Abstract
CD1d-restricted invariant (i)NKT cells are innate-like, lipid-reactive T lymphocytes implicated in the control of infections, cancer and autoimmunity. Our study suggests that the reconstitution of the peripheral iNKT cell compartment, following HLA-haploidentical hematopoietic stem cell transplantation, associates with leukemia control in children affected by different hematological malignancies.
Keywords: CD1d, NKT cells, immunesurveillance, immunotherapy, leukemia
Invariant natural killer T (iNKT) cells are innate-like T lymphocytes bearing conserved invariant Vα14-Jα18 Vα24-Jα18 TCR chains in mice and humans, respectively, paired with Vβ8, Vβ7 and Vβ2 in mice and Vβ11 in humans.1 iNKT cells recognize microbial and cell-endogenous lipids presented by the MHC class I-like molecule CD1d.1 iNKT cells acquire constitutive effector functions following lineage-dependent developmental cues, which enables them to rapidly respond to a variety of stimuli without prior Ag-sensitization. Upon activation, iNKT cells produce copious amounts of different cytokines and they have been implicated in the early control of cell stress and tissue damage related to infections, cancer and autoimmunity.1
The HLA-haploidentical hematopoietic stem cell transplantation (hHSCT) is a critical therapy for the great majority (75%) of leukemia patients lacking an HLA-identical sibling.2 The transfer of HSC from relatives that only share half HLA locus with the recipients permits to find an immediate donor for any patient.2 Purified donor-derived HSC are depleted of mature T cells to reduce the risk of GVHD, triggered by the donor/recipient histocompatibility barrier, and infused into patients who are profoundly myeloablated to eliminate both endogenous hemopoiesis and malignant cells.2 As a result, the immune system that reconstitutes in the recipients derives from the donor HSCs, which generate lymphoid precursors that seed in the thymus and give raise to the T and iNKT lymphocytes that are exported in the periphery.3 This process is particularly efficient in pediatric patients who maintain a functional thymus.3 A rapid immunoreconstitution is critical to control the major life threatening events that may occur following hHSCT, such as opportunistic infections and the leukemia recurrences.3 In light of these characteristics, we thought that hHSCT in pediatric patients with leukemia was a suitable model to investigate human iNKT cell reconstitution, to address the unsolved issues of their maturation dynamics and their role in leukemia relapse control. Due to the difficulty to follow longitudinally the iNKT cell development in healthy donors, only snapshots on the human iNKT cell development at initial (thymus and umbilical cord blood) or late (adult blood) stages were available from cross-sectional studies, showing that: (1) Thymic and neonatal iNKT cells display an immature CD4+ phenotype lacking CD161, the NK terminal differentiation marker, and poor effector cytokine production4; (2) adult mature circulating iNKT cells are divided into two main CD4+CD161+ TH0 helper/regulatory and CD4- CD161+ (mainly CD4-CD8-) TH1 effector/inflammatory subsets with a CD4- → CD4+ ratio.5,6 However, the dynamics by which an immature fetal CD4+161- precursor generates the two adult mature CD4+CD161+ and CD4-CD161+ iNKT cell subsets was unknown. On the other hand, evidence in mouse models and cancer patients suggested that iNKT cells participate in the immune surveillance of different solid tumors, lymphoma and multiple myeloma.7 Pre-clinical studies also elucidated the iNKT cell role in determining the positive balance between GVL and GVHD in mouse models of HSCT for leukemia.8 Hence, the potential control of leukemia relapses by human iNKT in the clinically relevant context of hHSCT was an important question.
In our study,9 we undertook a thorough longitudinal analysis of iNKT cell reconstitution in 22 consecutive pediatric patients undergoing hHSCT after a myeloablative regimen for the treatment of acute leukemia and myelodysplastic syndromes (Fig. 1A). CD4+ and CD4- iNKT cell frequency, number, phenotype and effector functions were assessed, in direct comparison with mainstream T cells, in the peripheral blood of recipients at monthly intervals up to 18 mo post-hHSCT, correlating their emergence with disease relapse and analyzing their reconstitution and maturation kinetics using mathematical models. The data on the reconstitution dynamics of iNKT cells was further reinforced by adding a cross-sectional analysis in a second group of 11 patients, who were in clinical remission for 2–6 y post-hHSCT.
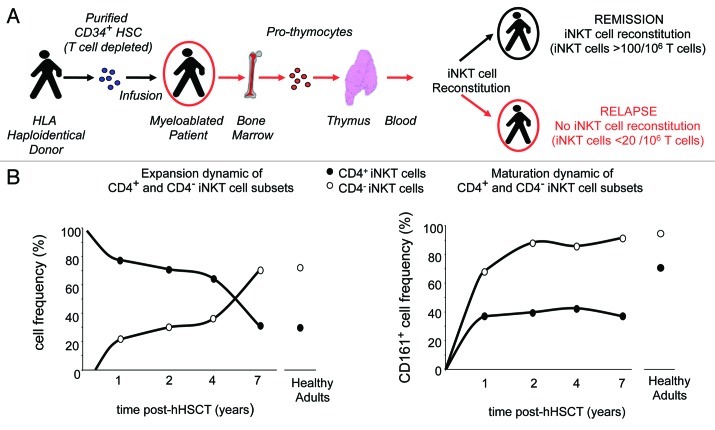
Figure 1. Schematic iNKT cell reconstitution dynamics and correlation with disease control following hHSCT in pediatric leukemia patients. A longitudinal analysis of iNKT cell reconstitution in relation to disease remission was performed monthly for 18 mo post-hHSCT in 22 children affected by leukemia and myelodisplasia. This data were integrated by a cross-sectional analysis of iNKT cell frequency and maturation in a second cohort of 11 leukemia patients that were in stable remission from 1 to 8 y after transplantation. (A) Schematic illustration of the transplantation physiology in case of HLA-haploidentical conditions. Donor CD34+ HSC are T cell depleted to reduce the risk of GVHD due to donor/recipient HLA barrier. Patients are myeloablated to eradicate malignant cells and eliminate the endogenous immune system and then infused with purified CD34+ HSC. Pro-thymocytes, originated from transplanted HSC that have reached the bone marrow, colonize the thymus and generate T and iNKT cells that are exported in the periphery. iNKT cells are detectable in periphery of patients maintaining remission as early as 3 mo post-hHSCT and reach an average of > 10 iNKT/106 T cells in the 18 mo observation time, whereas iNKT cells fail to reconstitute in relapsing patients, remaining on average < 20 iNKT/106 T cells. (B) Immunoreconstitution dynamics of human iNKT cell following hHSCT. The left panel depicts the different dynamics followed by the CD4+ and CD4- iNKT cell subsets. The CD4+ emerge before the CD4- ones in the periphery and remain the dominant subset up to 4–5 y post-transplant. The CD4- cells undergo a massive expansion around 7 y post-hHSCT, resulting in the CD4- > CD4+ iNKT cell ratio typical of adult healthy individuals. The right panel shows the dynamics of the terminal NK-maturation of the two iNKT cells subsets, as defined by the acquisition of CD161 (NKPR1a-c). Both CD4+ and CD4- subsets emerge in the periphery with an immature CD161- phenotype. The CD4- subset matures markedly more rapidly than the CD4+ cells and reaches adult CD161-expression levels already by 2 y post-hHSCT, whereas the CD4+ iNKT cells have not yet completed their NK-maturation after 7 y post-transplantation.
Two major findings were obtained (Fig. 1B). First, we identified a clearly different population dynamics of the CD4+ and CD4- iNKT cell subsets. The CD4+ emerged first and increased in the periphery at a rate twice faster than that of the CD4- cells. Both subsets appeared with an immature CD161- phenotype; however, the CD4- cells matured markedly more rapidly than the CD4+ iNKT cells, reaching adult-like frequency of CD61 and IFNγ expression already by 6 mo after their first detection. The CD4+ iNKT cells remained the most abundant subset up to 5–6 y post-hHSCT, when the CD4- cells underwent a brisk expansion resulting in the CD4- → CD4+ iNKT cell ratio typical of adults. These data suggested that the making of a mature iNKT cell repertoire is a slow process regulated by subset-specific mechanisms. Second, we observed a correlation between the presence of iNKT cells and leukemia remission. iNKT cells fully reconstituted in all 14 patients maintaining remission, whereas they completely failed to reconstitute in the 8 relapsing patients. Although the number of patients analyzed was relatively small, none of the best-known variables (female-to-male combination, donor NK alloreactivity, number of CD34+ cells infused) could predict the risk of disease recurrence. The critical parameter in the immune reconstitution was the iNKT/T cell ratio, which was on average 20 iNKT cells/106T cells in relapsed patients and 100 iNKT cells/106T cells in patients maintaining remission, during the same observation time. Although we do not yet have clear mechanistic insight, these findings suggested that, in the critical equilibrium conditions represented by the early post-transplantation period, iNKT cells are an essential part of the effector mosaic that maintains the control on the minimal residual leukemia cells that might have escaped the preparative regimen. This is consistent with other human critical cancer contexts, such as head and neck squamous cell carcinoma, where the disease progression following radiotherapy was found to correlate with a very low iNKT/T cell ratio (< 47 iNKT cells/106 T cells) present in the patients.10
Our findings have also potential clinical implications. The molecular determination of the iNKT cell reconstitution trend following hHSCT could become a predictive therapeutic marker. Furthermore, children who do not properly reconstitute the iNKT cell compartment within the first 2–6 mo after hHSCT could receive infusions of donor-derived iNKT cells to prevent possible leukemia recurrence, with minimal risk of inducing GVHD.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/18399
References
- 1.Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- 2.Aversa F, Tabilio A, Velardi A, Cunningham I, Terenzi A, Falzetti F, et al. Treatment of high-risk acute leukemia with T-cell-depleted stem cells from related donors with one fully mismatched HLA haplotype. N Engl J Med. 1998;339:1186–93. doi: 10.1056/NEJM199810223391702. [DOI] [PubMed] [Google Scholar]
- 3.Fry TJ, Mackall CL. Immune reconstitution following hematopoietic progenitor cell transplantation: challenges for the future. Bone Marrow Transplant. 2005;35(Suppl 1):S53–7. doi: 10.1038/sj.bmt.1704848. [DOI] [PubMed] [Google Scholar]
- 4.de Lalla C, Festuccia N, Albrecht I, Chang HD, Andolfi G, Benninghoff U, et al. Innate-like effector differentiation of human invariant NKT cells driven by IL-7. J Immunol. 2008;180:4415–24. doi: 10.4049/jimmunol.180.7.4415. [DOI] [PubMed] [Google Scholar]
- 5.Gumperz JE, Miyake S, Yamamura T, Brenner MB. Functionally distinct subsets of CD1d-restricted natural killer T cells revealed by CD1d tetramer staining. J Exp Med. 2002;195:625–36. doi: 10.1084/jem.20011786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee PT, Benlagha K, Teyton L, Bendelac A. Distinct functional lineages of human V(alpha)24 natural killer T cells. J Exp Med. 2002;195:637–41. doi: 10.1084/jem.20011908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vesely MD, Kershaw MH, Schreiber RD, Smyth MJ. Natural innate and adaptive immunity to cancer. Annu Rev Immunol. 2011;29:235–71. doi: 10.1146/annurev-immunol-031210-101324. [DOI] [PubMed] [Google Scholar]
- 8.Kohrt HE, Pillai AB, Lowsky R, Strober S. NKT cells, Treg, and their interactions in bone marrow transplantation. Eur J Immunol. 2010;40:1862–9. doi: 10.1002/eji.201040394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Lalla C, Rinaldi A, Montagna D, Azzimonti L, Bernardo ME, Sangalli LM, et al. Invariant NKT cell reconstitution in pediatric leukemia patients given HLA-haploidentical stem cell transplantation defines distinct CD4+ and CD4- subset dynamics and correlates with remission state. J Immunol. 2011;186:4490–9. doi: 10.4049/jimmunol.1003748. [DOI] [PubMed] [Google Scholar]
- 10.Molling JW, Langius JA, Langendijk JA, Leemans CR, Bontkes HJ, van der Vliet HJ, et al. Low levels of circulating invariant natural killer T cells predict poor clinical outcome in patients with head and neck squamous cell carcinoma. J Clin Oncol. 2007;25:862–8. doi: 10.1200/JCO.2006.08.5787. [DOI] [PubMed] [Google Scholar]