, within a few months after initial infection — whereas gambiense infection is usually a slower, chronic infection, with late CNS infection lasting months to years.
Early (hemolymphatic) stage.
The onset is variable but usually occurs 1–3 weeks after the bite. Episodes of fever lasting 1–7 days occur together with generalized lymphadenopathy. The early symptoms tend to be non-specific: malaise, headache, arthralgia, generalized weakness, and weight loss (11). Multiple organs may then be infected (5, 12), including the spleen, liver, skin, cardiovascular system, endocrine system, and eyes. This involvement underlies the wide spectrum of systemic dysfunction that may occur (5).
Late (encephalitic) stage.
The onset is insidious and the potential clinical phenotype is wide (5, 12). The broad neurologic spectrum has been detailed elsewhere (5), and the reported features can be grouped into general categories such as psychiatric, motor, and sensory abnormalities, and sleep disturbances. The mental disturbances may be subtle, and include irritability, lassitude, headache, apparent personality changes, and overt psychiatric presentations such as violence, hallucinations, suicidal tendencies, and mania (5, 12). Motor system involvement may include limb tremors, tongue and limb muscle fasciculation, limb hypertonia and pyramidal weakness, choreiform and athetoid movements, dysarthria, cerebellar ataxia, and polyneuritis (5, 13). Pout and palmar-mental reflexes may also be present. Sensory involvement may manifest as painful hyperaesthesia, pruritis, and also deep hyperaesthesia (Kerandel’s sign), the latter being reported as particularly common in Europeans (12). The characteristic sleep disturbances include lassitude, distractibility, and spontaneous, uncontrollable urges to sleep, along with a reversal of the normal sleep-wake cycle in which daytime somnolence alternates with nocturnal insomnia. While these various features, including the sleep abnormalities, are typical of HAT, they are not individually diagnostic, since some of them may also be seen during other CNS infections. If untreated, the patient progresses to the final stage of the disease, which is characterized by seizures, severe somnolence, double incontinence, cerebral edema, coma, systemic organ failure, and inevitable death.
Disease diagnosis
The diagnosis of HAT is based on a combination of clinical and investigative data. A typical clinical presentation in the context of a geographical location where HAT is known to be endemic is clearly the key diagnostic clue. However, the non-specific nature of many of the clinical features makes it imperative to exclude other infections such as malaria, tuberculosis, HIV infection, leishmaniasis, toxoplasmosis, hookworm infection, typhoid, and viral encephalitis (5). A particular pitfall is that inappropriate antimalarial treatment may actually reduce the fever due to HAT, thus confounding and delaying the correct diagnosis (5), and these two conditions may also co-exist.
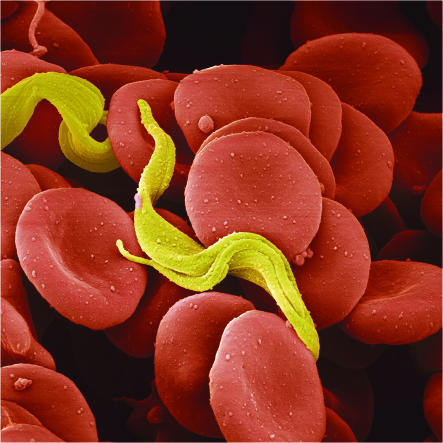
Specific diagnosis at the hemolymphatic stage ideally involves demonstration of the trypanosomes in the peripheral blood using stained thick and thin films (Figure 3), or in other infected tissues such as lymph node aspirates or occasionally bone marrow (5). While parasite detection in the blood is frequently successful in rhodesiense infection because of the permanent parasitaemia, this method is very difficult in gambiense infection, in which few parasites are present in the peripheral circulation other than at periods of cyclic parasitaemia, which reflects the chronicity of the disease. Therefore, serologic tests are of crucial importance in the diagnosis of gambiense infection. Currently the antibody-detecting card agglutination trypanosomiasis test (CATT) is in frequent use for serological gambiense diagnosis, being simple, easy to perform, and rapid (14).
Figure 3.
Colored scanning electron micrograph of Trypanosoma brucei in human blood. Image credit: Science Photo Library.
The key issue in HAT diagnosis and therapeutic decision making is to distinguish reliably the late encephalitic stage of HAT from the early stage. Accurate staging of HAT is critical because failure to treat a patient with CNS involvement will lead inevitably to death from the disease, yet inappropriate CNS treatment in an early-stage patient carries a high risk of unnecessary drug toxicity (see below). In patients with suspected late-stage disease it is imperative to perform a lumbar puncture, which typically shows a lymphocytic pleiocytosis and raised protein level of 40–200 mg/100 ml (5). Further, all CATT-positive patients also need to undergo a lumbar puncture, as there are no reliable clinical suspicion criteria for early-stage disease. The WHO criteria for CNS involvement, and therefore for CNS drug treatment, are demonstration of the parasites in the CSF or a white blood cell (WBC) count of >5/μl (15). However, these criteria have been challenged by some investigators. Thus, in Angola and the Ivory Coast the criterion used for CNS involvement is 20 WBCs/μl in the CSF (16). It has also been pointed out that concentration techniques for trypanosome detection in the CSF vary, and that a CSF pleiocytosis may be non-specific (17). Recently it has been shown that detection of intrathecal IgM synthesis is a very sensitive marker for CNS involvement in sleeping sickness (18). The latex agglutination assay for CSF IgM quantitation can be applied in the field and has considerable promise for both staging CNS sleeping sickness and monitoring the development of treatment relapses (18).
CSF PCR to detect trypanosome DNA has also been used in the diagnosis of HAT, but considerable care must be used in the correct choice of primers, and problems with assay reproducibility have been documented (19). It has recently been reported that CSF PCR has a sensitivity rate of 96%, although its value for therapeutic decision making has been questioned (17). Therefore, PCR has not yet superseded serological diagnosis and, crucially, it is not readily available in field conditions. It has recently been suggested by Lejon et al. that the WHO criteria should be replaced by the presence of intrathecal IgM synthesis or the presence of >20 WBCs/μl, independent of the presence of trypanosomes in the CSF (16). This author regards the presence of trypanosomes in the CSF as compelling evidence of CNS involvement. However, patients with gambiense disease who have trypanosomes in the CSF and <20 WBCs/μl have been treated successfully with pentamidine (16), so perhaps one might speculate that there is a kind of ‘intermediate stage’ in which the trypanosomes can cross the blood-brain barrier without invading and damaging brain structures at that stage. Thus there are two critical, and not necessarily congruent, issues involved, one being the biological definition of CNS involvement, and the other being the ground for therapeutic choices. This lack of a universal consensus on the operational definition of late-stage HAT remains very problematic, but the clear requirement is to develop robust surrogate markers to guide therapeutic choices. These diagnostics need to be novel, simple, and affordable.
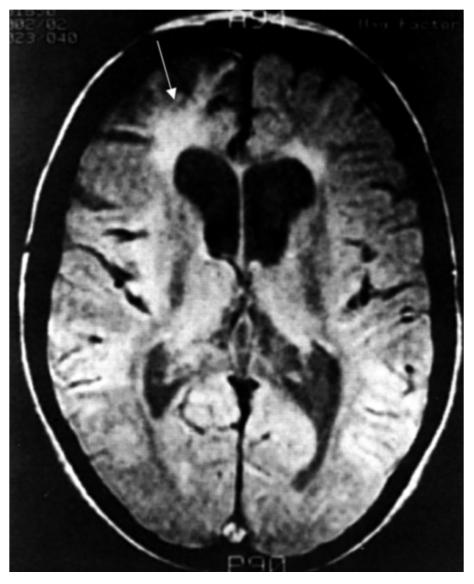
Electroencephalogram (EEG) and sophisticated neuroimaging are limited to specialist centers, but both have shown abnormalities in HAT. During the encephalitis stage the EEG shows non-specific abnormalities, which correlate with the severity of the disease. Changes include at least three different types of abnormal EEG patterns, which become normal after clinical improvement (5). Abnormalities reported on computed tomography scans and MRI are non-specific and not pathognomonic (Figure 4), but if available these tests should be carried out, partly to monitor the response to treatment, and also where the diagnosis is in doubt or where raised intracranial pressure is present. MRI of the brain may show diffuse asymmetric white matter abnormalities, diffuse hyperintensities in the basal ganglia, and ventricular enlargement (5, 20).
Figure 4.
MRI scan (proton density) of a 13-year-old patient with CNS trypanosomiasis 3 years after successful completion of multiple treatment regimens for numerous relapses of the disease. Ventricular enlargement (especially of the frontal horns) is seen as well as diffuse white matter changes, which are prominent in the right frontal (see arrow) and periventricular regions. Reproduced with permission from Butterworth-Heinemann (5).
Overview of current treatment
The current treatment of HAT is based on four main drugs, namely suramin, pentamidine, melarsoprol, and eflornithine (difluoromethylornithine, or DFMO), with nifurtimox undergoing evaluation. Table 1 summarizes their disease spectrum, stage-specificity, route of administration, postulated mode of action, and main side-effects (21–26). It should be appreciated that most of these drugs were developed in the first half of the twentieth century, some of them would probably not pass current high safety standards (26), and there have been no new registered drugs for HAT since 1981. Early-stage disease is treated with i.v. suramin in rhodesiense disease and with intramuscular (i.m.) pentamidine in gambiense disease according to established treatment protocols. Treatment is effective and prevents disease progression.
Table 1.
Drugs currently used for the treatment of human African trypanosomiasis
The trivalent organic arsenical melarsoprol is the only effective drug for late-stage disease in both forms of HAT, as the drug crosses the blood-brain barrier (5, 27). Specific treatment regimes vary considerably among different centers and depending on whether the infection is due to rhodesiense or gambiense. Typically, a course of 3–4 i.v. doses are given daily over a week for a total period of 3–4 weeks (27). Ideally, patients are then followed up every 6 months with clinical evaluation and CSF examination for a total of 2 years, at which point a cure has been established if the CSF is normal. However, this policy is very difficult to carry out in routine practice in the field. Although about 80–90% of patients are cured with standard treatment regimes (5), there is evidence of increasing drug resistance, with treatment failure rates of 30% reported among patients in Northern Uganda (27, 28). But the major problem with melarsoprol treatment is that it is followed by a severe post-treatment reactive encephalopathy (PTRE) in up to 10% of cases, with a fatality rate of about 50% (29). Thus the overall mortality rate from melarsoprol therapy is 5%, which is unacceptably high (30). A prospective, randomized, non-blinded trial involving 598 patients with gambiense disease showed that the incidence of melarsoprol-induced encephalopathy and death was reduced in patients who were given concurrent administration of prednisolone and melarsoprol compared with melarsoprol therapy alone. However, this combined treatment regime was not associated with a reduction in the incidence of the other complications of PTRE or the relapse rate after melarsoprol therapy (31). Treatment of PTRE is focussed on treating seizures, general management of the comatose patient with i.v. hydration, antipyretics, steroids, and reduction of cerebral edema (5). After recovery, melarsoprol has to be restarted, possibly with a smaller initial dose, and the course then completed (5). A new, shorter treatment regime consisting of a 10-day course of daily melarsoprol injections was recently found to be comparable to the standard longer treatment schedule over a period of 26 days, in terms of both cure and complication rates, and may be increasingly adopted in the future (32).
There has been great interest in developing safer drugs for late-stage HAT. DFMO has been used successfully to treat late-stage disease, especially melarsoprol-refractory gambiense infection (33), and also increasingly as first-line therapy, but is largely ineffective for rhodesiense infection. The problems with this drug’s availability will be mentioned later. Nifurtimox is the only other potential alternative treatment for late-stage disease, but well-documented evidence of its efficacy and safety is lacking, and its utility is more likely to be in the context of combination therapy.
Neuropathogenesis
The pathologic substrate of late-stage sleeping sickness is a meningoencephalitis in which cellular proliferation occurs in the leptomeninges, and a diffuse perivascular white matter infiltration consisting of lymphocytes, plasma cells, and macrophages is prominent (5, 34). The perivascular cuffs and adjacent parenchyma contain markedly activated astrocytes and macrophages, and the white matter contains pathognomonic morular or Mott cells, which are thought to be modified plasma cells containing eosinophilic inclusions comprising IgM (34) (Figure 5). PTRE shows an exacerbation of these pathologic features.
Figure 5.
Neuropathology of CNS human African trypanosomiasis. (a) Late-stage disease in a patient who died 3–5 months after first injection of melarsoprol. Many large astrocytes are located in white matter. Stained for glial fibrillary acidic protein by immunoperoxidase. Original magnification, ×400. (b) Morular cells (indicated by arrows) observed in the brain of a patient with CNS trypanosomiasis who had not received melarsoprol. Morular cells are plasma cells filled with immunoglobulin. H&E stain. Original magnification, ×400. (c) PTRE in a patient dying 9 days after receiving melarsoprol. Ischaemic cell changes (indicated by arrows) are seen in neurons in the hippocampus. H&E stain. Original magnification, ×250. (d) PTRE with acute haemorrhagic leukoencephalopathy in a patient 9 days after receiving melarsoprol. There is fibrinoid necrosis in an arteriole (indicated by arrow) and focal haemorrhage in the pons. Martius scarlet blue stain. Original magnification, ×250. Reproduced with permission from Neuropathol. Appl. Neurobiol. (34).
Current understanding of the highly complex pathogenesis of sleeping sickness is based mainly on studies carried out either on patients’ blood and CSF samples or in experimental animal models. In both cases, correlation of specific clinical features or stages with alterations of different biochemical or immunological parameters has often yielded interesting results, but caution must be used in assuming a cause-and-effect relationship between the investigation and the disease phenotype. Care must also be used in extrapolating results obtained in animal models to the human disease.
Alteration of cytokine levels has been detected in patients with CNS sleeping sickness. For example, significant elevations of IL-10 were detected in both the plasma and CSF in both early- and late-stage rhodesiense disease, and declined after treatment to the levels found in uninfected control persons (35). Total, but not free, plasma TNF-α levels were also higher in late-stage disease compared with levels obtained after treatment. However, the source of IL-10 elevation is unclear. Similar studies in patients with gambiense infection have also reported elevations of CSF IL-10 levels in late-stage disease, as well as a rise in IL-6 and IL-8 (36). Other abnormalities which have been reported in patients with CNS HAT include very raised CSF levels of prostaglandin D2 (37), which may be related to the marked somnolence, and raised blood and CSF endotoxin levels that may also contribute to the CNS pathology (38).
Several possible causes of PTRE have been suggested, including subcurative chemotherapy, abnormal immune responses to glial cell–attached antigens released from killed parasites following melarsoprol treatment, immune complex deposition, arsenical toxicity, and autoimmune mechanisms (39–42). PTRE has been studied in a reproducible mouse model that mirrors many of the pathologic features of the disease in humans (43). Injection of Trypanosoma brucei into mice via the intraperitoneal route leads to a chronic infection in which the parasites are detectable in the CNS after 21 days. If the drug berenil (diminazene aceturate), which does not cross the blood-brain barrier and therefore clears the parasites from the extravascular compartment but not the CNS, is given 21–28 days after infection, the mice develop a severe post-treatment meningoencephalitis, which persists after the parasitemic phase is over. This condition shows strong pathologic similarity to PTRE in humans. A consistent observation in this model is that astrocytes are activated 14–21 days after infection and prior to the development of the inflammatory response (43, 44), and that transcripts for several cytokines such as TNF-α, IL-1, IL-4, IL-6 and IFN-γ can be detected in the brain at this time (44, 45). Early astrocyte activation is therefore likely to be of central importance in generating the CNS inflammatory response.
Different types of drug have been shown to modulate the inflammatory response in this mouse model. The trypanostatic drug DFMO has the ability to prevent the development of PTRE or ameliorate it once it is established in terms of greatly reducing both the neuropathology and the degree of astrocyte activation (46). The immunosuppressant drug azathiaprine can prevent but not cure PTRE (47), and the non-peptide Substance P (SP) antagonist RP-67,580 has been shown to significantly ameliorate both the neuroinflammatory reaction and the level of astrocyte activation (48). Although this showed that SP plays a role in generating the inflammatory response in this PTRE, recent evidence has shown that this is complex, since infected SP knockout mice show a novel phenotype in which the clinical and neuroinflammatory responses were dissociated with evidence of alternative tachykinin receptor usage (49). There is also evidence for the role of various chemokines such as macrophage inflammatory protein (MIP)-2, RANTES, and MIP-1α produced by astrocytes, microglia, and T cells early in the CNS infection in a rat model (50). It should also be pointed out that in both human disease and animal models the cellular sources of these cytokines and neuropeptides are sometimes not known and are only inferred, with multiple stimuli for their secretion likely.
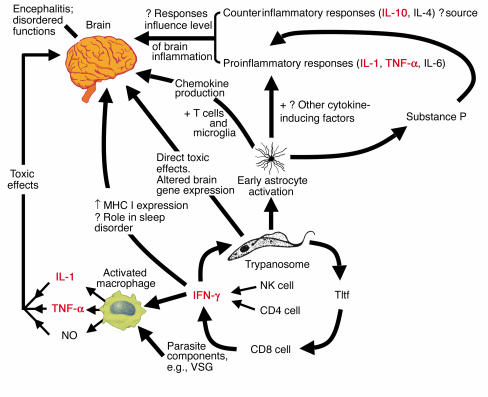
It is clear that macrophage activation by both parasite components and host-derived cytokines is central to HAT pathogenesis. Both VSG and GPI anchors are known to be potent macrophage activators (51, 52), as is IFN-γ, which itself may derive from several sources, including CD4+ and NK cells (53). A molecule called trypanosome-derived lymphocyte triggering factor has been described in mouse and rat models (54, 55). This molecule triggers the CD8+ T cell to produce IFN-γ, which both activates macrophages and apparently has growth-enhancing effects on trypanosomes (54, 56). The overall picture that is now emerging is a highly complex network of cytokine-brain interactions, with early astrocyte activation, macrophage activation, and, at least in animal models, an inflammatory cytokine response being prominent features (43, 52, 57, 58) (summarized schematically in Figure 6).
Figure 6.
Schematic representation of possible immunopathologic pathways leading to brain dysfunction in late-stage human African trypanosomiasis. Concepts are based on a combination of human and animal model data and ideas, particularly from refs. 43, 48, 51, 53, and 56. Cytokines shown in red probably have important roles in neuropathogenesis. The schematic emphasizes the central importance of early astrocyte activation, cytokine responses, and macrophage activation. One should note that there are likely to be multiple factors acting together to produce brain damage and also multiple potential sources of different cytokines. Tltf, trypanosome-derived lymphocyte triggering factor.
Prospects for CNS sleeping sickness
Advances in this field are likely to be made in several areas. The increasing use of animal models, including host and parasite gene knockouts, should help unravel the complex neuropathogenesis of sleeping sickness, in particular the role of specific neuropeptides and the importance of the balance of proinflammatory versus counterinflammatory cytokines. But such studies will need to be interpreted in the context of sophisticated analyses of the serum and CSF of human subjects.
Physicians need to reach a consensus as to what does and does not define late-stage HAT, and to be able to use reliable surrogate markers that will allow them to make rational therapeutic decisions, in particular when embarking on a treatment modality that currently has an overall mortality rate of 5%. There is a pressing need to developing a quick, easy to perform, reliable, and cheap diagnostic test that can be used in the field to diagnose and, crucially, to stage both gambiense and rhodesiense disease.
Control of sleeping sickness will require: (a) continuing and improved case surveillance with screening of humans in at-risk areas and also of domestic cattle; this will require both political will and stability and significantly increased funding to improve the screening infrastructure; (b) better treatment of human disease and of animal reservoirs; and (c) increased public health measures to significantly decrease, and ultimately eradicate, human/tsetse fly contact through the use of, for example, increasingly sophisticated fly traps in infected areas, spraying of insecticides, and molecular genetic approaches such as the replacement of susceptible insect phenotypes with their engineered refractory counterparts to result in decreased HAT transmission (7).
The unacceptable toxicity of the currently available drugs for HAT underpins the urgency of developing more effective and safer drug regimes. A safe drug that is effective in the treatment of CNS HAT would dramatically change the control and management of sleeping sickness, as it would obviate the current difficulties of staging with CSF analysis. However, in reality no new drugs are likely to appear within the next 5 years, and even that may be overly optimistic. An effective oral drug is required for early-stage disease, but several recent candidate compounds have been abandoned because of unacceptable toxicity or lack of efficacy. The best, indeed only, candidate is DB 289, which is a diamidine derivative and the oral prodrug of an active form called DB 75 (27). A phase IIa clinical trial with DB 289 has just been completed, with good results in terms of safety and efficacy, but the drug will probably only be effective in early-stage disease (C. Burri, personal communication). A phase IIb multi-center, randomized, controlled trial of 80 patients with DB 289 is currently under way (C. Burri, personal communication). DFMO is effective for late-stage gambiense disease and is far less toxic than melarsoprol, but it became an orphan drug, as it was expensive and non-profitable for pharmaceutical companies. Only as a result of the remarkable efforts of Médecins Sans Frontières, working with the WHO and the drug companies Aventis Pharma and Bristol-Myers, who had developed a renewed interest in this drug, is DFMO currently available for HAT treatment in Africa (59).
Another avenue of treatment is the use of combination therapy in order to increase efficacy, decrease toxicity, and delay the onset of drug resistance. Drug combinations also have the potential to solve the problems of complexity and high costs of current alternatives to melarsoprol. This approach can also be tested in the mouse model of HAT, which can provide valuable clues for novel treatment strategies (43). Current regimes of combination therapy which can be explored in humans with CNS HAT disease include DFMO/melarsoprol, melarsoprol/nifurtimox, and DFMO/nifurtimox. The latter regime, having shown lower toxicity in limited clinical studies, is currently under evaluation in a controlled clinical trial (G. Priotto, personal communication). A major hope for the future is that the morbidity and mortality from PTRE can be reduced from their current high level. Recent advances in our understanding of normal blood-brain barrier function and permeability have raised the possibility that existing or new trypanocidal drugs may be modified so as to cross the blood-brain barrier, thereby opening up a new therapeutic dimension for CNS sleeping sickness. A further approach is to modify dose regimes of currently available drugs, as has been the case with melarsoprol (32). More targeted approaches to treatment should also be possible, such as adjunct therapy of standard drug regimes with humanized neuropeptide antagonists to specifically block key components of the inflammatory response.
Acknowledgments
I wish to express my sincere gratitude to Jorge Atouguia, Els Torreele, Jeremy Sternberg, and Max Murray for their help with this article. Personal research described here was carried out with the financial support of the Wellcome Trust and the Sir Jules Thorne Charitable Trust.
Footnotes
The Science in Medicine series is supported by a generous grant from the Doris Duke Charitable Foundation.
Conflict of interest: The author has declared that no conflict of interest exists.
Nonstandard abbreviations used: human African trypanosomiasis (HAT); cerebrospinal fluid (CSF); variant surface glycoprotein (VSG); glycosylphosphatidylinositol (GPI); card agglutination trypanosomiasis test (CATT); white blood cell (WBC); electroencephalogram (EEG); difluoromethylornithine (DFMO); intramuscular (i.m.); post-treatment reactive encephalopathy (PTRE); Substance P (SP); macrophage inflammatory protein (MIP).
References
- 1.Vickerman, K. 1997. Landmarks in trypanosome research. In Trypanosomiasis and leishmaniasis. G. Hide, J.C. Mottram, G.H. Coombs, and P.H. Holmes, editors. Cab International. Oxford, United Kingdom. 1–37.
- 2.Williams, B.I. 1996. African trypanosomiasis. In The Wellcome Trust illustrated history of tropical diseases. F.E.A.G. Cox, editor. The Wellcome Trust. London, United Kingdom. 178–191.
- 3.Bentivoglio M, Grassi-Zucconi G, Kristensson K. From trypanosomes to the nervous system, from molecules to behavior: a survey, on the occasion of the 90th anniversary of Castellani’s discovery of the parasites in sleeping sickness. Ital. J. Neurol. Sci. 1994;15:77–89. doi: 10.1007/BF02340118. [DOI] [PubMed] [Google Scholar]
- 4.1986. Epidemiology and control of African trypanosomiasis. Report of a WHO expert committee. World Health Organization. Geneva, Switzerland. Technical Report Series, No. 739. 126 pp. [PubMed]
- 5.Atouguia, J.L.M., and Kennedy, P.G.E. 2000. Neurological aspects of human African trypanosomiasis. In Infectious diseases of the nervous system. L.E. Davis and P.G.E. Kennedy, editors. Butterworth-Heinemann. Oxford, United Kingdom. 321–372.
- 6.Kuzoe FA. Current situation of African trypanosomiasis. Acta Trop. 1993;54:153–162. doi: 10.1016/0001-706x(93)90089-t. [DOI] [PubMed] [Google Scholar]
- 7.Aksoy S. Control of tsetse flies and trypanosomes using molecular genetics. Vet. Parasitol. 2003;115:125–145. doi: 10.1016/s0304-4017(03)00203-6. [DOI] [PubMed] [Google Scholar]
- 8.El-Sayed, N.M.A., and Donelson, J.E. 1997. Sequencing and mapping the African trypanosome enome. In Trypanosomiasis and leishmaniasis. G. Hide, J.C. Mottram, G.H. Coombs, and P.H. Holmes, editors. Cab International. Oxford, United Kingdom. 51–55.
- 9.Donelson JE. Antigenic variation and the African trypanosome genome. Acta Tropica. 2002;85:391–404. doi: 10.1016/s0001-706x(02)00237-1. [DOI] [PubMed] [Google Scholar]
- 10.Barry, J.D. 1997. The biology of antigenic variation in African trypanosomes. In Trypanosomiasis and leishmaniasis. G. Hide, J.C. Mottram, G.H. Coombs, and P.H. Holmes, editors. Cab International. Oxford, United Kingdom. 89–107.
- 11.Apted, F.I.C. 1970. Clinical manifestations and diagnosis of sleeping sickness. In The African trypanosomiasis. H.W. Mulligan, editor. George Allen & Unwin. London, United Kingdom. 661–683.
- 12.Duggan AJ, Hutchington MP. Sleeping sickness in Europeans: a review of 109 cases. J. Trop. Med. Hyg. 1966;69:124–131. [PubMed] [Google Scholar]
- 13.Kristensson, K., Grassi-Zucconi, G., and Bentivoglio, M. 1995. Nervous system dysfunctions in African trypanosomiasis. In Recent advances in tropical neurology. F. Clifford Rose, editor. Elsevier Science BV. Oxford, United Kingdom. 165–174.
- 14.Truc P, et al. Evaluation of the micro-CATT, CATT/Trypanosoma brucei gambiense, and LATEX/T.b. gambiense methods for serodiagnosis and surveillance of human African trypanosomiasis in West and Central Africa. Bull. World Health Organ. 2002;80:882–886. [PMC free article] [PubMed] [Google Scholar]
- 15.1998. Control and surveillance of African trypanosomiasis. Report of a WHO expert committee. World Health Organization. Geneva, Switzerland. Technical Report Series, No. 881. 114 pp. [PubMed]
- 16.Lejon V, et al. Intrathecal immune response pattern for improved diagnosis of central nervous system involvement in trypanosomiasis. J. Infect. Dis. 2003;187:1475–1483. doi: 10.1086/374645. [DOI] [PubMed] [Google Scholar]
- 17.Jamonneau V, et al. Stage determination and therapeutic decision in human African trypanosomiasis: value of polymerase chain reaction and immunoglobulin M quantification on the cerebrospinal fluid of sleeping sickness patients in Côte d’Ivoire. Trop. Med. Int. Health. 2003;8:589–594. doi: 10.1046/j.1365-3156.2003.01079.x. [DOI] [PubMed] [Google Scholar]
- 18.Lejon V, et al. IgM quantification in the cerebrospinal fluid of sleeping sickness patients by a latex card agglutination test. Trop. Med. Int. Health. 2002;7:685–692. doi: 10.1046/j.1365-3156.2002.00917.x. [DOI] [PubMed] [Google Scholar]
- 19.Solano P, et al. Comparison of different DNA preparation protocols for PCR diagnosis of human African trypanosomosis in Côte d’Ivoire. Acta Trop. 2002;82:349–356. doi: 10.1016/s0001-706x(02)00029-3. [DOI] [PubMed] [Google Scholar]
- 20.Gill DS, Chatha DS, del Carpio-O’Donovan R. MR imaging findings in African trypansomiasis. Am. J. Neuroradiol. 2003;24:1383–1385. [PMC free article] [PubMed] [Google Scholar]
- 21.Baumann H, Strassmann G. Suramin inhibits the stimulation of acute phase plasma protein genes by IL-6-type cytokines in rat hepatoma cells. J. Immunol. 1993;151:1456–1462. [PubMed] [Google Scholar]
- 22.Benaim G, Lopez-Estrano C, Docampo R, Moreno SN. A calmodulin-stimulated Ca2+ pump in plasma-membrane vesicles from Trypanosoma brucei; selective inhibition by pentamidine. Biochem. J. 1993;296:759–763. doi: 10.1042/bj2960759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fairlamb AH, Henderson GB, Cerami A. Trypanothione is the primary target for arsenical drugs against African trypanosomes. Proc. Natl. Acad. Sci. U. S. A. 1989;86:2607–2611. doi: 10.1073/pnas.86.8.2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Metcalf BW, et al. Catalytic irreversible inhibition of mammalian ornithine decarboxylase by substrate and product analogues. J. Am. Chem. Soc. 1978;100:2551–2553. [Google Scholar]
- 25.Henderson GB, et al. “Subversive” substrates for the enzyme trypanothione disulfide reductase: alternative approach to chemotherapy of Chagas disease. Proc. Natl. Acad. Sci. U. S. A. 1988;85:5374–5378. doi: 10.1073/pnas.85.15.5374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fairlamb AH. Future prospects for the chemotherapy of human trypanosomiasis. 1. Novel approaches to the chemotherapy of trypanosomiasis. Trans. R. Soc. Trop. Med. Hyg. 1990;84:613–617. doi: 10.1016/0035-9203(90)90124-w. [DOI] [PubMed] [Google Scholar]
- 27.Legros D, et al. Treatment of human African trypanosomiasis — present situation and needs for research and development. Lancet Infect. Dis. 2002;2:437–440. doi: 10.1016/s1473-3099(02)00321-3. [DOI] [PubMed] [Google Scholar]
- 28.Legros D, Evans S, Maiso F, Enyaru JCK, Mbulamberi D. Risk factors for treatment failure after melarsoprol for Trypanosoma brucei gambiense trypanosomiasis in Uganda. Trans. R. Soc. Trop. Med. Hyg. 1994;93:439–442. doi: 10.1016/s0035-9203(99)90151-7. [DOI] [PubMed] [Google Scholar]
- 29.Pepin J, Milord F. The treatment of human African trypanosomiasis. Adv. Parasitol. 1994;33:1–47. doi: 10.1016/s0065-308x(08)60410-8. [DOI] [PubMed] [Google Scholar]
- 30.Pepin J, et al. Gambiense trypanosomiasis: frequency of, and risk factors for, failure of melarsoprol therapy. Trans. R. Soc. Trop. Med. Hyg. 1994;88:447–452. doi: 10.1016/0035-9203(94)90430-8. [DOI] [PubMed] [Google Scholar]
- 31.Pepin J, et al. Trial of prednisolone for prevention of melarsoprol-induced encephalopathy in gambiense sleeping sickness. Lancet. 1989;1:1246–1249. doi: 10.1016/s0140-6736(89)92340-4. [DOI] [PubMed] [Google Scholar]
- 32.Burri C, et al. Efficacy of new, concise schedule for melarsoprol in treatment of sleeping sickness caused by Trypanosoma brucei gambiense: a randomised trial. Lancet. 2000;355:1419–1425. doi: 10.1016/S0140-6736(00)02141-3. [DOI] [PubMed] [Google Scholar]
- 33.Burri C, Brun R. Eflornithine for the treatment of human African trypanosomiasis. Parasitol. Res. 2003;90(Suppl. 1):S49–S52. doi: 10.1007/s00436-002-0766-5. [DOI] [PubMed] [Google Scholar]
- 34.Adams JH, et al. Human African trypanosomiasis (T.b. gambiense): a study of 16 fatal cases of sleeping sickness with some observations on acute reactive arsenical encephalopathy. Neuropathol. Appl. Neurobiol. 1986;12:81–94. doi: 10.1111/j.1365-2990.1986.tb00682.x. [DOI] [PubMed] [Google Scholar]
- 35.MacLean L, Odiit M, Sternberg JM. Nitric oxide and cytokine synthesis in human African trypanosomiasis. J. Infect. Dis. 2001;184:1086–1090. doi: 10.1086/323479. [DOI] [PubMed] [Google Scholar]
- 36.Lejon V, et al. Interleukin (IL)-6, IL-8 and IL-10 in serum and CSF of Trypanosoma brucei gambiense sleeping sickness patients before and after treatment. Trans. R. Soc. Trop. Med. Hyg. 2002;96:329–333. doi: 10.1016/s0035-9203(02)90115-x. [DOI] [PubMed] [Google Scholar]
- 37.Pentreath VW. Trypanosomiasis and the nervous system. Pathology and immunology. Trans. R. Soc. Trop. Med. Hyg. 1995;89:9–15. doi: 10.1016/0035-9203(95)90637-1. [DOI] [PubMed] [Google Scholar]
- 38.Pentreath VW. Neurobiology of sleeping sickness. Parasitol. Today. 1989;5:215–218. doi: 10.1016/0169-4758(89)90273-1. [DOI] [PubMed] [Google Scholar]
- 39.Pepin J, Milord F. African trypanosomiasis and drug induced encephalopathy: risk factors and pathogenesis. Trans. R. Soc. Trop. Med. Hyg. 1991;85:222–224. doi: 10.1016/0035-9203(91)90032-t. [DOI] [PubMed] [Google Scholar]
- 40.Lambert PH, Berney M, Kazyumba GL. Immune complexes in serum and cerebrospinal fluid in sleeping sickness. Correlation with polyclonal B-cell activation and with intracerebral immunoglobulin synthesis. J. Clin. Invest. 1981;67:77–85. doi: 10.1172/JCI110035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hunter CA, Jennings FW, Adams JH, Murray M, Kennedy PGE. Sub-curative chemotherapy and fatal post-treatment reactive encephalopathies in African trypanosomiasis. Lancet. 1992;339:956–958. doi: 10.1016/0140-6736(92)91531-c. [DOI] [PubMed] [Google Scholar]
- 42.Poltera AA. Immunopathological and chemotherapeutic studies in experimental trypanosomiasis with special reference to the heart and brain. Trans. R. Soc. Trop. Med. Hyg. 1980;74:706–715. doi: 10.1016/0035-9203(80)90183-2. [DOI] [PubMed] [Google Scholar]
- 43.Kennedy PGE. The pathogenesis and modulation of the post-treatment reactive encephalopathy in a mouse model of human African trypanosomiasis. J. Neuroimmunol. 1999;100:36–41. doi: 10.1016/s0165-5728(99)00196-4. [DOI] [PubMed] [Google Scholar]
- 44.Hunter CA, Jennings FW, Kennedy PGE, Murray M. Astrocyte activation correlates with cytokine production in central nervous system of Trypanosoma brucei brucei-infected mice. Lab. Invest. 1992;67:635–642. [PubMed] [Google Scholar]
- 45.Hunter CA, Gow JW, Kennedy PGE, Jennings FW, Murray M. Immunopathology of experimental African sleeping sickness: detection of cytokine mRNA in the brains of Trypanosoma brucei brucei-infected mice. Infect. Immun. 1991;59:4636–4640. doi: 10.1128/iai.59.12.4636-4640.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jennings FW, et al. The role of the polyamine inhibitor eflornithine in the neuropathogenesis of experimental murine African trypanosomiasis. Neuropathol. Appl. Neurobiol. 1997;23:225–234. [PubMed] [Google Scholar]
- 47.Hunter CA, Jennings FW, Kennedy PGE, Murray M. The use of azathioprine to ameliorate post-treatment encephalopathy associated with African trypanosomiasis. Neuropathol. Appl. Neurobiol. 1992;18:619–625. doi: 10.1111/j.1365-2990.1992.tb00833.x. [DOI] [PubMed] [Google Scholar]
- 48.Kennedy PGE, et al. A substance P antagonist, RP-67,580, ameliorates a mouse meningoencephalitic response to Trypanosoma brucei brucei. Proc. Natl. Acad. Sci. U. S. A. 1997;94:4167–4170. doi: 10.1073/pnas.94.8.4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kennedy PGE, et al. Clinical and neuroinflammatory responses to meningoencephalitis in Substance P receptor knockout mice. Brain. 2003;16:1683–1690. doi: 10.1093/brain/awg160. [DOI] [PubMed] [Google Scholar]
- 50.Sharafeldin A, Eltayeb R, Pashendov M, Bakhiet M. Chemokines are produced in the brain early during the course of experimental African trypanosomiasis. J. Neuroimmunol. 2000;103:165–170. doi: 10.1016/s0165-5728(99)00238-6. [DOI] [PubMed] [Google Scholar]
- 51.Paulnock DM, Coller SP. Analysis of macrophage activation in African trypanosomiasis. J. Leukoc. Biol. 2001;69:685–690. [PubMed] [Google Scholar]
- 52.Magez S, et al. The glycosyl-inositol-phosphate and dimyristoylglycerol moieties of the glycosylphosphatidylinositol anchor of the trypanosome variant-specific surface glycoprotein are distinct macrophage-activating factors. J. Immunol. 1998;160:1949–1956. [PubMed] [Google Scholar]
- 53.Sternberg JM. Immunobiology of African trypanosomiasis. Chem. Immunol. 1998;70:186–199. doi: 10.1159/000058706. [DOI] [PubMed] [Google Scholar]
- 54.Olsson T, et al. CD8 is critically involved in lymphocyte activation by a T. brucei brucei-released molecule. Cell. 1993;72:715–727. doi: 10.1016/0092-8674(93)90400-k. [DOI] [PubMed] [Google Scholar]
- 55.Vaidya T, et al. The gene for a T lymphocyte triggering factor from African trypanosomes. J. Exp. Med. 1997;186:433–438. doi: 10.1084/jem.186.3.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bentivoglio M, Grassi-Zucconi G, Olsson T, Kristensson K. Trypanosoma brucei and the nervous system. Trends Neurosci. 1994;17:325–329. doi: 10.1016/0166-2236(94)90170-8. [DOI] [PubMed] [Google Scholar]
- 57.Hunter CA, Kennedy PGE. Immunopathology in central nervous system human African trypanosomiasis. J. Neuroimmunol. 1992;36:91–95. doi: 10.1016/0165-5728(92)90040-r. [DOI] [PubMed] [Google Scholar]
- 58.Schleifer KW, Filutowicz H, Schopf LR, Mansfield JM. Characterization of T helper cell responses to the trypanosome variant surface glycoprotein. J. Immunol. 1993;150:2910–2919. [PubMed] [Google Scholar]
- 59.Kennedy PGE, Murray M, Jennings F, Rodgers J. Sleeping sickness: new drugs from old? Lancet. 2002;359:1695–1696. doi: 10.1016/s0140-6736(02)08569-0. [DOI] [PubMed] [Google Scholar]