Abstract
Heat shock proteins (HSPs) play various stress-protective roles in plants. In this study, three HSP genes were isolated from a suppression subtractive hybridization (SSH) cDNA library of Ginkgo biloba leaves treated with cold stress. Based on the molecular weight, the three genes were designated GbHSP16.8, GbHSP17 and GbHSP70. The full length of the three genes were predicted to encode three polypeptide chains containing 149 amino acids (Aa), 152 Aa, and 657 Aa, and their corresponding molecular weights were predicted as follows: 16.67 kDa, 17.39 kDa, and 71.81 kDa respectively. The three genes exhibited distinctive expression patterns in different organs or development stages. GbHSP16.8 and GbHSP70 showed high expression levels in leaves and a low level in gynoecia, GbHSP17 showed a higher transcription in stamens and lower level in fruit. This result indicates that GbHSP16.8 and GbHSP70 may play important roles in Ginkgo leaf development and photosynthesis, and GbHSP17 may play a positive role in pollen maturation. All three GbHSPs were up-regulated under cold stress, whereas extreme heat stress only caused up-regulation of GbHSP70, UV-B treatment resulted in up-regulation of GbHSP16.8 and GbHSP17, wounding treatment resulted in up-regulation of GbHSP16.8 and GbHSP70, and abscisic acid (ABA) treatment caused up-regulation of GbHSP70 primarily.
Keywords: heat shock proteins, SSH, cold stress, heat stress, abiotic stress, Ginkgo biloba
1. Introduction
Heat shock protein (HSP) is a type of specific stress protein produced by living organisms in response to high temperatures and other environmental stresses. Abundant HSP expression could remarkably improve the survivability and endurance of cells to environmental stress or damage [1]. HSPs can be classified into five families based on molecular weight, namely, HSP100, HSP90, HSP70, HSP60, and small molecule sHSP [2].
Previous studies had shown that the accumulation of HSPs plays a pivotal role in abiotic stress responses in plants [3–5]. Most HSPs function as molecular chaperones in maintaining homeostasis of protein folding and are thought to be responsible for the acquisition of thermo tolerance [6]. Aside from high temperature, low temperature [7], drought stress [8], heavy metal ions [9], high salinity [10], anaerobic environments [11], diseases and pests [12], ultraviolet light [13], superoxide ions [14,15], mechanical injury, SA [16,17] and abscisic acid (ABA) treatment [5] can all induce HSP generation. Moreover, HSPs were shown to be involved in many steps of cell apoptosis [18,19]; the protective effects of the chaperone machinery, in which different HSPs or chaperones acted cooperatively [2]. In the absence of environmental stresses, the expressions of some HSPs were shown to be developmentally [20] or tissue-specifically regulated [5]. Transgenic plants overexpressing HSP genes exhibited improved tolerance to heat, cold stress and drought stress [21–23].
Currently, Ginkgo biloba is one of the most popular functional plants, particularly as a medicinal plant. Extracts of G. biloba leaves contained active compounds such as flavonoids and terpene lactones (ginkgolides and bilobalide), which may induce an increase in peripheral and cerebral blood flow [24,25]. Ginkgo has important economic and medicinal values, and its plantation scope is expanding gradually. The extreme temperature in G. biloba’s natural habitat does not exceed 40 °C or fall below 4 °C, which affects its regional expansion. G. biloba has survived all kinds of complex climatic environments for millions of years; it has shown strong adaptability and has changed little in morphology. The survival of G. biloba is more or less related to its strong tolerance to environmental stresses [26]. In this study we isolated three novel HSP genes after cold treatment of Ginkgo leaves. Expression analysis indicated that the selected Ginkgo biloba heat shock protein genes from low temperature seedling could be induced by heat stress and other abiotic stress. A study of the cloning and expression of the heat shock protein genes in G. biloba will help reveal the coping mechanism of this plant to the environment and the physiological mechanism of resistance. In addition, it can provide the theoretical foundation and gene resources for cultivating resilient forests using gene engineering.
2. Results and Discussion
2.1. Results
2.1.1. Cloning and Sequence Analyses of Three Genes Encoding Heat Shock Protein from Ginkgo biloba
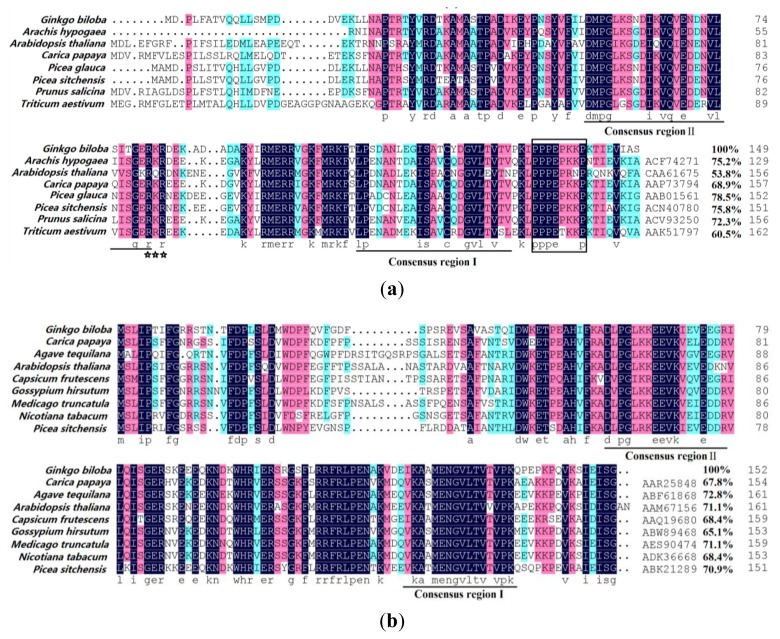
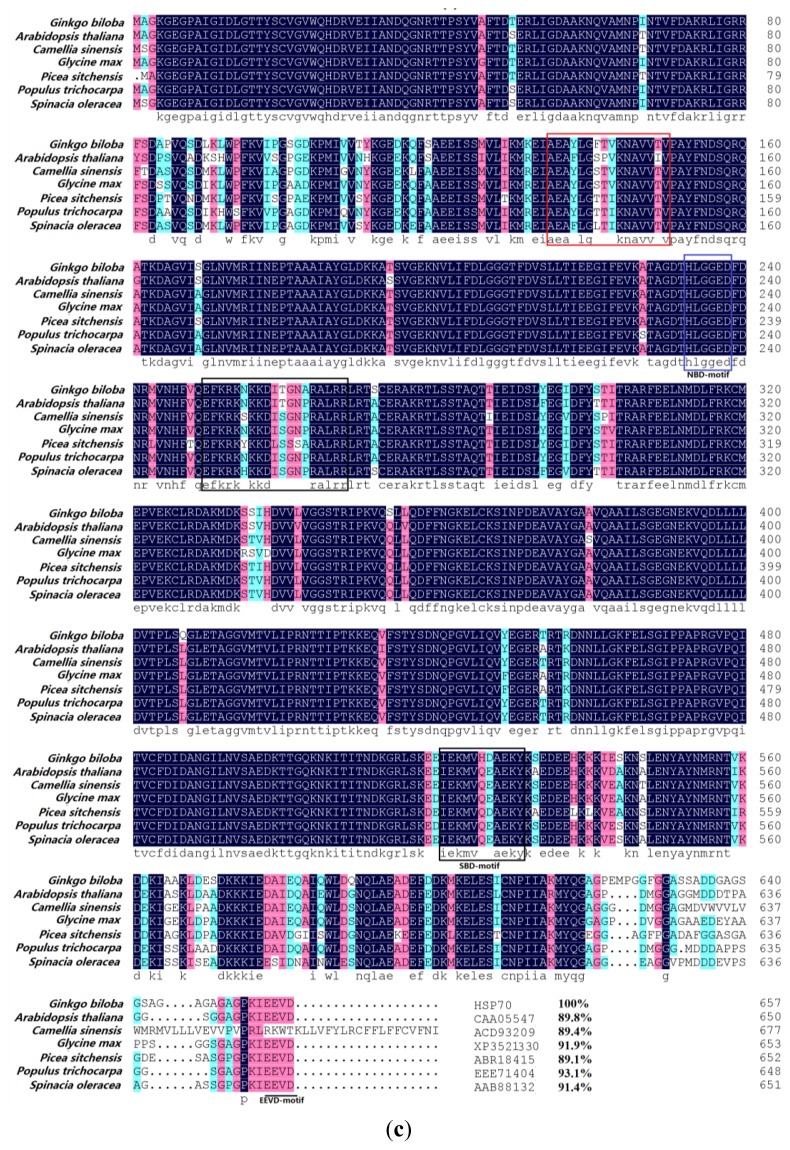
To investigate the molecular events of Ginkgo development and environments, two cDNA libraries were constructed using mRNAs isolated from Ginkgo leaves after cold shock treatment or in normal growing conditions. Three ESTs (expressed sequence tag) were isolated from the cold-treated cDNA library and the full-length cDNA was obtained by RACE. Sequencing revealed that the full length of the three HSP genes were 905 bp, 623 bp, and 2408 bp (Figure S1), respectively. According to their approximate molecular weight, the three GbHSPs studied in this work were named GbHSP16.8, GbHSP17, GbHSP70. The ORFs of the full length GbHSP16.8, GbHSP17, and GbHSP70 were 450, 459, and 1974 bp, respectively. Three polypeptide chains containing 149 amino acids (Aa), 152 Aa, and 657 Aa were respectively encoded and their corresponding molecular weights were predicted as follows: 16.67 kDa, 17.39 kDa, and 71.81 kDa. The pIs were predicted as: 6.55, 5.83, 5.12, respectively. The three HSPs exhibited a high similarity with the amino acids of other HSPs (Figure 1). The comparison of BLASTP results in NCBI showed that GbHSP70 had more than 93.1% similarity with HSP70 of Populus trichocarpa (EEE71404), 91.9% similarity with Glycine max (XP3521330) and 91.4% similarity with Spinacia oleracea (AAB88132); The amino acid sequence of the GbHSP16.8 shared 78.5% identity with Picea glauca (AAB01561), 75.8% with Picea sitchensis (ACN40780), 75.2% with Arachis hypogaea (ACF74271), 72.3% with Prunus salicina (ACV93250), 68.9% with Carica papaya (AAP73794), 60.5% with Triticum aestivum (AAK51797), 53.8% with Arabidopsis thaliana (CAA61675); The GbHSP17 shared 72.8% homology with Agave tequilana (ABF61868), 71.1% with Arabidopsis thaliana (AAM67156) and Medicago truncatula (AES90474), 70.9% with Picea sitchensis (ABK21289), 68.4% with Capsicum frutescens (AAQ19680) and Nicotiana tabacum (ADK36668), 67.8% with Carica papaya (AAR25848), 65.1% with Gossypium hirsutum (ABW89468).
Figure 1.
Protein sequence alignment of Ginkgo heat shock protein (HSP) with other HSPs. Two consensus regions are underlined and a putative nuclear localization signal is indicated by asterisks. A polyproline motif at the carboxyl end of proteins is boxed. Following the aligned sequences is the accession numbers and homology percentage. (a) HSP 16.8; (b) HSP 17, (c) HSP 70.
An alignment of the deduced protein sequence of GbHSP16.8 and GbHSP17 with other plant cytosolic class I and class II sHSPs are shown in Figure 1a,b. Comparison with other plant sHSPs representing the two cytoplasmic subfamilies revealed that GbHSP17 cDNAs coded for cytoplasmic class I sHSP subfamily members, GbHSP16.8 for class II. As shown in Figure 2, within each class there was more similarity to proteins of other organisms belonging to the same class than to proteins of the other class. The sequence of the N-terminal domain of plant cytosolic class II sHSP was divergent, which may partially account for their functional multiplicity among different plant species. By contrast, all plant cytosolic sHSP share a conserved C-terminal domain of about 90 Aa called ACD or heat shock domain, which can be further divided into two subdomains, Consensus I Pro-A(14)-Gly-Val-Leu and Consensus II Pro-A(14)-Val/Leu/Ile-Val/Leu/Ile of the carboxyl terminal. A putative nuclear localization signal (RKR) and a polyproline motif (PPPEPKKP) are only found at the C-terminal of GbHSP16.8.
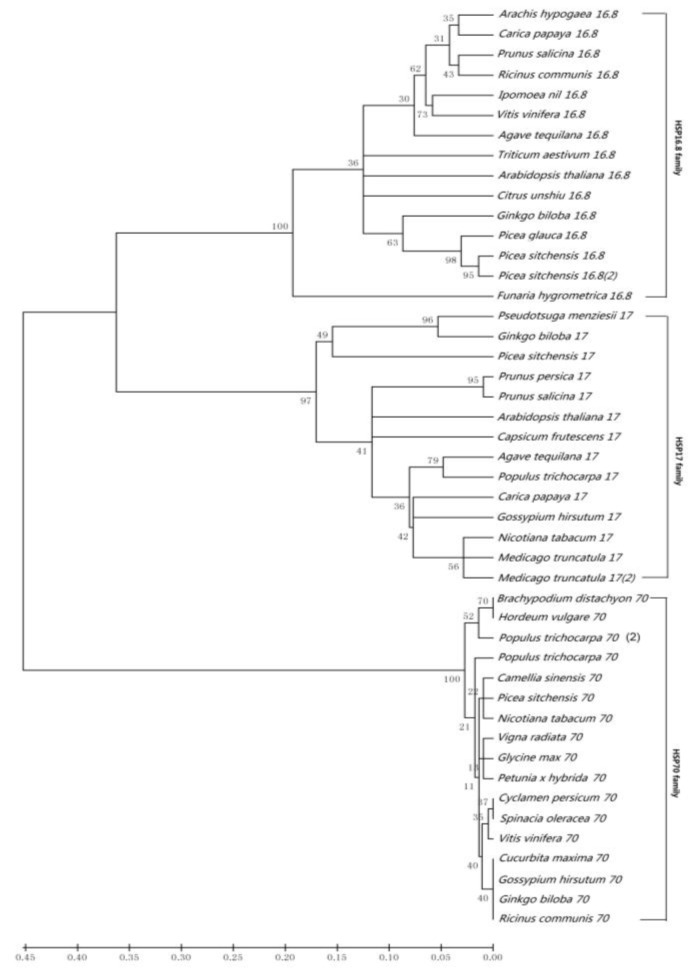
Figure 2.
Gene tree of the three Ginkgo biloba HSPs. Gene trees were constructed using the software Molecular Evolutionary Genetics Analysis (MEGA) Version 4.0 by the neighbor-joining method with pairwise deletion and the poisson correction model. Bootstrap support values for each node are shown (percentage of bootstrap trees supporting the node, out of 1000 trees). Accession numbers for all sequences are as listed here. Hsp16.8 family: Arabidopsis thaliana CAA61675, Arachis hypogaea ACF74271, Carica papaya AAP73794, Citrus unshiu BAK61844, Picea glauca AAB01561, Picea sitchensis ACN40780, Picea sitchensis(2) ABK26390, Prunus salicina ACV93250, Triticum aestivum AAK51797, Ipomoea nil AAB39335, Vitis vinifera XP 2280485, Agave tequilana ABF61870, Funaria hygrometrica CAC81966, Ricinus communis, XP 2516106; HSP17 family: Agave tequilana ABF61868, Arabidopsis thaliana AAM67156, Capsicum frutescens AAQ19680, Carica papaya AAR25848, Gossypium hirsutum ABW89468, Medicago truncatula AES90474, Medicago truncatula(2) AES75921, Nicotiana tabacum ADK36668, Picea sitchensis ABK21289, Populus trichocarpa EEE89499, Prunus persica AAR99375, Prunus salicina, ACV93249, Pseudotsuga menziesii CAA63570; HSP70 family: Brachypodium distachyon XP 3558228, Hordeum vulgare BAJ86014, Populus trichocarpa EEE71403, Populus trichocarpa EEE71404, Camellia sinensis ACD93209, Picea sitchensis ABR18415, Nicotiana tabacum AAR17080, Vigna radiata AAS57912, Glycine max XP 3521330, Petunia hybrida CAA30018, Cyclamen persicum ABP35942, Spinacia oleracea AAB88132, Vitis vinifera CAN81694, Cucurbita maxima AAN86274, Gossypium hirsutum ACJ11741, Ricinus communis EEF34649.
The secondary structure of HSP70 has α-helical segments, immediately followed by a β-sandwich subdomain. The C-terminal 10-kDa subdomain is α-helical while the other subdomains are β-sheets. Two regions are mutually dependent. The structure of the β-sandwich is consistent with the β-bridge at the N-end. A detailed analysis of the ATPase domain (nucleotide binding domain, NBD) and polypeptide-binding (substrate binding domain, SBD) [27] demonstrated that the side chains of amino acids critical for protein functions have conserved positions (Figure 2c). In particular, within the N-terminal NBD, the HLGGED motif in positions 234–239, critical for ATP/ADP binding, is conserved [28]. The same holds true for residues V415, M416, L419, I420, A496 and D498 and the motif IEKMVHDAEKY (521–531) within the C-terminal SBD, critical for peptide binding [29]. Furthermore, within the N-terminal, the EEVD motif in positions 654–657 is the signature cytosolic HSP70-specific motif [30].
To classify the three GbHSPs, we generated a phylogenetic tree using Molecular Evolutionary Genetics Analysis (MEGA) Version 4.0 by the neighbor-joining method (Figure 2). Phylogenetic analysis and amino acid sequence alignment indicated that the Aa sequence of GbHSP16.8 and GbHSP17 has high homology with the cytoplasmic II sHSPs of other plants. As shown in Figure 3, based on the amino acid sequence homology, the three GbHSPs genes were divided into three classes. GbHSP70 belonged to HSP70 family while GbHSP16.8 and GbHSP17 belonged to two cytoplasmic sHSP subfamilies.
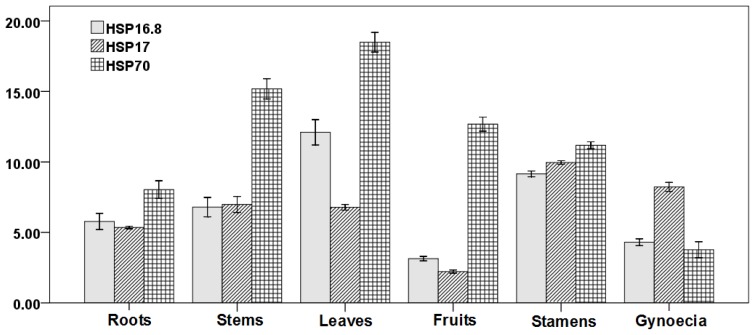
Figure 3.
Analysis of QRT-PCR for tissue-specific transcription of GbHSP accumulation after 2 h heat shock.
2.1.2. Expression Analysis of Three GbHSPs Genes in Different Tissues
The expression of the three GbHSPs genes was analyzed using QRT-PCR. The three GbHSPs genes exhibited diverse expression in different organs, although expression could be detected in all organs we checked (Figure 3). GbHSP16.8 was highly expressed in Ginkgo leaves, stamens, stalk, root, and pistil, and was less abundant in the fruit section. GbHSP17 was predominantly expressed in stamens, pistil, stalk, leaves, and roots, and moderately expressed in the fruit. GbHSP70 had the highest expression level among the three HSP genes with the highest expression level in leaves, followed by stalks, fruits, stamens, and roots. The lowest expression level of GbHSP70 was observed in pistil. In the current study, GbHSP16.8 and GbHSP17 were predominantly expressed in stamens, and would play roles in pollen development and pollen maturation. Usually reproductive organs are much more sensitive to heat stress than other organs [5]. GbHSP70 was more highly expressed in leaves and stems than other organs, indicating that these genes may play some roles in maintaining the normal leaf functions, such as respiration and photosynthesis.
2.1.3. Expression Profiles of the Three HSPs under Low Temperature or Heat Shock Treatments at Seedling Stage
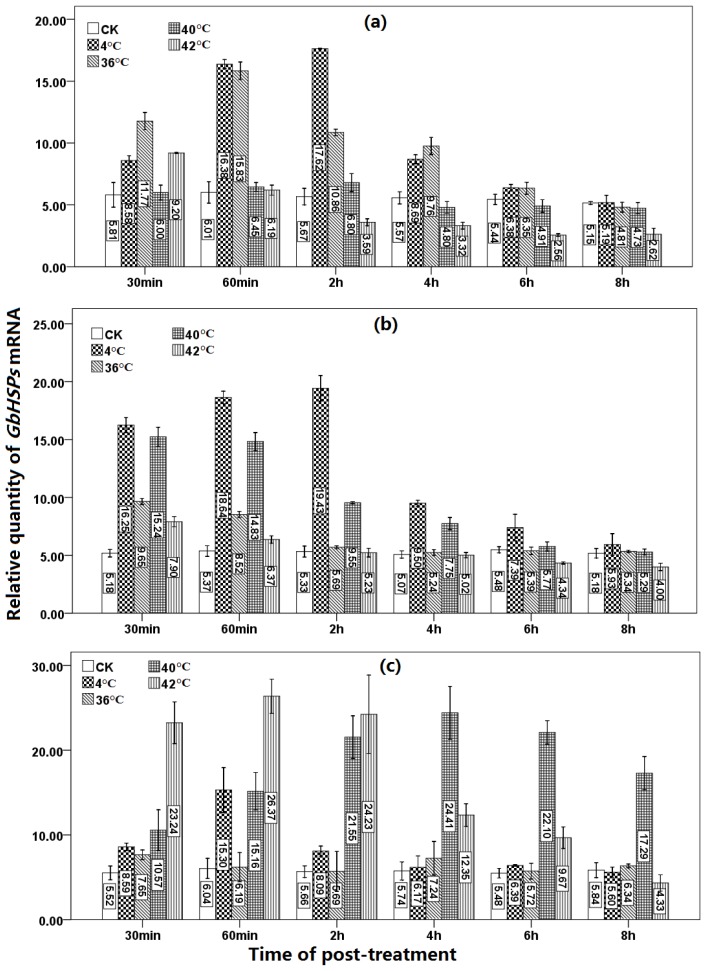
The changes in expressions levels of the three GbHSP genes from G. biloba were determined in response to low and high temperatures (Figure 4, Table S1). Specific primers for the three GbHSPs were selected and used in QRT-PCR to measure the dynamic changes in expression. The expressions of HSP16.8 and HSP17 were induced by low temperature (4 °C); whereas, the transcription level of HSP70 was only slightly affected. The transcription level of HSP16.8 increased sharply to approximately 1.48 times higher than the control level (CK on Figure 4) after 30 min at low temperature, and to approximately 2.73 times after 60 min. The highest level, 3.11 times that of the control, was achieved at 2 h. After 8 h, the transcription level of GbHSP16.8 decreased rapidly until it reached the control level. For GbHSP17 at 4 °C, the transcript level rose rapidly to approximately three times that of the control after 30 min, reaching a peak of 3.65 times the control level at 2 h. After 8 h, the transcription level of GbHSP17 decreased sharply to 1.15 times that of the control. The response of GbHSP70 to low temperature was much slower than the other two HSPs. After 30 min at low temperature, the transcription level rose to 1.56 times that of the control and then peaked after 60 min to 2.53 times that of the control. The transcription level then decreased rapidly to that of the control level.
Figure 4.
Under different stress temperatures, mRNA expression levels of the three GbHSPs were analyzed by real-time quantitative RT-PCR, G. biloba GAPDH gene was used as the internal control. Relative expression levels are shown for: (a) The relative expression levels of GbHSP16.8 at different times after stress induction. (b) The relative expression levels of GbHSP17 at different times after stress induction. (c) GbHSP70 genes expression levels.
High temperatures (36, 40 and 42 °C) had different effects on the expression of the three HSPs in G. biloba. At 36 °C, the transcription level of GbHSP16.8 rose to twice that of the control after 30 min and reached a peak after 60 min (Figure 4a). The transcription level decreased slowly to a level slightly lower than that of the control at 8 h. At 40 °C, the transcription level of GbHSP16.8 showed no significant difference after 30 min; however, at 2 h, a slight increase was observed, after which the transcription level decreased slowly to a level slightly lower than the control. At 42 °C, the GbHSP16.8 transcription level rose to about 1.58 times higher than the control after 30 min, and then decreased to about 0.47 times that of the control at 6 h. At 8 h, the transcription level was slightly increased; however, the level was still lower than that of the control. It appears that a high temperature (42 °C) inhibits the expression of GbHSP16.8 (Table S1).
GbHSP17 was quite sensitive to high temperature. At 36 °C, the transcription level was about 1.58 times that of the control within the first 60 min; after which it dropped to the control level and reached equilibrium. At 40 °C, the transcription level reached a peak after 30 min at 2.94 times that of the control (Figure 4b). The transcription level then decreased slowly after 2 h. At 42 °C, the transcription level rose to 1.52 times that of the control after 30 min and then decreased to 0.77 times that of the control after 8 h (Table S2).
GbHSP70 was also quite sensitive to high temperatures (Figure 4c). In particular, a high temperature of 42 °C induced a rapid increase in GbHSP70 transcription. After 30 min, the transcription level rose to 4.21 times that of the control and reached a peak of 4.37 that of the control at 60 min. By 8 h, the transcription level had decreased to a level slightly lower than that of the control. At 40 °C, GbHSP70 exhibited a slow and constant induction. After 30 min, the transcription level started to increase and reached a peak of 4.26 times that of the control at 4 h. Thereafter, the transcription level decreased slowly and maintained a high transcription level (about 2.96 times that of the control) at 8 h. However, 36 °C had no apparent inductive effect on GbHSP70. The transcription level rose slightly at the 30 min time point; however, at 4 h and subsequent time points, the expression level of GbHSP70 was similar to the control level (Table S3).
Based on these results, GbHSP16.8 and GbHSP17 might be induced by low temperature signals. In addition, the two genes might play a key role in the adaptation of Ginkgo leaves to low temperatures. GbHSP70 might also participate in the response to low temperature signals; however, it may have a more important role in the heat stress resistance of Ginkgo.
2.1.4. Expression Patterns of the Three GbHSPs under Abiotic Stresses
Numerous abiotic stresses are known to trigger alteration in the transcription of HSP genes. Therefore, we determined the expression profiles of the three HSP genes under diverse abiotic stress treatments, using QRT-PCR.
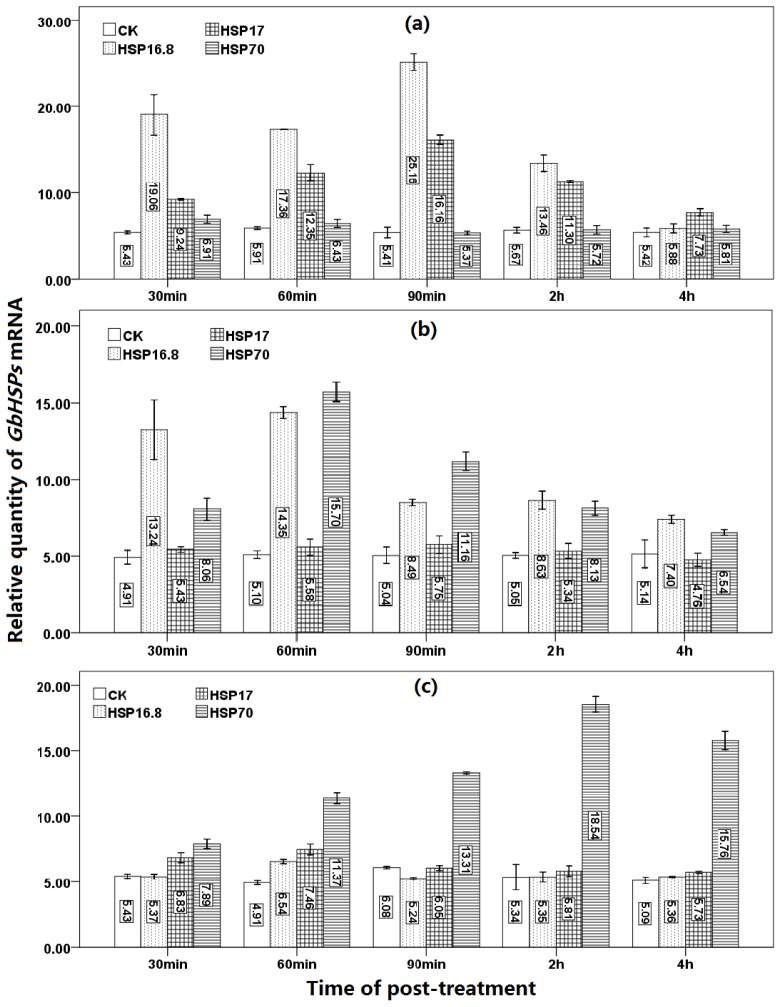
Irradiation with UV-B upregulated the transcription of the smaller HSPs (Figure 5b). The transcription level of GbHSP16.8 under ultraviolet treatment varied only slightly after rapidly increasing to 3.51 times that of the control at 30 min. GbHSP16.8 reached a peak of 4.65 times that of the control level after 90 min, and then slowly decreased. GbHSP17 was also induced by UV-B, but less efficiently. It reached a peak of 2.98 times higher than the control level after 90 min of treatment. UV-B did not appear to induce the expression of GbHSP70. Its expression increased slightly during the early period of treatment and thereafter maintained a similar level to the control (Table S4).
Figure 5.
Real-time PCR analysis of the relative expression levels of the GbHSP genes in Ginkgo biloba under abiotic stress treatment. The G. biloba GAPDH gene was used as an internal control. The bars are means of the relative fold change of three biological and two technical replicates obtained by real-time RT-PCR. The standard errors of the biological replicates are shown as error bars. Relative expression levels are shown for (a) UV-B treatment, (b) wounding treatment and (c) ABA treatment.
Plants are often injured by environmental factors during their growth and development, and these injuries stimulate plant responses. GbHSP16.8 and GbHSP70 were apparently induced by mechanical injuries (Figure 5c). The transcription level of GbHSP16.8 in leaves rose rapidly 2 h after being cut and reached a peak after 60 min of 2.82 times higher than the control level. The expression level then decreased to 1.44 times lower than the control level, and this level was maintained throughout the remaining experimental period. Injury also plays a key role in the induction of GbHSP70. The transcription level rose to 1.64 times higher than the control at 30 min and reached a peak of 3.08 times that of the control after 60 min, after which the expression level slowly decreased. Injury treatment had no apparent role on the induction of GbHSP17; the expression level at several time points after treatment did not increase significantly (Table S5).
ABA is one of the five major plant hormones that facilitates the maturity and abscission of fruits and suppresses plant growth. The expression level of the three HSPs increased after ABA treatment (Figure 5a), particularly GbHSP70. After 1 h, the transcription level of GbHSP70 slowly increased to 1.3 times higher than the control level. It further increased to 2.18 times higher than the control level after 90 min and reached a peak of 3.47 times that of the control after 2 h. The expression level then decreased slowly to 3.10 times lower than the control level after 4 h (Table S6). Treatment with ABA had no apparent role in the induction of GbHSP16.8 (Table S6). The transcription level after about 30 min of treatment was not significantly different to the control level. The transcription level increased slightly after 1 h to 1.33 times higher than the control level, after which it decreased to the control level. The expression profile of GbHSP17 was similar to that of GbHSP16.8.
2.2. Discussion
Like other long-lived woody species native to subtropical zone regions, G. biloba exhibits a remarkable abiotic stress tolerance. In this study, we cloned three HSP genes, particularly GbHSP16.8, GbHSP17, and GbHSP70, from G. biloba using SSH cDNA library of Ginkgo leaves treated with cold stress and the full sequence was isolated by 5′ and 3′-RACE according to the user manual.
Small heat shock proteins are chaperones that play an important role in stress tolerance [31]. According to their Aa sequence, all cytoplasmic sHSPs in plants described so far belong to two different classes: class I and class II [32]. GbHSP17 and GbHSP16.8 belong to cytosolic class I and class II sHSPs and this was validated by the results of alignment and phylogenetic analysis with sHSP sequences of other plants. sHSPs share an evolutionarily conserved sequence of 80–100 amino acids, located in the C-terminal region, and called α-crystallin domain or heat shock domain contributing to subunits interactions [33]. The small heat shock domain can be further subdivided into two regions, consensus region I and II separated by a hydrophilic region of variable length. The type of sHSP related with α-crystalline is extremely abundant in higher plants. Though the middle and N-terminal amino acids of GbHSP17 and GbHSP16.8 were very different from others, this difference may partially explain why the two small HSPs could increase resistance to a broader array of stressors than other sHSPs that have been reported.
Ginkgo HSP16.8 and HSP17 show more homology in the ACD domain, which has two conserved regions. This domain within HSPs is very highly conserved and, as a molecule chaperone, can help to stabilize unfolding proteins because of its propensity to associate with denaturing proteins [34,35]. The conservative sequence (Pro-X-Gly-Val-Leu) is the typical structural feature of most HSPs. The structural domain of GbHSP70 is mainly divided into two parts: the 40 kDa region at the N-terminus acts as ATPase and the 30 kDa region at the C-terminus functions in the formation of non-foldable polypeptide into protein conformation. Based on HSP16.5 [36] crystal structure of Methanococcus jannaschii, we infer that the dimer of HSP is formed by the folding of a large amount of beta sub-units, which are basic structures formed by oligomers. After heat shock, HSP17 (I) and HSP17 (II) will form a particle complex with high molecular weight and a size of 40 nm. These compounds can store housekeeping genes and some degraded protein, e.g., luciferase [37]. HSP70 has a calmodulin binding domain, which contains the important conditioning signal during a living organism’s adaptation to stress [38].
2.2.1. GbHSPs Are Expressed under Normal Conditions with Tissue Preference
Most studies have shown that HSPs are needed for basic plant growth and development. However, some previous studies revealed that HSPs may only be expressed in a specific tissue under normal growth conditions, partially because of the lesser sensitivity of the Northern blot for very low expression [39]. In the present study, transcripts of the three GbHSPs genes were observed in every tissue under general high temperature, although they were only slightly detected in some tissues. The three GbHSP genes exhibited diverse expression in different organs, although the expression could be detected in almost all organs we studied. Tissue-specific expression patterns differed from gene to gene even though they belong to the same family as observed in the current study.
In Arabidopsis, HSP70b transcripts were not detected in any organ; transcripts of HSP70 were abundant in roots but hardly detectable in other organs; and expression of HSP70-1 and HSP70-2 were more abundant in leaves than other organs [40]. Different HSP members of the same family can have diverse preferred tissue-specific expression patterns. In rice, OsHSP80.2 transcripts were detected more abundantly in roots, suggesting a specific role of OsHSP80.2 in root growth or function. The other two OsHSP90 genes and OsHSP70 genes were highly expressed in leaves and sheaths than the other organs [5]. The expression patterns of the three HSPs genes in Ginkgo were also different. GbHSP70 and HSP16.8 were expressed at higher levels in the leaves, which may contribute to maintaining normal leaf functions including respiration and photosynthesis [5]. The other gene was mainly expressed in gynoecia and stamens. GbHSP70 and GbHSP16.8 were also expressed at higher levels in stamens, which indicated that they played certain roles in G. biloba stamen development.
2.2.2. GbHSPs Expression Is Generally Enhanced under Cold or Heat Stress
Expression of HSP genes has been shown to be enhanced by elevated temperatures in many plant species [5,40,41]. In Arabidopsis, the expression of some HSP70s was elevated 2- to 20-fold by 30 min heat stress at 40 °C [40]. In rice, transcripts of some sHSP-Cl genes were detected as early as 5 min after exposure to 41 °C treatment. The transcripts of all nine sHSP-Cl genes were detected within 15 min [42]. In our present study, transcripts of all three GbHSPs genes were increased under heat shock treatment, although they exhibited different response patterns. The transcripts of GbHSP70 were increased rapidly and kept at constantly high levels during the 40 °C and 42 °C heat stress. The expression of GbHSP17 was increased 30 min after the 40 °C heat treatment but was reduced after 1 h. The expression of GbHSP16.8 was increased 30 to 60 min later but was reduced after 4 h. Based on these variations, the different HSPs are regulated in different patterns or by different signals. In addition, they may have different assigned functions in response to heat stress. The accumulation of HSPs was believed to play a major role in the heat stress response and in acquired thermotolerance in plants [2,43]. The protective effects of HSPs/chaperones can be attributed to the network of the chaperone machinery, in which different HSPs/chaperones acted cooperatively [2].
Although heat shock and cold shock trigger different adaptive responses and induce the production of unique stress proteins, several studies proved that low temperatures could induce HSPs involved in the resistance of cells against extreme temperatures [44]. Some HSP genes were observed to be induced by low temperature in spinach [45], Arabidopsis [40], Sweet chestnut [46], and Brassica napus [47]. The study of Soto [48] showed that treatment with low temperature could induce the expression of CsHSP17.5 in Class I gene in the chestnut cytoplasm, and it could be expressed in both roots and stalks. The expression of HSP genes under cold temperature conditions further proved the important role of HSP in boosting plant resistance to cold injury [47], Excessive expression of sHSPs in the chloroplasts of tomato showed that the symptoms of cold injury in tomato were lesser than the tomato with unexpressed sHSPs. The resistance of tomato against cold temperature has improved [49]. However, the specific mechanism of HSPs in inducing resistance of plants to low temperature remains unclear. Guo [22,50] found that the cytoplasmic sHSP18 gene could be expressed in sweet pepper leaves by subjecting the plant to low temperature, but chloroplast sHSP26 gene could not be induced by cold stress; Zhu [51] found that CaHsp24 could be induced by heat stress and also weakly expressed by cold stress. In our study, we found that GbHSP16.8 and GbHSP17 could be induced by cold stress, but the HSP70 gene could not be induced by cold stress. One of the possible reasons may be that only some members of the sHSP family respond to low temperature and this does not include in HSP70. HSP or other compounds containing HSPs of different molecular weights may be involved in transporting the polypeptide synthesized under cold-induced stress to the plasma membrane, nucleus, and other organelles. Moreover, physiological changes induced by low temperature could denature some functional proteins like the proteins related with membrane fluidity. HSP could induce refolding and restoring the functions of a denatured protein caused by low temperatures [32]. Morimoto [52] proposed the regulating pattern in the expression of HSP gene (autoregulation) involving three key steps: HSF’s activity is altered by changing internal and external elements; activated HSF identifies and binds with HSE in the promoter region of the HSP gene; and the transcriptionally active region of the HSP gene opens and facilitates the transcription.
2.2.3. GbHSPs Can Also Be Induced by Stresses Other than Temperature Stress
The three HSPs genes isolated from low temperature seedling could be induced by other abiotic stress. Stress induced by UV-B will cause easy accumulation of free radicals and other oxygen derivatives inside the plants. Reactive oxygen-induced stress is an important type of plant stress. In rice seedlings, resistance to UV-B irradiation in rice improved greatly after heat stress treatment. Moreover, rice seedlings with genetically modified HSP17.7 could improve its resistance to UV-B irradiation, which was related with the transcription level of HSP17.7 [13]. High temperature and UV-B stress readily cause the formation and accumulation of free oxygen species inside the plants, and further result in oxidation of liposomes, proteins, and other large molecules thereby damaging the plants [53,54]. The expression of sHSPs in cytoplasm and mitochondria as induced by active oxygen has been reported [43,55]. When living organisms were subjected to salt tolerance test, sHSPs in the mitochondria could protect the electron transport complex I and prevent its damage by free oxygen. Its role was similar to glutathione, APX, SOD, CAT, and other enzymes [56].
The induction of HSPs by mechanical injury in plants has also been studied, although it has not been fully elucidated. In rice, Oshsp18.0-CII was induced by mechanical injury and SA to a much lower level compared with heat shock. However, mechanical injury and SA did not induce OsHSP 18.0-CII protein accumulation. Mechanical injury could induce the synthesis of enzymes related with the metabolism of phenolic compounds and the production of abundant HSPs in plants. Meanwhile, HSPs could suppress the synthesis of enzymes for phenolic metabolism and prevent the local browning of tissues [57]. In plant roots, sHSPs may contribute to managing periapical lesions, including their influence on the migration of epithelial cell rests and their role in increasing the resistance against necrotic and apoptotic cell death [58]. In Ginkgo, damage could induce the synthesis of high amounts of HSP70 and HSP17, indicating that HSPs could be induced solely by mechanical injury without heat stress.
ABA could regulate the adaptation of many plants to environmental stress. The function of ABA in regulating the expression of HSP genes in many plants has been widely studied [5,59,60]. In response to drought and temperature stress, ABA levels in plants changed remarkably [61]. When ABA receptor-deficient mutants of maize were subjected to drought, high temperature or both, ABA could improve the endurance of plants by regulating the level of HSP70 synthesis [60]. The transcription level of HSP70 rose remarkably in response to extreme heat stress. In this study, GbHSP70 was up-regulated by ABA. The expression of the other two sHSP was not affected by ABA, which implied that ABA may improve plant tolerance to extreme heat stress by increasing HSP70 expression. In rice exposed to heat stress, the promoter structure of all nine OsHSP genes has a ABRELATERD1 cis-acting element. The adaptation of plants to heat stress was possibly related with the regulation of ABA level [62]. These observations may indicate that both ABA-dependent and independent stress signal transduction pathways were involved in HSP70, HSP16.8 and HSP17 expression regulation. When Ginkgo was under extreme high temperature conditions, the transcription levels of HSP70s were related with the regulation of endogenous ABA. HSP16.8 and HSP17 transcription in Ginkgo could be induced by high temperatures (36 °C and 40 °C); however, ABA cannot induce the same effect, which implied that HSP16.8 and HSP17 genes involved in response to high temperature has no direct relationship with the mechanism involved in regulating ABA.
3. Experimental Section
3.1. Plant Materials and Treatments
Two-year old grafted G. biloba seedlings, growing in a greenhouse in Huanggang (E, 114°54′–116°8′, N, 29°45′–31°35′, Hubei province, central of China), were sampled as cDNA library construction materials. For tissue expression analysis, diverse tissues, including young leaves, mature leaves, ovules, stamens, albumen, gynoecia, stems and roots were collected for RNA extraction as described by Xu [63]. Tissues were immediately frozen in liquid nitrogen and kept at −80 °C prior to total RNA extraction.
Two-year old cuttings from the same genotypic strain of G. biloba were subjected to treatments with UV-B, heat-shock, ABA (abscisic acid) and wounding treatment. For UV-B treatment, seedlings were exposed to 1500 μJ/m2 UV-B irradiation in a closed chamber, and the control cuttings were placed in a dark closed chamber. The ABA (10 mM) was dissolved in 0.01% Tween 20 and sprayed onto young leaves. The control leaves were sprayed with an equivalent volume of 0.01% (v/v) Tween 20. The edges of the Ginkgo leaves were cut by about 0.6 cm with scissors for wounding treatment, the intact leaves of Ginkgo were as control. For cold and heat shock treatments, seedlings were exposed to 4 °C and 36 °C, 40 °C, 42 °C. Leaves were collected after exposure for 30 min, 60 min, 90 min, 2 h, 4 h, 6 h, 8 h.
3.2. Subtractive Hybridization
Total RNA was isolated from Ginkgo seedling leaves of cold treated (4 °C, 1 h, designed as S1) and normal growing conditions (25 °C, designed as S2) using Xu’s method [63]. mRNA was isolated from total RNA with an mRNA Isolation kit (Tiandz, Beijing, China) according to the manufacturer’s instructions. cDNA synthesis, digestion with Rsa I, hybridization, and PCR amplification were carried out using the PCR-Select cDNA subtraction Kit (Clontech, Mountain View, CA, USA) according to the manufacturer’s instructions. Forward subtraction was performed using S2 cDNA as a tester and S1 cDNA as a driver. Reverse subtraction was performed using S1 cDNA as a tester and S2 as a driver. PCR products were ligated into pMD18-T vectors (TaKaRa, Dalian, China) to obtain forward and reverse subtraction libraries. About 2300 colonies each were obtained using a portion of PCR products by SSH in both directions and these ESTs fragments were sequenced by Sangon (Sangon Biotech, Shanghai, China).
3.3. Molecular Cloning of the GbHSPs cDNA
Through EST analysis, a 274 bp fragment of GbHSPs16.8, 363 bp fragment of GbHSPs17 and 522 bp fragment of GbHSP70 were obtained from the heat-treated cDNA library. Based on the sequence, the specific primer pairs (H70R5, H17R5, H16R5 and H70R3, H17R3, H16R3) and the nested primer pairs (H70N5, H17N5, H16N5 and H70N3, H17N3, H16N3) were designed to amplify the 5′ and 3′ end of GbHSPs using the SMART™ RACE cDNA Amplification Kit (Clontech, Mountain View, CA, USA). Table 1 lists the primer sequence for each gene. The PCR products were purified and cloned into the pMD18-T vector for sequencing. After comparing and aligning the sequence of 5′RACE, 3′RACE, and the internal fragment, the full-length cDNA sequence of GbHSPs were obtained. The full-length cDNA of GbHSP16.8, GbHSP17 and GbHSP70 were obtained when the 5′ and 3′ fragments were assembled by Vector NTI 10.0 software.
Table 1.
Primers used in the present study.
| Primer | Sequence (5′–3′) | Description |
|---|---|---|
| H70R5 | TGTTCCGCATGTTGTAGGCATAGTT | Reverse primer for 5′RACE, outer |
| H70R3 | CTATTCCCACAAAGAAAGAGCAGGTT | Forward primer for 3′RACE, outer |
| H17R5 | GGTTTAGGTTCAGGTTGTTTAGGCAC | Reverse primer for 5′RACE, outer |
| H17R3 | CCAGGTTTGAAGAAAGAGGAGGTTA | Forward primer for 3′RACE, outer |
| H16R5 | TGCCGACTCTTCGCTCCATTCTTAT | Reverse primer for 5′RACE, outer |
| H16R3 | TAAGAATGGAGCGAAGAGTCGGCAAAT | Forward primer for 3′RACE, outer |
| H70N5 | CTTTGTGGGAATAGTAGTGTTT | Reverse primer for 5′RACE, nested |
| H70N3 | ATGGCATCCTTAATGTCTCA | Forward primer for 3′RACE, nested |
| H17N5 | CGATGCCATTTGTCATTCTT | Reverse primer for 5′RACE, nested |
| H17N3 | CCTGAGAATGCCAAGGTAGA | Forward primer for 3′RACE, nested |
| H16N5 | GCTTTCTCATCTCGCTTTCG | Reverse primer for 5′RACE, nested |
| H16N3 | CGTTACGGTTCCCAAGATTC | Forward primer for 3′RACE, nested |
| H70T1 | ACCAATGACAAGGGTAGG | Primer for QRT-PCR, forward |
| H70T2 | TGTAGGCATAGTTCTCCAAT | Primer for QRT-PCR, reverse |
| H17T1 | CTCACATCTTCAAGGCTGATC | Primer for QRT-PCR, forward |
| H17T2 | CTTCTTCTTTGCTGCGTTCT | Primer for QRT-PCR, reverse |
| H16T1 | GAGCGAAGAGTCGGCAAATT | Primer for QRT-PCR, forward |
| H16T2 | TAGCACGCCATCGTAACACG | Primer for QRT-PCR, reverse |
| GAPU | TGTCACGGTTTTCGGTTGTAG | Control Primer for QRT-PCR, forward |
| GAPD | ACCTTTTTGGCACCTCCCTTA | Control Primer for QRT-PCR, reverse |
3.4. Relative Quantification by QRT-PCR
The transcription levels of GbHSPs were determined in different G. biloba tissues, as well as in young seedling leaf samples collected at different time points after stress and hormone treatments. QRT-PCR was carried out using an ABI PRISM 7500 Sequence Detection System (Applied Biosystems, Foster City, CA, USA) with SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA, USA) according to the manufacturer’s protocol. The G. biloba glyceraldehydes-3-phosphate dehydrogenase gene (GbGAPDH, L26924) [64] was used as the reference gene as described by Xu [63].
The gene-specific primers (H70T1, H70T2, H17T1, H17T2, H16T1, H16T2) and reference primers (GAPU, GAPD) for QRT-PCR are listed in Table 1. The QRT-PCR conditions were: 10 min at 95 °C, and 40 cycles (95 °C for 15 s, 60 °C for 1 min). Before performing QRT-PCR, primer efficiency was evaluated using both GbHSP70 and GbGAPDH at 100 nM, 150 nM, 200 nM, 250 nM and 300 nM combinations. A 150 nM concentration was chosen as the most suitable combination for both genes. For each plant sample, aliquots of 150 ng total RNA was analyzed for each gene and the four genes (GbHSP16.8, GbHSP17, GbHSP70 and GbGAPDH) were always analyzed simultaneously. Each sample was amplified 3 times and all reactions were performed on an ABI PRISM 7500 Sequence Detection System. With a housekeeping gene GbGAPDH, the relative amount of the three GbHSP transcriptsw is presented as 2(-ddCt) according to the CT method (dCt = Ctsample-Ctcontrol) described in the QRT-PCR Application Guide (Applied Biosystems). When comparing the expression of GbHSPs in different tissues, the relative expression of GbHSPs was achieved by calibrating its transcription level to that of the reference gene, GbGAPDH.
3.5. Statistics
Similarity search of the three GbHSP proteins were performed with the Blastx or Blastp program [65]. Multiple sequence alignment of the deduced GbHSPs with other congeneric HSPs was conducted using the clustlx program. The phylogenetic tree was constructed by a neighbor-joining (NJ) method and measured by bootstrap analysis with 1000 replicates. Phylogenetic tree analysis of GbHSPs and known HSPs from other plant species retrieved from GenBank were aligned with Mega 4.0 [66]. Vector NTI Suite 10 was used for sequence alignment and analysis. SPSS 17 was used for statistical analysis and graphing.
4. Conclusions
In summary, the analysis of the tissue and environment stress expression profiles of the three HSP genes has improved the functional dissection of Ginkgo HSP genes. It is possible that appropriate low-temperature treatment will improve the adaptation of Ginkgo to other abiotic stresses. Elucidation of the precise role of each GbHSP gene, however, requires other experimental approaches including overexpression or RNAi strategies.
Acknowledgments
This work was supported by Economic Forest Germplasm Improvement and Comprehensive Utilization of Resources of Hubei Key Laboratory (20011BLKF238), the Natural Science Foundation of China (30971974), and University-industry Cooperation Fund of Hubei Educational Office (CXY2009B009).
References
- 1.Ballinger D.G., Pardue M.L. The control of protein synthesis during heat shock in Drosophila cells involves altered polypeptide elongation rates. Cell. 1983;33:103–113. doi: 10.1016/0092-8674(83)90339-2. [DOI] [PubMed] [Google Scholar]
- 2.Wang W., Vinocur B., Shoseyov O., Altman A. Role of plant heat-shock proteins and molecular chaperones in the abiotic stress response. Trends Plant Sci. 2004;9:244–252. doi: 10.1016/j.tplants.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 3.Sørensen J.G., Kristensen T.N., Loeschcke V. The evolutionary and ecological role of heat shock proteins. Ecol. Lett. 2003;6:1025–1037. [Google Scholar]
- 4.Sun W., van Montagu M., Verbruggen N. Small heat shock proteins and stress tolerance in plants. Biochim. Biophys. Acta. 2002;1577:1–9. doi: 10.1016/s0167-4781(02)00417-7. [DOI] [PubMed] [Google Scholar]
- 5.Zou J., Liu A., Chen X., Zhou X., Gao G., Wang W., Zhang X. Expression analysis of nine rice heat shock protein genes under abiotic stresses and ABA treatment. J. Plant Physiol. 2009;166:851–861. doi: 10.1016/j.jplph.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 6.Vierling E. The roles of heat shock proteins in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1991;42:579–620. [Google Scholar]
- 7.Sejerkilde M., Sørensen J.G., Loeschcke V. Effects of cold-and heat hardening on thermal resistance in Drosophila melanogaster. J. Insect Physiol. 2003;49:719–726. doi: 10.1016/s0022-1910(03)00095-7. [DOI] [PubMed] [Google Scholar]
- 8.Tammariello S.P., Rinehart J.P., Denlinger D.L. Desiccation elicits heat shock protein transcription in the flesh fly, Sarcophaga crassipalpis, but does not enhance tolerance to high or low temperatures. J. Insect Physiol. 1999;45:933–938. doi: 10.1016/s0022-1910(99)00073-6. [DOI] [PubMed] [Google Scholar]
- 9.Tedengren M., Olsson B., Bradley B., Zhou L. Heavy metal uptake, physiological response and survival of the blue mussel (Mytilus edulis) from marine and brackish waters in relation to the induction of heat-shock protein 70. Hydrobiologia. 1999;393:261–269. [Google Scholar]
- 10.Spees J.L., Chang S.A., Snyder M.J., Chang E.S. Osmotic induction of stress-responsive gene expression in the lobster Homarus americanus. Biol. Bull. 2002;203:331–337. doi: 10.2307/1543575. [DOI] [PubMed] [Google Scholar]
- 11.Ma E., Haddad G.G. Anoxia regulates gene expression in the central nervous system of Drosophila melanogaster. Mol. Brain Res. 1997;46:325–328. doi: 10.1016/s0169-328x(97)00074-0. [DOI] [PubMed] [Google Scholar]
- 12.Rinehart J.P., Denlinger D.L., Rivers D.B. Upregulation of transcripts encoding select heat shock proteins in the flesh fly Sarcophaga crassipalpis in response to venom from the ectoparasitoid wasp Nasonia vitripennis. J. Invertebr. Pathol. 2002;79:62–63. doi: 10.1016/S0022-2011(02)00002-2. [DOI] [PubMed] [Google Scholar]
- 13.Murakami T., Matsuba S., Funatsuki H., Kawaguchi K., Saruyama H., Tanida M., Sato Y. Over-expression of a small heat shock protein, sHSP17.7, confers both heat tolerance and UV-B resistance to rice plants. Mol. Breed. 2004;13:165–175. [Google Scholar]
- 14.Niedzwiecki A., Reveillaud I., Fleming J.E. Changes in superoxide dismutase and catalase in aging heat-shocked Drosophila. Free Radic. Res. Commun. 1992;17:355–367. doi: 10.3109/10715769209083140. [DOI] [PubMed] [Google Scholar]
- 15.Musch M.W., Kapil A., Chang E.B. Heat shock protein 72 binds and protects dihydrofolate reductase against oxidative injury. Biochem Biophys. Res. Commun. 2004;313:185–192. doi: 10.1016/j.bbrc.2003.11.096. [DOI] [PubMed] [Google Scholar]
- 16.Campbell J.L., Klueva N.Y., Zheng H., Nieto-Sotelo J., Ho T.H., Nguyen H.T. Cloning of new members of heat shock protein HSP101 gene family in wheat (Triticum aestivum (L.) Moench) inducible by heat, dehydration, and ABA. Biochim. Biophys. Acta. 2001;1517:270–277. doi: 10.1016/s0167-4781(00)00292-x. [DOI] [PubMed] [Google Scholar]
- 17.Chang P.F., Jinn T.L., Huang W.K., Chen Y., Chang H.M., Wang C.W. Induction of a cDNA clone from rice encoding a class II small heat shock protein by heat stress, mechanical injury, and salicylic acid. Plant Sci. 2007;172:64–75. [Google Scholar]
- 18.Lewis J., Devin A., Miller A., Lin Y., Rodriguez Y., Neckers L., Liu Z. Disruption of hsp90 function results in degradation of the death domain kinase, receptor-interacting protein (RIP), and blockage of tumor necrosis factor-induced nuclear factor-κB activation. J. Biol. Chem. 2000;275:10519–10526. doi: 10.1074/jbc.275.14.10519. [DOI] [PubMed] [Google Scholar]
- 19.Pandey P., Saleh A., Nakazawa A., Kumar S., Srinivasula S.M., Kumar V., Weichselbaum R., Nalin C., Alnemri E.S., Kufe D. Negative regulation of cytochrome c-mediated oligomerization of Apaf-1 and activation of procaspase-9 by heat shock protein 90. EMBO J. 2000;19:4310–4322. doi: 10.1093/emboj/19.16.4310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Volkov R.A., Panchuk I.I., Schöffl F. Small heat shock proteins are differentially regulated during pollen development and following heat stress in tobacco. Plant Mol. Biol. 2005;57:487–502. doi: 10.1007/s11103-005-0339-y. [DOI] [PubMed] [Google Scholar]
- 21.Sanmiya K., Suzuki K., Egawa Y., Shono M. Mitochondrial small heat-shock protein enhances thermotolerance in tobacco plants. FEBS. Lett. 2004;557:265–268. doi: 10.1016/s0014-5793(03)01494-7. [DOI] [PubMed] [Google Scholar]
- 22.Guo S.J., Zhou H.Y., Zhang X.S., Li X.G., Meng Q.W. Overexpression of CaHSP26 in transgenic tobacco alleviates photoinhibition of PSII and PSI during chilling stress under low irradiance. J. Plant Physiol. 2007;164:126–136. doi: 10.1016/j.jplph.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 23.Sato Y., Yokoya S. Enhanced tolerance to drought stress in transgenic rice plants overexpressing a small heat-shock protein, sHSP17.7. Plant Cell Rep. 2008;27:329–334. doi: 10.1007/s00299-007-0470-0. [DOI] [PubMed] [Google Scholar]
- 24.Smith J., Luo Y. Studies on molecular mechanisms of Ginkgo biloba extract. Appl. Microbiol. Biotechnol. 2004;64:465–472. doi: 10.1007/s00253-003-1527-9. [DOI] [PubMed] [Google Scholar]
- 25.van Beek T. Chemical analysis of Ginkgo biloba leaves and extracts. J. Chromatogr. A. 2002;967:21–55. doi: 10.1016/s0021-9673(02)00172-3. [DOI] [PubMed] [Google Scholar]
- 26.Deng Z., Wang Y., Jiang K., Liu X., Wu W., Gao S., Lin J., Sun X., Tang K. Molecular cloning and characterization of a novel dehydrin gene from Ginkgo biloba. Biosci. Rep. 2006;26:203–215. doi: 10.1007/s10540-006-9016-x. [DOI] [PubMed] [Google Scholar]
- 27.Mayer M., Bukau B. Hsp70 chaperones: Cellular functions and molecular mechanism. Cell Mol. Life Sci. 2005;62:670–684. doi: 10.1007/s00018-004-4464-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu X., Yano M., Washida H., Kido H. The second metal-binding site of 70 kDa heat-shock protein is essential for ADP binding, ATP hydrolysis and ATP synthesis. Biochem. J. 2004;378:793–799. doi: 10.1042/BJ20031680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morshauser R.C., Wang H., Flynn G.C., Zuiderweg E.R. The peptide-binding domain of the chaperone protein Hsc70 has an unusual secondary structure topology. Biochemistry. 1995;34:6261–6266. doi: 10.1021/bi00019a001. [DOI] [PubMed] [Google Scholar]
- 30.Morshauser R.C., Hu W., Wang H., Pang Y., Flynn G.C., Zuiderweg E.R. High-resolution solution structure of the 18 kDa substrate-binding domain of the mammalian chaperone protein Hsc701. J. Mol. Biol. 1999;289:1387–1403. doi: 10.1006/jmbi.1999.2776. [DOI] [PubMed] [Google Scholar]
- 31.Bondino H.G., Valle E.M. Evolution and functional diversification of the small heat shock protein α-crystallin family in higher plants. Planta. 2011 doi: 10.1007/s00425-011-1575-9. [DOI] [PubMed] [Google Scholar]
- 32.Waters E.R., Lee G.J., Vierling E. Evolution, structure and function of the small heat shock proteins in plants. J. Exp. Bot. 1996;47:325–338. [Google Scholar]
- 33.Plesofsky-Vig N., Vig J., Brambl R. Phylogeny of the α-crystallin-related heat-shock proteins. J. Mol. Evol. 1992;35:537–545. doi: 10.1007/BF00160214. [DOI] [PubMed] [Google Scholar]
- 34.Horwitz J. Alpha-crystallin can function as a molecular chaperone. Proc. Natl. Acad. Sci. USA. 1992;89:10449–10453. doi: 10.1073/pnas.89.21.10449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Merck K., Groenen P., Voorter C., de Haard-Hoekman W., Horwitz J., Bloemendal H., De J.W. Structural and functional similarities of bovine α-crystallin and mouse small heat-shock protein A family of chaperones. J. Biol. Chem. 1993;268:1046–1062. [PubMed] [Google Scholar]
- 36.Kim K.K., Kim R., Kim S.H. Crystal structure of a small heat-shock protein. Nature. 1998;394:595–599. doi: 10.1038/29106. [DOI] [PubMed] [Google Scholar]
- 37.Löw D., Brändle K., Nover L., Forreiter C. Cytosolic heat-stress proteins Hsp17.7 class I and Hsp17.3 class II of tomato act as molecular chaperones in vivo. Planta. 2000;211:575–582. doi: 10.1007/s004250000315. [DOI] [PubMed] [Google Scholar]
- 38.Stevenson M., Calderwood S. Members of the 70-kilodalton heat shock protein family contain a highly conserved calmodulin-binding domain. Mol. Cell Biol. 1990;10:1234–1238. doi: 10.1128/mcb.10.3.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang Z., Quick M.K., Kanelakis K.C., Gijzen M., Krishna P. Characterization of a plant homolog of hop, a cochaperone of hsp90. Plant Physiol. 2003;131:525–535. doi: 10.1104/pp.011940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sung D.Y., Vierling E., Guy C.L. Comprehensive expression profile analysis of the Arabidopsis Hsp70 gene family. Plant Physiol. 2001;126:789–800. doi: 10.1104/pp.126.2.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koo H.J., Xia X., Hong C.B. Genes and expression pattern of tobacco mitochondrial small heat shock protein under high-temperature stress. J. Plant Biol. 2003;46:204–210. [Google Scholar]
- 42.Guan J.C., Jinn T.L., Yeh C.H., Feng S.P., Chen Y.M., Lin C.Y. Characterization of the genomic structures and selective expression profiles of nine class I small heat shock protein genes clustered on two chromosomes in rice (Oryza sativa L.) Plant Mol. Biol. 2004;56:795–809. doi: 10.1007/s11103-004-5182-z. [DOI] [PubMed] [Google Scholar]
- 43.Sun W., Bernard C., Van De., Cotte B., Van Montagu M., Verbruggen N. At-HSP17.6A encoding a small heat-shock protein in Arabidopsis, can enhance osmotolerance upon overexpression. Plant J. 2001;27:407–415. doi: 10.1046/j.1365-313x.2001.01107.x. [DOI] [PubMed] [Google Scholar]
- 44.Collins G.G., Nie X.L., Saltveit M.E. Heat shock proteins and chilling sensitivity of mung bean hypocotyls. J. Exp. Bot. 1995;46:795–802. [Google Scholar]
- 45.Anderson J.V., Li Q.B., Haskell D.W., Guy C.L. Structural organization of the spinach endoplasmic reticulum-luminal 70-kilodalton heat-shock cognate gene and expression of 70-kilodalton heat-shock genes during cold acclimation. Plant Physiol. 1994;104:1359–1370. doi: 10.1104/pp.104.4.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lopez-Matas M.A., Nuñez P., Soto A., Allona I., Casado R., Collada C., Guevara M.A., Aragoncillo C., Gomez L. Protein cryoprotective activity of a cytosolic small heat shock protein that accumulates constitutively in chestnut stems and is up-regulated by low and high temperatures. Plant Physiol. 2004;134:1708–1717. doi: 10.1104/pp.103.035857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Krishna P., Sacco M., Cherutti J.F., Hill S. Cold-induced accumulation of hsp90 transcripts in Brassica napus. Plant Physiol. 1995;107:915–923. doi: 10.1104/pp.107.3.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Soto A., Allona I., Collada C., Guevara M.A., Casado R., Rodriguez-Cerezo E., Aragoncillo C., Gomez L. Heterologous expression of a plant small heat-shock protein enhances Escherichia coli viability under heat and cold stress. Plant Physiol. 1999;120:521–528. doi: 10.1104/pp.120.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang L., Zhao C.M., Wang Y.J., Liu J. Overexpression of chloroplast-localized small molecular heat-shock protein enhances chilling tolerance in tomato plant. J. Plant Physiol. Mol. Biol. 2005;31:167–174. [PubMed] [Google Scholar]
- 50.Guo S.J., Chen N., Guo P., Meng Q.W. cDNA cloning and expression of a cytosolic small heat shock protein gene (CaHSP18) from Capsicum annuum. J. Plant Physiol. Mol. Biol. 2005;31:409–416. [PubMed] [Google Scholar]
- 51.Zhu W., Lu M., Gong Z., Chen R. Cloning and expression of a small heat shock protein gene CaHSP24 from pepper under abiotic stress. Afr. J. Biotechnol. 2011;10:4968–4975. [Google Scholar]
- 52.Morimoto R. Cells in stress: Transcriptional activation of heat shock genes. Science. 1993;259:1409–1450. doi: 10.1126/science.8451637. [DOI] [PubMed] [Google Scholar]
- 53.Krizek D.T., Kramer G.F., Upadhyaya A., Mirecki R.M. UV-B response of cucumber seedlings grown under metal halide and high pressure sodium-deluxe lamps. Physiol. Plant. 1993;88:350–358. [Google Scholar]
- 54.Strid Å. Alteration in expression of defence genes in Pisum sativum after exposure to supplementary ultraviolet-B radiation. Plant Cell Physiol. 1993;34:949–953. [Google Scholar]
- 55.Banzet N., Richaud C., Deveaux Y., Kazmaier M., Gagnon J., Triantaphylidès C. Accumulation of small heat shock proteins, including mitochondrial HSP22, induced by oxidative stress and adaptive response in tomato cells. Plant J. 1998;13:519–527. doi: 10.1046/j.1365-313x.1998.00056.x. [DOI] [PubMed] [Google Scholar]
- 56.Hamilton E.W., Heckathorn S.A. Mitochondrial adaptations to NaCl. Complex I is protected by anti-oxidants and small heat shock proteins, whereas complex II is protected by proline and betaine. Plant Physiol. 2001;126:1266–1274. doi: 10.1104/pp.126.3.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kang H.M., Saltveit M.E. Wound-induced PAL activity is suppressed by heat-shock treatments that induce the synthesis of heat-shock proteins. Physiol. Plant. 2003;119:450–455. [Google Scholar]
- 58.Leonardi R., Villari L., Caltabiano M., Travali S. Heat shock protein 27 expression in the epithelium of periapical lesions. J. Endod. 2001;27:89–92. doi: 10.1097/00004770-200102000-00007. [DOI] [PubMed] [Google Scholar]
- 59.Cho E.K., Hong C.B. Over-expression of tobacco NtHSP70-1 contributes to drought-stress tolerance in plants. Plant Cell Rep. 2006;25:349–358. doi: 10.1007/s00299-005-0093-2. [DOI] [PubMed] [Google Scholar]
- 60.Hu X., Liu R., Li Y., Wang W., Tai F., Xue R., Li C. Heat shock protein 70 regulates the abscisic acid-induced antioxidant response of maize to combined drought and heat stress. Plant Growth Regul. 2010;60:225–235. [Google Scholar]
- 61.Daie J., Campbell W.F. Response of tomato plants to stressful temperatures: Increase in abscisic acid concentrations. Plant Physiol. 1981;67:26–29. doi: 10.1104/pp.67.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ye S.F., Yu S.W., Shu L.B., Wu J.H., Wu A.Z., Luo L.J. Expression profile analysis of 9 heat shock protein genes throughout the life cycle and under abiotic stress in rice. Chin. Sci. Bull. 2011;57:336–343. [Google Scholar]
- 63.Xu F., Cheng H., Cai R., Li L.L., Chang J., Zhu J., Zhang F.X., Chen L.J., Wang Y., Cheng S.H. Molecular cloning and function analysis of an anthocyanidin synthase gene from Ginkgo biloba, and its expression in abiotic stress responses. Mol. Cells. 2008;26:536–547. [PubMed] [Google Scholar]
- 64.Jansson S., Meyer-Gauen G., Cerff R., Martin W. Nucleotide distribution in gymnosperm nuclear sequences suggests a model for GC-content change in land-plant nuclear genomes. J. Mol. Evol. 1994;39:34–46. doi: 10.1007/BF00178247. [DOI] [PubMed] [Google Scholar]
- 65.NCBI Basic Local Alignment Search Tool. [accessed on 5 October 2011]. Available online: http://www.ncbi.nlm.nih.gov/blast/Blast.cgi.
- 66.Tamura K., Dudley J., Nei M., Kumar S. MEGA4: Molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol. Biol. Evol. 2007;24:1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]