Abstract
The success of anticancer chemotherapy relies at least in part on the induction of an immune response against tumor cells. Thus, tumors growing on mice that lack the pattern recognition receptor TLR4 or the purinergic receptor P2RX7 fail to respond to chemotherapy with anthracyclins or oxaliplatin in conditions in which the same neoplasms growing on immunocompetent mice would do so. Similarly, the therapeutic efficacy (measured as progression-free survival) of adjuvant chemotherapy with anthracyclins is reduced in breast cancer patients bearing loss-of-function alleles of TLR4 or P2RX7. TLR4 loss-of-function alleles also have a negative impact on the therapeutic outcome of oxaliplatin in colorectal cancer patients. Here, we report that loss-of-function TLR4 and P2RX7 alleles do not affect overall survival in non-small cell lung cancer (NSCLC) patients, irrespective of the administration and type of chemotherapy. The intrinsic characteristics of NSCLC (which near-to-always is chemoresistant and associated with poor prognosis) and/or the type of therapy that is employed to treat this malignancy (which near-to-always is based on cisplatin) may explain why two genes that affect the immune response to dying cells fail to influence the clinical progression of NSCLC patients.
Keywords: IALT, calreticulin, immunogenic cell death, necrosis factor α, rs3751143, rs4986790, tumor
Introduction
There is ever expanding evidence suggesting that the escape from immune surveillance is a fundamental hallmark of cancer1,2 and that therapeutic interventions with cytotoxic compounds or targeted anticancer agents are successful only when they re-establish an immune control on cancer growth.3-5 Transplantable or primary murine cancers respond to chemotherapy with anthracyclins or oxaliplatin much more efficiently when they grow in syngenic immunocompetent mice than in immunodeficient hosts.6,7 In line with this finding, clinical studies revealed that severe lymphopenia negatively affects the response of solid tumors to chemotherapy.8 Moreover, while tumors growing on immunocompetent mice respond to chemotherapy with anthracyclins or oxaliplatin, tumors that grow on hosts lacking T cells, important cytokines [such as interleukin 1β (IL-1β), IL-17A and interferon γ] or their receptors continue to proliferate in an unperturbed fashion.7,9,10 Thus, at least in some settings, immune defects are negative predictors of the response to chemotherapy.
Successful chemotherapeutics can induce a type of tumor cell death that is immunogenic,11-13 implying that the patient’s dying cancer cells function as a therapeutic vaccine, thereby eliciting an antitumor immune response that controls or eliminates the residual (chemotherapy-resistant) disease.5,14 We have reported in the past that immunogenic cell death (ICD) is characterized by the pre-apoptotic exposure of calreticulin (CRT) on the cell surface,15-17 the active secretion of ATP during the blebbing phase of apoptosis,9,18,19 and the post-apoptotic release of the chromatin-binding non-histone protein high mobility group B1 (HMGB1).20 CRT, ATP and HMGB1 interact with CD91, purinergic P2RX7 receptors and Toll-like receptor 4 (TLR4), respectively, on the surface of dendritic cells, thus promoting the engulfment of dying cells or their debris,21,22 the production of IL-1β9,23 and cross-presentation of tumor antigens to T cells,7,24 respectively.
Mice lacking P2rx7 or Tlr4 phenocopy mice devoid of T cells (such as athymic nu/nu mice or mice injected with antibodies that deplete CD4+ and CD8+ T cells) in thus far that tumors growing on P2rx7−/− or Tlr4−/− mice do not respond to chemotherapy with anthracyclins or oxaliplatin in conditions in which the same neoplasms growing on normal, immunocompetent animals do so.7,9,10,20 Similarly, adjuvant chemotherapy exhibits a reduced efficacy in patients bearing loss-of-function alleles of P2RX7 or TLR4. This has been shown for patients with the single-nucleotide polymorphism (SNP) rs4986790 in TLR4 (1307A→G; Asp299Gly; NM_138554.3:c.896A > G) and rs3751143 in P2RX7 (1513A→C; Glu496Ala; NM_002562.4:c.1487A > C).7,9,10,20
The Asp299Gly substitution (corresponding to SNP rs4986790) impairs the affinity of TLR4 for lipopolysaccharide (LPS) and reportedly reduces the LPS-driven tumor necrosis factor α (TNFα) production by monocytes in vitro.25 At a clinical level, SNP rs4986790 has been associated with a reduced frequency of chronic obstructive pulmonary disease,26 but increased incidence of chronic sarcoidosis.27 Operable breast cancer patients with one single lymph node invasion (but no distant metastasis) that are homozygous or heterozygous for SNP rs4986790 exhibit accelerated relapse upon anthracyclin-based chemotherapy as compared with patients bearing the wild type genotype.7 Similarly, in a cohort of patients with colorectal cancer treated with oxaliplatin, homozygous or heterozygous carriers of SNP rs4986790 exhibited a more rapid relapse than age- and sex-matched patients bearing the wild type allele.10 The Glu496Ala substitution (corresponding to SNP rs3751143) limits the affinity of P2RX7 for ATP and renders patient-derived, Mycobacterium-infected macrophages resistant against ATP-induced cell death. This is most pronounced for macrophages derived from homozygous subjects,28 although some effect is also seen in the context of heterozygosity.29 Subjects that carry one or two copies of SNP rs3751143 exhibit an enhanced susceptibility to extrapulmonary tuberculosis29 and toxoplasmic retinochoroiditis,30 confirming the functional impact of this polymorphism at the clinical level. Moreover, among breast cancer patients bearing two copies of the wild type TLR4 allele, homozygous or heterozygous carriers of the P2RX7 rs3751143 exhibited a more rapid relapse than age- and sex-matched patients who carried the wild type allele only.9
Based on these premises, we decided to evaluate the impact of the aforementioned TLR4 and P2RX7 alleles on the survival of patients with non-small cell lung cancer (NSCLC).
Results and Discussion
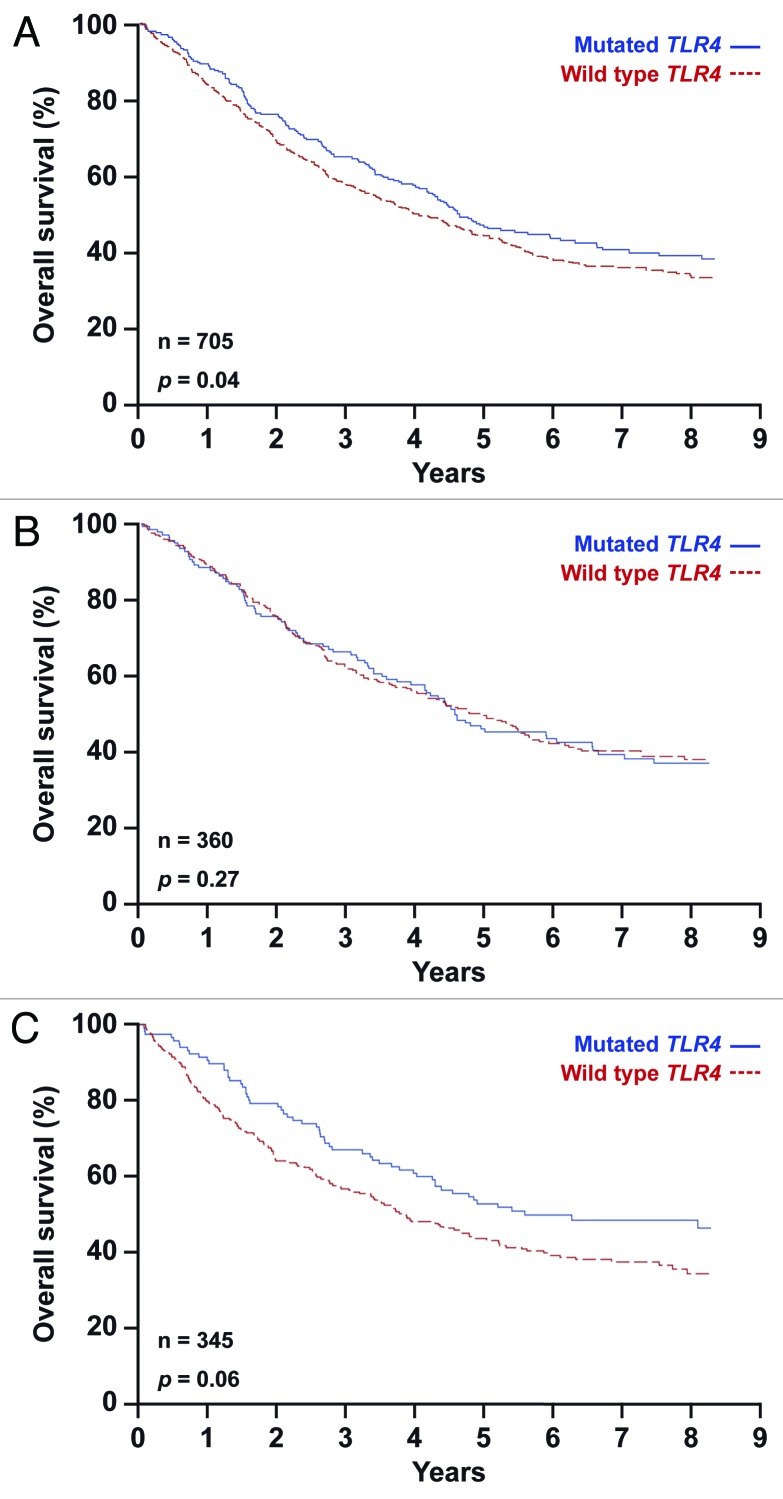
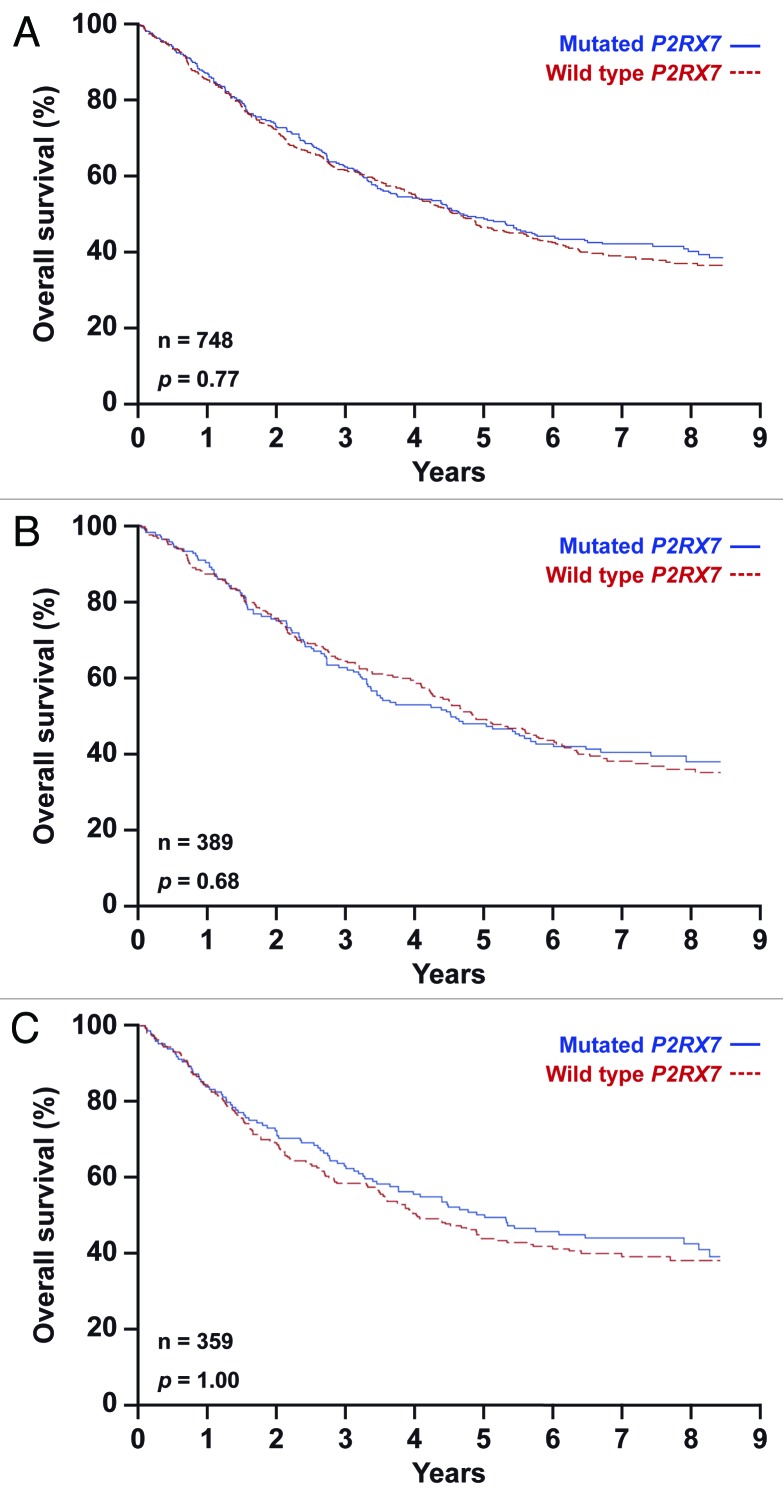
We took advantage of patient material from the phase III International Adjuvant Lung Cancer Trial (IALT), which compared cisplatin-based chemotherapy to no treatment in patients with resected stage-I-IIIA NSCLC, leading to the conclusion that adjuvant therapy can delay a substantial number of deaths,31 at least in a subset of patients.32,33 DNA from tumor specimens was extracted, yielding sufficient material to analyze the absence or presence (homo- or heterozygosity) of TLR4 rs4986790 and P2RX7 rs3751143 in 705 and 748 patients, respectively (Table 1). Subsequently, we compared patients that were homozygous for the wild type alleles of TLR4 (Fig. 1; Table 2) or P2RX7 (Fig. 2; Table 2) with those bearing one or two copies of the loss-of-function alleles, and then plotted the Kaplan-Meier survival curves for all patients included in the study (Figs. 1A and 2A), for patients that received chemotherapy (Figs. 1B and 2B) and for patients that did not (Figs. 1C and 2C). We found that neither SNP rs4986790 in TLR4 nor SNP rs3751143 in P2RX7 impact on the overall survival of NSCLC patients included in the IALT study. This held true when the entire patient population was analyzed (Figs. 1A and 2A), as well as upon the stratification of patients based on their allocation to chemotherapy (Fig. 1B and C; Fig. 2B and C). Stratification according to additional criteria at the level of the genotype (such as heterozygosity vs. homozygosity) or the treatment (cisplatin plus etoposide vs. cisplatin plus microtubular inhibitors) did not reveal any consistent impact of the analyzed TLR4 or P2RX7 polymorphisms on patient survival (not shown). Along similar lines, a combined analysis in which patients were classified into groups bearing one or several loss-of-function alleles failed to uncover any predictive or prognostic impact of TLR4 SNP rs4986790 and P2RX7 SNP rs3751143 (Fig. 3, Table 2). Intriguingly both TLR4 and P2RX7 mutational statuses were found to be significantly associated with age, and that of P2RX7 also with World Health Organization (WHO) performance status and lymphoid infiltration (Table 1).
Table 1. Statistical analysis of the IALT cohort.
| |
TLR4 |
P2RX7 |
||||||
|---|---|---|---|---|---|---|---|---|
| Variable |
Wild type (n = 460) |
Mutated (n = 245) |
Total (n = 705) |
Univariate p (trend test p) |
Wild type (n = 445) |
Mutated (n = 303) |
Total (n = 748) |
Univariate p (trend test p) |
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | |||
| Sex |
|
|
|
0.48 |
|
|
|
0.97 |
| Female |
88 (19.1) |
43 (17.6) |
131 (18.6) |
|
80 (18.0) |
58 (19.1) |
138 (18.4) |
|
| Male |
372 (80.9) |
202 (82.4) |
574 (81.4) |
|
365 (82.0) |
245 (80.9) |
610 (81.6) |
|
| Age (years) |
|
|
|
< 0.001* |
|
|
|
0.07 |
| < 55 |
150 (32.6) |
69 (28.2) |
219 (31.1) |
|
122 (27.4) |
103 (34.0) |
225 (30.1) |
(0.02) |
| 55–64 |
218 (47.4) |
88 (35.9) |
306 (43.4) |
|
194 (43.6) |
132 (43.6) |
326 (43.6) |
|
| > 64 |
92 (20.0) |
88 (35.9) |
180 (25.5) |
|
129 (29.0) |
68 (22.4) |
197 (26.3) |
|
| Stage |
|
|
|
0.93 |
|
|
|
0.43 |
| I |
151 (32.8) |
89 (36.3) |
240 (34.0) |
(0.71) |
145 (32.6) |
110 (36.3) |
255 (34.1) |
(0.55) |
| II |
110 (23.9) |
56 (22.9) |
166 (23.5) |
|
102 (22.9) |
74 (24.4) |
176 (23.5) |
|
| III |
199 (43.3) |
100 (40.8) |
299 (42.4) |
|
198 (44.5) |
119 (39.3) |
317 (42.4) |
|
| N of TNM |
|
|
|
0.61 |
|
|
|
0.20 |
| 0 |
202 (43.9) |
122 (49.8) |
324 (46.0) |
(0.34) |
202 (45.4) |
142 (46.9) |
344 (46.0) |
(0.83) |
| 1 |
136 (29.6) |
70 (28.6) |
206 (29.2) |
|
124 (27.9) |
94 (31.0) |
218 (29.1) |
|
| 2 |
122 (26.5) |
53 (21.6) |
175 (24.8) |
|
119 (26.7) |
67 (22.1) |
186 (24.9) |
|
| T of TNM |
|
|
|
0.83 |
|
|
|
1.00 |
| 1 |
69 (15.0) |
37 (15.1) |
106 (15.0) |
(0.68) |
68 (15.3) |
48 (15.8) |
116 (15.5) |
(0.93) |
| 2 |
278 (60.4) |
142 (58.0) |
420 (59.6) |
|
266 (59.8) |
178 (58.7) |
444 (59.4) |
|
| 3 |
113 (24.6) |
66 (26.9) |
179 (25.4) |
|
111 (24.9) |
77 (25.4) |
188 (25.1) |
|
| Histology |
|
|
|
0.70 |
|
|
|
0.77 |
| Adenocarcinoma |
157 (34.1) |
75 (30.6) |
232 (32.9) |
|
141 (31.7) |
98 (32.3) |
239 (32.0) |
|
| Other NSCLC |
55 (12.0) |
32 (13.1) |
87 (12.3) |
|
59 (13.3) |
35 (11.6) |
94 (12.6) |
|
| Squamous cell carcinoma |
248 (53.9) |
138 (56.3) |
386 (54.8) |
|
245 (55.1) |
170 (56.1) |
415 (55.5) |
|
| Surgery |
|
|
|
0.49 |
|
|
|
0.29 |
| Lobe- or segmentectomy |
278 (60.4) |
144 (58.8) |
422 (59.9) |
|
270 (60.7) |
178 (58.7) |
448 (59.9) |
|
| Pneumonectomy |
182 (39.6) |
101 (41.2) |
283 (40.1) |
|
175 (39.3) |
125 (41.3) |
300 (40.1) |
|
| WHO PS** |
|
|
|
0.54 |
|
|
|
0.01 |
| 0 |
259 (56.3) |
132 (53.9) |
391 (55.5) |
(0.33) |
220 (49.4) |
19 (63.7) |
413 (55.2) |
(< 0.001) |
| 1 |
163 (35.4) |
93 (38.0) |
256 (36.3) |
|
182 (40.9) |
94 (31.0) |
276 (36.9) |
|
| 2 |
38 (8.3) |
20 (8.2) |
58 (8.2) |
|
43 (9.7) |
16 (5.3) |
59 (7.9) |
|
| Lymphoid infiltration |
|
|
|
0.55 |
|
|
|
0.04 |
| Intense |
52 (11.3) |
25 (10.2) |
77 (10.9) |
|
40 (9.0) |
43 (14.2) |
83 (11.1) |
|
| Weak |
408 (88.7) |
220 (89.8) |
628 (89.1) |
|
405 (91.0) |
260 (85.8) |
665 (88.9) |
|
| Pleural invasion |
|
|
|
0.80 |
|
|
|
0.61 |
| No |
422 (91.7) |
223 (91.0) |
645 (91.5) |
|
406 (91.2) |
279 (92.1) |
685 (91.6) |
|
| Yes |
38 (8.3) |
22 (9.0) |
60 (8.5) |
|
39 (8.8) |
24 (7.9) |
63 (8.4) |
|
| Vascular invasion |
|
|
|
0.73 |
|
|
|
0.74 |
| No |
318 (69.1) |
172 (70.2) |
490 (69.5) |
|
313 (70.3) |
215 (71.0) |
528 (70.6) |
|
| Yes |
142 (30.9) |
73 (29.8) |
215 (30.5) |
|
132 (29.7) |
88 (29.0) |
220 (29.4) |
|
| Lymphatic invasion |
|
|
|
0.39 |
|
|
|
0.42 |
| No |
139 (30.2) |
81 (33.1) |
220 (31.2) |
|
133 (29.9) |
99 (32.7) |
232 (31.0) |
|
| Yes |
321 (69.8) |
164 (66.9) |
485 (68.8) |
|
312 (70.1) |
204 (67.3) |
516 (69.0) |
|
| Quality after final H&E |
|
|
|
0.30 |
|
|
|
0.98 |
| Average |
46 (10.0) |
32 (13.1) |
78 (11.1) |
|
47 (10.6) |
34 (11.2) |
81 (10.8) |
|
| Good | 414 (90.0) | 213 (86.9) | 627 (88.9) | 398 (89.4) | 269 (88.8) | 667 (89.2) | ||
Significant p values are indicated in italic. p values were calculated by two-sided χ2 tests, using logistic regressions stratified on center.
World Health Organization (WHO) scores for performance status (PS) range from 0 to 2, with score of 0 indicating no symptoms, 1 mild symptoms and 2 moderate symptoms.
Abbreviations: H&E, hematoxylin and eosin; NSCLC, non small cell lung cancer; TNM, tumor node metastasis.
Figure 1. Kaplan-Meier estimates of overall survival in non-small cell lung carcinoma (NSCLC) patients bearing one or two copies of the TLR4 rs4986790 SNP (mutated TLR4) or the wild type allele only (wild type TLR4). Overall survival according to TLR4 status in all 705 patients (A), in patients subjected to chemotherapy (B) and in untreated patients (C).
Table 2. Overall survival according to treatment and mutational status.
| Group | All patients | Treated patients | Untreated patients |
|---|---|---|---|
|
Population with wild type TLR4 |
|
|
|
| Deaths/total n° of patients |
298/460 |
149/230 |
149/230 |
| Median OS - months |
4.1 |
4.4 |
3.8 |
|
Population with mutated TLR4 |
|
|
|
| Deaths/total n° of patients |
144/245 |
85/130 |
59/115 |
| Median OS - months |
4.6 |
4.5 |
5.6 |
| HR* for death (95% CI) |
0.81 (0.66–0.99) |
0.85 (0.64–1.13) |
0.73 (053–1.01) |
|
p value |
0.04 |
0.27 |
0.06 |
|
Population with wild type P2RX7 |
|
|
|
| Deaths/total n° of patients |
281/445 |
150/232 |
131/213 |
| Median OS - months |
4.4 |
4.6 |
3.9 |
|
Population with mutated P2RX7 |
|
|
|
| Deaths/total n° of patients |
187/303 |
99/157 |
88/146 |
| Median OS - months |
4.5 |
4.3 |
4.7 |
| HR* for death (95% CI) |
1.03 (0.85–1.24) |
1.06 (0.81–1.38) |
1.00 (0.76–1.32) |
|
p value |
0.77 |
0.68 |
1.00 |
|
Population with wild type TLR4 and P2RX7 |
|
|
|
| Deaths/total n° of patients |
162/241 |
83/124 |
79/117 |
| Median OS - months |
4.0 |
4.3 |
3.5 |
|
Population with at least one TLR4 or P2RX7 mutation |
|
|
|
| Deaths/total n° of patients |
263/436 |
145/228 |
118/208 |
| Median OS - months |
4.6 |
4.5 |
5.2 |
| HR* for death (95% CI) |
0.84 (0.68–1.03) |
0.86 (0.65–1.15) |
0.80 (0.59–1.08) |
| p value | 0.09 | 0.32 | 0.14 |
Hazard ratios (HR) reflect the comparison between the mutated and the wild type groups. All hazard ratios were adjusted for sex, age, tumor stage, histology type and the presence or absence of pleural invasion, and were stratified according to clinical center.
Abbreviations: CI, confidence interval; OS, overall survival.
Figure 2. Kaplan-Meier estimates of overall survival in non-small cell lung carcinoma (NSCLC) patients bearing one or two copies of the P2RX7 rs3751143 SNP (mutated P2RX7) or the wild type allele only (wild type P2RX7). Overall survival according to P2RX7 status in all 748 patients (A), in patients subjected to chemotherapy (B) and in untreated patients (C).
Figure 3. Kaplan-Meier estimates of overall survival in non-small cell lung carcinoma (NSCLC) patients bearing the wild type alleles for both TLR4 and P2RX7 (wild type TLR4 and P2RX7) or at least one variant for either TLR4 or P2RX7. Overall survival according to TLR4 and P2RX7 status in all 677 patients (A), in patients subjected to chemotherapy (B) and in untreated patients (C).
Altogether, the data presented here indicate that loss-of-function alleles in TLR4 and P2RX7 do not affect overall survival in NSCLC patients, irrespective of the administration and type of chemotherapy.
What may be the reasons for this finding, which clearly differs from our previous observations on anthracyclin-based adjuvant chemotherapy in breast cancer and oxaliplatin-based adjuvant chemotherapy in colorectal cancer?5,7,9,10 There are at least two possible explanations for this discrepancy. First, the prognosis of NSCLC is intrinsically dismal,34 and bronchial carcinomas may be subjected to a less vigorous immunosurveillance than tumors located in other organs such as the mammary gland or the colic mucosa.35 NSCLC may also be particularly efficient in suppressing the function of local innate immune effectors such as natural killer (NK) cells,36 or in escaping CD8+ T cell-mediated adaptive immunity (for instance due to impaired expression and/or function of FAS).37 Irrespective of this, which remains a mere matter of speculation, the literature reporting a clinical benefit from the infiltration of NSCLC by effector T cells is relatively scarce as compared with the plethora of reports demonstrating that mammary and colorectal cancers are controlled by a clinically relevant level of immunosurveillance.5,38,39 Of note, it appears that the infiltration of the tumor mass by dendritic cells has a more positive impact on NSCLC prognosis than that by effector T cells.40 These latter findings, together with recent results from the largest multicentric NSCLC gene profiling study to date, the NIH Director’s Challenge Study,41 support further investigations on the role of the immune system in NSCLC and on its relevance during the response to chemotherapy. Second, the standard treatment for NSCLC is based on cisplatin, a DNA damaging agent that, besides being associated with a high rate of relapse due to the development of chemoresistance,42 induces non-immunogenic cell death.10 This means that cells succumbing to cisplatin in vitro fail to elicit specific anticancer immune responses when inoculated in vivo.43,44 Accordingly, the response to cisplatin of experimental cancers in vivo is not influenced by the absence or presence of TLR4.10 Of note, cisplatin is usually combined with other chemotherapeutic agents that are also unable to induce ICD, such as etoposide,15,45 or with microtubular inhibitors, whose capacity to induce ICD has not yet been thoroughly evaluated. Importantly, our findings do not rule out a role for TLR4 and P2XR7 in the pathogenesis and response to therapy of NSCLC, as multiple epigenetic mechanisms, including promoter hypermethylation, alternative splicing and microRNA-mediated gene regulation, might be responsible for the functional inhibition of these two proteins. Future studies investigating the how the innate and cognate immune systems influence the clinical progression of NSCLC will surely provide deeper insights into the results of our retrospective analysis.
Materials and Methods
Patients and study design
One-thousand-eight-hundred-sixty-seven patients were enrolled in the IALT study, upon informed consent. The clinical features of the patient cohort can be found elsewhere.31 Paraffin-embedded tumor blocks (obtained at the time of surgery) were collected in 14 distinct countries by 28 medical centers that participated into the IALT study with more than ten patients.32 Approval was obtained by local institutional review boards according to the national regulations. A total of 867 samples were reviewed at the Centre Hospitalier Universitaire Albert Michallon (Grenoble, France) and histopathologically classified according to the system adopted by the World Health Organization (WHO) in 2004. The amount and quality of 822 among the 867 paraffin blocks were judged adequate for experimental procedures. Finally, 776 samples were identified as non-small cell lung cancer (NSCLC), but only 705 and 748 were available for TLR4 and P2RX7 genotyping, respectively.
Genotyping
Genomic DNA was isolated from paraffin-embedded tumors by means of the DNeasy Blood and Tissue Kit (Qiagen). Gene-specific Taqman® primers and genotype-specific probes (Applied Biosystems) were used to amplify a TLR4 fragment containing the Asp299Gly single nucleotide polymorphism (rs4986790) site and a P2RX7 fragment containing the Glu496Ala polymorphism (rs3751143). Genotypes were determined by comparing the signals from fluorescent probes (FAM and VIC) and by calculating the natural logarithm of the ratio between the FAM and VIC signals [log (FAM/VIC)].
Statistical analysis
All statistical analyses were performed with long-term survival data,46 by means of the SAS software, version 9.2 (SAS Institute). Conditional logistic regression on an aggregate center variable was used for both univariate and multivariate analyses. Survival rates were estimated using the Kaplan-Meier method. The prognostic values of the TLR4 and P2RX7 status and chemotherapy were studied using a Cox model taking into account every factor used in the stratified random assignment (center, tumor stage, type of surgery) plus clinical and histological prognostic factors (age, sex, WHO performance status, nodal status, lymphoid infiltration and revised histopathological type), as in previous IALT-based studies,32 as well as all factors that were statistically related to the biomarker status in the multivariate logistic model (p < 0.05). All reported p values were two sided and only p values < 0.001 were considered statistically significant, to minimize the risk of false-positive results.
Acknowledgments
A.D. is supported by an unrestricted research grant from Eli Lilly, and by grants from Programme Hospitalier de Recherche Clinique 2005 and Cance´ropôle Rhône-Alpes. G.K. is financed by the Ligue Nationale contre le Cancer (Equipes labelise´e), Agence Nationale pour la Recherche (ANR), Fondation Axa (Chair For Longevity Research), Fondation Bettencourt-Schueller, European Commission (Apo-Sys, ArtForce, ChemoRes), Fondation pour la Recherche Me´dicale (FRM), Institut National du Cancer (INCa), Cance´ropôle Ile-de-France and the Labex Immuno-Oncology.
Glossary
Abbreviations:
- CRT
calreticulin
- HMGB1
high mobility group B1
- IALT
International Adjuvant Lung Cancer Trial
- ICD
immunogenic cell death
- IL
interleukin
- LPS
lipopolysaccharide
- NSCLC
non-small cell lung cancer
- P2RX7
purinergic receptor P2X, ligand-gated ion channel 7
- SNP
single nucleotide polymorphism
- TLR4
Toll-like receptor 4
- TNFα
tumor necrosis factor α
- WHO
World Health Organization
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/18684
References
- 1.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science. 2011;331:1565–70. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 3.Delahaye NF, Rusakiewicz S, Martins I, Menard C, Roux S, Lyonnet L, et al. Alternatively spliced NKp30 isoforms affect the prognosis of gastrointestinal stromal tumors. Nat Med. 2011;17:700–7. doi: 10.1038/nm.2366. [DOI] [PubMed] [Google Scholar]
- 4.Balachandran VP, Cavnar MJ, Zeng S, Bamboat ZM, Ocuin LM, Obaid H, et al. Imatinib potentiates antitumor T cell responses in gastrointestinal stromal tumor through the inhibition of Ido. Nat Med. 2011;17:1094–100. doi: 10.1038/nm.2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zitvogel L, Kepp O, Kroemer G. Immune parameters affecting the efficacy of chemotherapeutic regimens. Nat Rev Clin Oncol. 2011;8:151–60. doi: 10.1038/nrclinonc.2010.223. [DOI] [PubMed] [Google Scholar]
- 6.Casares N, Pequignot MO, Tesniere A, Ghiringhelli F, Roux S, Chaput N, et al. Caspase-dependent immunogenicity of doxorubicin-induced tumor cell death. J Exp Med. 2005;202:1691–701. doi: 10.1084/jem.20050915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13:1050–9. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- 8.Ray-Coquard I, Cropet C, Van Glabbeke M, Sebban C, Le Cesne A, Judson I, et al. Lymphopenia as a prognostic factor for overall survival in advanced carcinomas, sarcomas, and lymphomas. Cancer Res. 2009;69:5383–91. doi: 10.1158/0008-5472.CAN-08-3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghiringhelli F, Apetoh L, Tesniere A, Aymeric L, Ma Y, Ortiz C, et al. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1beta-dependent adaptive immunity against tumors. Nat Med. 2009;15:1170–8. doi: 10.1038/nm.2028. [DOI] [PubMed] [Google Scholar]
- 10.Tesniere A, Schlemmer F, Boige V, Kepp O, Martins I, Ghiringhelli F, et al. Immunogenic death of colon cancer cells treated with oxaliplatin. Oncogene. 2010;29:482–91. doi: 10.1038/onc.2009.356. [DOI] [PubMed] [Google Scholar]
- 11.Green DR, Ferguson T, Zitvogel L, Kroemer G. Immunogenic and tolerogenic cell death. Nat Rev Immunol. 2009;9:353–63. doi: 10.1038/nri2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zappasodi R, Pupa SM, Ghedini GC, Bongarzone I, Magni M, Cabras AD, et al. Improved clinical outcome in indolent B-cell lymphoma patients vaccinated with autologous tumor cells experiencing immunogenic death. Cancer Res. 2010;70:9062–72. doi: 10.1158/0008-5472.CAN-10-1825. [DOI] [PubMed] [Google Scholar]
- 13.Ferguson TA, Choi J, Green DR. Armed response: how dying cells influence T-cell functions. Immunol Rev. 2011;241:77–88. doi: 10.1111/j.1600-065X.2011.01006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Finn OJ. Cancer immunology. N Engl J Med. 2008;358:2704–15. doi: 10.1056/NEJMra072739. [DOI] [PubMed] [Google Scholar]
- 15.Obeid M, Tesniere A, Ghiringhelli F, Fimia GM, Apetoh L, Perfettini JL, et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med. 2007;13:54–61. doi: 10.1038/nm1523. [DOI] [PubMed] [Google Scholar]
- 16.Panaretakis T, Joza N, Modjtahedi N, Tesniere A, Vitale I, Durchschlag M, et al. The co-translocation of ERp57 and calreticulin determines the immunogenicity of cell death. Cell Death Differ. 2008;15:1499–509. doi: 10.1038/cdd.2008.67. [DOI] [PubMed] [Google Scholar]
- 17.Panaretakis T, Kepp O, Brockmeier U, Tesniere A, Bjorklund AC, Chapman DC, et al. Mechanisms of pre-apoptotic calreticulin exposure in immunogenic cell death. EMBO J. 2009;28:578–90. doi: 10.1038/emboj.2009.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martins I, Tesniere A, Kepp O, Michaud M, Schlemmer F, Senovilla L, et al. Chemotherapy induces ATP release from tumor cells. Cell Cycle. 2009;8:3723–8. doi: 10.4161/cc.8.22.10026. [DOI] [PubMed] [Google Scholar]
- 19.Michaud M, Martins I, Sukkurwala AQ, Adjemian S, Ma Y, Pellegatti P, et al. Autophagy dictates anticancer immune responses induced by chemotherapeutic agents in mice. Science. 2011;334:1573–7. doi: 10.1126/science.1208347. [DOI] [PubMed] [Google Scholar]
- 20.Apetoh L, Ghiringhelli F, Tesniere A, Criollo A, Ortiz C, Lidereau R, et al. The interaction between HMGB1 and TLR4 dictates the outcome of anticancer chemotherapy and radiotherapy. Immunol Rev. 2007;220:47–59. doi: 10.1111/j.1600-065X.2007.00573.x. [DOI] [PubMed] [Google Scholar]
- 21.Gardai SJ, McPhillips KA, Frasch SC, Janssen WJ, Starefeldt A, Murphy-Ullrich JE, et al. Cell-surface calreticulin initiates clearance of viable or apoptotic cells through trans-activation of LRP on the phagocyte. Cell. 2005;123:321–34. doi: 10.1016/j.cell.2005.08.032. [DOI] [PubMed] [Google Scholar]
- 22.Chao MP, Jaiswal S, Weissman-Tsukamoto R, Alizadeh AA, Gentles AJ, Volkmer J, et al. Calreticulin is the dominant pro-phagocytic signal on multiple human cancers and is counterbalanced by CD47. Sci Transl Med. 2010;2:63ra94. doi: 10.1126/scitranslmed.3001375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mutini C, Falzoni S, Ferrari D, Chiozzi P, Morelli A, Baricordi OR, et al. Mouse dendritic cells express the P2X7 purinergic receptor: characterization and possible participation in antigen presentation. J Immunol. 1999;163:1958–65. [PubMed] [Google Scholar]
- 24.Bianchi ME. HMGB1 loves company. J Leukoc Biol. 2009;86:573–6. doi: 10.1189/jlb.1008585. [DOI] [PubMed] [Google Scholar]
- 25.Arbour NC, Lorenz E, Schutte BC, Zabner J, Kline JN, Jones M, et al. TLR4 mutations are associated with endotoxin hyporesponsiveness in humans. Nat Genet. 2000;25:187–91. doi: 10.1038/76048. [DOI] [PubMed] [Google Scholar]
- 26.Rohde G, Klein W, Arinir U, Hagedorn M, Duerig N, Bauer TT, et al. Association of the ASP299GLY TLR4 polymorphism with COPD. Respir Med. 2006;100:892–6. doi: 10.1016/j.rmed.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 27.Pabst S, Baumgarten G, Stremmel A, Lennarz M, Knufermann P, Gillissen A, et al. Toll-like receptor (TLR) 4 polymorphisms are associated with a chronic course of sarcoidosis. Clin Exp Immunol. 2006;143:420–6. doi: 10.1111/j.1365-2249.2006.03008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saunders BM, Fernando SL, Sluyter R, Britton WJ, Wiley JS. A loss-of-function polymorphism in the human P2X7 receptor abolishes ATP-mediated killing of mycobacteria. J Immunol. 2003;171:5442–6. doi: 10.4049/jimmunol.171.10.5442. [DOI] [PubMed] [Google Scholar]
- 29.Fernando SL, Saunders BM, Sluyter R, Skarratt KK, Goldberg H, Marks GB, et al. A polymorphism in the P2X7 gene increases susceptibility to extrapulmonary tuberculosis. Am J Respir Crit Care Med. 2007;175:360–6. doi: 10.1164/rccm.200607-970OC. [DOI] [PubMed] [Google Scholar]
- 30.Jamieson SE, Peixoto-Rangel AL, Hargrave AC, Roubaix LA, Mui EJ, Boulter NR, et al. Evidence for associations between the purinergic receptor P2X(7) (P2RX7) and toxoplasmosis. Genes Immun. 2010;11:374–83. doi: 10.1038/gene.2010.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arriagada R, Bergman B, Dunant A, Le Chevalier T, Pignon JP, Vansteenkiste J. Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N Engl J Med. 2004;350:351–60. doi: 10.1056/NEJMoa031644. [DOI] [PubMed] [Google Scholar]
- 32.Olaussen KA, Dunant A, Fouret P, Brambilla E, Andre F, Haddad V, et al. DNA repair by ERCC1 in non-small-cell lung cancer and cisplatin-based adjuvant chemotherapy. N Engl J Med. 2006;355:983–91. doi: 10.1056/NEJMoa060570. [DOI] [PubMed] [Google Scholar]
- 33.Olaussen KA, Fouret P, Kroemer G. ERCC1-specific immunostaining in non-small-cell lung cancer. N Engl J Med. 2007;357:1559–61. doi: 10.1056/NEJMc072007. [DOI] [PubMed] [Google Scholar]
- 34.Herbst RS, Heymach JV, Lippman SM. Lung cancer. N Engl J Med. 2008;359:1367–80. doi: 10.1056/NEJMra0802714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kroemer G, Cuende E, Martinez C. Compartmentalization of the peripheral immune system. Adv Immunol. 1993;53:157–216. doi: 10.1016/S0065-2776(08)60500-3. [DOI] [PubMed] [Google Scholar]
- 36.Platonova S, Cherfils-Vicini J, Damotte D, Crozet L, Vieillard V, Validire P, et al. Profound coordinated alterations of intratumoral NK cell phenotype and function in lung carcinoma. Cancer Res. 2011;71:5412–22. doi: 10.1158/0008-5472.CAN-10-4179. [DOI] [PubMed] [Google Scholar]
- 37.Viard-Leveugle I, Veyrenc S, French LE, Brambilla C, Brambilla E. Frequent loss of Fas expression and function in human lung tumours with overexpression of FasL in small cell lung carcinoma. J Pathol. 2003;201:268–77. doi: 10.1002/path.1428. [DOI] [PubMed] [Google Scholar]
- 38.Fridman WH, Galon J, Pages F, Tartour E, Sautes-Fridman C, Kroemer G. Prognostic and predictive impact of intra- and peritumoral immune infiltrates. Cancer Res. 2011;71:5601–5. doi: 10.1158/0008-5472.CAN-11-1316. [DOI] [PubMed] [Google Scholar]
- 39.Zitvogel L, Kepp O, Aymeric L, Ma Y, Locher C, Delahaye NF, et al. Integration of host-related signatures with cancer cell-derived predictors for the optimal management of anticancer chemotherapy. Cancer Res. 2010;70:9538–43. doi: 10.1158/0008-5472.CAN-10-1003. [DOI] [PubMed] [Google Scholar]
- 40.Dieu-Nosjean MC, Antoine M, Danel C, Heudes D, Wislez M, Poulot V, et al. Long-term survival for patients with non-small-cell lung cancer with intratumoral lymphoid structures. J Clin Oncol. 2008;26:4410–7. doi: 10.1200/JCO.2007.15.0284. [DOI] [PubMed] [Google Scholar]
- 41.Suzuki K, Kachala SS, Kadota K, Shen R, Mo Q, Beer DG, et al. Prognostic immune markers in non-small cell lung cancer. Clin Cancer Res. 2011;17:5247–56. doi: 10.1158/1078-0432.CCR-10-2805. [DOI] [PubMed] [Google Scholar]
- 42.Galluzzi L, Senovilla L, Vitale I, Michels J, Martins I, Kepp O, et al. Molecular mechanisms of cisplatin resistance. Oncogene. 2011;Epub ahead of press doi: 10.1038/onc.2011.384. [DOI] [PubMed] [Google Scholar]
- 43.Tesniere A, Apetoh L, Ghiringhelli F, Joza N, Panaretakis T, Kepp O, et al. Immunogenic cancer cell death: a key-lock paradigm. Curr Opin Immunol. 2008;20:504–11. doi: 10.1016/j.coi.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 44.Martins I, Kepp O, Schlemmer F, Adjemian S, Tailler M, Shen S, et al. Restoration of the immunogenicity of cisplatin-induced cancer cell death by endoplasmic reticulum stress. Oncogene. 2011;30:1147–58. doi: 10.1038/onc.2010.500. [DOI] [PubMed] [Google Scholar]
- 45.Obeid M, Tesniere A, Panaretakis T, Tufi R, Joza N, van Endert P, et al. Ecto-calreticulin in immunogenic chemotherapy. Immunol Rev. 2007;220:22–34. doi: 10.1111/j.1600-065X.2007.00567.x. [DOI] [PubMed] [Google Scholar]
- 46.Arriagada R, Dunant A, Pignon JP, Bergman B, Chabowski M, Grunenwald D, et al. Long-term results of the international adjuvant lung cancer trial evaluating adjuvant Cisplatin-based chemotherapy in resected lung cancer. J Clin Oncol. 2010;28:35–42. doi: 10.1200/JCO.2009.23.2272. [DOI] [PubMed] [Google Scholar]