Abstract
The ability of the liver, small intestine, and kidney to synthesize and subsequently eliminate dinitrophenyl-S-glutathione (DNP-SG), a substrate for multidrug resistance-associated protein 2 (Mrp2), was assessed in rats treated with glucagon-like peptide 2 (GLP-2, 12 μg/100 g b.wt. s.c. every 12 h for 5 consecutive days). An in vivo perfused jejunum model with simultaneous bile and urine collection was used. A single intravenous dose of 30 μmol/kg b.wt. 1-chloro-2,4-dinitrobenzene (CDNB) was administered, and its conjugate, DNP-SG, and dinitrophenyl cysteinyl glycine (DNP-CG), resulting from the action of γ-glutamyltransferase on DNP-SG, were determined in bile, intestinal perfusate, and urine by high-performance liquid chromatography. Tissue content of DNP-SG was also assessed in liver, intestine, and kidneys. Biliary excretion of DNP-SG+DNP-CG was decreased in GLP-2 rats with respect to controls. In contrast, their intestinal excretion was substantially increased, whereas urinary elimination was not affected. Western blot and real-time polymerase chain reaction studies revealed preserved levels of Mrp2 protein and mRNA in liver and renal cortex and a significant increase in intestine in response to GLP-2 treatment. Tissue content of DNP-SG detected 5 min after CDNB administration was decreased in liver, increased in intestine, and unchanged in kidney in GLP-2 versus control group, consistent with GLP-2-induced down-regulation of expression of glutathione transferase (GST) Mu in liver and up-regulation of GST-Alpha in intestine at both protein and mRNA levels. In conclusion, GLP-2 induced selective changes in hepatic and intestinal disposition of a common GST and Mrp2 substrate administered systemically that could be of pharmacological or toxicological relevance under therapeutic treatment conditions.
Introduction
Glucagon-like peptide-2 (GLP-2) is a 33-amino acid hormone secreted by L enteroendocrine cells at the distal intestine (L'Heureux and Brubaker, 2001; Drucker, 2002). Its action was first reported in mice in 1996 (Drucker et al., 1996) and consisted of stimulation of intestinal growth and cell proliferation. Several subsequent studies demonstrated that GLP-2 is capable of restoring mucosal integrity, barrier function, and absorption of nutrients in different experimental models of intestinal injury (Brubaker et al., 1997; Scott et al., 1998; Benjamin et al., 2000; Cani et al., 2009). Because of these properties, GLP-2 was proposed as a potential therapeutic agent to restore intestinal function in different human pathologies, including inflammatory bowel diseases (IBD) (L'Heureux and Brubaker, 2001; Drucker, 2002; Yazbeck, 2010; Jeppesen et al., 2011). Either GLP-2 or its protease-resistant analog [Gly2]GLP-2 increased mesenteric blood flow in healthy volunteers (Bremholm et al., 2009) and in patients with jejunostomy short bowel syndrome (Bremholm et al., 2011). In addition, [Gly2]GLP-2 improved nutrient absorption, decreased fecal energy losses, and reduced parenteral nutrition and intravenous fluid requirements in patients with short bowel syndrome (Jeppesen et al., 2011). In addition, its use was suggested for treatment of patients with moderate to severe Crohn's disease (Buchman et al., 2010) and chemotherapy-induced intestinal mucositis (Yazbeck, 2010).
Multidrug resistance-associated protein 2 (Mrp2, Abcc2) is a member of the ATP-binding cassette family of export pumps (Büchler et al., 1996; Paulusma et al., 1996; Keppler et al., 1997). In the intestine, it is located at the brush-border membrane of the enterocyte. It is mainly expressed in the proximal small intestine and acts coordinately with conjugating enzymes such as glutathione transferase (GST; EC 2.5.1.18) and UDP-glucuronosyltransferase (EC 2.4.1.17) to metabolize and eliminate common substrates into the intestinal lumen (Mottino et al., 2000). We have recently demonstrated in rats that GLP-2 increases the expression of Mrp2 in jejunum in association with increased prevention of mucosal to serosal absorption of 1-chloro-2,4-dinitrobenzene (CDNB) as an increased amount of its glutathione derivative, dinitrophenyl-glutathione (DNP-SG), is pumped back to the luminal side (Villanueva et al., 2010). The stimulatory effect of GLP-2 was exerted on both GST and Mrp2 and was associated with increased capability for protection against absorption of potentially toxic xenobiotics from the lumen and for prevention of enterocyte toxicity. We proposed these findings to be of pathophysiological relevance as GLP-2 may exert a cytoprotective action under conditions of intestinal damage or during development, lactation, or tissue regeneration in addition to its trophic action. This important role in regulation of membrane chemical barrier gives additional support to therapeutic application of GLP-2 in human intestinal disease.
GLP-2 is normally secreted by the distal intestine, and its action is probably restricted to the digestive tract, mostly to the proximal small intestine, whereas in patients, GLP2 or [Gly2]GLP-2 is preferentially administered subcutaneously (Thulesen et al., 2000; Marier et al., 2008, 2010; Buchman et al., 2010; Jeppesen et al., 2011). Thus, an effect on major epithelial tissues other than the intestine cannot be ruled out under therapeutic treatment conditions. Patients receiving GLP-2 exhibit alterations in intestinal morphology, absorption, and immunological status, and in consequence, they usually receive nutrients and medications parenterally. Thus, the absence of presystemic clearance would give the liver and kidneys a more relevant participation in drug disposition than following oral administration. The effect of subcutaneous administration of GLP-2 on hepatic and renal-conjugating enzymes and Mrp2 has never been explored. In addition, although our previous study demonstrated that GLP-2 improved the ability of the jejunum to restrict absorption of luminal xenobiotics, it remains uncertain whether GLP-2 would similarly improve the elimination of xenobiotics after their systemic administration. In the current study, we explored the effect of GLP-2 on hepatic, renal, and intestinal excretion of DNP-SG after in vivo intravenous single administration of CDNB. Because of the relevance of GST in converting CDNB to the Mrp2 substrate, DNP-SG, we also determined GST activity in cytosol from these tissues. Expression of Mrp2 and major classes of GST were additionally assessed to establish a potential correlation with functional findings. The data show that biliary excretion of DNP-SG was decreased, whereas intestinal excretion was increased by GLP-2. Changes in expression of GST in liver and of GST and Mrp2 in the intestine probably accounted for the reported specific functional alterations.
Materials and Methods
Chemicals.
Leupeptin, phenylmethylsulfonyl fluoride, pepstatin A, sucrose, CDNB, and glutathione were obtained from Sigma-Aldrich (St. Louis, MO). Rat GLP-2 was obtained from American Peptide Company (Sunnyvale, CA). All other chemicals and reagents were commercial products of analytical grade purity.
Animals, Treatment, and Specimen Collection.
Adult female Wistar rats weighing 200 to 230 g (National University of Rosario, Rosario, Argentina) were used. Animals had free access to food and water and received human care as outlined in the National Institutes of Health Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, 2010). The rats were randomly divided in two experimental groups. GLP-2-treated rats (GLP-2 group) were administered GLP-2 dissolved in sterile phosphate-buffered saline (PBS) (12 μg/100 g b.wt.) by subcutaneous injection every 12 h for 5 consecutive days. Control rats (C group) received injections of the vehicle (PBS, subcutaneously) according to this same schedule. Experiments were performed 18 h after the last GLP-2 injection.
The common bile duct was cannulated with polyethylene tubing (PE10) under urethane anesthesia. After a 30-min stabilization period, bile was collected for 10 min in preweighed tubes containing 0.1 ml of 10% sulfosalicylic acid for determination of total and oxidized glutathione and for a further 10 min in dried preweighed tubes to determine the endogenous bilirubin output. Bile flow was determined gravimetrically, assuming a density of 1 g/ml. Immediately after bile collection, animals were sacrificed by exsanguination. The liver was perfused in situ with ice-cold saline through the portal vein and used for preparation of crude plasma membranes by differential centrifugation, as described previously (Meier et al., 1984). The proximal jejunum (∼30-cm length) was removed, carefully rinsed with ice-cold saline, and homogenized as described previously (Mottino et al., 2000). Brush-border membranes from the intestinal mucosa were prepared as described previously (Mottino et al., 2000). Renal cortex was isolated and homogenized, and brush-border membranes were obtained by Mg/EGTA precipitation as described previously (Ohoka et al., 1993), with some modifications (Torres et al., 2003). Jejunum and renal cortex homogenates were alternatively solubilized with Triton X-100 as described previously (Cao et al., 2002). Cytosolic fractions from these same tissues were obtained by ultracentrifugation as described previously (Siekevitz et al., 1962). Protein concentration in plasma membrane, homogenate, and cytosol preparations was measured using bovine serum albumin as a standard (Lowry et al., 1951).
Assessment of Expression of Mrp1, Mrp2, Mrp3, and Major GST Classes.
Expression of basolateral Mrp1 and Mrp3 proteins was assessed by Western blotting in liver crude plasma membranes and in jejunum- and renal cortex-solubilized homogenates using goat polyclonal antibodies to human MRP1 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) and MRP3 (Santa Cruz Biotechnology, Inc.). Detection of apical Mrp2 was performed in liver crude plasma membranes and in intestinal and renal brush-border membranes using a monoclonal antibody to human MRP2 (M2 III-6; Alexis Biochemicals, Carlsbad, CA). Western blot studies of the different GST classes were performed in cytosolic fractions using goat antisera against rat GST-Alpha and -Mu (GS9 and GS23, respectively; Oxford Biomedical Research, Oxford, MI) and rabbit antiserum against human GST-Pi (Immunotech, Marseille, France) as described previously (Catania et al., 2000). Equal loading and transfer of protein were checked by detection of β-actin using a monoclonal antibody to rat β-actin (Sigma-Aldrich) and by Ponceau S staining of the membranes. The immunoreactive bands were quantified with the Gel-Pro Analyzer (MediaCybernetics, Inc., Bethesda, MD) software.
Quantitative real-time PCR studies of Mrp2 and GST mRNAs were performed as described previously (Villanueva et al., 2010) using the following primer pairs: forward, 5′-accttccacgtagtgatcct-3′ and reverse, 5′-acctgctaagatggacggtc-3′ for Mrp2 (Villanueva et al., 2010); forward, 5′-gattgacatgtattcagagggt-3′ and reverse, 5′-tttgcatccatggctggctt-3′ for GSTYa2 belonging to GST class Alpha (Villanueva et al., 2010); forward, 5′-tttgagcccaagtgcctgga-3′ and reverse, 5′-gcaggatccaatgtggacag-3′, and forward, 5′-ttcgcctgttcctggagtat-3′, and reverse, 5′-ttgctctgggtgatcttgtg-3′ for GSTYb1 (McBride et al., 2005) and GSTYb2 (Wiegand et al., 2009), respectively, belonging to GST class Mu; and forward, 5′-gtaacccgttgaaccccatt-3′ and reverse, 5′-ccatccaatcggtagtagcg-3′ for 18S rRNA (housekeeping gene) (Villanueva et al., 2010).
Assessment of Mrp2 and GST Activities.
Mrp2 activity was evaluated in vivo through determination of DNP-SG, a model substrate of Mrp2, and its derivative DNP-CG in bile, urine, and intestinal perfusate. DNP-CG is the result of γ-glutamyltransferase action on DNP-SG at the luminal side of secretory epithelia (Hinchman et al., 1991). The rats were anesthetized with urethane (1000 mg/kg b.wt. i.p.) and thus maintained throughout. Body temperature was measured with a rectal probe and maintained at 37°C with a heating lamp. The femoral vein, the common bile duct, and the urinary bladder were cannulated as described previously (Villanueva et al., 2005). Intestinal excretion studies were performed using the in situ single-pass perfusion technique (Gotoh et al., 2000). In brief, the intestine was perfused with isotonic PBS, pH 7.35, from the upper jejunum to the end of distal jejunum with a peristaltic pump at a rate of 0.4 ml/min. After a 30-min stabilization period, a single bolus of CDNB (30 μmol/kg b.wt. i.v. in 1:19 dimethyl sulfoxide/saline) was administered. Bile, urine, and intestinal perfusate were collected for 90 min at 10-, 30- and 15-min intervals, respectively. Their volumes were estimated gravimetrically. DNP-SG and DNP-CG content was assessed in all samples by high-performance liquid chromatography, as described previously (Mottino et al., 2001). Saline was administered intravenously throughout the experiment to replenish body fluids. In a different set of rats, the animals were sacrificed by exsanguinations 5 min after administration of CDNB, and the liver, proximal jejunum, and kidneys were removed, rinsed with ice-cold saline, and homogenized in two volumes of PBS, pH 7.35. DNP-SG was determined in serum as well as in liver, jejunum, and renal cortex homogenates (Mottino et al., 2001). Glutathione-conjugating activity toward CDNB was assayed in vitro in cytosol from liver, jejunum, and renal cortex by a reported procedure (Catania et al., 2000).
Analytical Procedures.
Total glutathione (reduced+oxidized) and oxidized glutathione in bile were determined spectrophotometrically by the recycling method of Tietze (1969), as modified by Griffith (1980). Total bilirubin in bile was determined using a commercial kit (Wiener Lab, Rosario, Argentina) following the manufacturer's instructions.
Statistical Analysis.
Data are presented as mean ± S.D. Statistical analysis was performed using the Student's t test. Values of P < 0.05 were considered statistically significant.
Results and Discussion
GLP-2 induced a significant increase (+36%) in the weight of the portion of small intestine perfused in vivo relative to body weight compared with controls (0.019 ± 0.002 versus 0.014 ± 0.001, respectively; P < 0.05, n = 4). This portion of the small intestine (∼50-cm long) corresponds mainly to jejunum where the highest expression and activity of Mrp2 were reported (Gotoh et al., 2000; Mottino et al., 2000). In contrast, the relative masses of the liver and the two kidneys (taken together) did not differ between GLP-2 and control rats (0.033 ± 0.002 versus 0.032 ± 0.001 and 0.008 ± 0.001 versus 0.007 ± 0.001 for liver and kidneys, respectively, P > 0.05, n = 4). GLP-2 did not induce any significant change in basal biliary or urinary flows with respect to control group (see Table 1).
TABLE 1.
Effect of GLP-2 on basal biliary and urinary secretory function and on serum and tissue levels of DNP-SG
Basal bile and urine were collected for 10 min, and corresponding flows were estimated gravimetrically. Serum and tissue contents of DNP-SG were assessed by high-performance liquid chromatography 5 min after administration of CDNB (30 μmol/kg b.wt.). Data are means ± S.D.
| Parameters | C (n = 4) | GLP2 (n = 4) | |
|---|---|---|---|
| Basal conditions | Bile flow (ml · min−1 · g liver−1) | 1.61 ± 0.15 | 1.69 ± 0.22 |
| Urinary flow (ml · min−1 · g kidney−1) | 8.7 ± 0.5 | 8.2 ± 0.3 | |
| Biliary excretion of total glutathione (nmol · min−1 · g liver−1) | 3.42 ± 0.35 | 3.36 ± 0.29 | |
| Biliary excretion of oxidized glutathione (nmol · min−1 · g liver−1) | 1.22 ± 0.30 | 1.18 ± 0.25 | |
| Biliary excretion of bilirubin (nmol · min−1 · g liver−1) | 0.132 ± 0.005 | 0.136 ± 0.004 | |
| Serum concentration of DNP-SG (mM) | 0.008 ± 0.001 | 0.009 ± 0.002 | |
| After CDNB administration | Liver content of DNP-SG (nmol/g liver) | 7.1 ± 1.1 | 4.5 ± 0.3* |
| Intestinal content of DNP-SG (nmol/g intestine) | 2.8 ± 1.0 | 9.8 ± 5.2* | |
| Renal content of DNP-SG (nmol/g kidney) | 3.4 ± 0.7 | 3.1 ± 0.6 |
Significantly different from control (C), P < 0.05.
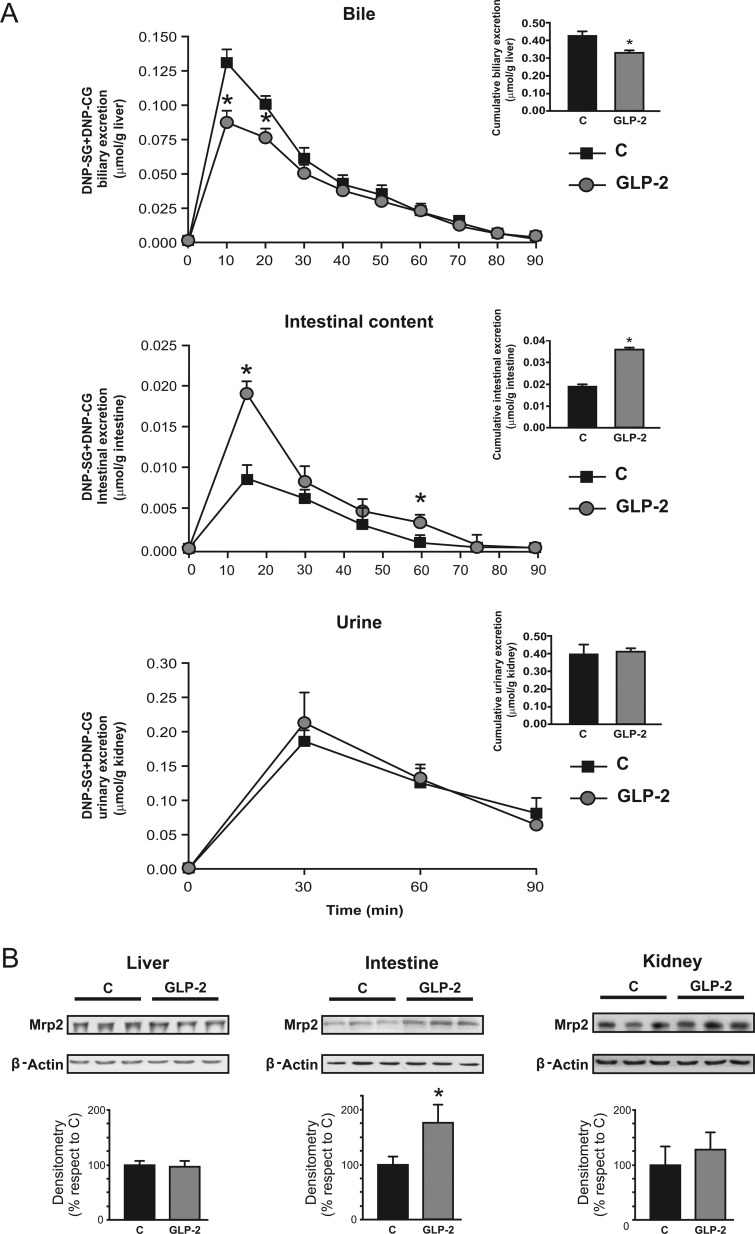
DNP-SG and DNP-CG were the major metabolites of CDNB detected in bile, intestinal perfusate, or urine. DNP-SG is a high-affinity, prototypical Mrp2 substrate (Keppler et al., 2000). Because DNP-CG is formed from DNP-SG once it reaches the extracellular compartment, excretion rate of these two derivatives properly estimates Mrp2 activity as measured in vivo. As shown in Fig. 1A, the biliary excretion rate of DNP-SG+DNP-CG was decreased in GLP-2 rats, particularly during the first periods of bile collection, so that their cumulative excretion was significantly decreased (−20%; Fig. 1A, inset). In contrast, intestinal excretory rate was substantially increased by GLP-2, particularly at the excretion peak, and as a result, their cumulative excretion increased by 103% (Fig. 1A, inset). Urinary elimination of these same compounds was not affected by GLP-2 (Fig. 1A). Excretion of DNP-SG+DNP-CG was also calculated as a percentage of the dose of CDNB. Under normal conditions, biliary and urinary routes accounted for elimination of 50 and 10% of the total CDNB dose, respectively, with a minor contribution from intestinal excretion (∼1%). The contribution of the biliary route was decreased by GLP-2 (−24%, P < 0.05, n = 4), whereas the intestinal counterpart was substantially increased (+240%, P < 0.05, n = 4), and that of the renal route was not affected. Thus, the total amount of DNP-SG+DNP-CG eliminated by the three tissues was lower in the GLP-2 group relative to the control group, indicating that the impaired excretion of DNP-SG+DNP-CG described for the liver was only partially compensated by their exacerbated intestinal excretion.
Fig. 1.
Effect of GLP-2 on activity and expression of hepatic, intestinal, and renal Mrp2. A, excretion rate of DNP-SG, prototypical substrate for Mrp2, and its derivative DNP-CG was assessed in bile, intestinal perfusate, and urine at 10-, 15-, and 30-min collection periods, respectively, for 90 min. Insets depict cumulative excretion of DNP-SG by 90 min. The data represent means ± S.D. of four rats per group. *, significantly different from control (C), P < 0.05. B, Mrp2 protein was detected by Western blot of hepatic, intestinal, and renal membranes from GLP-2 and control rats. Protein (5 and 30 μg) from liver crude plasma membranes and brush-border membranes from proximal jejunum and renal cortex, respectively, were loaded in the gels. Uniformity of loading and transfer from gel to PVDF membrane was controlled with Ponceau S. Mrp2 expression was normalized relative to β-actin expression; data on densitometric analysis are presented as percentages relative to control, considered as 100%, and were expressed as means ± S.D. of six rats per group. *, significantly different from control (C), P < 0.05.
Metabolism and transport processes, such as those involving sequential participation of GST and Mrp2, affect a wide variety of naturally occurring xenobiotics and therapeutic drugs (Catania et al., 2004). To further explore the bases for changes in excretion of DNP-SG+DNP-CG by the liver and intestine, we assessed the expression of Mrp2 in crude plasma membranes from liver and apical membranes from intestine and renal cortex. Figure 1B shows that neither the liver nor the kidneys exhibited any change in Mrp2 expression in response to GLP-2 administration, as detected by Western blotting. In contrast, intestinal Mrp2 expression was significantly increased in GLP-2 versus control rats (+75%), in agreement with our previous report (Villanueva et al., 2010). Induction of Mrp2 probably occurred at the transcriptional level, since expression of its mRNA was increased by 123% (P < 0.05, n = 4) in response to GLP-2.
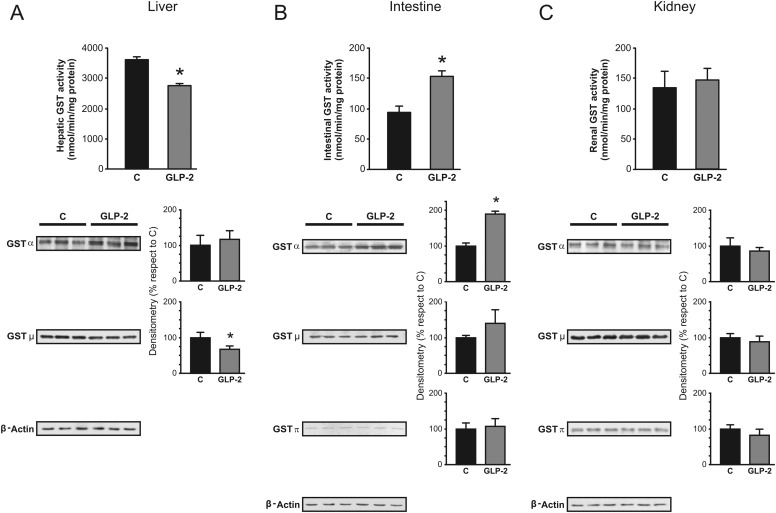
Liver contribution toward DNP-SG elimination was decreased by GLP-2 (Fig. 1A). It is noteworthy that this occurred in spite of preserved expression of Mrp2 in crude plasma membranes. To rule out the possibility of inactivation of Mrp2 in response to GLP-2 treatment, we assessed biliary excretion of glutathione and bilirubin, endogenous substrates with low and high affinities toward Mrp2 (Keppler et al., 2000). The data in Table 1 indicate that neither total nor oxidized glutathione or bilirubin exhibited any changes between GLP-2 and controls. DNP-SG is also a substrate for Mrp1 (Abcc1) and Mrp3 (Abcc3), and their up-regulation at the basolateral membrane of the liver could exacerbate secretion of their substrates into blood, thus influencing their biliary elimination. We found that GLP-2 did not affect the expression of these transporters in any of the tissues studied, either at protein or mRNA levels (data not shown). Furthermore, serum concentration of DNP-SG, detected 5 min after CDNB administration, was not affected by GLP-2 (Table 1). Thus, it seems unlikely that decreased biliary excretion of DNP-SG results from increased transport at the basolateral membrane. Because of its highly hydrophobic nature, it is assumed that CDNB freely enters the cells by diffusion; thus, GST activity becomes the major step determining CDNB conjugation by the different tissues. We found that hepatic GST activity was reduced by 24% in the GLP-2 group (Fig. 2A), in association with reduced expression of GST-Mu (−30%; Fig. 2A), as detected by Western blotting. Thus, an alteration in intrahepatic conjugation of CDNB could be responsible for the impaired hepatic disposition of CDNB shown in Fig. 1A. In support of this possibility, we observed less intrahepatic accumulation of DNP-SG in GLP-2 group than in controls, as detected 5 min after CDNB administration (Table 1). Mrp2-mediated transport, rather than GST conjugation, is the probable rate-limiting step in the overall disposition of CDNB in intestine (Mottino et al., 2001). However, the situation could be different in other tissues with high constitutive Mrp2 expression such as the liver, where overall transport of DNP-SG could rather depend on its formation, particularly if its subsequent secretion to bile is not saturated. This needs experimental demonstration.
Fig. 2.
Effect of GLP-2 on activity and expression of hepatic, intestinal, and renal GST. GST activity toward CDNB (top) and Western blot studies of main GST classes (bottom) were performed in cytosol isolated from liver (A), jejunal mucosa (B), and renal cortex (C). For Western blots, equal amounts of protein (10 μg) were loaded in all lanes. Expression of GST-Alpha, -Mu, and -Pi was calculated relative to β-actin expression. Uniformity of protein loading and transfer from gel to PVDF membrane was controlled with Ponceau S. Data on densitometric analysis are presented as percentages relative to control, considered as 100%, and were expressed as means ± S.D. of six rats per group. Hepatic GST-Pi was not detected in either control or GLP-2 groups.*, significantly different from control (C), P < 0.05.
In contrast to GLP-2-induced down-regulation of hepatic GST expression and activity, the rate of intestinal CDNB conjugation was increased (+64%) by hormonal treatment, in association with increased expression of GST-Alpha (+91%) (Fig. 2B). Consistent with these findings, accumulation of DNP-SG in intestinal tissue was also higher in GLP-2 group (Table 1). GLP-2 was unlikely to affect CDNB conjugation by the kidneys (Table 1), consistent with unaffected levels of GST expression and activity in renal cortex (Fig. 2C).
GSTYb1, GSTYb2, and GSTYa2 are the only isoforms of GST, which belongs to the Mu and Alpha classes, respectively, to be detected in rat liver and intestine (Hayes and Pulford, 1995). GLP-2-induced down-regulation of expression of GST-Mu in liver correlated well with decreased expression of GSTYb1 and GSTYb2 mRNAs (−61 and −59%, respectively) compared with controls (P < 0.05, n = 4). In contrast, intestinal GSTYa2 mRNA was increased by GLP-2 (+121%; P < 0.05, n = 4), in agreement with GST-Alpha protein up-regulation (Fig. 2B). Taken together, these data suggest transcriptional regulation of GST-Mu and -Alpha classes by GLP-2.
The up-regulation of intestinal GST expression by GLP-2, in association with up-regulation of Mrp2 expression at the brush-border membrane, could accelerate the inactivation and elimination of toxic compounds absorbed systemically that, as in the case of CDNB, critically depend on sequential participation of biotransformation and transport processes. More importantly, IBD are fairly common chronic inflammatory conditions of the gastrointestinal tract. Although the exact etiology of IBD remains uncertain, reactive oxygen species are produced in abnormally high levels, and their destructive effects may contribute to the initiation and/or propagation of the disease (Rezaie et al., 2007). Because of the known beneficial action of GSTs in detoxifying many electrophilic compounds and fatty acid hydroperoxides, up-regulation of GSTs after GLP-2 treatment could be of clinical relevance in IBD therapy. In line with this, it is noteworthy that patients with ulcerative colitis exhibited deficient expression of GST, as detected in serum, which was related to an early age of onset and more severe clinical course, leading to colectomy (Hertervig et al., 1994). The mechanism by which GST-Alpha and Mrp2 are concomitantly increased after GLP-2 treatment is not totally understood. It is possible that GLP-2 interacts with the enterocyte through a yet unidentified member of the glucagon receptor family, leading to G protein activation, and subsequently, to activation of adenylyl cyclase. Increased formation of cAMP then activates transcription factors, perhaps via protein kinase A, ultimately leading to induction of transcription of selective genes such as Mrp2 and GST, as previously postulated (Villanueva et al., 2010).
GLP-2 and [Gly2]GLP-2 are considered safe drugs based on evidence from basic and clinical studies, indicating that they do not exhibit significant effects in organs other than intestine and, to a lesser extent, stomach (Drucker et al., 1999). This is supported by the lack of detection of GLP-2R in tissues other than these. We demonstrate for the first time an action on liver detoxification function which, in spite of its low magnitude, may be of particular significance for substrates selectively conjugated by GST. For example, thiopurine is widely used in the management of IBD and is a substrate for GST (Dewit et al., 2010); its efficacy and/or side effects could be changed under GLP-2 treatment. The mechanism underlying down-regulation of hepatic GST by GLP-2 is not known. The liver is the major tissue registering radioactive GLP-2 accumulation 5 min after its intravenous administration to rats, although it was thought not to be associated with specific interaction with GLP-2R (Thulesen et al., 2000). Interaction with other members of the superfamily of glucagon receptors is also possible, because hormone-receptor cross-interactions and inhibitions were demonstrated for glucagon, GLP-1, and GLP-2 (MacNeil et al., 1994; Körner et al., 2007). Although no reports describe an action of GLP-1 on the GST system, glucagon was found to decrease GST-Mu in rat hepatocytes (Kim et al., 2003). A more direct evaluation of the effects of GLP-2 on liver cells should be performed to confirm such possibility.
Whether the currently reported effects are applicable to humans resulting in potential pharmacological or toxicological derivations remains unknown. Daily GLP-2 doses used therapeutically for treatment of short bowel syndrome and moderate to severe Crohn's disease reach values of up to 0.01 mg/100 g b.wt. (Jeppesen et al., 2011), 0.02 mg/100 g b.wt. (Buchman et al., 2010), or even as high as a total single dose of 80 mg (Marier et al., 2010), with duration of treatments varying between a few to more than 20 weeks. Our total daily dose of 0.024 mg/100 g b.wt. is well within this same range; consequently, an action on patients receiving such high doses of GLP-2 cannot be ruled out.
In summary, we demonstrated that GLP-2 treatment decreased hepatic and increased intestinal disposition of a prototypical xenobiotic, CDNB, administered systemically by reducing expression of hepatic GST-Mu and increasing expression of intestinal GST-Alpha and Mrp2, respectively.
This work was supported by the National Institutes of Health Eunice Kennedy Shriver National Institute of Child Health and Human Development [Grant R01-HD58299] (to M.V.); Agencia Nacional de Promoción Científica y Tecnológica [Grant PICT 2007-1637]; Consejo Nacional de Investigaciones Científicas y Técnicas [Grant PIP 112-200801-00691] (to A.D.M.); and Agencia Nacional de Promoción Científica y Tecnológica [Grant PICT 2010-1475] (to S.S.M.V.).
Article, publication date, and citation information can be found at http://dmd.aspetjournals.org.
- GLP-2
- glucagon-like peptide 2
- GST
- glutathione transferase
- Mrp2
- multidrug resistance-associated protein 2
- IBD
- inflammatory bowel diseases
- GLP-2R
- glucagon-like peptide 2 receptor
- CDNB
- 1-chloro-2,4-dinitrobenzene
- DNP-CG
- dinitrophenyl cysteinyl glycine
- DNP-SG
- dinitrophenyl-S-glutathione
- PBS
- phosphate-buffered saline.
Authorship Contributions
Participated in research design: Villanueva, Vore, Catania, and Mottino.
Conducted experiments: Villanueva, Perdomo, Ruiz, Rigalli, Arias, and Luquita.
Contributed new reagents or analytic tools: Vore.
Performed data analysis: Villanueva, Perdomo, Ruiz, Rigalli, and Arias.
Wrote or contributed to the writing of the manuscript: Villanueva, Mottino, Catania, and Vore.
References
- Benjamin MA, McKay DM, Yang PC, Cameron H, Perdue MH. (2000) Glucagon-like peptide-2 enhances intestinal epithelial barrier function of both transcellular and paracellular pathways in the mouse. Gut 47:112–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremholm L, Hornum M, Henriksen BM, Larsen S, Holst JJ. (2009) Glucagon-like peptide-2 increases mesenteric blood flow in humans. Scand J Gastroenterol 44:314–319 [DOI] [PubMed] [Google Scholar]
- Bremholm L, Hornum M, Andersen UB, Hartmann B, Holst JJ, Jeppesen PB. (2011) The effect of glucagon-like peptide-2 on mesenteric blood flow and cardiac parameters in end-jejunostomy short bowel patients. Regul Pept 168:32–38 [DOI] [PubMed] [Google Scholar]
- Brubaker PL, Izzo A, Hill M, Drucker DJ. (1997) Intestinal function in mice with small bowel growth induced by glucagon-like peptide-2. Am J Physiol 272:E1050–E1058 [DOI] [PubMed] [Google Scholar]
- Büchler M, König J, Brom M, Kartenbeck J, Spring H, Horie T, Keppler D. (1996) cDNA cloning of the hepatocyte canalicular isoform of the multidrug resistance protein, cMrp, reveals a novel conjugate export pump deficient in hyperbilirubinemic mutant rats. J Biol Chem 271:15091–15098 [DOI] [PubMed] [Google Scholar]
- Buchman AL, Katz S, Fang JC, Bernstein CN, Abou-Assi SG, and Teduglutide Study Group (2010) Teduglutide, a novel mucosally active analog of glucagon-like peptide-2 (GLP-2) for the treatment of moderate to severe Crohn's disease. Inflamm Bowel Dis 16:962–973 [DOI] [PubMed] [Google Scholar]
- Cani PD, Possemiers S, Van de Wiele T, Guiot Y, Everard A, Rottier O, Geurts L, Naslain D, Neyrinck A, Lambert DM, et al. (2009) Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut 58:1091–1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J, Stieger B, Meier PJ, Vore M. (2002) Expression of rat hepatic multidrug resistance-associated proteins and organic anion transporters in pregnancy. Am J Physiol Gastrointest Liver Physiol 283:G757–G766 [DOI] [PubMed] [Google Scholar]
- Catania VA, Luquita MG, Sánchez Pozzi EJ, Mottino AD. (2000) Quantitative and qualitative gender-related differences in jejunal glutathione S-transferase in the rat effect of testosterone administration. Life Sci 68:467–474 [DOI] [PubMed] [Google Scholar]
- Catania VA, Sánchez Pozzi EJ, Luquita MG, Ruiz ML, Villanueva SS, Jones B, Mottino AD. (2004) Co-regulation of expression of phase II metabolizing enzymes and multidrug resistance-associated protein 2. Ann Hepatol 3:11–17 [PubMed] [Google Scholar]
- Dewit O, Starkel P, Roblin X. (2010) Thiopurine metabolism monitoring: implications in inflammatory bowel diseases. Eur J Clin Invest 40:1037–1047 [DOI] [PubMed] [Google Scholar]
- Drucker DJ, Erlich P, Asa SL, Brubaker PL. (1996) Induction of intestinal epithelial proliferation by glucagon-like peptide 2. Proc Natl Acad Sci 93:7911–7916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drucker DJ, Boushey RP, Wang F, Hill ME, Brubaker PL, Yusta B. (1999) Biologic properties and therapeutic potential of glucagon-like peptide-2. J Parenter Enteral Nutr 23:S98–S100 [DOI] [PubMed] [Google Scholar]
- Drucker DJ. (2002) Biological actions and therapeutic potential of the glucagon-like peptides. Gastroenterology 122:531–544 [DOI] [PubMed] [Google Scholar]
- Gotoh Y, Suzuki H, Kinoshita S, Hirohashi T, Kato Y, Sugiyama Y. (2000) Involvement of an organic anion transporter (canalicular multispecific organic anion transporter/multidrug resistance-associated protein 2) in gastrointestinal secretion of glutathione conjugates in rats. J Pharmacol Exp Ther 292:433–439 [PubMed] [Google Scholar]
- Griffith OW. (1980) Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal Biochem 106:207–212 [DOI] [PubMed] [Google Scholar]
- Hayes JD, Pulford DJ. (1995) The glutathione S-transferase supergene family: regulation of GST and the contribution of the isoenzymes to cancer chemoprotection and drug resistance. Crit Rev Biochem Mol Biol 30:445–600 [DOI] [PubMed] [Google Scholar]
- Hertervig E, Nilsson A, Seidegård J. (1994) The expression of glutathione transferase mu in patients with inflammatory bowel disease. Scand J Gastroenterol 29:729–735 [DOI] [PubMed] [Google Scholar]
- Hinchman CA, Matsumoto H, Simmons TW, Ballatori N. (1991) Intrahepatic conversion of a glutathione conjugate to its mercapturic acid. Metabolism of 1-chloro-2,4-dinitrobenzene in isolated perfused rat and guinea pig livers. J Biol Chem 266:22179–22185 [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources (2010) Guide for the Care and Use of Laboratory Animals 8th ed Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council, Washington DC [Google Scholar]
- Jeppesen PB, Gilroy R, Pertkiewicz M, Allard JP, Messing B, O'Keefe SJ. (2011) Randomised placebo-controlled trial of teduglutide in reducing parenteral nutrition and/or intravenous fluid requirements in patients with short bowel syndrome. Gut 60:902–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keppler D, König J, Büchler M. (1997) The canalicular multidrug resistance protein, cMRP/MRP2, a novel conjugate export pump expressed in the apical membrane of hepatocytes. Adv Enzyme Regul 37:321–333 [DOI] [PubMed] [Google Scholar]
- Keppler D, Kamisako T, Leier I, Cui Y, Nies AT, Tsujii H, König J. (2000) Localization, substrate specificity, and drug resistance conferred by conjugate export pumps of the MRP family. Adv Enzyme Regul 40:339–349 [DOI] [PubMed] [Google Scholar]
- Kim SK, Woodcroft KJ, Novak RF. (2003) Insulin and glucagon regulation of glutathione S-transferase expression in primary cultured rat hepatocytes. J Pharmacol Exp Ther 305:353–361 [DOI] [PubMed] [Google Scholar]
- Körner M, Stöckli M, Waser B, Reubi JC. (2007) GLP-1 receptor expression in human tumors and human normal tissues: potential for in vivo targeting. J Nucl Med 48:736–743 [DOI] [PubMed] [Google Scholar]
- L'Heureux MC, Brubaker PL. (2001) Therapeutic potential of the intestinotropic hormone, glucagon-like peptide-2. Ann Med 33:229–235 [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275 [PubMed] [Google Scholar]
- MacNeil DJ, Occi JL, Hey PJ, Strader CD, Graziano MP. (1994) Cloning and expression of a human glucagon receptor. Biochem Biophys Res Commun 198:328–334 [DOI] [PubMed] [Google Scholar]
- Marier JF, Beliveau M, Mouksassi MS, Shaw P, Cyran J, Kesavan J, Wallens J, Zahir H, Wells D, Caminis J. (2008) Pharmacokinetics, safety, and tolerability of teduglutide, a glucagon-like peptide-2 (GLP-2) analog, following multiple ascending subcutaneous administrations in healthy subjects. J Clin Pharmacol 48:1289–1299 [DOI] [PubMed] [Google Scholar]
- Marier JF, Mouksassi MS, Gosselin NH, Beliveau M, Cyran J, Wallens J. (2010) Population pharmacokinetics of teduglutide following repeated subcutaneous administrations in healthy participants and in patients with short bowel syndrome and Crohn's disease. J Clin Pharmacol 50:36–49 [DOI] [PubMed] [Google Scholar]
- McBride MW, Brosnan MJ, Mathers J, McLellan LI, Miller WH, Graham D, Hanlon N, Hamilton CA, Polke JM, Lee WK, et al. (2005) Reduction of Gstm1 expression in the stroke-prone spontaneously hypertension rat contributes to increased oxidative stress. Hypertension 45:786–792 [DOI] [PubMed] [Google Scholar]
- Meier PJ, Sztul ES, Reuben A, Boyer JL. (1984) Structural and functional polarity of canalicular and basolateral plasma membrane vesicles isolated in high yield from rat liver. J Cell Biol 98:991–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mottino AD, Hoffman T, Jennes L, Vore M. (2000) Expression and localization of multidrug resistant protein mrp2 in rat small intestine. J Pharmacol Exp Ther 293:717–723 [PubMed] [Google Scholar]
- Mottino AD, Hoffman T, Jennes L, Cao J, Vore M. (2001) Expression of multidrug resistance-associated protein 2 in small intestine from pregnant and postpartum rats. Am J Physiol Gastrointest Liver Physiol 280:G1261–G1273 [DOI] [PubMed] [Google Scholar]
- Ohoka K, Takano M, Okano T, Maeda S, Inui K, Hori R. (1993) p-Aminohippurate transport in rat renal brush-border membranes: a potential-sensitive transport system and an anion exchanger. Biol Pharm Bull 16:395–401 [DOI] [PubMed] [Google Scholar]
- Paulusma CC, Bosma PJ, Zaman GJ, Bakker CT, Otter M, Scheffer GL, Scheper RJ, Borst P, Oude Elferink RP. (1996) Congenital jaundice in rats with a mutation in a multidrug resistance-associated protein gene. Science 271:1126–1128 [DOI] [PubMed] [Google Scholar]
- Rezaie A, Parker RD, Abdollahi M. (2007) Oxidative stress and pathogenesis of inflammatory bowel disease: an epiphenomenon or the cause? Dig Dis Sci 52:2015–2021 [DOI] [PubMed] [Google Scholar]
- Scott RB, Kirk D, MacNaughton WK, Meddings JB. (1998) GLP-2 augments the adaptive response to massive intestinal resection in rat. Am J Physiol 275:G911–G921 [DOI] [PubMed] [Google Scholar]
- Siekevitz P. (1962) Preparation of microsomes and submicrosomal fractions. Methods Enzymol 5:61–68 [Google Scholar]
- Thulesen J, Hartmann B, Orskov C, Jeppesen PB, Holst JJ, Poulsen SS. (2000) Potential targets for glucagon-like peptide 2 (GLP-2) in the rat: distribution and binding of i.v. injected (125)I-GLP-2. Peptides 21:1511–1517 [DOI] [PubMed] [Google Scholar]
- Tietze F. (1969) Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem 27:502–522 [DOI] [PubMed] [Google Scholar]
- Torres AM, MacLaughlin M, Quaglia NB, Stremmel W. (2003) Role of BSP/bilirubin binding protein on p-aminohippurate transport in rat kidney. Mol Cell Biochem 245:149–156 [DOI] [PubMed] [Google Scholar]
- Villanueva SS, Ruiz ML, Luquita MG, Sánchez Pozzi EJ, Catania VA, Mottino AD. (2005) Involvement of Mrp2 in hepatic and intestinal disposition of dinitrophenyl-S-glutathione in partially hepatectomized rats. Toxicol Sci 84:4–11 [DOI] [PubMed] [Google Scholar]
- Villanueva SS, Arias A, Ruiz ML, Rigalli JP, Pellegrino JM, Vore M, Catania VA, Mottino AD. (2010) Induction of intestinal multidrug resistance-associated protein 2 by glucagon-like Peptide 2 in the rat. J Pharmacol Exp Ther 335:332–341 [DOI] [PubMed] [Google Scholar]
- Wiegand H, Wagner AE, Boesch-Saadatmandi C, Kruse HP, Kulling S, Rimbach G. (2009) Effect of dietary genistein on Phase II and antioxidant enzymes in rat liver. Cancer Genomics Proteomics 6:85–92 [PubMed] [Google Scholar]
- Yazbeck R. (2010) Teduglutide, a glucagon-like peptide-2 analog for the treatment of gastrointestinal diseases, including short bowel syndrome. Curr Opin Mol Ther 12:798–809 [PubMed] [Google Scholar]