Abstract
The aim of this study was to investigate the contribution of reduced apparent clearance to the enhanced exposure reported for biodegradable nanoparticles after extravascular and intravascular routes of administration. Plasma concentration profiles for drug and nanoparticle formulations after administration by intravenous, intraduodenal, and oral routes were extracted from the literature. Data were fit to pharmacokinetic models using BOOMER. The compartmental pharmacokinetic analysis of literature data for six drugs (camptothecin, 9-nitrocamptothecin, epirubicin, vinpocetine, clozapine, and cyclosporine) showed that the encapsulation of drug molecules in nanoparticles significantly reduced the apparent clearance and prolonged the apparent circulation half-life compared with those for the plain drug. Positively charged nanoparticles assessed in this study had lower apparent clearance, lower elimination rate constant values, and longer apparent circulation half-life than neutral and negatively charged nanoparticles. After oral administration, a reduction in apparent clearance contributed substantially to elevations in plasma drug exposure with nanoparticles. For the drugs and delivery systems examined, the nano-advantage in drug delivery enhancement can be explained, in part, by reduced clearance.
Introduction
Drug-encapsulating nanoparticles or nanosystems in general have been reported to enhance delivery to target tissue because of increased drug permeability or absorption (Gelperina et al., 2005; Mishra et al., 2008), thereby reducing the dosing frequency and improving patient compliance (Gelperina et al., 2005; Emerich and Thanos, 2006; Bawa, 2008; Zhang et al., 2009). Nanoparticles also minimize side effects (Sinha et al., 2006; Zhang et al., 2008) and sustain drug release over a prolonged period of time. Nanosystems are being developed for various drug molecules. Although several of the above advantages are feasible in general, enhanced permeability-based absorption with nanoparticles is debatable as the sole or primary contributor to the nanoparticle delivery advantage observed in several of the previous studies. Typical studies based on nanosystems assess a single time point or an incomplete concentration versus time profile and conclude enhanced drug absorption or delivery. In such studies, dissecting the contribution of enhanced absorption versus reduced clearance is not possible. Even in studies in which the entire concentration-time course is obtained, the assumption of enhanced absorption is made without fully considering the contribution of reduced clearance (El-Shabouri, 2002; Italia et al., 2007). Drug exposure is usually measured as area under the concentration versus time curve (AUC) in target tissues or in a surrogate tissue such as plasma. AUC can be increased owing to an increase in fraction absorbed or a decrease in clearance rate. Reduction in clearance contributes to elevated AUC but may be incorrectly attributed to enhanced absorption. Therefore, the purpose of this study was to determine the contribution of reduced clearance to enhanced delivery or drug exposure measured as AUC in several previously reported studies (El-Shabouri, 2002; Manjunath and Venkateswarlu, 2005; Luo et al., 2006; Schluep et al., 2006; Dadashzadeh et al., 2008; Li et al., 2010).
Nanosystems may enhance oral absorption by increasing the gastric residence time through mucosal adhesion (Takeuchi et al., 1996) or by increasing cell or tissue entry (e.g., Peyer's patches and M cell-mediated uptake) (Florence et al., 1995a,b; Torché et al., 2000; Florence, 2005). An additional barrier for drug absorption is chemical and metabolic instability of the drug. The gastrointestinal (GI) tract is rich in phase I and phase II metabolic enzymes. Drugs such as cyclosporine (Italia et al., 2007), estradiol (Mittal et al., 2007), and curcumin (Anand et al., 2007) exhibit chemical or enzymatic instability in the GI tract. Many drug molecules have lower systemic bioavailability after oral administration, because of enzymatic and nonenzymatic degradation in the GI tract and first-pass metabolism in the liver before the drug reaches the systemic circulation (Italia et al., 2007; Mittal et al., 2007). Nanoparticle formulations can reduce drug exposure to the adverse conditions in the GI tract, thereby minimizing enzymatic and nonenzymatic degradations; this can lead to an increase in AUC or drug exposure.
Intravenously administered hydrophilic drug molecules typically undergo rapid renal clearance because of poor reabsorption after glomerular filtration(Lin and Lu, 1997), whereas lipophilic drugs tend to undergo biotransformation in the liver to hydrophilic metabolites before biliary or renal excretion (Parkinson et al., 2010). Encapsulation of drug in nanoparticles reduces renal clearance because of increased size (cutoff for renal clearance is <15 nm) (Choi et al., 2007). Furthermore, nanoparticle formulations may protect lipophilic drugs from metabolizing enzymes in the liver (Li and Huang, 2008).
Most of the reported studies observed an increase in the AUC or drug exposure for drugs encapsulated in nanoparticles compared with that for the plain drug solution or suspension administered by the same route. After intravenous administration, it is possible to identify the contribution of reduced total drug clearance to enhanced drug exposure by nanoparticle formulation. For extravascular routes of administration, assessing the contribution of reduced clearance versus enhanced absorption becomes more difficult. Previous literature reports indicated that an increase in delivery with nanoparticles compared with that of plain drug solution or suspension was due to enhanced uptake from the site of administration (Hussain et al., 1997; El-Shabouri, 2002; Qian et al., 2006; Italia et al., 2007). Although these conclusions may be true in part, these studies did not consider the effect of reduced clearance from plasma or at the site of administration as a contributor to enhanced drug exposure. There are no reports that systematically determined the contribution of reduced clearance to nanoparticle-mediated enhancement in drug exposure. Part of the difficulty, as elaborated through this study, includes a lack of available methods to analyze free drug, nanoparticle-bound drug, and protein-bound drug in the tissues of interest.
The objective of the present study was to develop pharmacokinetic (PK) models for plain drug and nanoparticle-encapsulated drug after oral, intraduodenal, and intravenous routes of administration and to understand the influence of altered drug clearance on enhanced drug exposure with nanoparticle formulations. Furthermore, efforts were made to understand the influence of nanoparticle surface charge on plasma pharmacokinetics.
Materials and Methods
Data Set for Modeling.
The data sets used in this study were collected from the literature for six drug molecules. Only data pertaining to the plasma concentration profile after single-dose administration of the plain drug formulation and nanoparticle formulation from the same study were considered for analysis. The plasma concentration data were extracted from the plasma concentration profile graphs provided in the literature, and S.D.s were not used/available. Because of the limitation of currently available bioanalytical methods, published reports used for this study did not distinguish free drug, nanoparticle-bound drug, and protein-bound drug. These earlier studies reported total drug concentration, which was used in the present study. The parameter estimates obtained for nanoparticles are a combination of both released plain drug and drug in nanoparticles. Therefore, the parameters estimated for the nanosystems should be considered as apparent clearance, apparent volume of distribution, and apparent circulation half-life. Nanoparticle literature commonly contains the term circulation half-life as a synonym for terminal half-life (Petros and DeSimone, 2010). Hence, we use the term circulation half-life in this report.
Modeling Software.
The mathematical compartmental modeling was performed using BOOMER, a differential and integrated equation-based modeling program (Bourne, 1989). The parameters for each compartmental model were estimated using the simplex damping Gauss-Newton curve-fitting procedure in this software. Numerical integration was performed using the Runge-Kutta-Fehlberg 45 method for all analyses.
Model Development.
PK model development was started as a forward stepwise approach as in the multiple linear regression. The initial model was developed as the simplest model based on the route of administration. For the oral route of administration, both absorption and elimination processes were considered as first-order processes. A distribution compartment was added to the initial model when necessary to improve the fit to the data. Data were weighted using two weighting schemes, equal weight and 1/Cp(i)2. The final best-fit model was selected on the basis of the Akaike information criterion (AIC), parameter coefficients of variation (%CV), visual inspection of the weighted residual plots and concentration versus time plots, and coefficient of regression (R2). Lower AIC values, lower coefficient of variation (%CV), weighted residual plot with random distribution, and higher coefficient of regression (R2) indicated a better fit to the model. Between the two weighting schemes used, 1/Cp(i)2 showed better random distribution of data in weighted residual plots for all drugs compared with the equal weight scheme; hence, the 1/Cp(i)2 weighting scheme was used for all selected models.
Results
Data Collection.
Literature reports on the plasma concentration profiles for plain drug and nanoparticle formulations were chosen for six drug molecules administered by three different routes of administration, i.e., oral, intraduodenal, and intravenous. Table 1 summarizes the drug name, particle size, surface charge, route of administration, species of study, and dose of drug. Except for camptothecin IT-101, which is a soluble conjugate, all other nanoformulations are particle dispersions. To compare the effect of nanoformulation on pharmacokinetic parameters, compartmental modeling was performed after intravenous, intraduodenal, and oral administrations and compared with plain drug formulation. To study the effect of surface charge of nanoparticles on pharmacokinetic parameters, the pharmacokinetic profiles of positive, negative, and neutral nanoparticles were assessed. The data set contained only the small drug molecules and not the macromolecules such as proteins and plasmids because of the paucity of PK profile data for macromolecules encapsulated in nanoparticles.
TABLE 1.
Drug name, nanoparticle composition, particle size, surface charge, route of administration, species of study, and dose of nanoparticles used for pharmacokinetic parameter estimations in this study
| Drug Name | Matrix Material of Nanoparticles | Particle Size | Particle Charge | Route | Species | Dose Administered | Reference |
|---|---|---|---|---|---|---|---|
| nm | mg/kg | ||||||
| Camptothecin IT-101 | Polymer conjugate of camptothecin and β-cyclodextrin-based polymer | 78 | N.A. | Intravenous | Female Sprague-Dawley rats | 1 | Schluep et al., 2006 |
| 9-Nitrocamptothecin | Nanoparticles made with PLGA (50:50) and polyvinyl alcohol (PVA) | 207 ± 26 | N.A. | Intravenous | Male Wistar rats | 2 | Dadashzadeh et al., 2008 |
| Epirubicin | Self-assembled system made with carboxymethyl curdlan coupled with cholesterol chitosan | 208 | Negative (−32.1) | Intravenous | Male Wistar rats | 10 | Li et al., 2010 |
| Vinpocetine | Solid lipid nanoparticles made with glycerol monostearate, polysorbate 80, and soya lecithin | 70.3 ± 7.8 | Negative (−33.8 ± 0.9) | Oral | Male Wistar rats | 10 | Luo et al., 2006 |
| Clozapine | Solid lipid nanoparticles made with triglyceride, phosphatidylcholine, poloxamer 188, and tripalmitin along with stearylamine | 233 ± 13 | Positive (+23.2 ± 0.9) | Duodenal and intravenous | Male Wistar rats | 20 | Manjunath and Venkateswarlu, 2005 |
| Clozapine | Solid lipid nanoparticles made with triglyceride, phosphatidylcholine, poloxamer 188, and tripalmitin | 163 ± 0.7 | Neutral (+0.2 ± 0.1) | Duodenal and intravenous | Male Wistar rats | 20 | Manjunath and Venkateswarlu, 2005 |
| Cyclosporine (positively charged NP) | Nanoparticles made with lecithin, poloxamer 188, and chitosan | 148 ± 29 | Positive (+31.2 ± 1.6) | Oral | Male beagle dogs | 7.5 | El-Shabouri, 2002 |
| Cyclosporine (negatively charged NP) | Nanoparticles made with lecithin, poloxamer 188, and sodium glycocholate | 104 ± 18 | Negative (−41.6 ± 1.1) | Oral | Male beagle dogs | 7.5 | El-Shabouri, 2002 |
N.A., not available; NP, nanoparticles.
Pharmacokinetic Modeling after Intravenous Administration.
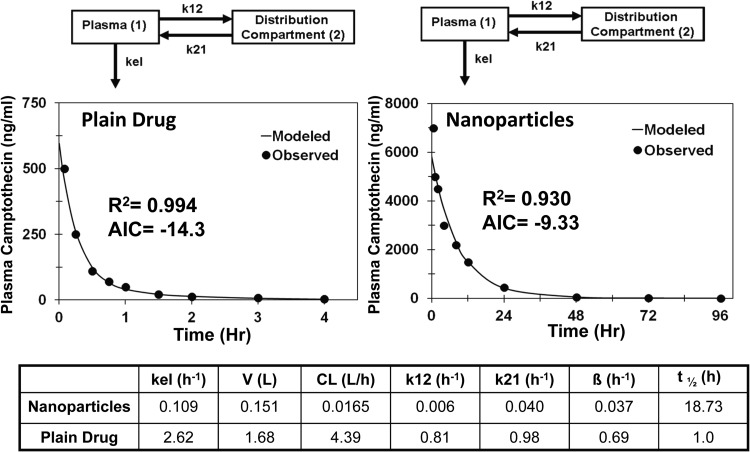
Intravenous data were available for camptothecin solution and its polymer conjugate, 9-nitrocamptothecin solution and nanoparticles, and epirubicin solution and nanoparticles. For camptothecin, a single-compartment model showed a poor fit to the data for both drug solution and the polymer conjugate (AIC = 6.6 and −6.6, respectively). Addition of a second distribution compartment improved the fit significantly for drug solution and nanoparticles (AIC = −14.3 and −9.3, respectively). The best-fit model along with a PK parameter summary is given in Fig. 1. The polymer-drug conjugate showed 266-fold lower apparent plasma clearance, 11-fold lower apparent volume of distribution, and 19 times longer apparent circulation half-life than the plain drug.
Fig. 1.
Model predicted and observed concentrations of camptothecin in plasma after intravenous administration in female Sprague-Dawley rats. Two-compartment model for camptothecin solution and polymer conjugated camptothecin (IT-101). kel, elimination rate constant from the plasma compartment; k12, rate constant for transfer of drug from the plasma compartment to the distribution compartment; k21, rate constant for transfer of drug from the distribution compartment to the plasma compartment.
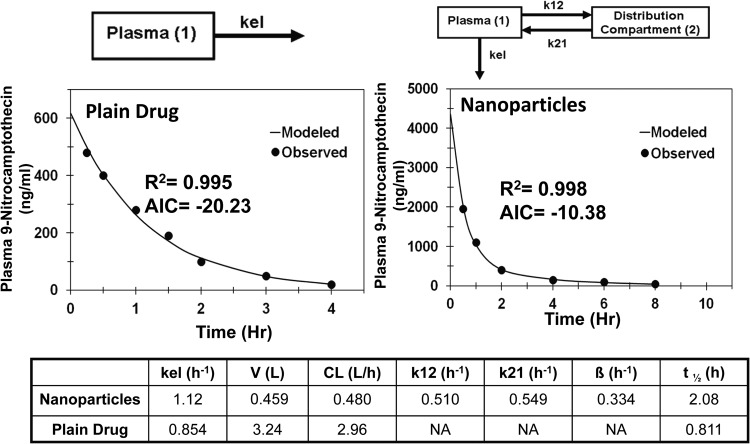
For 9-nitrocamptothecin, a single-compartment model provided a good fit for drug solution (AIC = −20.2) and a reasonable fit for nanoparticles (AIC = 1.16). Addition of a distribution compartment resulted in a significant improvement in the fit to the data for nanoparticles with a significant reduction in AIC value (AIC = −10.4). Comparison of PK parameters showed that nanoparticles had a 5.4-fold lower apparent clearance, 7.1-fold lower apparent volume of distribution, and 2.6 times longer apparent circulation half-life (Fig. 2).
Fig. 2.
Model predicted and observed concentrations of 9-nitocamptothecin in plasma after intravenous administration in male Wistar rats. One-compartment model for 9-nitocamptothecin solution and two-compartment model for polymeric nanoparticles of 9-nitrocamptothecin. kel, elimination rate constant from the plasma compartment; k12, rate constant for transfer of drug from the plasma compartment to the distribution compartment; k21, rate constant for transfer of drug from the distribution compartment to the plasma compartment.
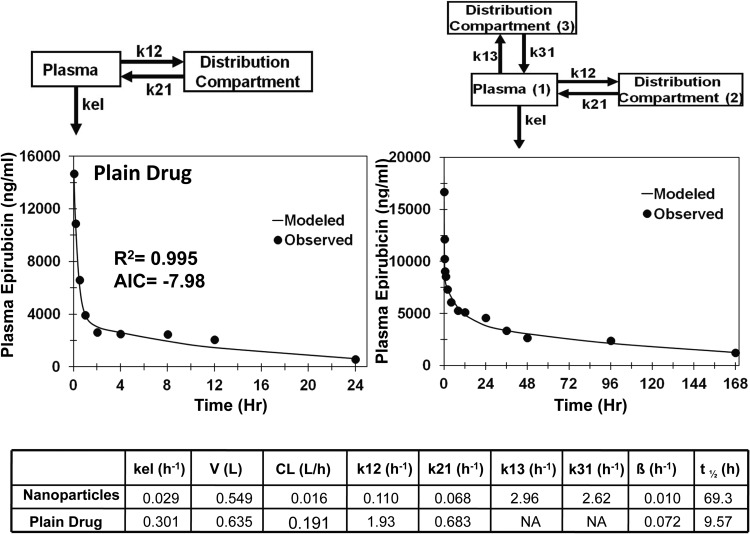
For epirubicin solution and nanoparticles, the single-compartment model showed a poor fit to the data (AIC = 1.2 and 6.6, respectively); however, addition of distribution compartment improved the fit significantly for the solution (AIC = −7.7). For epirubicin nanoparticles, addition of a second compartment improved the fit (AIC = −12.6), but visual observation of the weighted residual plot and concentration time plot suggested that a model with three compartments might be a better model. Addition of a second distribution compartment resulted in a significant improvement in the fit (AIC = −23.3) for epirubicin nanoparticles compared with the one- and two-compartment models (Fig. 3). Comparison of PK parameters for the final selected models showed that the nanoparticles had 11.9-fold lower apparent clearance, 1.2-fold lower apparent volume of distribution, and 7.2 times longer apparent circulation half-life (Fig. 3).
Fig. 3.
Model predicted and observed concentrations of epirubicin in plasma after intravenous administration in male Wistar rats. Two-compartment model for epirubicin solution and three-compartment model for self-assembled curdlan and cholesterol nanoparticles of epirubicin. kel, elimination rate constant from the plasma compartment; k12, rate constant for transfer of drug from the plasma compartment to the distribution compartment; k21, rate constant for transfer of drug from the distribution compartment to the plasma compartment; k13, rate constant for transfer of drug from the plasma compartment to the second distribution compartment; k31, rate constant for transfer of drug from the second distribution compartment to the plasma compartment.
Pharmacokinetic Modeling after Oral Administration.
Data sets for two drug molecules administered either orally or intraduodenally as solution or suspension and their nanoparticles were used for PK modeling. The model development was started with one-compartment extravascular administration, with drug dosing in the GI compartment. A distribution compartment was added when necessary to achieve the best fit.
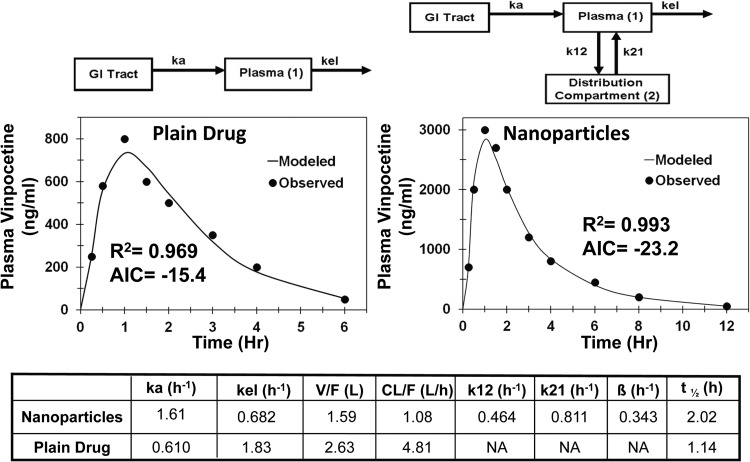
For vinpocetine drug solution and nanoparticles, the pharmacokinetic compartmental modeling was started with a one-compartment model describing the GI tract and blood compartment and elimination from the blood compartment. For vinpocetine solution, a one-compartment model showed a better fit than a two-compartment model (AIC = −10.8 and −6.8, respectively), but the predicted maximum concentration (Cmax) was lower than the observed one. Addition of a lag time component to the one-compartment model for vinpocetine solution improved the fit significantly (AIC = −15.4) and the predicted Cmax value was closer to the observed value (Fig. 4). For vinpocetine nanoparticles, a two-compartment model with lag time (Fig. 4) provided a better fit to the data than a one-compartment model (AIC = −23.1 versus −16.4). Comparisons of the PK parameters for the final selected models showed that incorporation of drug in nanoparticles results in 4.5-fold lower apparent clearance (CL/F), 1.7-fold lower apparent volume of distribution (V/F), and 1.8 times longer apparent circulation half-life (Fig. 4) than the plain drug.
Fig. 4.
Model predicted and observed concentrations of vinpocetine in plasma after oral administration in male Wistar rats. One-compartment model for vinpocetine solution and two compartment model for vinpocetine solid-lipid nanoparticles. ka, absorption rate constant from GI tract to the plasma; kel, elimination rate constant from the plasma compartment; k12, rate constant for transfer of drug from the plasma compartment to the distribution compartment; k21, rate constant for transfer of drug from the distribution compartment to the plasma compartment.
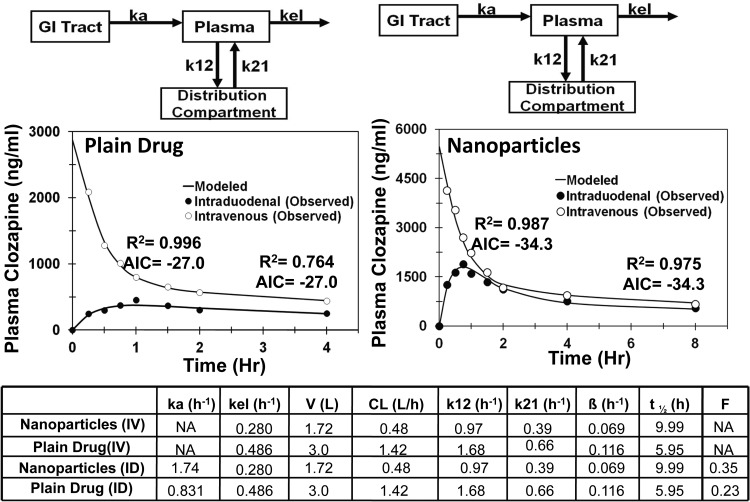
For clozapine drug suspension and nanoparticles, the data were available for both intravenous and intraduodenal administration. The compartmental modeling was performed using simultaneous fit to the intraduodenal and intravenous plasma data. All of the PK parameters except the absorption rate constant and fraction absorbed were common for both intraduodenal and intravenous data. After intravenous administration, both nanoparticles and suspension could be explained well by a two-compartmental model, and, therefore, simultaneous fit was performed using a two-compartmental model. Model predicted and observed data fit along with comparisons of PK parameters for the final selected models are given in Fig. 5. As shown in Fig. 5, incorporation of drug in nanoparticles results in 2.95-fold lower apparent clearance (CL), 1.74-fold lower apparent volume of distribution (V), 1.7 times longer apparent circulation half-life, 2.1-fold higher apparent absorption rate constant(ka), and 1.5-fold higher fraction absorbed (F).
Fig. 5.
Model predicted and observed concentrations of clozapine in plasma after intraduodenal (ID) and intravenous (IV) administration in male Wistar rats. Two-compartment model for clozapine suspension and clozapine solid-lipid nanoparticles. ka, absorption rate constant from GI tract to the plasma; kel, elimination rate constant from the plasma compartment; k12, rate constant for transfer of drug from the plasma compartment to the distribution compartment; k21, rate constant for transfer of drug from the distribution compartment to the plasma compartment.
Effect of Surface Charge of Nanoparticles on Absorption and Elimination.
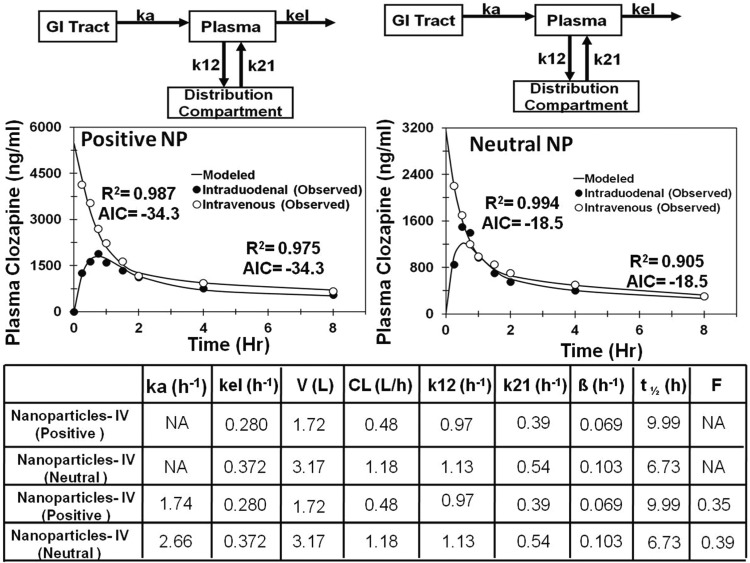
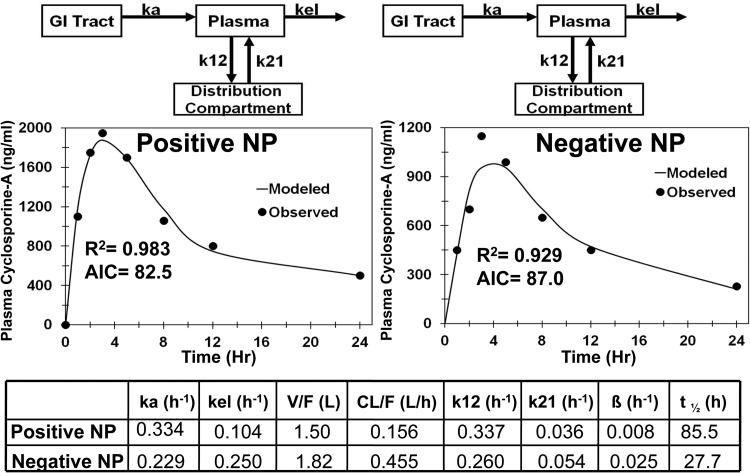
To investigate the effect of surface charge of nanoparticles on PK profile, compartmental modeling was performed on positively and negatively charged cyclosporine nanoparticles administered orally and positively and neutrally charged clozapine nanoparticles administered intraduodenally and intravenously. The two-compartment models showed a better fit to the data after the oral route of administration for both clozapine nanoparticles and cyclosporine nanoparticles (Figs. 6 and 7). Comparisons of PK parameters for the final selected model for clozapine nanoparticles are given in Fig. 6. After intravenous administration, neutral clozapine nanoparticles showed 2-fold higher apparent clearance and 2-fold lower apparent circulation half-life compared with positively charged nanoparticles (Fig. 6). After intraduodenal administration, negatively charged nanoparticles had 2.3-fold higher apparent clearance and 5.3-fold lower apparent circulation half-life than positively charged clozapine nanoparticles. Comparisons of PK parameters for the final selected models for cyclosporine nanoparticles are given in Fig. 7. For cyclosporine, negatively charged nanoparticles exhibited 3-fold higher apparent plasma oral clearance and 3-fold lower apparent circulation half-life than the positively charged nanoparticles. Differences in the apparent absorption rate constant were not as large (1.45-fold higher for positively charged nanoparticles).
Fig. 6.
Model predicted and observed concentrations of clozapine in plasma after intraduodenal and intravenous administration of nanoparticles (NP) in male Wistar rats. Two-compartment model for neutral charged solid-lipid nanoparticles and positively charged solid-lipid nanoparticles. ka, absorption rate constant from GI tract to the plasma; kel, elimination rate constant from the plasma compartment; k12, rate constant for transfer of drug from the plasma compartment to the distribution compartment; k21, rate constant for transfer of drug from the distribution compartment to the plasma compartment.
Fig. 7.
Model predicted and observed concentrations of cyclosporine in plasma after oral administration of nanoparticles (NP) in male beagle dogs. Two-compartment model for negatively charged and positively charged nanoparticles. ka, absorption rate constant from the GI tract to the plasma; kel, elimination rate constant from the plasma compartment; k12, rate constant for transfer of drug from the plasma compartment to the distribution compartment; k21, rate constant for transfer of drug from the distribution compartment to the plasma compartment.
Discussion
The relative role of enhanced uptake/absorption versus reduced clearance on enhanced delivery and efficacy achieved by biodegradable, drug-loaded nanoparticles is unclear. It is generally assumed that the enhanced uptake/absorption is responsible for the enhanced delivery and efficacy observed with nanoparticles. In this study, on the basis of compartmental pharmacokinetic analysis of literature data for drug disposition after administration of plain drug and nanoparticle formulations by oral, intraduodenal, and intravenous routes of administration, we assessed the contribution of changes in absorption and clearance to the delivery of six drug molecules. We observed that the total body apparent clearance and the elimination rate constants were severalfold lower for the drug encapsulated in nanoparticle formulations than for the plain drug after oral, intraduodenal, and intravenous administrations. The effect of nanoparticle formulation on the absorption rate constant after oral administration was less substantial. Furthermore, our analysis also shows that positively charged nanoparticles evaluated in this study offer lower apparent clearance and elimination rate constants and hence longer apparent circulation half-life and higher plasma AUC. These findings are further elaborated on below.
Some of the previous literature reports showed rapid clearance and very short circulation half-lives for nano- and microsystems after intravenous administration compared with those for the plain drug (Grislain et al., 1983; Verrecchia et al., 1995). Keeping the rapid clearance of nanoparticles in mind, many investigators may have interpreted enhanced efficacy of nanosystems to be a result of enhanced uptake in the target organ. Various attempts were made in the literature to prolong the circulation half-life for nanoparticles. Some examples include the use of polyethylene glycol, polyvinyl alcohol, polysaccharides, and surfactants such as poloxamer to coat the surface of nanosystems to reduce the opsonization and clearance by the reticuloendothelial system (Papahadjopoulos et al., 1991; Gref et al., 1994; Slepushkin et al., 1997). On the basis of these developments and pharmacokinetic principles, it is critical to dissect the contributions of reduced drug clearance (measured as changes in total body clearance and/or apparent elimination rate constant) and enhanced absorption (measured as apparent rate constant for absorption or fraction absorbed) to enhanced drug delivery achieved by nanosystems.
To determine the contribution of change in apparent clearance to plasma pharmacokinetics, compartmental modeling was performed on plain drug as well as on nanosystems of camptothecin, 9-nitrocamptothecin, and epirubicin after intravenous administration (Figs. 1, 2, and 3). Entrapment of these drugs in nanosystems resulted in reduced apparent clearance, longer apparent circulation half-life, and lower apparent volume of distribution, leading to protracted drug exposure or AUC in the plasma. Because apparent volume of distribution is lower for nanoparticles compared with that for the drug, longer apparent circulation half-life does not appear to be a result of rapid deposition of nanoparticles in tissues followed by slow drug redistribution to blood. Furthermore, distribution compartment parameters k12 and k21 were lower for nanoparticles than for plain drug after intravenous administrations, indicating slower distribution of drug to peripheral tissues for nanoparticles than for the plain drug (Figs. 1 and 3). Because of the large size of nanoparticles, small gaps between endothelial cells of blood vessels in peripheral tissues hinder the delivery of nanoparticles, resulting in reduced apparent volume of distribution for nanoparticles compared with that for plain drugs. For camptothecin and 9-nitorcamptothecin, nanosystems exhibited 11- and 7-fold lower apparent volumes of distribution compared with those for the plain drugs (Figs. 1 and 2).
Clinical applicability of camptothecin was limited by instability of the lactone ring, which rapidly hydrolyzes in vivo to inactive carboxylate metabolite (Scott et al., 1993; Schluep et al., 2006). Conjugation of camptothecin to a cyclodextrin-based polymer (IT-101) reduces metabolic clearance by increasing chemical stability of the lactone ring and reduces renal clearance by increasing the size, thereby prolonging the circulation half-life (Schluep et al., 2006). Similar to camptothecin, 9-nitrocamptothecin has the lactone ring instability (Chow et al., 2000). Furthermore, 9-nitrocamptothecin undergoes phase I and phase II hepatic metabolism (Li et al., 2003). Encapsulation of drug in polymeric nanoparticles imparted both chemical and metabolic stability to 9-nitrocamptothecin, resulting in reduced hepatic clearance and prolonged circulation half-life (Dadashzadeh et al., 2008). Epirubicin, an antineoplastic drug, undergoes extensive hepatic metabolism (Gurney et al., 1998), and encapsulation of drug in nanoparticles reduces its hepatic clearance.
Nanoparticles are widely gaining attention as drug delivery systems for oral administration of drugs having poor oral bioavailability. In addition to enhancing drug absorption through cell-mediated uptake (Florence, 2005), drug-loaded nanoparticles can reduce transporter-mediated efflux (Ling et al., 2010). Furthermore, drug entrapment in nanoparticles protects the drug from degradation by adverse conditions in the gastrointestinal tract (Italia et al., 2007; Mittal and Kumar, 2009) and reduces hepatic first-pass metabolism (Anand et al., 2007; Mittal et al., 2007). Protection of drug from degradation/metabolism in the gastrointestinal tract and liver reduces the metabolic clearance and enhances the apparent rate constant for absorption and fraction absorbed. At times in the literature, the enhanced delivery with nanoparticles after oral administration was translated as a consequence of enhanced permeability processes (El-Shabouri, 2002; Gelperina et al., 2005; Luo et al., 2006; Mittal et al., 2007). We performed plasma pharmacokinetic analysis for orally administered nanoparticles and plain drug formulations of vinpocetine and clozapine to determine the contribution of reduced clearance to enhanced drug delivery observed with nanoparticles (Figs. 4 and 5). With oral or duodenal administration, the nanoparticles showed lower apparent clearance, lower apparent volume of distribution, lower elimination rate constant, longer apparent circulation half-life, and higher plasma AUC than the plain drug administered as solutions or suspensions of vinpocetine and clozapine, respectively (Figs. 4 and 5). For vinpocetine nanoparticles, the plasma concentration profile was available only for the oral route of administration; hence, dissection of clearance and volume of distribution from fraction observed (F) was not possible. Nanoparticle encapsulation of vinpocetine results in a 4.5-fold reduction in apparent clearance and 1.7-fold lower apparent volume of distribution, whereas there was a 2.6-fold increase in apparent absorption rate constant. After oral administration, vinpocetine undergoes extensive first-pass metabolism, resulting in low bioavailability (<7%) (El-Laithy et al., 2011). Furthermore, absorbed drug undergoes extensive hepatic metabolism and unchanged vinpocetine could not be detected in urine (Vereczkey et al., 1979). Incorporation of vinpocetine in solid lipid nanoparticles protected it from enzymatic degradation and reduced its metabolic clearance (Luo et al., 2006). For clozapine, plasma concentration profiles were available after both intravenous and intraduodenal administration of nanoparticles and plain drug suspension. Simultaneous model fitting of intraduodenal and intravenous plasma concentration profile gives fraction absorbed, apparent clearance, and apparent volume of distribution (Fig. 6). Clozapine nanoparticles showed 1.5-fold higher fraction absorbed, 3.0-fold lower apparent clearance, and 1.7-fold lower apparent volume of distribution than plain drug suspension after intraduodenal administration (Fig. 5). Clozapine is a lipophilic molecule that is rapidly absorbed after oral administration but undergoes extensive first-pass hepatic metabolism, resulting in poor oral bioavailability (<30%). Solid lipid nanoparticles of clozapine can be absorbed through the lymphatic duct and bypass the presystemic hepatic metabolism to an extent after oral administration (Bargoni et al., 1998). However, clozapine in blood circulation undergoes extensive metabolic clearance, resulting in low plasma half-life (4.8 h for plain clozapine versus 8.7 h for nanoparticles). Results from clozapine nanoparticle pharmacokinetics clearly show that orally administered clozapine nanoparticles enhance drug exposure to the system, in part, by reducing metabolic clearance.
Nanoparticle surface properties including surface charge, hydrophobicity, and functional groups are key determinants of their biological fate. Levchenko et al. (2002) showed that negatively charged liposomes (200 nm) have higher plasma clearance than the neutral liposomes. On the other hand, according to Kataoka and colleagues (Yamamoto et al., 2001) and Roser et al. (1998), surface charge has no effect on plasma clearance of poly(ethylene glycol)-poly(d,l-lactide) micelles (37–39 nm) and albumin nanoparticles (500–600 nm). Piskin et al. (1994) showed that positively charged polystyrene microparticles with primary amines have higher phagocytosis and nonspecific internalization compared with the negatively charged microparticles. In these prior reports, it was hypothesized that positively charged nanoparticles of have high nonspecific internalization and short blood circulation half-life (Alexis et al., 2008). Many attempts have been made to investigate the effect of surface charges of nanoparticle on pharmacokinetics and tissue distribution, but the controversy remains. To study the effect of surface charge of nanoparticles on absorption versus clearance, PK modeling was performed for positively and negatively charged cyclosporine nanoparticles after oral administration in dogs and for positively and neutrally charged solid lipid nanoparticles of clozapine after intraduodenal and intravenous administrations in rats. Compartmental modeling of these two drugs with three different routes of administration unambiguously showed that positive surface charge resulted in reduced apparent clearance and longer apparent circulation half-life compared with the neutral and negatively charged nanoparticles (Figs. 6 and 7). After the oral route of administration, positively charged nanoparticles of clozapine and cyclosporine exhibited 3.0- to 5.3-fold longer apparent circulation half-life, respectively, than negative and neutral nanoparticles of these drugs (Fig. 6 and 7). After intravenous administration, positively charged clozapine nanoparticles have 2.0-fold longer apparent circulation half-life and 1.9-fold lower apparent clearance than neutral charged nanoparticles. Xu et al. (2009) also showed that cationic nanoparticles have 9- and 31-fold longer circulation half-life than untreated and anionic polymeric nanoparticles after intravenous administration in rats. Another literature report also showed the accumulation of anionic liposomes in liver and spleen (Chonn et al., 1992).
Although we tried to dissect the contribution of various pharmacokinetic parameters to enhanced delivery with nanosystems, our study has its limitations. The plasma concentration profiles used in this study do not distinguish free drug, nanoparticle-bound drug, and protein-bound drug. Thus, the pharmacokinetic measures of this study are apparent values based on the total drug concentrations measured in the nanosystem groups. Furthermore, the amount of drug bound to plasma proteins may be reduced by the nanoparticle formulation. Alternatively, particles may bind plasma proteins, altering particle size, charge, as well as drug release profile in vivo. Drug encapsulated in nanoparticles is usually inactive and requires drug release for activity at the target site (Li and Huang, 2008). Failure to release the active drug molecule at the target site is one possible reason for clinical failure of nanoparticle formulations. One example is the cisplatin liposomal formulation (SPI-077). Tumor cisplatin levels were 4-fold higher for SPI-077 than for plain cisplatin, but the formulation failed to exert its anticancer activity because of failure to release cisplatin from the liposomes (Harrington et al., 2001; Andresen et al., 2005). Thus, precautions need to be taken when enhanced delivery of nanoparticle formulation is correlated to efficacy.
Dissection of the contribution of released versus nanoparticle-bound drug on pharmacokinetics using simulation is one approach to understanding in vivo pharmacokinetics; however, development of simulation models for nanoparticle pharmacokinetics is limited by in vivo complexity. In addition to the drug being present in multiple forms including free drug, plasma protein- or tissue-bound drug, and nanoparticle-bound drug, drug release from polymeric nanoparticles generally exhibits a triphasic release profile. The first phase is burst release governed by fast release of surface adsorbed drug, the second phase is first-order diffusion of drug from the polymeric matrix, and the third phase is first-order release due to degradation of the polymeric matrix) (Zweers et al., 2006). Because the drug released from the nanoparticle will probably exhibit pharmacokinetic parameters similar to those with plain drug dosing, the pharmacokinetic parameters reported for nanoparticle groups in this study should be considered as apparent values. Furthermore, in vivo clearance and distribution of nanoparticles is governed by various factors including nanoparticle material, size, shape, hydrophilicity, surface charge, and surface chemistry. Alexis et al. (2008) have reviewed the factors affecting clearance and distribution of polymeric nanoparticles in vivo.
In summary, we have shown the influence of reduced apparent clearance on enhanced exposure reported for various nanoparticles using pharmacokinetic modeling. We have suggested that encapsulation of drug molecules in nanoparticles significantly reduces the apparent drug clearance from plasma, thereby enhancing the apparent drug circulation half-life and potential cumulative drug delivery to the target tissues. Furthermore, we have suggested that some positively charged nanoparticles have longer apparent circulation half-life and reduced plasma clearance than neutral and negatively charged nanoparticles, resulting in better delivery. In addition to reduced clearance in the lumen of the gastrointestinal tract, liver, and circulation, it is anticipated that reduced clearance from tissues at the site of administration or absorption may also contribute to enhanced drug exposure with nanoparticles.
This work was supported by the National Institutes of Health National Eye Institute [Grants R01-EY017533, R01-EY018940, R0-1EY017045].
Article, publication date, and citation information can be found at http://dmd.aspetjournals.org.
- AUC
- area under the plasma concentration versus time curve
- GI
- gastrointestinal
- PK
- pharmacokinetic
- AIC
- Akaike information criterion.
Authorship Contributions
Participated in research design: Kadam and Kompella.
Conducted experiments: Kadam, Bourne, and Kompella.
Performed data analysis: Kadam, Bourne, and Kompella.
Wrote or contributed to the writing of the manuscript: Kadam, Bourne, and Kompella.
References
- Alexis F, Pridgen E, Molnar LK, Farokhzad OC. (2008) Factors affecting the clearance and biodistribution of polymeric nanoparticles. Mol Pharm 5:505–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. (2007) Bioavailability of curcumin: problems and promises. Mol Pharm 4:807–818 [DOI] [PubMed] [Google Scholar]
- Andresen TL, Jensen SS, Jørgensen K. (2005) Advanced strategies in liposomal cancer therapy: problems and prospects of active and tumor specific drug release. Prog Lipid Res 44:68–97 [DOI] [PubMed] [Google Scholar]
- Bargoni A, Cavalli R, Caputo O, Fundarò A, Gasco MR, Zara GP. (1998) Solid lipid nanoparticles in lymph and plasma after duodenal administration to rats. Pharm Res 15:745–750 [DOI] [PubMed] [Google Scholar]
- Bawa R. (2008) Nanoparticle-based therapeutics in humans: a survey. Nanotechnol Law Bus 5:135–155 [Google Scholar]
- Bourne DW. (1989) BOOMER, a simulation and modeling program for pharmacokinetic and pharmacodynamic data analysis. Comput Methods Programs Biomed 29:191–195 [DOI] [PubMed] [Google Scholar]
- Choi HS, Liu W, Misra P, Tanaka E, Zimmer JP, Itty Ipe B, Bawendi MG, Frangioni JV. (2007) Renal clearance of quantum dots. Nat Biotechnol 25:1165–1170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chonn A, Semple SC, Cullis PR. (1992) Association of blood proteins with large unilamellar liposomes in vivo. Relation to circulation lifetimes. J Biol Chem 267:18759–18765 [PubMed] [Google Scholar]
- Chow DS, Gong L, Wolfe MD, Giovanella BC. (2000) Modified lactone/carboxylate salt equilibria in vivo by liposomal delivery of 9-nitro-camptothecin. Ann NY Acad Sci 922:164–174 [DOI] [PubMed] [Google Scholar]
- Dadashzadeh S, Derakhshandeh K, Shirazi FH. (2008) 9-Nitrocamptothecin polymeric nanoparticles: cytotoxicity and pharmacokinetic studies of lactone and total forms of drug in rats. Anticancer Drugs 19:805–811 [DOI] [PubMed] [Google Scholar]
- El-Laithy HM, Shoukry O, Mahran LG. (2011) Novel sugar esters proniosomes for transdermal delivery of vinpocetine: preclinical and clinical studies. Eur J Pharm Biopharm 77:43–55 [DOI] [PubMed] [Google Scholar]
- El-Shabouri MH. (2002) Positively charged nanoparticles for improving the oral bioavailability of cyclosporin-A. Int J Pharm 249:101–108 [DOI] [PubMed] [Google Scholar]
- Emerich DF, Thanos CG. (2006) The pinpoint promise of nanoparticle-based drug delivery and molecular diagnosis. Biomol Eng 23:171–184 [DOI] [PubMed] [Google Scholar]
- Florence AT. (2005) Nanoparticle uptake by the oral route: fulfilling its potential? Drug Discov Today 2:75–81 [DOI] [PubMed] [Google Scholar]
- Florence AT, Hillery AM, Hussain N, Jani PU. (1995a) Factors affecting the oral uptake and translocation of polystyrene nanoparticles: histological and analytical evidence. J Drug Target 3:65–70 [DOI] [PubMed] [Google Scholar]
- Florence AT, Hillery AM, Hussain N, Jani PU. (1995b) Nanoparticles as carriers for oral peptide absorption: studies on particle uptake and fate. J Control Release 36:39–46 [Google Scholar]
- Gelperina S, Kisich K, Iseman MD, Heifets L. (2005) The potential advantages of nanoparticle drug delivery systems in chemotherapy of tuberculosis. Am J Respir Crit Care Med 172:1487–1490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gref R, Minamitake Y, Peracchia MT, Trubetskoy V, Torchilin V, Langer R. (1994) Biodegradable long-circulating polymeric nanospheres. Science 263:1600–1603 [DOI] [PubMed] [Google Scholar]
- Grislain L, Couvreur P, Lenaerts V, Roland M, Deprez-Decampeneere D, Speiser P. (1983) Pharmacokinetics and distribution of a biodegradable drug-carrier. Int J Pharm 15:335–345 [Google Scholar]
- Gurney HP, Ackland S, Gebski V, Farrell G. (1998) Factors affecting epirubicin pharmacokinetics and toxicity: evidence against using body-surface area for dose calculation. J Clin Oncol 16:2299–2304 [DOI] [PubMed] [Google Scholar]
- Harrington KJ, Lewanski CR, Northcote AD, Whittaker J, Wellbank H, Vile RG, Peters AM, Stewart JS. (2001) Phase I–II study of pegylated liposomal cisplatin (SPI-077) in patients with inoperable head and neck cancer. Ann Oncol 12:493–496 [DOI] [PubMed] [Google Scholar]
- Hussain N, Jani PU, Florence AT. (1997) Enhanced oral uptake of tomato lectin-conjugated nanoparticles in the rat. Pharm Res 14:613–618 [DOI] [PubMed] [Google Scholar]
- Italia JL, Bhatt DK, Bhardwaj V, Tikoo K, Kumar MN. (2007) PLGA nanoparticles for oral delivery of cyclosporine: nephrotoxicity and pharmacokinetic studies in comparison to Sandimmune Neoral. J Control Release 119:197–206 [DOI] [PubMed] [Google Scholar]
- Levchenko TS, Rammohan R, Lukyanov AN, Whiteman KR, Torchilin VP. (2002) Liposome clearance in mice: the effect of a separate and combined presence of surface charge and polymer coating. Int J Pharm 240:95–102 [DOI] [PubMed] [Google Scholar]
- Li K, Chen X, Zhong D, Li Y. (2003) Identification of the metabolites of 9-nitro-20(S)-camptothecin in rats. Drug Metab Dispos 31:792–797 [DOI] [PubMed] [Google Scholar]
- Li L, Gao FP, Tang HB, Bai YG, Li RF, Li XM, Liu LR, Wang YS, Zhang QQ. (2010) Self-assembled nanoparticles of cholesterol-conjugated carboxymethyl curdlan as a novel carrier of epirubicin. Nanotechnology 21:265601. [DOI] [PubMed] [Google Scholar]
- Li SD, Huang L. (2008) Pharmacokinetics and biodistribution of nanoparticles. Mol Pharm 5:496–504 [DOI] [PubMed] [Google Scholar]
- Lin JH, Lu AY. (1997) Role of pharmacokinetics and metabolism in drug discovery and development. Pharmacol Rev 49:403–449 [PubMed] [Google Scholar]
- Ling G, Zhang P, Zhang W, Sun J, Meng X, Qin Y, Deng Y, He Z. (2010) Development of novel self-assembled DS-PLGA hybrid nanoparticles for improving oral bioavailability of vincristine sulfate by P-gp inhibition. J Control Release 148:241–248 [DOI] [PubMed] [Google Scholar]
- Luo Y, Chen D, Ren L, Zhao X, Qin J. (2006) Solid lipid nanoparticles for enhancing vinpocetine's oral bioavailability. J Control Release 114:53–59 [DOI] [PubMed] [Google Scholar]
- Manjunath K, Venkateswarlu V. (2005) Pharmacokinetics, tissue distribution and bioavailability of clozapine solid lipid nanoparticles after intravenous and intraduodenal administration. J Control Release 107:215–228 [DOI] [PubMed] [Google Scholar]
- Mishra VK, Mohammad G, Mishra SK. (2008) Downregulation of telomerase activity may enhanced by nanoparticle mediated curcumin delivery. Dig J Nanomater Biostruct 3:163–169 [Google Scholar]
- Mittal G, Kumar MN. (2009) Impact of polymeric nanoparticles on oral pharmacokinetics: a dose-dependent case study with estradiol. J Pharm Sci 98:3730–3734 [DOI] [PubMed] [Google Scholar]
- Mittal G, Sahana DK, Bhardwaj V, Ravi Kumar MN. (2007) Estradiol loaded PLGA nanoparticles for oral administration: effect of polymer molecular weight and copolymer composition on release behavior in vitro and in vivo. J Control Release 119:77–85 [DOI] [PubMed] [Google Scholar]
- Papahadjopoulos D, Allen TM, Gabizon A, Mayhew E, Matthay K, Huang SK, Lee KD, Woodle MC, Lasic DD, Redemann C. (1991) Sterically stabilized liposomes: improvements in pharmacokinetics and antitumor therapeutic efficacy. Proc Natl Acad Sci USA 88:11460–11464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson A, Ogilive BW, Paris BL, Hensely TN, Loewen GJ. (2010) Human biotransformation, in Biotransformation and Metabolite Elucidation of Xenobiotics. John Wiley & Sons, New York [Google Scholar]
- Petros RA, DeSimone JM. (2010) Strategies in the design of nanoparticles for therapeutic applications. Nat Rev Drug Discov 9:615–627 [DOI] [PubMed] [Google Scholar]
- Piskin E, Tuncel A, Denizli A, Ayhan H. (1994) Monosize microbeads based on polystyrene and their modified forms for some selected medical and biological applications. J Biomater Sci Polym Ed 5:451–471 [DOI] [PubMed] [Google Scholar]
- Qian F, Cui F, Ding J, Tang C, Yin C. (2006) Chitosan graft copolymer nanoparticles for oral protein drug delivery: preparation and characterization. Biomacromolecules 7:2722–2727 [DOI] [PubMed] [Google Scholar]
- Roser M, Fischer D, Kissel T. (1998) Surface-modified biodegradable albumin nano- and microspheres. II: effect of surface charges on in vitro phagocytosis and biodistribution in rats. Eur J Pharm Biopharm 46:255–263 [DOI] [PubMed] [Google Scholar]
- Schluep T, Cheng J, Khin KT, Davis ME. (2006) Pharmacokinetics and biodistribution of the camptothecin-polymer conjugate IT-101 in rats and tumor-bearing mice. Cancer Chemother Pharmacol 57:654–662 [DOI] [PubMed] [Google Scholar]
- Scott DO, Bindra DS, Stella VJ. (1993) Plasma pharmacokinetics of lactone and carboxylate forms of 20(S)-camptothecin in anesthetized rats. Pharm Res 10:1451–1457 [DOI] [PubMed] [Google Scholar]
- Sinha R, Kim GJ, Nie S, Shin DM. (2006) Nanotechnology in cancer therapeutics: bioconjugated nanoparticles for drug delivery. Mol Cancer Ther 5:1909–1917 [DOI] [PubMed] [Google Scholar]
- Slepushkin VA, Simões S, Dazin P, Newman MS, Guo LS, Pedroso de Lima MC, Düzgüneş N. (1997) Sterically stabilized pH-sensitive liposomes. Intracellular delivery of aqueous contents and prolonged circulation in vivo. J Biol Chem 272:2382–2388 [DOI] [PubMed] [Google Scholar]
- Takeuchi H, Yamamoto H, Niwa T, Hino T, Kawashima Y. (1996) Enteral absorption of insulin in rats from mucoadhesive chitosan-coated liposomes. Pharm Res 13:896–901 [DOI] [PubMed] [Google Scholar]
- Torché AM, Jouan H, Le Corre P, Albina E, Primault R, Jestin A, Le Verge R. (2000) Ex vivo and in situ PLGA microspheres uptake by pig ileal Peyer's patch segment. Int J Pharm 201:15–27 [DOI] [PubMed] [Google Scholar]
- Vereczkey L, Czira G, Tamás J, Szentirmay Z, Botár Z, Szporny L. (1979) Pharmacokinetics of vinpocetine in humans. Arzneimittelforschung 29:957–960 [PubMed] [Google Scholar]
- Verrecchia T, Spenlehauer G, Bazile DV, Murry-Brelier A, Archimbaud Y, Veillard M. (1995) Non-stealth (poly(lactic acid/albumin)) and stealth (poly(lactic acid-polyethylene glycol)) nanoparticles as injectable drug carriers. J Control Release 36:49–61 [Google Scholar]
- Xu F, Yuan Y, Shan X, Liu C, Tao X, Sheng Y, Zhou H. (2009) Long-circulation of hemoglobin-loaded polymeric nanoparticles as oxygen carriers with modulated surface charges. Int J Pharm 377:199–206 [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Nagasaki Y, Kato Y, Sugiyama Y, Kataoka K. (2001) Long-circulating poly(ethylene glycol)-poly(d,l-lactide) block copolymer micelles with modulated surface charge. J Control Release 77:27–38 [DOI] [PubMed] [Google Scholar]
- Zhang C, Newsome JT, Mewani R, Pei J, Gokhale PC, Kasid UN. (2009) Systemic delivery and pre-clinical evaluation of nanoparticles containing antisense oligonucleotides and siRNAs. Methods Mol Biol 480:65–83 [DOI] [PubMed] [Google Scholar]
- Zhang L, Gu FX, Chan JM, Wang AZ, Langer RS, Farokhzad OC. (2008) Nanoparticles in medicine: therapeutic applications and developments. Clin Pharmacol Ther 83:761–769 [DOI] [PubMed] [Google Scholar]
- Zweers ML, Engbers GH, Grijpma DW, Feijen J. (2006) Release of anti-restenosis drugs from poly(ethylene oxide)-poly(dl-lactic-co-glycolic acid) nanoparticles. J Control Release 114:317–324 [DOI] [PubMed] [Google Scholar]