Abstract
Purpose
To investigate the prophylactic effect of Tamsulosin, a super-selective alpha-1a adrenergic blocking agent, on the development of urinary retention in men undergoing elective inguinal herniorrhaphy.
Materials and Methods
From May 2010 through November 2011, a total of 80 males who underwent elective inguinal herniorrhaphy in a university hospital were included in this study. Patients were randomly assigned to one of two groups. In group one (control), the patients were given two doses of placebo orally, 6 hours before surgery and 6 to 12 hours after surgery. Patients in group two were given 0.4 mg of Tamsulosin orally in the same manner as the placebo. All patients were closely followed for 24 hours post-operatively, and any voiding difficulties or urinary retention was recorded.
Results
There were 40 patients in group one (control group) and 40 patients in group two (Tamsulosin group). The patients' mean age was 64 years. In group one, 6 patients and in group two, 1 patient required catheterization. Thus, 15% of patients in group I and 2.5% of patients in group II had urinary retention. The difference in the requirement for catheterization was statistically significant (p=0.04). The technique of herniorrhaphy, the side of the body in which the hernia was located, the type of anesthesia, the duration of the surgery, and the severity of pre-operative urinary symptoms had no significant effect on the incidence of urinary retention.
Conclusions
The use of perioperative Tamsulosin represents an effective strategy to reduce the risk of post-operative urinary retention following inguinal herniorrhaphy.
Keywords: Adrenergic alpha-Antagonists, Herniorrhaphy, Tamsulosin, Urinary retention
INTRODUCTION
Postoperative urinary retention (POUR) is a common and potentially serious morbidity with a reported incidence of 3 to 25% [1]. POUR has generally been defined as the inability to pass any urine in the presence of a palpable or percussible bladder after surgery, but the definition varies widely.
POUR occurs in patients of both sexes and all age groups and after all types of surgical procedures. It occurs more frequently after lower urinary tract, perineal, gynecologic, and anorectal surgeries [1].
Both the health and financial costs of retention are considerable, because it can cause urinary tract infections and necessitate catheterization, which can in turn result in urethral strictures, prolonged hospital stays, and additional operations. Because of the necessity to seek immediate medical attention in order to relieve the severe discomfort, renal insufficiency is not often a complicating factor.
In several clinical studies, alpha-adrenergic blocking agents have been shown to have prophylactic and therapeutic potential on POUR [2,3]. The alpha-1 receptor antagonist acts by reducing tone in the bladder outlet, thereby decreasing outflow resistance and facilitating micturition.
The aim of this randomized, prospective study was to investigate the prophylactic effect of tamsulosin, a super-selective alpha-1a adrenergic blocking agent, on the development of urinary retention in men undergoing elective inguinal herniorrhaphy.
MATERIALS AND METHODS
From May 2010 through November 2011, a total of 80 males who underwent elective inguinal herniorrhaphy in a university hospital were included in this study. Female patients were not studied because of the small sample size.
The criteria for exclusion were as follows: active urinary tract infection, recurrent or bilateral hernia, age younger than 50 years, cardiopulmonary or other significant systemic diseases, previous neurological disease, medications that could affect voiding function such as cholinergic drugs, previous urological surgery, previous urological disease such as urethral stricture or bladder or prostatic cancer, serum creatinine greater than 1.6 mg/dl, urinary incontinency, and those with an indwelling Foley catheter.
All patients had a complete physical examination preoperatively, electrocardiogram, chest X-ray, and baseline blood analysis and urinalysis. In addition, all underwent preoperative ultrasonographic investigation (measurement of residual urine and prostatic volume). Residual urine volume was measured just before the operation.
After informed consent was obtained from the patients, they were randomly assigned to one of two groups by the selection of an envelope in the preoperative holding area. In group one (control), the patients were given two doses of placebo orally, 6 hours before surgery and 6 to 12 hours after surgery. Patients in group two were given 0.4 mg of tamsulosin orally in the same manner as the placebo.
In all cases, administration of lactated Ringer's solution (1.5 ml/kg/h) was begun in the operating room before the anesthetic was given and was continued postoperatively during the NPO period (for 4 to 6 hours).
Surgery was performed after the administration of either a general endotracheal anesthetic agent (halothane) or a short-acting spinal anesthetic (5% lidocaine). The attending anesthesiologist chose the type of anesthetic without consulting the surgeon.
The hernia repairs were done by use of either the Bassini procedure or McWay.
Postoperatively, the patients were initially given either pentazocine or morphine and then diclofenac suppository for pain control. The dosage of analgesia administered was adjusted until the patients reported zero to mild pain scores on a visual analogue scale.
All patients were closely followed for 24 hours postoperatively, and any voiding difficulties or urinary retention was recorded.
Urinary retention was diagnosed when a patient had a palpable mass in the suprapubic area, felt discomfort, and failed to pass urine within 24 hours after the operation despite a sufficient fluid intake and when conservative efforts such as warming the suprapubic region and encouraging the patient to stand up and walk were unsuccessful and bladder catheterization seemed inevitable.
Statistical analysis was accomplished by use of the chi-square test and Fisher's exact test with a p-value of less than 0.05 considered significant.
RESULTS
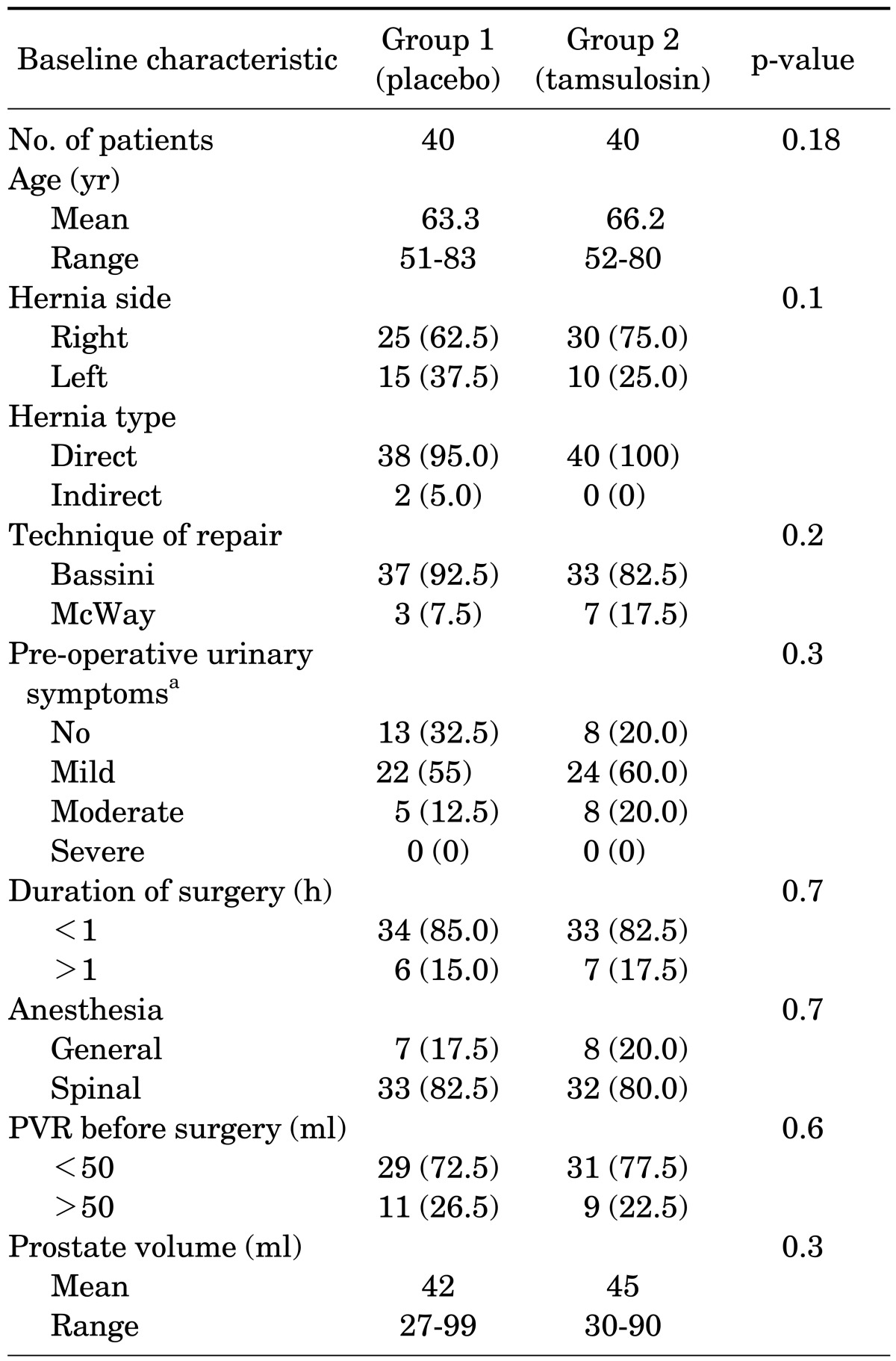
There were 40 patients in group one (control group) and 40 patients in group two (tamsulosin group). Fifty-five hernias were right-sided and 25 were left-sided. The type of repair performed was modified Bassini in 70 patients and Cooper ligament repair (McWay) in 10 patients. Six patients in group one and 4 in group two had diabetes mellitus. The characteristics of the patients are listed in Table 1.
TABLE 1.
Patient characteristics and operative parameters
Values are presented as number (%).
AUA, American Urological Association; PVR, post-void urinary retention.
a:According to AUA symptom score.
No statistically significant differences were found between the two groups in terms of age (p=0.18), severity of preoperative urinary symptoms (p=0.3), post-voiding residual urine volume (p=0.6), type of anesthesia (p=0.7), or duration of surgery (p=0.7). The average nonsteroidal anti-inflammatory drug use in the two groups was similar (200 mg diclofenac suppository).
In group one, 6 patients required catheterization with a mean urine volume of 800 ml at catheterization. In group two, 1 patient required catheterization with a 600-ml mean urine volume. Thus, 15% of patients in group 1 and 2.5% of patients in group 2 had urinary retention.
The difference in the requirement for catheterization was statistically significant (p=0.04).
Too few patients had indirect inguinal hernia, thus precluding meaningful evaluation of the type of hernia.
The technique of herniorrhaphy (p=0.4), the side of the body in which the hernia was located (p=0.3), the type of anesthesia (p=0.2), and the duration of the surgery (p=0.1) had no significant effect on the incidence of urinary retention and the necessity for catheterization (data not shown). Also, there was no statistically significant difference between the incidence of urinary retention and the severity of pre-operative urinary symptoms (p=0.4).
No complications or side effects of therapy were encountered during the treatment with tamsulosin or placebo.
DISCUSSION
Urinary retention is a common complication after any surgical procedure. It occurs more frequently after lower urinary tract, perineal, gynecologic, and anorectal surgeries. The incidence of urinary retention after herniorrhaphy ranges from 0.2 to 30% [2]. The widely varying reported incidence of POUR reflects differences in patient characteristics, the lack of uniform defining criteria, and the multifactorial etiology of POUR, including age, gender, inadequate perioperative fluids, type of anesthesia, and type of surgery [4].
Urinary retention produces discomfort and it can lead to urethral injury and urinary tract infection following catheterization. In addition, with increasing financial constraints and the trend toward early discharge, avoidance of this perioperative complication is particularly important. Although most patients may recover from POUR with a trial without catheter (TWOC) after a few days of catheterization, some patients with POUR can have persistent urinary retention after failure of TWOC and this prolongs or complicates the postoperative recovery phase. Therefore, efforts should be made to prevent POUR, especially in men at high risk for the condition.
Many factors contribute to the development of POUR. These include the direct effects of anesthetic agents on the bladder, traumatic instrumentation, pelvic dissection, overzealous intravenous hydration resulting in bladder distension, diminished awareness of bladder sensation, increased outlet resistance, immobilization after the procedure, postoperative pain (nociceptive inhibitory reflex), use of narcotics for analgesia, and patient age and sex [5].
Toyonaga et al. [6] showed that female sex, preoperative urinary symptoms, diabetes mellitus, large amounts of intravenous fluid administered perioperatively, and postoperative pain are independent risk factors for urinary retention in selected cases of anorectal surgery such as hemorrhoidectomy and fistulectomy.
Petros et al. [5] retrospectively reviewed 295 inguinal herniorrhaphies in men. They found age less than 53 years, spinal anesthesia, and perioperative fluids less than 1,200 ml all significantly reduced the incidence of POUR.
The contractility of the detrusor decreases with advancing age. Accordingly, in light of previous studies, it was generally assumed that POUR increases with age, with the risk increasing by 2.4 to 2.8 times in patients over 50 years of age [7]. Age is an independent factor predicting successful TWOC for POUR and patients older than 70 years are at a 1.8 times higher risk of failure of TWOC than are those younger than 70 years [8].
Controversy exists about the impact of gender on the development of POUR. Some studies have shown that gender is not significantly correlated with the development of POUR [5], whereas other studies reported a higher incidence of POUR in men than in women [9].
Many different methods have been tried to prevent this complication, including the use of parasympathomimetic agents, use of α-adrenergic blockers, use of anxiolytic agents, restriction of perioperative fluid intake, avoidance of anal packing, sitz baths, use of local anesthesia, use of short-acting anesthesia, and outpatient surgery.
Some precautions, such as limitation of fluid intake, early mobilization, warm compress to the suprapubic area, and the use of short-acting local or spinal anesthesia have been reported to prevent this complication.
The overwhelming majority of inguinal herniorrhaphies are performed as elective outpatient procedures without intra-operative catheterization. Therefore, limiting the perioperative volume of fluids and controlling pain are reasonable measures to reduce POUR [10].
In a randomized prospective study of perioperative fluid restriction in anorectal surgery, Bailey and Ferguson were able to reduce urinary retention from 14.9 to 3.5% [11].
The avoidance of acute bladder overdistention in order to prevent POUR is supported by the experimental observation of a reduced bladder response to sacral neural stimulation during overdistention (>80% reduction) as well as after overdistention (19% reduction) [12].
The type of anesthesia during surgery is another important factor in the development of POUR. In a group of 880 patients undergoing inguinal herniorrhaphy using local anesthesia, Finley et al. [13] noticed that the incidence of POUR was 0.2%. During the same period, a similar group of 200 patients had their hernias repaired with the use of general or spinal anesthesia. The incidence of POUR in that group was 13%. The authors contended that the use of local anesthesia in inguinal hernia repair almost eliminates POUR.
Jensen et al. [14] recently reported a Medline-based search intended to determine the incidence of POUR following herniorrhaphy. The incidences of POUR following inguinal herniorrhaphies performed under local anesthesia, regional anesthesia, and general anesthesia were 0.37%, 2.4%, and 3.0%, respectively. The investigators concluded that the type of anesthesia significantly influenced the risk of POUR.
An alternative explanation for the low risk of POUR following herniorrhaphies performed under local anesthesia is superior pain control [15].
Efforts toward the pharmacological prevention and treatment of POUR have focused on increasing detrusor muscle activity or decreasing the opening pressure of the internal sphincter at the bladder neck.
It is well documented that adrenergic receptors are present throughout the bladder. Beta-adrenergic receptors are predominantly in the body and dome, and alpha-adrenergic receptors are in the base and neck of the bladder.
In the acute postoperative setting, sympathetic nerve discharge causes catecholamine release and α-adrenergic-mediated contraction of the bladder neck, resulting in functional obstruction of the bladder outlet.
Administration of sympathomimetic and anticholinergic agents (for example, phenylephrine and atropine) during anesthesia can inhibit contraction of the detrusor muscle in the bladder. This relaxes the bladder and decreases the urge to void and the recognition that the bladder is full.
The pain in the inguinal area can stimulate the α-receptors in the bladder neck and proximal urethra, thereby increasing urethral resistance and bladder outlet tone. The end result is that attempts to void encounter increased output resistance [16].
In a review article published by Buckley and Lapitan in 2010 to find the best drug for the treatment of POUR in adults, they concluded that no statistically significant associations were reported between successful treatment or any other outcome and cholinergic agents, alpha-blockers, and sedatives as monotherapies. A statistically significant association between intravesically administered prostaglandin and successful voiding was detected (risk ratio, 3.07). A statistically significant association was detected between cholinergic agents combined with sedative and an improved likelihood of spontaneous voiding compared with placebo (risk ratio, 1.39) [17].
Bethanechol (a parasympathomimetic agent) acts primarily on the postganglionic effector cells, with a relatively selective muscarinic action on the smooth muscle of the bladder. Thus, bethanechol increases the intravesical pressure but produces no relaxation in the bladder outlet. Indeed, bethanechol has been shown to be virtually useless in humans and to do no more than increase bladder discomfort and distress. Accordingly, bethanechol cannot be recommended as the drug of choice in the management of POUR [18].
α-Adrenergic blockade with phenoxybenzamine historically has seemed effective prophylactically in decreasing the incidence of postoperative retention. Velanovich performed a meta-analysis on the use of phenoxybenzamine and concluded that this agent reduced the occurrence by 29.1% [14].
Goldman et al. [2] performed a randomized, placebo-controlled trial to determine the role of alpha-blockers in reducing the risk of POUR after hernioplasty. The study included 102 men older than 60 years who were randomly assigned to receive phenoxybenzamine or a control. POUR developed in 26% of men in the control group and 0% of men who received phenoxybenzamine.
Prazosin, a selective alpha 1-adrenoceptor antagonist, produces clinical effects similar to those of phenoxybenzamine with less significant side effects. Prazosin has little selectivity for pre-synaptic alpha-2 adrenergic receptors but has marked selectivity for post-synaptic (alpha-1) adrenergic receptors. Gonullu et al. [3] demonstrated that the incidence of urinary retention after herniorrhaphy was 25% in the placebo group, of whom 13.8% required catheterization. By contrast, 10.8% in the prazosin group developed urinary retention, of whom 3.5% required catheterization.
Tamsulosin is a superselective adrenoceptor antagonist (alpha-1a). Its preventive effect has not been previously studied in reducing the risk of POUR after herniorrhaphy. Patel et al. [19] investigated the potential efficacy of alpha-blockers for facilitating early removal of the urinary catheter following radical prostatectomy. A consecutive group of 250 men undergoing radical prostatectomy received tamsulosin, 0.4 mg, 3 days before a cystography planned for postoperative day 8. Tamsulosin was administered for an additional 4 days following the catheter removal. The incidence of POUR in the men who received tamsulosin was only 2.6% compared with 10% in control group.
Djavan et al. [20] reported a randomized study evaluating neoadjuvant and adjuvant alpha-blockade as a strategy to decrease the risk of acute urinary retention following transurethral microwave thermotherapy (TUMT). In that study, 41 men with benign prostatic hyperplasia underwent TUMT with neoadjuvant and adjuvant tamsulosin therapy (0.4 mg daily) and 40 men underwent TUMT alone. Urinary retention was observed in 12% of the TUMT-alone group and 2% of the tamsulosin-treated group. The use of neoadjuvant and adjuvant tamsulosin represents an effective strategy to reduce the risk of catheter dependency following TUMT and provides immediate symptom relief.
In the present study, 1 of 40 patients (2.5%) in the tamsulosin group developed urinary retention. In the placebo group, 6 of 40 patients (15%) had urinary retention and required catheterization. The incidence of acute urinary retention was significantly greater in men who did not receive tamsulosin before and after surgery.
The incidence of urinary retention showed no statistically significant difference when we considered the technique of herniorrhaphy, the side of the body in which the hernia was located, the type of anesthesia, or the duration of the surgery. The difference was also insignificant when the severity of preoperative urinary symptoms was compared between the two groups. Therefore, the use of tamsulosin can be recommended in adult male patients over 50 years of age who undergo inguinal hernia repair, regardless of their baseline characteristics.
The current study is the only prospective evaluation of tamsulosin as a means of avoiding urinary retention after inguinal herniorrhaphy. This protocol resulted in the reduction of urinary retention from 15% in the controls to 2.5% in the treatment group.
CONCLUSIONS
To the best of our knowledge, the effect of prophylactic tamsulosin has not been investigated in inguinal hernia operations. Our data suggest that tamsulosin significantly reduces the risk of acute urinary retention after surgery. Therefore, we recommend administering a perioperative course of tamsulosin therapy when performing inguinal herniorrhaphy.
Footnotes
The authors have nothing to disclose.
References
- 1.Stallard S, Prescott S. Postoperative urinary retention in general surgical patients. Br J Surg. 1988;75:1141–1143. doi: 10.1002/bjs.1800751128. [DOI] [PubMed] [Google Scholar]
- 2.Goldman G, Leviav A, Mazor A, Kashtan H, Aladgem D, Greenstein A, et al. Alpha-adrenergic blocker for posthernioplasty urinary retention. Prevention and treatment. Arch Surg. 1988;123:35–36. doi: 10.1001/archsurg.1988.01400250037005. [DOI] [PubMed] [Google Scholar]
- 3.Gonullu NN, Dulger M, Utkan NZ, Canturk NZ, Alponat A. Prevention of postherniorrhaphy urinary retention with prazosin. Am Surg. 1999;65:55–58. [PubMed] [Google Scholar]
- 4.Baldini G, Bagry H, Aprikian A, Carli F. Postoperative urinary retention: anesthetic and perioperative considerations. Anesthesiology. 2009;110:1139–1157. doi: 10.1097/ALN.0b013e31819f7aea. [DOI] [PubMed] [Google Scholar]
- 5.Petros JG, Rimm EB, Robillard RJ, Argy O. Factors influencing postoperative urinary retention in patients undergoing elective inguinal herniorrhaphy. Am J Surg. 1991;161:431–433. doi: 10.1016/0002-9610(91)91105-r. [DOI] [PubMed] [Google Scholar]
- 6.Toyonaga T, Matsushima M, Sogawa N, Jiang SF, Matsumura N, Shimojima Y, et al. Postoperative urinary retention after surgery for benign anorectal disease: potential risk factors and strategy for prevention. Int J Colorectal Dis. 2006;21:676–682. doi: 10.1007/s00384-005-0077-2. [DOI] [PubMed] [Google Scholar]
- 7.Lee SJ, Kim YT, Lee TY, Woo YN. Analysis of risk factors for acute urinary retention after non-urogenital surgery. Korean J Urol. 2007;48:1277–1284. [Google Scholar]
- 8.Lee KS, Lim KH, Kim SJ, Choi HJ, Noh DH, Lee HW, et al. Predictors of successful trial without catheter for postoperative urinary retention following non-urological surgery. Int Neurourol J. 2011;15:158–165. doi: 10.5213/inj.2011.15.3.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zaheer S, Reilly WT, Pemberton JH, Ilstrup D. Urinary retention after operations for benign anorectal diseases. Dis Colon Rectum. 1998;41:696–704. doi: 10.1007/BF02236255. [DOI] [PubMed] [Google Scholar]
- 10.Lepor H. Managing and preventing acute urinary retention. Rev Urol. 2005;7(Suppl 8):S26–S33. [PMC free article] [PubMed] [Google Scholar]
- 11.Bailey HR, Ferguson JA. Prevention of urinary retention by fluid restriction following anorectal operations. Dis Colon Rectum. 1976;19:250–252. doi: 10.1007/BF02590913. [DOI] [PubMed] [Google Scholar]
- 12.Bross S, Schumacher S, Scheepe JR, Zendler S, Braun PM, Alken P, et al. Effects of acute urinary bladder overdistension on bladder response during sacral neurostimulation. Eur Urol. 1999;36:354–359. doi: 10.1159/000019999. [DOI] [PubMed] [Google Scholar]
- 13.Finley RK, Jr, Miller SF, Jones LM. Elimination of urinary retention following inguinal herniorrhaphy. Am Surg. 1991;57:486–488. [PubMed] [Google Scholar]
- 14.Jensen P, Mikkelsen T, Kehlet H. Postherniorrhaphy urinary retention--effect of local, regional, and general anesthesia: a review. Reg Anesth Pain Med. 2002;27:612–617. doi: 10.1053/rapm.2002.37122. [DOI] [PubMed] [Google Scholar]
- 15.Mulroy MF. Hernia surgery, anesthetic technique, and urinary retention-apples, oranges, and kumquats? Reg Anesth Pain Med. 2002;27:587–589. doi: 10.1053/rapm.2002.37326. [DOI] [PubMed] [Google Scholar]
- 16.Wein AJ, Dmochowski RR. Neuromuscular dysfunction of the lower urinary tract. In: Wein AJ, Kavoussi LR, Novick AC, Partin AW, Peters CA, editors. Campbell-Walsh urology. 10th ed. Philadelphia: Saunders; 2012. p. 1940. [Google Scholar]
- 17.Buckley BS, Lapitan MC. Drugs for treatment of urinary retention after surgery in adults. Cochrane Database Syst Rev. 2010;(10):CD008023. doi: 10.1002/14651858.CD008023.pub2. [DOI] [PubMed] [Google Scholar]
- 18.Finkbeiner AE. Is bethanechol chloride clinically effective in promoting bladder emptying? A literature review. J Urol. 1985;134:443–449. doi: 10.1016/s0022-5347(17)47234-x. [DOI] [PubMed] [Google Scholar]
- 19.Patel R, Fiske J, Lepor H. Tamsulosin reduces the incidence of acute urinary retention following early removal of the urinary catheter after radical retropubic prostatectomy. Urology. 2003;62:287–291. doi: 10.1016/s0090-4295(03)00333-9. [DOI] [PubMed] [Google Scholar]
- 20.Djavan B, Shariat S, Fakhari M, Ghawidel K, Seitz C, Partin AW, et al. Neoadjuvant and adjuvant alpha-blockade improves early results of high-energy transurethral microwave thermotherapy for lower urinary tract symptoms of benign prostatic hyperplasia: a randomized, prospective clinical trial. Urology. 1999;53:251–259. doi: 10.1016/s0090-4295(98)00538-x. [DOI] [PubMed] [Google Scholar]