Abstract
Here we discuss a recently published study from our group on how a continuous CD4 T-cell dependent immune clearance of premalignant senescent cells, designated “senescence surveillance,” restricts liver cancer development.
Keywords: cancer, carcinoma, hepatocellular, immune, premalignant, senescence, surveillance
Upon the aberrant activation of oncogenes cells can either undergo apoptosis or cellular senescence, a state of stable cell cycle arrest.1 Like apoptosis, cellular senescence was shown to also represent an important failsafe mechanism against tumor development in vivo (reviewed in ref. 2). While cellular senescence so far has been regarded a solely cell intrinsic mechanism of tumor suppression, i.e., suppressing tumor development through the induction of a stable cell cycle arrest, recent data from our laboratory suggest that the cellular senescence program also plays a crucial role in the induction of immune responses against pre-cancerous cells.3 To mimic aberrant oncogene activation, which can occur in hepatocytes of chronically damaged human livers, we stably delivered oncogenic NrasG12V into mouse livers in vivo. NrasG12V expression in hepatocytes was accompanied by the induction of a cellular senescence response. Senescent NrasG12V expressing hepatocytes were found to express a myriad of cytokines and chemokines, which led to the attraction of innate as well as adaptive immune cells. These different immune cell populations were found in close proximity to senescent, NrasG12V expressing hepatocytes. In accordance with the hypothesis that attracted immune cells could mediate the clearance of senescent cells, time course analyses revealed a progressive loss of NrasG12V expressing senescent cells within two months after stable intrahepatic delivery of oncogenic NrasG12V. Importantly, all analyses were paralleled using a NrasG12V effector loop mutant (NrasG12V/D38A) incapable of signaling to downstream pathways and therefore for senescence induction. Importantly, NrasG12V/D38A expressing hepatocytes could be detected long-term after intrahepatic delivery, thus ruling out immune responses against overexpressed Nras peptide per se. To address the significance of the observed immune cell populations for the clearance of senescent cells, we took a genetic approach: NrasG12V expressing transposable elements were intrahepatically delivered into mice harboring genetically defined immune defects and the number of NrasG12V expressing senescent cells was followed over time. We found that the immune clearance of pre-cancerous NrasG12V expressing hepatocytes was abrogated in severe combined immunodeficient (SCID) mice and also in CD4 knockout mice. Strikingly, the missing immune clearance of NrasG12V expressing pre-cancerous cells in these mice resulted in the development of hepatocellular carcinomas at later time points. Enzyme-linked immunospot (ELISPOT) assays revealed the presence of mutant Nras-specific CD4 T-cells in mice harboring senescent NrasG12V expressing hepatocytes but not in NrasG12V-transduced p19Arf knockout mice, in which induction of the cellular senescence program is genetically disabled. Collectively, our data showed that oncogene induced senescence (OIS) plays an important role in the induction of specific immune responses against antigens expressed in pre-cancerous cells. Further mechanistic studies characterized the observed CD4 T-cell response as a Th1 type response and we found that CD4 T-cells depended on monocytes and freshly replenished macrophages to efficiently kill senescent hepatocytes (Fig. 1). Interestingly, liver resident macrophages (Kupffer cells) were found not to be involved in the killing of NrasG12V expressing senescent hepatocytes.
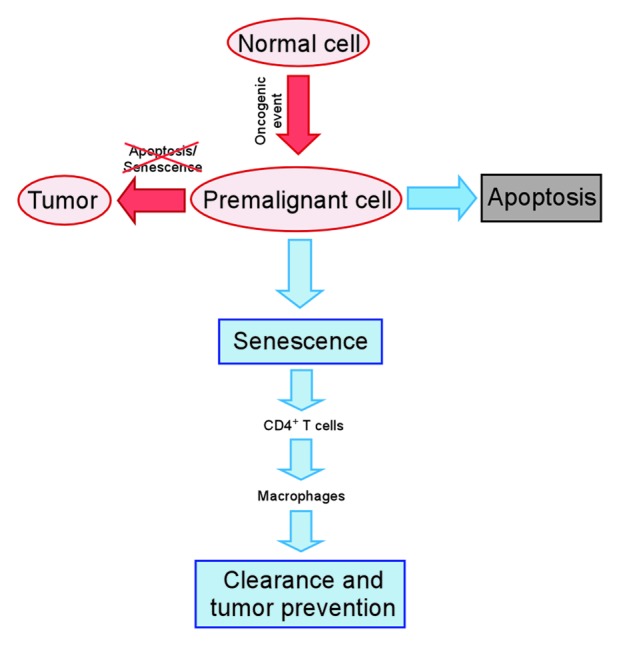
Figure 1. Clearance of pre-malignant senescent cells through a CD4 T-cell mediated immune response. Upon the aberrant activation of oncogenes cells can either undergo apoptosis or cellular senescence. Pre-malignant senescent hepatocytes are subject to a continuous CD4 T-cell mediated immune clearance which is crucial for liver cancer suppression. CD4 T-cells depend on monocytes/macrophages to execute the killing of pre-malignant senescent hepatocytes.
Taken together, our study shows that a continuous, CD4 T-cell mediated immune clearance of pre-malignant senescent cells is crucial to suppress liver cancer development. Conversely, an impaired immune clearance of premalignant senescent hepatocytes was shown to result in the development of liver carcinomas. Our study suggests that a small subfraction of senescent hepatocytes can escape their cell cycle arrest if there is no immune clearance of these cells. However, a direct demonstration of this senescence escape remains subject for future studies.
Finally we also addressed whether there is evidence for immune surveillance of senescent hepatocytes in humans. For this purpose, we quantified the numbers of senescent hepatocytes in patients under immunosuppressive therapy (due to organ transplantation) or in patients with HIV infection, both conditions that impact CD4 T-cell function. Increased numbers of senescent hepatocytes were found in both cohorts, thus supporting that immune surveillance of senescent cells also occurs in humans.
There has been a long scientific debate regarding the significance of a T-cell dependent immune surveillance mechanism of pre-cancerous cells (reviewed in ref. 4). For example, an elegant recent study showed that spontaneous intrahepatic activation of the Simian virus 40 (SV40) large T antigen did not result in efficient immune responses against these hepatocytes5 but rather resulted in the growth of liver carcinomas and tumor-specific T cells were found to produce TGFβ rather than IFNγ. Our data may help to resolve some of the controversy, as it suggests that the induction of a specific immune response against pre-cancerous cells is dependent on an intact cellular senescence response. Therefore, immune surveillance against pre-cancerous cells may occur if the senescence program is engaged after the occurrence of an oncogenic event. However, if the induction of the cellular senescence program is affected, e.g., due to impaired p53 function or reduced levels of p14Arf (p19Arf in mice), a specific immune response against such tumor prone cells may not occur. The fact that SV40 is known to disable senescence induction may explain the lack of immune surveillance of SV40 expressing hepatocytes in the study by Blankenstein and colleagues.5
Future studies are needed to characterize whether immune surveillance of pre-cancerous senescent cells also occurs in other organs. Also, a deeper characterization of the underlying mechanisms of senescence surveillance, especially the individual contribution of involved cyto- and chemokines secreted from senescent cells is needed. Such studies may pave the way to eventually harness this process for cancer prevention and therapy.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/19128
References
- 1.Lowe SW, Cepero E, Evan G. Intrinsic tumour suppression. Nature. 2004;432:307–15. doi: 10.1038/nature03098. [DOI] [PubMed] [Google Scholar]
- 2.Narita M, Lowe SW. Senescence comes of age. Nat Med. 2005;11:920–2. doi: 10.1038/nm0905-920. [DOI] [PubMed] [Google Scholar]
- 3.Kang TW, Yevsa T, Woller N, Hoenicke L, Wuestefeld T, Dauch D, et al. Senescence surveillance of pre-malignant hepatocytes limits liver cancer development. Nature. 2011;479:547–51. doi: 10.1038/nature10599. [DOI] [PubMed] [Google Scholar]
- 4.Swann JB, Smyth MJ. Immune surveillance of tumors. J Clin Invest. 2007;117:1137–46. doi: 10.1172/JCI31405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Willimsky G, Blankenstein T. Sporadic immunogenic tumours avoid destruction by inducing T-cell tolerance. Nature. 2005;437:141–6. doi: 10.1038/nature03954. [DOI] [PubMed] [Google Scholar]


