Abstract
The first gene profiling study of cancer-specific CD8+ T-cells demonstrates that lymphocyte dysfunction in cancer tissue is due to multiple molecular alterations,1 similar as in “exhausted” T-cells in chronic infection.2 The data suggest novel drug targets, and show that T-cell exhaustion is reversible and limited to anatomical sites of disease.
Keywords: T-cell dysfunction, Tumor biology, immune deficiency, immunotherapy, vaccination
Until the 1990s, it was thought that clonal deletion eliminates most cancer-specific T-cells by negative selection in the thymus. Indeed, mature high-affinity CD8+ T-cells specific for self/cancer-antigens are relatively infrequent. Nevertheless, one can find large numbers of mature cancer-specific T-cells in cancer patients, in part with good functional avidity conferring efficient cancer cell recognition. These T-cells can reach high functional competence, in patients treated with state-of-the-art cancer vaccines3 or after adoptive T-cell transfer.4 Several studies have shown that CD8+ T-cells have the potential to effectively destroy cancer and stroma cells. However, in the tumor microenvironment, cancer-specific T-cells become dysfunctional, showing insufficient cytokine production when analyzed directly ex vivo.1,5
Major research efforts are undertaken to identify the reasons for T-cell dysfunction in cancer tissues. It has long been discussed whether it is due to cellular senescence, and/or resembles features described in models of T-cell anergy or T-cell exhaustion. Mechanisms of T-cell dysfunction have primarily been characterized in infectious diseases. As compared with cancer-specific T-cells, virus-specific T-cells are more frequent and more active, allowing in depth investigations. Infection of mice with the Armstrong strain of the Lymphocytic Chorio-Meningitis Virus (LCMV) causes acute viral infection, and induces fully functional cytotoxic CD8+ T-cells that are capable of rapid virus elimination. In contrast, infection with LCMV clone-13 leads to chronic/protracted infection. Here, T-cells are also activated but rapidly become dysfunctional precluding viral clearance. T-cell dysfunction is characterized by the progressive loss of production capabilities, first of IL-2, then TNFα and finally IFNγ.6-8
Studying mice infected with LCMV clone-13 facilitated the characterization of important negative regulatory T-cell pathways. Inhibitory lymphocyte receptors (such as PD-1) play prominent roles, but immune regulatory cells and soluble factors are also involved (reviewed in ref. 9). Studies in patients with infectious diseases demonstrated that similar mechanisms are responsible for T-cell dysfunction in HIV and HCV patients, providing important insight in situations of immune deficiency causing major pathology. These results emphasize the need for a profound understanding of T-cell dysfunction. In 2007, Wherry et al. published a molecular “resource” paper presenting the results from gene expression profiling studies of T cells from mice infected with LCMV clone-13.2 This data provided a molecular “exhaustion profile” and showed that T-cell exhaustion has numerous characteristics, beyond deficient effector functions and enhanced expression of inhibitory lymphocyte receptors. Indeed, exhausted virus-specific T cells show multiple molecular alterations, affecting genes regulating chemotaxis, adhesion, co-receptors, migration, metabolism and energy household.
Until recently, much less was known about mechanisms of cancer-specific T-cell dysfunction. In order to obtain comprehensive insight in T cells from cancer patients, we performed gene expression profiling of antigen-specific T cells directly ex vivo.1 Previously, we had validated an approach for gene expression profiling of low numbers of sorted human T cells. Good reproducibility of microarray data was obtained with numbers as low as 100 to 1000 lymphocytes. With this technique, we sorted 1000 antigen-specific T-cells per sample, directly ex vivo, from 19 melanoma patients and four healthy donors. In parallel to cancer-antigen (Melan-A/MART-1) specific T cells, we analyzed T cells specific for two herpes viruses (Epstein-Barr Virus and Cytomegalovirus), and naïve CD8+ T cells for comparison. These T cells were isolated from peripheral blood, after vaccination of patients with Melan-A peptide and CpG-oligodeoxynucleotides. In addition, we recovered large T-cell numbers directly ex vivo from melanoma metastases (i.e., from tumor-infiltrated lymph nodes), enabling the analysis of cancer-specific T cells from the tumor microenvironment. As expected, cluster analysis of molecular data revealed that gene expression differences between naïve T cells and antigen experienced T cells were much larger than between antigen experienced T cells specific for viral vs. cancer antigens. More importantly, clustering was “clean” (i.e., with complete segregations) also for the T-cell populations specific for cancer vs. viral antigens, and of cancer-specific T cells from peripheral blood vs. metastases. These results were remarkable, given the high genetic heterogeneity in humans.
In cancer-specific T cells from blood vs. metastases, we found differential expression of 332 genes.1 Detailed comparative analysis with the microarray data from mice infected with LCMV clone-13 virus showed that the gene set described for exhausted murine T cells2 was significantly enriched in cancer-specific T cells from metastases from melanoma patients. Our data represent the first comprehensive molecular characterization of functional T-cell impairment in cancer tissue of any species, and provide mechanistic explanation for T-cell immune deficiency in cancer. Moreover, the similarity to a well-defined animal model offers new opportunities to identify essential pathways and novel drug target candidates.
While the gene expression profile of metastases derived cancer-specific T-cells was characteristic for exhaustion, there was only insignificant overlap with what was found in models of T-cell anergy or cellular senescence.1 Furthermore, the data are compatible with previous findings showing that loss of cancer-specific T-cell clonotypes was rare in melanoma patients, and occurred due to passive attrition (competition) rather than active attrition (cellular senescence).10
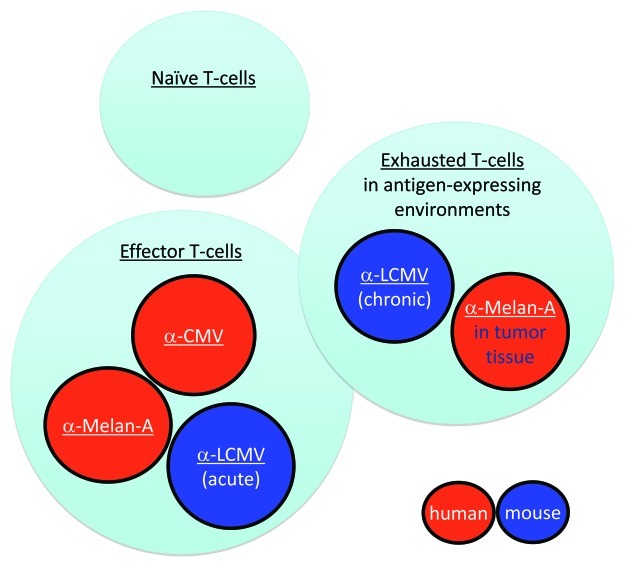
Exhaustion of cancer-specific T cells seems largely reversible.1,5 Very interestingly, it occurs primarily in metastases, whereas cancer-specific T cells in circulation become fully functional after appropriate immunotherapy,3 demonstrating a striking co-existence of “unfit” and “fit” T cells in the same patient (Fig. 1). These findings further support the notion that powerful vaccination can mobilize T cells up to the level where they become clinically useful for cancer patients. At present, developing efficient T-cell vaccines still remains a great challenge. Most clinically available vaccines activate T cells much less efficiently than natural infection or the rare live replicating vaccines (essentially smallpox and yellow fever vaccines). Recently, we and others found that novel formulations of synthetic vaccines can overcome this deficit. For example, vaccines with antigen and CpG-oligodeoxynucleotides are capable of inducing CD8+ T-cell responses with similar properties as protective virus-specific T cells. Preliminary data suggest that these T-cells may be clinically beneficial for melanoma patients.3
Figure 1. Functional competence vs. deficiency of virus- and cancer-specific CD8+ T cells. Once naive T-cells are activated, most of their progeny become protective effector T cells, such as in mice acutely infected with LCMV Armstrong strain, or humans infected with cytomegalovirus (CMV). Cancer (Melan-A/MART-1)-specific T cells induced by vaccination or after adoptive T-cell transfer can also become fully functional effector cells in peripheral blood, much like CMV-specific T cells. In chronic infection such as in mice infected with LCMV clone-13, T cells are functionally impaired (“exhausted”). Cancer-specific T cells from metastases show a similar exhaustion profile, with numerous molecular alterations of multiple cellular systems, resulting in impairment of T-cell function and local immune deficiency.
Strong T-cell inhibition is conferred by the inhibitory receptor CTLA-4, but there are conflicting data with regard to the role of CTLA-4 in T-cell exhaustion.9 Nevertheless, the recent clinical success and FDA/EMA approval of treating melanoma patients with anti-CTLA-4 blocking antibody emphasizes the high potential of T-cell based cancer therapy. Unfortunately, patients with metastatic disease frequently become therapy-resistant, calling for better understanding and more efficient treatments. Public availability of basic and clinical data, and intense bio-medical research and development will enhance and accelerate innovation.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/18342
References
- 1.Baitsch L, Baumgaertner P, Devevre E, Raghav SK, Legat A, Barba L, et al. Exhaustion of tumor-specific CD8+ T cells in metastases from melanoma patients. J Clin Invest. 2011;121:2350–60. doi: 10.1172/JCI46102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wherry EJ, Ha SJ, Kaech SM, Haining WN, Sarkar S, Kalia V, et al. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity. 2007;27:670–84. doi: 10.1016/j.immuni.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 3.Baumgaertner P, Jandus C, Rivals JP, Derre L, Lovgren T, Baitsch L, et al. Vaccination-induced functional competence of circulating human tumor-specific CD8 T-cells. Int J Cancer. 2011;130:2607–17. doi: 10.1002/ijc.26297. [DOI] [PubMed] [Google Scholar]
- 4.Rosenberg SA, Yang JC, Sherry RM, Kammula US, Hughes MS, Phan GQ, et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin Cancer Res. 2011;17:4550–7. doi: 10.1158/1078-0432.CCR-11-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang SF, Fouquet S, Chapon M, Salmon H, Regnier F, Labroquere K, et al. Early T cell signalling is reversibly altered in PD-1+ T lymphocytes infiltrating human tumors. PLoS ONE. 2011;6:e17621. doi: 10.1371/journal.pone.0017621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moskophidis D, Lechner F, Pircher H, Zinkernagel RM. Virus persistence in acutely infected immunocompetent mice by exhaustion of antiviral cytotoxic effector T cells. Nature. 1993;362:758–61. doi: 10.1038/362758a0. [DOI] [PubMed] [Google Scholar]
- 7.Zajac AJ, Blattman JN, Murali-Krishna K, Sourdive DJ, Suresh M, Altman JD, et al. Viral immune evasion due to persistence of activated T cells without effector function. J Exp Med. 1998;188:2205–13. doi: 10.1084/jem.188.12.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–7. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 9.Wherry EJ. T cell exhaustion. Nat Immunol. 2011;131:492–9. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- 10.Derre´ L, Bruyninx M, Baumgaertner P, Devevre E, Corthesy P, Touvrey C, et al. In vivo persistence of codominant human CD8+ T cell clonotypes is not limited by replicative senescence or functional alteration. J Immunol. 2007;179:2368–79. doi: 10.4049/jimmunol.179.4.2368. [DOI] [PubMed] [Google Scholar]