Abstract
Association between chronic inflammation and cancer development is exemplified by inflammatory bowel disease (IBD) where patients with chronic uncontrolled colitis have a significantly increased risk of developing colitis-associated colorectal cancer (CACC). CACC appears to progresses through the inflammation-dysplasia-carcinoma sequence. This highlights the need to identify targets and interventions that reduce inflammation and prevent development of dysplasia in the context of IBD. Using the dextran sulfate sodium (DSS) mouse model of chronic colitis and CACC, we show that an important target of intervention in human disease would be the epithelial cell molecule MUC1 that is aberrantly expressed on inflamed colonocytes and promotes inflammation and progression to CACC. We show that a MUC1 vaccine can ameliorate chronic colitis and prevent development of dysplasia in the colon and thus extend survival in human MUC1 transgenic mice. This study supports the potential of prophylactic vaccines to target antigens that become aberrantly expressed in chronic inflammation (e.g., IBD) and continue to be expressed on the associated cancers (e.g., colon cancer), to prevent and/or treat both diseases.
Keywords: Inflammatory Bowel Disease, MUC1, cancer vaccine, colon cancer, inflammation
Introduction
It is well established that chronic inflammation can serve as a carcinogenic event resulting in cancer development at the affected site.1 This association is exemplified in individuals with inflammatory bowel disease (IBD) where patients with chronic uncontrolled colitis have a significantly increased risk of developing colitis-associated colorectal cancer (CACC).2 The etiology of IBD is unknown, but manifestations of the disease include severe disruption in the epithelium and its associated mucus layer. The chronically inflamed mucosa progresses through inflammation to dysplasia to eventual adenocarcinoma.3 This sequence suggests that it might be possible to identify targets and interventions directed at various stages of the disease progression. In this study we tested the potential of an immune response, elicited through vaccination, to reduce chronic inflammation and prevent progression to dysplasia in IBD, and thus reduce the risk of CACC.
MUC1 mucin is a large glycoprotein that is expressed at low levels on the apical surface of ductal epithelial cells in several organs including the colonic epithelium. Its biological functions include lubrication and hydration of epithelia and protection against colonization by pathogens. The extracellular domain of MUC1 is comprised of varying number of tandem repeats region (VNTR) composed of 20-amino acid (HGVTSAPDTRPAPGSTAPPA) long repeats containing five potential O-glycosylation sites that on healthy epithelia are heavily glycosylated.4,5 MUC1 is abnormally expressed in all stages of the development of adenocarcinoma and in various chronic inflammatory diseases including IBD.6-13 Abnormal MUC1 expression is characterized by overexpression and loss of apical polarization. In addition, the extracellular VNTR region has fewer carbohydrates per repeat and truncation of carbohydrate chains exposing the peptide backbone. The mouse homolog (Muc-1) shares only 34% homology with human MUC1in the VNTR and thus does not play the same role in IBD and CACC as the human MUC1 molecule. In order to study human MUC1, we have taken advantage of the availability of human MUC1 transgenic (MUC1.tg) mice. These mice carry the human MUC1 transgene expressed under its own promoter and express the full-length glycoprotein in the same spatial and tissue distribution as in humans.14 This includes low expression on the apical surface of healthy epithelia and overexpression of the abnormal, hypoglycosylated form on epithelial tumor cells.
We have previously shown that in the MUC1/interleukin-10 knockout (MUC1+/IL-10−/−) mouse model of human IBD and CACC, derived by crossing human MUC1 transgenic mouse (MUC1.tg) with the IL-10−/− mouse, MUC1 expression has a profound effect on the time of IBD occurrence, degree of inflammation, and progression to colon cancer.9 In addition, we showed that early intervention with vaccination against abnormal MUC1 altered the immunosuppressive microenvironment of chronic inflammation and led to lessening of inflammation and protection from CACC development.8 One of the caveats of the spontaneous IL-10−/− mouse model of IBD is that the time when the inflammatory process begins varies between animals, and thus early treatment may be prophylactic in one animal but therapeutic in another animal. Furthermore, the lack of IL-10 makes this model less reflective of human IBD. In the current study, we used the dextran sulfate sodium (DSS) model of colitis where mice are fully immunocompetent and the time of initiation of inflammation can be controlled. We added to the model the human MUC1 molecule known to be expressed in human disease but lacking in all previous studies employing DSS. We compared results obtained in MUC1.tg mice with those in WT mice to confirm the importance of MUC1 in disease development and to test the efficacy of anti-MUC1 immunotherapy in a new model of MUC1+ human IBD.
Results
Human MUC1 expression accelerates colonic inflammation in DSS-induced colitis
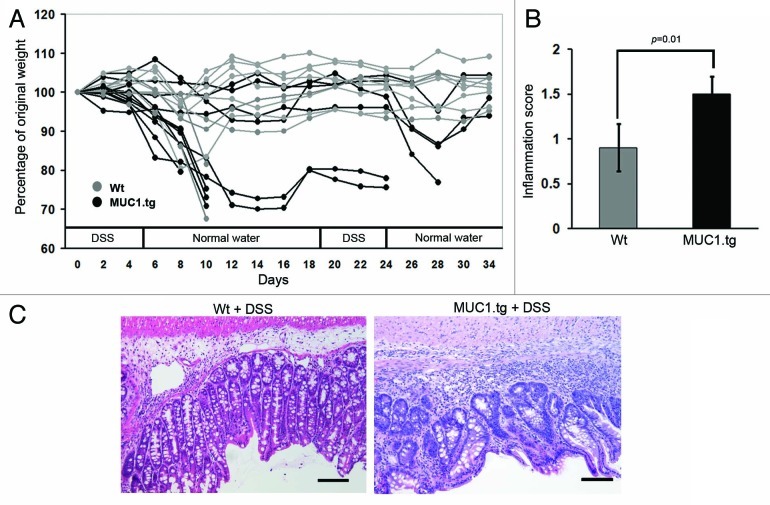
To induce chronic colitis, 2.5% DSS was repeatedly administered in the drinking water to wild-type (Wt) and MUC1.tg mice. During the course of DSS treatment, mice were monitored for general signs of malaise, body weight loss and diarrhea. Shortly after the first cycle of DSS, MUC1.tg mice experienced more severe disease symptoms including greater body weight loss compared with Wt mice. This resulted in increased mortality in MUC1.tg mice (5 of 11) compared with Wt mice (one of nine), with most deaths occurring at day ten after colitis induction (Fig. 1A). Colons from mice that succumbed after one DSS treatment were examined by a pathologist. Severe colonic inflammation and mild ulceration were found in all mice confirming DSS water consumption and excluding dehydration as a cause of early mortality. To establish chronic inflammation, a second cycle of DSS was administered to surviving MUC1.tg and Wt mice. This resulted in additional mortality in theMUC1.tg mice (three of six) and no deaths in the Wt mice (Fig. 1A). These results suggest that in DSS-induced colitis, the expression of human MUC1 is a contributing factor in accelerating colonic inflammation leading to increased mortality.
Figure 1. Expression of human MUC1 accelerates colonic inflammation and dysplasia in DSS-treated mice. (A) Human MUC1.tg mice (black) and Wt mice (gray) were given two cycles of DSS in drinking water and body weight was monitored over time. (B) After two cycles of DSS, MUC1.tg and Wt mice were sacrificed, colons removed and paraffin embedded, and stained with H&E. H&E stained colon sections were examined by pathologist and given inflammation scores (see Materials and Methods) and assessed for dysplasia. p was determined by two-tailed unpaired t-test. (C) Representative H&E stained colon sections showing high-grade dysplasia in MUC1.tg and no dysplasia in Wt mice. Scale bars are 100 µm.
Colon samples taken from MUC1.tg and Wt mice that had received two cycles of DSS were assessed by pathologist blinded to the sample groups. The evaluation gave significantly increased inflammation scores for the colons from MUC1.tg compared with Wt mice (Fig. 1B). In addition, high grade dysplasia was found in the colons from the MUC1.tg mice (Fig. 1C, right) and none in Wt mice (Fig. 1C, left).
DSS-induced colitis increases the expression of abnormal MUC1 in the colon
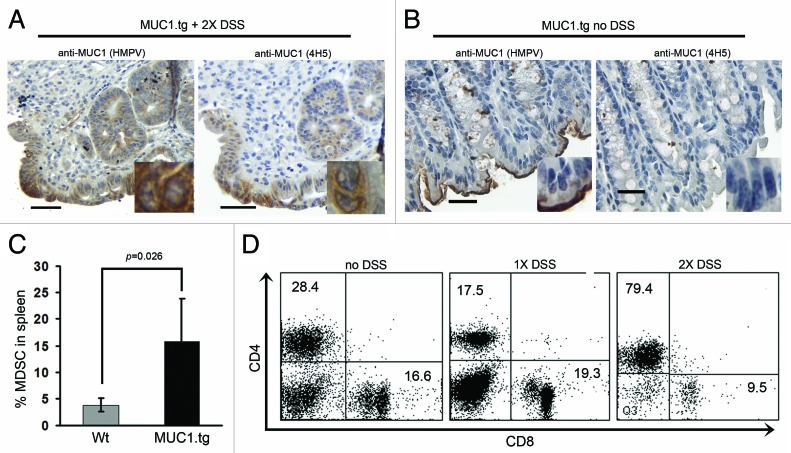
Colonic sections taken from MUC1.tg mice after two cycles of DSS were immunostained with two different anti-MUC1-specific monoclonal antibodies (mAb). Antibody HMPV is MUC1 glycosylation independent and recognizes all forms of MUC1. In contrast, antibody VU4H5 is MUC1 glycosylation dependent and recognizes only the abnormal hypoglycosylated form of MUC1. Analysis of immunostained colonic sections revealed increased expression of total MUC1 in inflamed colons of treated mice (Fig. 2A, left) compared with untreated MUC1.tg mice with no inflammation (Fig. 2B, left). In addition, the MUC1 in treated mice was the abnormal hypoglycosylated form (Fig. 2A, right) not found in control mice (Fig. 2B, right). Higher magnification insets show cytoplasmic MUC1 expression as well as loss of MUC1 apical polarization in the colonic epithelial cells.
Figure 2.DSS-induced colitis in MUC1tg mice is characterized by increased expression of abnormal MUC1, increased percentage of MDSC and loss of CD8+ T cells. Immunostaining of colon tissue sections from MUC1.tg mice that received two cycles of DSS (A) and no DSS control mice (B). HMPV antibody stains all MUC1; 4H5 antibody stains hypoglycosylated MUC1. Scale bars are 100 µm. Inserts were taken with 100x oil-immersion objective. (C) Isolated spleen cells from Wt (n-4) and MUC1.tg (n = 4) mice were stained for Gr-1 and CD11b expression and analyzed by flow cytometry. p was determined by two-tailed unpaired t-test. (D) Isolated spleen cells from control MUC1.tg mice that received no DSS, MUC1.tg mice that received one cycle of DSS, and MUC1.tg mice that received two cycles of DSS were labeled for CD4 and CD8 and analyzed by flow cytometry. Representative dot plots from two separate experiments.
DSS-induced colitis in MUC1.tg mice results in increased percentages of myeloid derived suppressor cells (MDSC) and loss of CD8+ T cells
We previously published that in the MUC1+/IL-10−/− mouse model of IBD there was accumulation of MDSC in the spleens of mice that had dysplasia and cancer.8 We asked whether there was a similar accumulation of MDSC in the DSS-induced colonic inflammation and CACC. Spleen cells isolated from MUC1.tg and Wt mice that had received two cycles of DSS were stained for expression of Gr-1 and CD11b, cell surface markers of mouse MDSC. Using flow cytometry we found a statistically significant increase in the percentage of Gr1+CD11b+ cells in the spleens from DSS-treated MUC1.tg mice compared with Wt mice (Fig. 2C).
Next, we examined the T-cell composition in MUC1.tg mice with DSS-induced colitis. Isolated lymph node and spleen cells from MUC1.tg mice that received either one or two cycles of DSS were stained for expression of T-cell markers CD3, CD4, and CD8. Flow cytometry revealed that after one cycle of DSS, MUC1.tg mice experienced an increase in CD8/CD4 T-cell ratio suggesting that CD8+ T cells were expanding in the secondary lymphoid organs. In contrast, after two cycles of DSS the majority of cells were CD3+CD4+ T cells and the CD3+CD8+ T cells had disappeared (Fig. 2D). For comparison, a representative dot plot from MUC1.Tg mice that did not receive DSS is shown.
This experiment recapitulates our previous results in the MUC1+/IL-10−/− mouse model of colitis and suggests that acceleration and severity of inflammation, and development of dysplasia in the presence of human MUC1 is not unique to the MUC1+/IL-10−/− model of colonic inflammation, but rather a characteristic of MUC1+ IBD.
MUC1 vaccine slows down IBD progression
We wanted to know if an immune response against the abnormal form of MUC1 can decrease inflammation and prevent chronic inflammation thus preventing development of dysplasia and progression to CACC. Furthermore, we questioned if preexisting immune response would prevent the loss of CD8+ T cells observed in MUC1.tg mice that received two cycles of DSS. As always, we looked for any signs of autoimmunity that might result from anti-MUC1 immunity in this particular disease setting.
The MUC1 vaccine we used to elicit anti-MUC1 immune response was our previously published synthetic MUC1–100-mer peptide or glycopeptide TnMUC1–100mer with E6020 adjuvant, a synthetic, attenuated Toll-like receptor-4 agonist that promotes both systemic and mucosal immunity.15 The synthetic MUC1–100-mer peptide consists of five 20-amino-acid-long tandem repeats from the VNTR region of MUC1. The glycopeptide TnMUC1–100mer consists of the same five tandem repeats glycosylated in vitro to express the truncated tumor-associated glycan GalNAc.16 Mice were primed and followed by two boosts given two weeks apart. The vaccine was administered nasally to promote mucosal immunity capable of homing to the colonic mucosa.
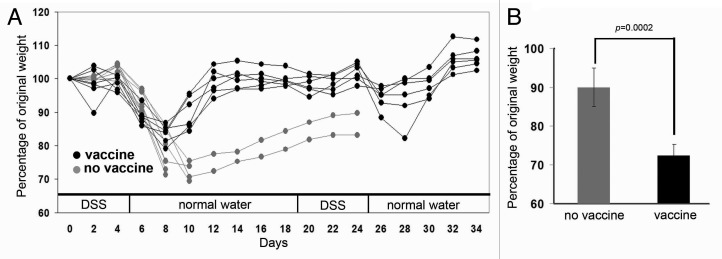
Two weeks following the second boost, vaccinated and age-matched MUC1.tg mice that did not receive vaccine were started on 2.5% DSS in their drinking water. Between days four and six, both vaccinated and unvaccinated mice experienced an average of 10% loss of their pre-DSS treatment weight (Fig. 3A). Between days six and eight, the two groups began to dramatically separate where unvaccinated mice experienced an additional drop in weight (15%) that required of two mice to be removed from the protocol due to the severity of disease symptoms. In contrast, vaccinated mice only lost an additional 5% of their pre-DSS weight. At day ten, there was a statistically significant difference in body weight loss between the vaccinated and unvaccinated mice. Vaccinated mice were clearly beginning to rebound with amelioration of disease symptoms and weight gain of 6.5% while unvaccinated mice continued to lose body weight with an additional three mice removed from the protocol due to severity of disease symptoms (Fig. 3B).
Figure 3. Vaccination against abnormal MUC1 ameliorates DSS-induced weight loss. (A) Weight loss over time in vaccinated (black) and unvaccinated (gray) MUC1.tg mice that received DSS in drinking water. (B) Percentage of original body weight in individual mice at day 10 following one cycle of DSS. Unvaccinated MUC1.tg mice, n = 6. Vaccinated MUC1.tg mice, n = 4. p was determined by two-tailed unpaired t-test.
Vaccine induces MUC1-specific immunity
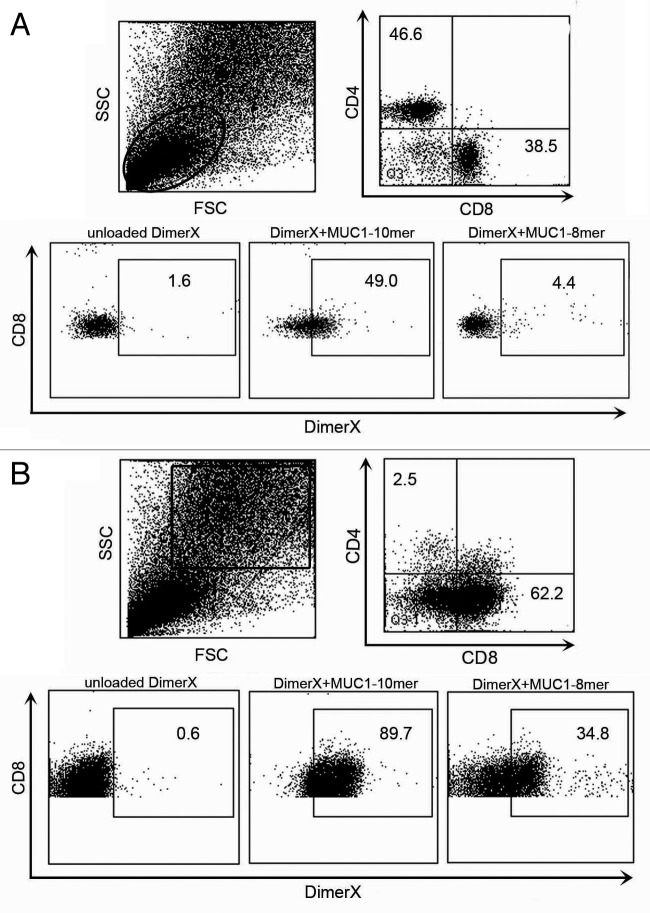
One vaccinated MUC1.tg mouse was removed from the protocol following one cycle of DSS to determine if the vaccine elicited a MUC1-specific immune response. We looked for MUC1-specific CD8+ T cells in secondary lymphoid organs. Isolated spleen and lymph node cells were stained with CD3, CD4, CD8 and peptide loaded MHC class I H2Kb DimerX (BD DimerX), MHC-Ig fusion protein that when loaded with antigen can bind to antigen-specific T cells.17 DimerX was incubated overnight with either the MUC1–8mer peptide (SAPDTRPA) or glycopeptide MUC1–10mer (SAPDTRPAPG), both derived from the VNTR region of MUC1. The MUC1–10mer glycopeptide has the GalNAc glycan attached to the threonine at position five. Unloaded DimerX was used as a negative control. Using flow cytometry, we first gated on the lower lymphocyte population in the forward- and side-scatter plot (Fig. 4A). We determined the percentage of DimerX+ MUC1-specific T cells by gating on CD3+CD8+ T cells. We found that 49.0% of CD8+ T cells were specific for the MUC1–10mer glycopeptide loaded DimerX and 4.4% of CD8+ T cells were specific for the MUC1–8mer peptide loaded DimerX (Fig. 4A). Next, we examined the larger more granular population of cells in the forward- and side-scatter plots (Fig. 4B). The majority of cells in this gate were also CD3+ and almost entirely comprised of CD8+ T cells (Fig. 4B). We found 89.7% of CD3+CD8+ T cells were specific for the MUC1–10mer glycopeptide loaded DimerX and 34.8% of CD8+ T cells were specific for the MUC1–8mer peptide loaded DimerX (Fig. 4B). This larger, more granular CD3+CD8+ T cell population in the forward- and side-scatter plot is indicative of blasting T cells. We found no DimerX+ T cells in unvaccinated MUC1.tg mice after one cycle of DSS (data not shown).
Figure 4.
Vaccine induces MUC1-specific CD8+ T cells. MHC class I H2Kb DimerX (BD DimerX) was incubated overnight with either the MUC1–8mer peptide (SAPDTRPA) or glycopeptide MUC1–10mer (SAPD(T)RPAPG). Isolated spleen cells from vaccinated MUC1.tg mouse that received one cycle of DSS were stained for expression of CD3, CD4 and CD8, mixed with peptide-loaded DimerX and analyzed by flow cytometry. (A) Gated on the small CD3+CD8+ lymphocyte population (circle). (B) Gated on the CD3+CD8+ blasting lymphocyte population (square).
After two cycles of DSS isolated spleen cells were stained for expression of Gr-1 and CD11b, MDSC cell surface markers. Using flow cytometry, we observed difference in the percentage of MDSC in individual mice, but this difference was not associated with the vaccinated or unvaccinated groups. Instead, mice that had experienced greater body weight loss during the course of DSS treatments had increased percentage of Gr1+CD11b+ and that was true for unvaccinated mice as well as vaccinated mice (data not shown).
Lastly, after two cycles of DSS, we failed to see the high percentage of MUC1-specific T cells that we saw after just one cycle in vaccinated mice but they did maintain an average one to two CD8+/CD4+ T cell ratio. This was in contrast to the loss of CD8+ T cells we found in unvaccinated MUC1.tg mice (Fig. 2C).
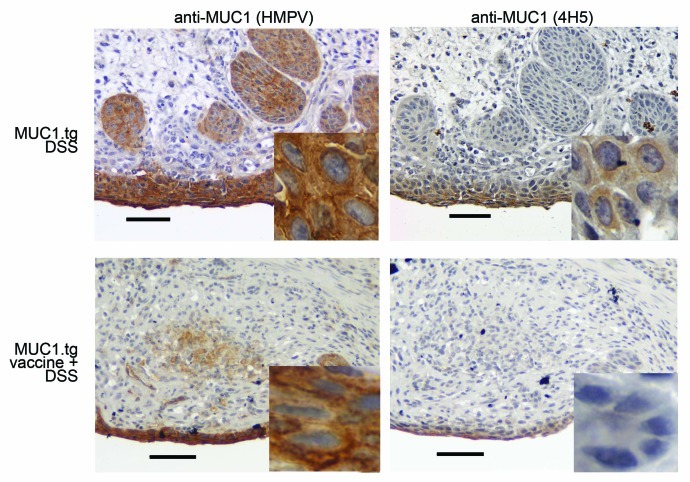
Colonic sections taken from vaccinated and unvaccinated MUC1.tg mice that received two cycles of DSS were immunostained with anti-MUC1 antibodies HMPV and anti-MUC1 vu4H5. Analysis revealed lower level of MUC1 expression in vaccinated compared with unvaccinated mice. Importantly, vaccinated mice showed little or no expression of hypoglycosylated MUC1 (Fig. 5, bottom right). Colon section from healthy, unmanipulated MUC1.tg mice is shown for comparison (Fig. 2B). We found no dysplasia in colons from vaccinated MUC1.tg mice. In contrast, dysplasia was observed in colons from unvaccinated MUC1.tg that received DSS.
Figure 5. Vaccination results in elimination of abnormal MUC1-expressing epithelial cells. Representative immunostaining of colon tissue sections from unvaccinated and vaccinated MUC1.tg mice that received DSS. HMPV antibody stains all MUC1; 4H5 antibody stains hypoglycosylated MUC1. Scale bars are 100 µm. Inserted images were taken with 100× oil-immersion objective.
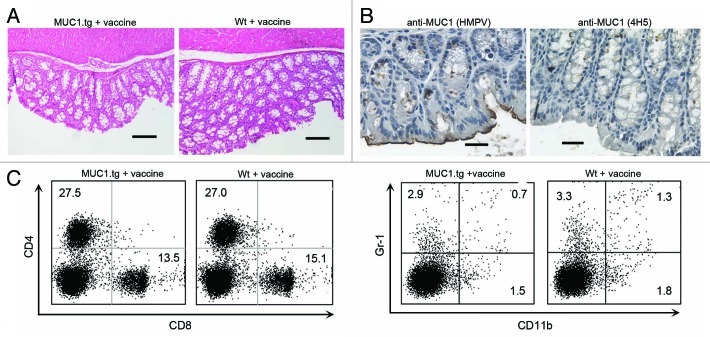
In addition to the experimental groups we also examined MUC1.tg and Wt mice that received vaccine but did not receive DSS. Colons from both groups were examined by pathologist and no unwanted vaccine induced side effects were found (Fig. 6A). Colons from the MUC1.tg mice were stained with both anti-MUC1 antibodies. We found the typical low MUC1 expression with apical polarization (Fig. 6B, left) and no evidence of abnormal hypoglycosylated MUC1 (Fig. 6B, right). In addition, we examined the T-cell composition in spleens and lymph nodes and found healthy CD8+/CD4+ T-cell ratio with no accumulation of MDSC (Fig. 6C).
Figure 6. MUC1 vaccine does not induce autoimmunity. (A) Representative H&E stained colon tissue sections from vaccinated MUC1.tg and Wt mice. (B) Representative immunostaining of colon tissue sections from vaccinated MUC1.tg mice. HMPV antibody stains all MUC1; 4H5 antibody stains hypoglycosylated MUC1. Scale bars are 100 µm. (C) Isolated spleen cells from vaccinated MUC1.tg and Wt mice were stained for expression of CD3, CD4, CD8, Gr1, CD11b. Representative dot plots show percentage of CD4+ and CD8+ T cells (left panel) from the gated CD3+ cells and the percentage of Gr1+CD11b+ MDSC cells (right panel) from the gated non-CD3+ cells.
Discussion
We show that human MUC1 plays a pro-inflammatory role in the establishment of chronic colitis and CACC in the DSS mouse model of IBD. In addition, we show that early intervention with a MUC1vaccine can ameliorate chronic colitis and prevent development of dysplasia in the colon and thus extend survival in MUC1.tg mice. The vaccine is safe with no autoimmune side effects.
We and others have shown that abnormal MUC1 expression is not limited to fully transformed cells but can also be seen in chronic inflammation.8,9,11,13,18 Although we have only recently begun to address mechanistically how MUC1 drives and perpetuates chronic inflammation, we and others have shown that MUC1 plays a role in tumorigenesis through changes in intracellular signaling, adhesion and migration, and by increasing resistance to apoptosis.19-22 During chronic inflammation, increased MUC1 expression is most likely due to the inflammatory cytokine milieu such as TNFα, IL-6, and IFNγ that we know can induce MUC1 expression in epithelial cells.23,24 Changes in MUC1 expression could perpetuate inflammation as we have previously shown that abnormal forms of MUC1 are chemotactic for immature dendritic cells and this is mediated by the epitopes in the tandem repeat region of the hypoglycosylated MUC1.25 In addition, we recently reported that the tandem repeat region of MUC1 upregulated inflammatory cytokines IL-6 and TNFα expression by binding to their promoter regions in an NFκB p65-dependent manner.26
Our data suggest that MUC1 vaccination induces a strong MUC1-specific CD8+ T-cell response. Given that we see complete prevention of dysplasia and less hypoglycosylated MUC1 on the epithelia of vaccinated animals, we propose that the MUC1-specific CD8+ T cells can target and eliminate dysplastic epithelial cells that are expressing abnormal MUC1. This will prevent tumor formation and most likely alter the cytokine milieu resulting in an amelioration of colonic inflammation.
Given the established association between chronic inflammation and cancer, we can consider early stages of chronic inflammation as a premalignant condition. Molecules, such as MUC1, that are abnormally expressed in both chronically inflamed tissues and the cancers that arise from those tissues, are most likely playing significant roles in both diseases. As such, they can be used as targets for immunotherapy for chronic inflammation and cancer prevention.
Material and Methods
Mice
C57BL/6 mice were purchased from the Jackson Laboratory. MUC1 transgenic mice were originally purchased from Dr. S.J. Gendler (The Mayo Clinic) and are bred at the University of Pittsburgh. Colitis was induced with DSS (M.W. = 36,000–50,000; MP Biomedicals) in sterilized water, with each cycle consisting of 5 d of 2.5% DSS (w/v) in the drinking water, followed by 14 d of sterilized normal tap water. All experiments were approved by the Institutional Animal Care and Use Committee of the University of Pittsburgh.
Peptide synthesis and in vitro glycosylation
The TnMUC1 100-mer peptide used for immunization corresponds to five tandem repeats of a 20-amino acid sequence HGVTSAPDTRPAPGSTAPPA from the extracellular VNTR region of MUC1. Enzymatic addition of GalNAc to the peptide was done using recombinant UDP-GalNAc poplypeptide N-acetyl-galactosaminyltransferases rGalNAc-T1 as previously described.16 The MUC1 100-mer peptide corresponds to five tandem repeats of the same 20-amino acid sequence with no sugars. The MUC1–8mer and glycopeptide MUC1–10–5GalNAc correspond to the extracellular VNTR region of MUC1. The peptides were synthesized at the University of Pittsburgh Genomics and proteomics Core Laboratories.
Vaccine protocol
Mice in the vaccine group received 20 µl intranasally (10 µl/nare) of 30 µg of either TnMUC1 100-mer or MUC1–100-mer peptide mixed with 3 µg of adjuvant E6020 in PBS. The adjuvant E6020 is a synthetic, attenuated Toll-like receptor-4 agonist provided by Eisai Research Institute, Andover, MA.15
Histology and inflammation scores
Colons were removed immediately after sacrifice, cut longitudinally, washed in cold 1X PBS, fixed in 5% buffered formalin, and embedded in paraffin. Sections (5 µm thick) were stained with H&E. Inflammation scores (0–4) were determined by a pathologist who was blinded to the experimental protocol using the criteria previously reported.9 Briefly 100–200 separate microscopic fields were evaluated for each mouse and graded 0 to 4. The total inflammation score for each sample was determined by taking the sum of the fields divided by the number of fields.
Immunohistochemistry
Tissue paraffin sections were deparaffinized by baking overnight at 59°C. Endogenous peroxidase activity was eliminated by treatment with 30% hydrogen peroxide for 15 min at room temperature. Antigen retrieval was done by microwave heating in 0.1% citrate buffer. Nonspecific binding sites were blocked with 1% BSA. The anti-MUC1 antibody HMPV (BD PharMingen) recognizes all forms of MUC1 by binding to the epitope APDTR in the VNTR region in a glycosylation-independent manner. The anti-MUC1 antibody vu-4H5 (Santa Cruz Biotechnology) recognizes the epitope APDTRPAP in the VNTR region of hypoglycosylated MUC1. Staining was done by the avidin-biotin peroxidase method with a commercial kit (Vectastain ABC kit, Vector Laboratories). Color development was done using a 3,3′-diaminobenzidine kit (BD PharMingen).
Microscopy and image acquisition
Histology sections were observed using an Olympus BX40 microscope. Images were acquired using a Leica DFC420 camera and Leica Application Suite version 2.7.1 R1.
Flow cytometry
Isolated cells from spleens and lymph nodes were washed and resuspended in fluorescence-activated cell sorting buffer (2% fetal bovin serum in PBS) and plated at 0.5 × 106 to 1 × 106 per well. Surface Fc receptors were blocked with the addition of anti-CD16 (BD Bioscience) and incubated for 20 min. Cells were stained with anti-CD3, anti-CD4, anti-CD8, anti-Gr-1, and anti-CD11b (BD Bioscience). DimerX soluble dimeric mouse H2kb:Ig fusion protein (BD PharMingen) was used according to manufacturer’s protocol to detect MUC1-specific CD8+ T cells. Cells were analyzed on an LSR II flow cytometer (BD Bioscience), running FACSDiva software.
Acknowledgments
This work was supported by NIH grant RO1 CA56103.
Glossary
Abbreviations:
- DSS
dextran sulfate sodium
- IBD
inflammatory bowel disease
- CACC
colitis-associated colon cancer
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/18950
References
- 1.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–7. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feagins LA, Souza RF, Spechler SJ. Carcinogenesis in IBD: potential targets for the prevention of colorectal cancer. Nat Rev Gastroenterol Hepatol. 2009;6:297–305. doi: 10.1038/nrgastro.2009.44. [DOI] [PubMed] [Google Scholar]
- 3.Ullman TA, Itzkowitz SH. Intestinal inflammation and cancer. Gastroenterology. 2011;140:1807–16. doi: 10.1053/j.gastro.2011.01.057. [DOI] [PubMed] [Google Scholar]
- 4.Finn OJ, Gantt KR, Lepisto AJ, Pejawar-Gaddy S, Xue J, Beatty PL. Importance of MUC1 and spontaneous mouse tumor models for understanding the immunobiology of human adenocarcinomas. Immunol Res. 2011;50:261–8. doi: 10.1007/s12026-011-8214-1. [DOI] [PubMed] [Google Scholar]
- 5.Vlad AM, Kettel JC, Alajez NM, Carlos CA, Finn OJ. MUC1 immunobiology: from discovery to clinical applications. Adv Immunol. 2004;82:249–93. doi: 10.1016/S0065-2776(04)82006-6. [DOI] [PubMed] [Google Scholar]
- 6.Adsay NV, Merati K, Andea A, Sarkar F, Hruban RH, Wilentz RE, et al. The dichotomy in the preinvasive neoplasia to invasive carcinoma sequence in the pancreas: differential expression of MUC1 and MUC2 supports the existence of two separate pathways of carcinogenesis. Mod Pathol. 2002;15:1087–95. doi: 10.1097/01.MP.0000028647.98725.8B. [DOI] [PubMed] [Google Scholar]
- 7.Ajioka Y, Allison LJ, Jass JR. Significance of MUC1 and MUC2 mucin expression in colorectal cancer. J Clin Pathol. 1996;49:560–4. doi: 10.1136/jcp.49.7.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beatty PL, Narayanan S, Gariepy J, Ranganathan S, Finn OJ. Vaccine against MUC1 antigen expressed in inflammatory bowel disease and cancer lessens colonic inflammation and prevents progression to colitis-associated colon cancer. Cancer Prev Res (Phila); 3:438-46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beatty PL, Plevy SE, Sepulveda AR, Finn OJ. Cutting edge: transgenic expression of human MUC1 in IL-10−/− mice accelerates inflammatory bowel disease and progression to colon cancer. J Immunol. 2007;179:735–9. doi: 10.4049/jimmunol.179.2.735. [DOI] [PubMed] [Google Scholar]
- 10.Ho SB, Ewing SL, Montgomery CK, Kim YS. Altered mucin core peptide immunoreactivity in the colon polyp-carcinoma sequence. Oncol Res. 1996;8:53–61. [PubMed] [Google Scholar]
- 11.Jerome KR, Kirk AD, Pecher G, Ferguson WW, Finn OJ. A survivor of breast cancer with immunity to MUC-1 mucin, and lactational mastitis. Cancer Immunol Immunother. 1997;43:355–60. doi: 10.1007/s002620050344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kadayakkara DK, Beatty PL, Turner MS, Janjic JM, Ahrens ET, Finn OJ. Inflammation driven by overexpression of the hypoglycosylated abnormal mucin 1 (MUC1) links inflammatory bowel disease and pancreatitis. Pancreas. 2010;39:510–5. doi: 10.1097/MPA.0b013e3181bd6501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Longman RJ, Poulsom R, Corfield AP, Warren BF, Wright NA, Thomas MG. Alterations in the composition of the supramucosal defense barrier in relation to disease severity of ulcerative colitis. J Histochem Cytochem. 2006;54:1335–48. doi: 10.1369/jhc.5A6904.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rowse GJ, Tempero RM, VanLith ML, Hollingsworth MA, Gendler SJ. Tolerance and immunity to MUC1 in a human MUC1 transgenic murine model. Cancer Res. 1998;58:315–21. [PubMed] [Google Scholar]
- 15.Ishizaka ST, Hawkins LD. E6020: a synthetic Toll-like receptor 4 agonist as a vaccine adjuvant. Expert Rev Vaccines. 2007;6:773–84. doi: 10.1586/14760584.6.5.773. [DOI] [PubMed] [Google Scholar]
- 16.Brokx RD, Revers L, Zhang Q, Yang S, Mal TK, Ikura M, et al. Nuclear magnetic resonance-based dissection of a glycosyltransferase specificity for the mucin MUC1 tandem repeat. Biochemistry. 2003;42:13817–25. doi: 10.1021/bi0353070. [DOI] [PubMed] [Google Scholar]
- 17.Schneck JP, Slansky JE, O'Herrin SM, Greten TF. Monitoring antigen-specific T cells using MHC-Ig dimers. Curr Protoc Immunol 2001; Chapter 17:Unit 17 2. [DOI] [PubMed] [Google Scholar]
- 18.Andrianifahanana M, Moniaux N, Batra SK. Regulation of mucin expression: mechanistic aspects and implications for cancer and inflammatory diseases. Biochim Biophys Acta 2006; 1765:189-222 [DOI] [PubMed] [Google Scholar]
- 19.Li Y, Bharti A, Chen D, Gong J, Kufe D. Interaction of glycogen synthase kinase 3beta with the DF3/MUC1 carcinoma-associated antigen and beta-catenin. Mol Cell Biol. 1998;18:7216–24. doi: 10.1128/mcb.18.12.7216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pandey P, Kharbanda S, Kufe D. Association of the DF3/MUC1 breast cancer antigen with Grb2 and the Sos/Ras exchange protein. Cancer Res. 1995;55:4000–3. [PubMed] [Google Scholar]
- 21.Ren J, Agata N, Chen D, Li Y, Yu WH, Huang L, et al. Human MUC1 carcinoma-associated protein confers resistance to genotoxic anticancer agents. Cancer Cell. 2004;5:163–75. doi: 10.1016/S1535-6108(04)00020-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yin L, Huang L, Kufe D. MUC1 oncoprotein activates the FOXO3a transcription factor in a survival response to oxidative stress. J Biol Chem. 2004;279:45721–7. doi: 10.1074/jbc.M408027200. [DOI] [PubMed] [Google Scholar]
- 23.Gaemers IC, Vos HL, Volders HH, van der Valk SW, Hilkens J. A stat-responsive element in the promoter of the episialin/MUC1 gene is involved in its overexpression in carcinoma cells. J Biol Chem. 2001;276:6191–9. doi: 10.1074/jbc.M009449200. [DOI] [PubMed] [Google Scholar]
- 24.Shirasaki H, Kanaizumi E, Watanabe K, Konno N, Sato J, Narita S, et al. Tumor necrosis factor increases MUC1 mRNA in cultured human nasal epithelial cells. Acta Otolaryngol. 2003;123:524–31. doi: 10.1080/00016480310001268. [DOI] [PubMed] [Google Scholar]
- 25.Carlos CA, Dong HF, Howard OM, Oppenheim JJ, Hanisch FG, Finn OJ. Human tumor antigen MUC1 is chemotactic for immature dendritic cells and elicits maturation but does not promote Th1 type immunity. J Immunol. 2005;175:1628–35. doi: 10.4049/jimmunol.175.3.1628. [DOI] [PubMed] [Google Scholar]
- 26.Cascio S, Zhang L, Finn OJ. MUC1 expression in tumor cells regulates transcription of proinflammatory cytokines by forming a complex with NF-{kappa}B p65 and binding to cytokine promoters: importance of the extracellular domain. J Biol Chem 2011; 286:42248-56; 10.1074/jbc.M111.297630 [DOI] [PMC free article] [PubMed] [Google Scholar]