Abstract
Effective repression of cI transcription from PRM by the bacteriophage λ CI repressor requires binding sites (OL) located 2.4 kb from the promoter. A CI tetramer bound to OL1.OL2 interacts with a tetramer bound near PRM (OR1.OR2), looping the intervening DNA. We previously proposed that in this CI octamer:DNA complex, the distant OL3 operator and the weak OR3 operator overlapping PRM are juxtaposed so that a CI dimer at OL3 can cooperate with a CI dimer binding to OR3. Here we show that OL3 is necessary for effective repression of PRM and that the repressor at OL3 appears to interact specifically with the repressor at OR3. The OL3-CI-OR3 interaction involves the same CI interface used for short-range dimer-dimer interactions and does not occur without the other four operators. The long-range interactions were incorporated into a physicochemical model, allowing estimation of the long-range interaction energies and showing the lysogenic state to be ideally poised for CI negative autoregulation. The results establish the λ system as a powerful tool for examining long-range gene regulatory interactions in vivo.
Keywords: DNA looping, protein-protein interactions, multimerization, transcriptional control, lysogeny
In complex organisms, activation or repression of promoter activity by proteins bound to enhancer or silencer elements located several kilobases away from the promoter has been recognized for many years; however, a precise molecular understanding of any of these multi-protein interactions is still lacking(see Carter et al. 2002). In prokaryotes, although some gene regulators, such as DeoR (Dandanell et al. 1987) and NtrC (Reitzer and Magasanik 1986) are able to work over large distances, most regulatory protein binding sites lie within 300 bp of the promoter (Gralla and Collado-Vides 1996). The discovery that the well studied CI protein of bacteriophage λ interacts over DNA distances of up to 3.8 kb (Révet et al. 1999; Dodd et al. 2001) therefore provides a unique opportunity for the characterization of long-range gene regulation. The wealth of available biochemical data and the relative ease of testing theoretical predictions have also made the λ system ideal for the development of models of gene regulatory networks (Shea and Ackers 1985; Arkin et al. 1998; Aurell et al. 2002), and an important tool in the emerging field of gene circuit engineering (Hasty et al. 2002). Information about the OL-OR interaction should contribute to the refinement of these models and tools.
The CI protein of λ has been the subject of intensive genetic, molecular biological, biochemical, physical and structural study (for reviews, see Johnson et al. 1981; Ptashne 1998; Hochschild 2002; see also Bell et al. 2000). CI binds to two operator regions, OR and OL, located 2.4 kb apart on the phage chromosome, with each region containing three individual CI operators spaced ∼two DNA turns apart (Fig. 1A). Each operator is contacted by a CI dimer, with dimers binding cooperatively to adjacent pairs of operators and preferentially occupying OR1.OR2 and OL1.OL2. This represses the early lytic promoters PR and PL and activates the weak promoter for the cI gene, PRM (Johnson et al. 1981; Ptashne 1998). At high concentrations, CI can also repress PRM by occupying OR3 (negative autoregulation; Maurer et al. 1980).
Figure 1.
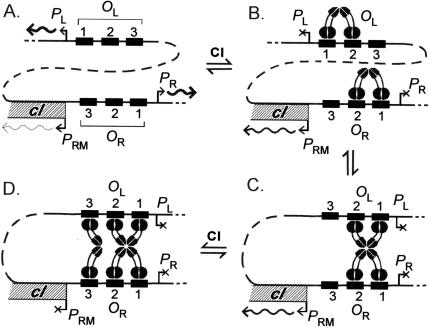
Model of CI regulation with long-range DNA looping. Cartoon depicting the major predicted CI:DNA complexes at OL and OR on the λ chromosome and their effects on transcription as CI concentration increases (A to B, C to D).
The CI protein consists of two domains joined by a protease sensitive linker of ∼40 amino acids (Pabo et al. 1979). The N-terminal domain (NTD) is responsible for DNA binding and contains the amino acids that contact the σ subunit of RNA polymerase to activate PRM (Nickels et al. 2002 and references therein). The C-terminal domain (CTD) is responsible for CI self-assembly and cooperative DNA binding(Bell et al. 2000). Full-length CI associates to tetramers and octamers in the absence of DNA, although such oligomers are rare in solution at physiological CI concentrations (Senear et al. 1993). Genetic studies have shown that cooperative DNA binding of CI dimers is mediated by a group of residues in the CTD (Bell et al. 2000 and references therein). It is likely that the same CTD interface is used for tetramerization on DNA and in solution, because mutations that disrupt cooperativity on the DNA also disrupt tetramerization in solution (Burz and Ackers 1996), and the amino acids involved in cooperativity interact at the dimer-dimer interface in the crystal structure of the CTD tetramer (Bell et al. 2000).
The CI tetramerization reaction is capable of mediating the interaction of CI dimers bound to DNA sites spaced up to a few helical turns apart (Hochschild and Ptashne 1986) but becomes undetectable at distances beyond 20 turns (A. Hochschild and M. Ptashne, unpubl.). However, Révet et al. (1999) showed that CI dimers bound to pairs of adjacent operators could interact over much larger distances in vitro and in vivo. Repression of PR by CI binding to OR1 and OR2 was enhanced fourfold by the presence of OL1 and OL2 (but not OL1 alone) at a distance of 3.6 kb. Révet et al. (1999) thus proposed that the interaction involved the formation of a CI octamer (Fig. 1C). Although this has not been tested directly, octamerization of DNA-bound CI is consistent with the ability of CI to octamerize in solution (Senear et al. 1993) and with the crystal structure of the CTD octamer (Bell and Lewis 2001).
Whether the improvement in PR repression caused by the OL-OR interaction is physiologically significant is not clear. However, repression of PRM by CI in a λ lysogen is dependent on the presence of OL. This negative autoregulation by CI limits the CI concentration in the lysogen and facilitates efficient induction of the prophage in response to UV light (Dodd et al. 2001). To explain this effect of OL on PRM repression, it was proposed that the CI-mediated OL-OR loop juxtaposes OR3 and OL3 so that a CI dimer bound at OL3 (a higher-affinity binding site than OR3) can assist a CI dimer to bind at OR3 and repress PRM (Fig. 1D; Dodd et al. 2001). Although this model is plausible and provides a rationale for retention of OL3 in the evolution of λ, it has not been tested.
In this paper, we confirm a number of aspects of the proposed model for CI-mediated negative autoregulation (see Fig. 1D). Specifically, we show that (1) OL3 is necessary for repression of PRM at physiological CI concentrations, (2) the primary role of OL3 is to foster CI binding at OR3 and so provide CI negative autoregulation, (3) the interaction between OR3 and OL3 is mediated by the CI CTD, and (4) the long-range OR3-OL3 interaction only occurs in the presence of the other four operators. Finally, we incorporate the proposed CI interactions between OR and OL into a physicochemical model of CI regulation and find that a good approximation to our in vivo promoter reporter data is achieved by using some fairly simple assumptions about the energetics of the long-range interactions.
Results
Mutation of OL3 blocks CI repression of PRM
In the model shown in Figure 1D, CI binding to OR3, and thus repression of PRM, is enhanced by a cooperative interaction with CI bound at OL3. To test this, we eliminated CI binding to OL3 by altering four base pairs in the operator (creating the OL3-4 mutation). The predicted effect of the changes on CI binding is a ΔΔG of +10.3 kcal/mole (Sarai and Takeda 1989).
The OL3-4 mutation was introduced into the chromosomal PRM reporter construct shown in Figure 2, in which OL (OL1.OL2.OL3) is located downstream of lacZ expressed under the control of PRM.OR (OR3.OR2.OR1). This PRM.lacZ fusion construct contains the +62 to -123 region of PRM with an OL-OR spacing of 3.8 kb. A range of CI concentrations was supplied to the reporters from a plasmid that directed the synthesis of CI under the control of an IPTG-inducible promoter. The CI levels relative to those in a wild-type λ lysogen (wild-type lysogenic units, WLU) were determined previously (Dodd et al. 2001).
Figure 2.
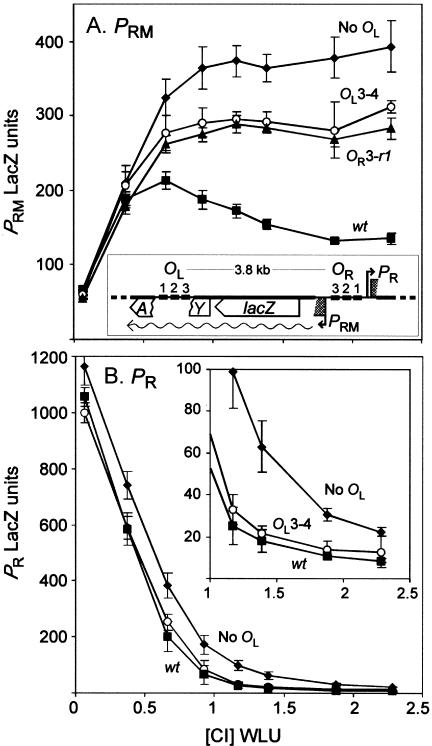
OL3 is needed for efficient CI repression of PRM but not PR. Activities of PRM::lacZ (A) and PR::lacZ (B) operon fusions over a range of CI concentrations (in wild-type lysogenic units, WLU) supplied using plasmids pZC320cI and pUHA1 (providing lac repressor) with IPTG (0 to 200 μM). The wt PRM reporter fusion is depicted (A); in the PR reporters the orientation of the OR fragment is reversed. Each reporter carried three operators at OR; these were wt except for the OR3-r1 PRM::lacZ fusion (OR3-r1). Downstream of lacZ there was either no λ sequences (no OL) or a 70-bp wt OL1.OL2.OL3 fragment (wt) or one carrying the OL3-4 mutation (OL3-4). Inset in (B) expands the rightmost portion of the PR graph. Error bars indicate 95% confidence limits (n > 5).
When OL3 and OR3 were intact, PRM was activated at low CI concentrations but became repressed at higher CI concentrations (Fig. 2A). As expected, repression was lost when OR3 carried the r1 mutation or when OL was not present (Dodd et al. 2001). (As seen previously, maximal PRM activation was ∼35% greater in the absence of OL than in its presence. It seems that octamer formation slightly inhibits the ability of the CI dimer bound at OR2 to activate PRM). CI repression of PRM was also abolished by the OL3-4 mutation. Thus, the OL3 CI binding site, located 3.8 kb away from PRM, was necessary for repression of PRM by physiological levels of CI.
OL3 is not needed for CI repression of PR
These results support the idea of a specific interaction between OL3 and OR3 for CI repression of PRM. However, an alternative possibility is that the CI dimer at OL3 plays a more indirect role in facilitating PRM repression, for example by interacting with the CI dimer bound at OR1 and thereby enabling the CI dimer bound at OR2 to cooperate with a dimer bound at OR3. We investigated the possibility that the CI dimer bound at OL3 interacts with CI bound at OR1 or OR2 by testing whether the OL3-4 mutation interfered with CI repression of PR.
The OR fragment in the reporter construct of Figure 2A was reversed so that lacZ was expressed from PR (fragment is +42 to -143 of PR). Assays in the presence of a range of CI concentrations showed that CI repression of PR was most efficient when wild-type OL was present (Fig. 2B). Removal of OL weakened repression by up to fourfold, depending on the CI concentration, consistent with the finding of Révet et al. (1999). However, the OL3-4 mutation weakened repression of PR only slightly, indicating that the OL-OR loop was not disrupted.
These results show that, despite CI binding at OL3 being critical for repression of PRM, it does not contribute strongly to the OL-OR interaction, supporting the idea of a specific interaction between OL3 and OR3.
Whole phage studies—the role of OL3 in CI autoregulation and prophage induction
The function of OL3 in λ development has been a puzzle because the binding of either CI or Cro to this operator should not affect PL activity (Johnson et al. 1981). Our results showing a need for OL3 in CI repression of PRM now suggest a role for OL3, because repression of PRM is necessary for efficient switching into lytic development in response to UV (i.e., prophage induction; Dodd et al. 2001). To test whether or not OL3 actually facilitates prophage induction, the OL3-4 mutation was introduced into wild-type λ to give λOL3-4. The mutation had no detectable effect on plaque morphology or phage production kinetics after infection (data not shown). We measured CI levels in NK7049 monolysogens of λr1 (carries OR3-r1 mutation) and λOL3-4 using our gel mobility shift assay for CI DNA binding activity (Dodd et al. 2001). Combining these and previous gel shift assay results gave estimated CI levels of 2.8 WLU (95% confidence limits: 2.51-2.99, n = 5) and 3.0 WLU (95% confidence limits: 2.94-3.14, n = 4) for the λr1 and λOL3-4 lysogens, respectively. Thus, the OL3-4 and OR3-r1 mutations each caused a similar increase in the lysogenic CI concentration, confirming that OL3 is needed for CI repression of PRM in the native λ context.
We then compared the UV-inducibility of the wild type and mutant prophages by measuring the fraction of lysogens induced versus dose of UV (Dodd et al. 2001). The λOL3-4 lysogen displayed a defect in prophage induction very similar to that of the λr1 lysogen, with only 25%-33% as many lysogens induced compared to the wild-type control at three different UV doses (data not shown). This defect is presumably due to the high level of CI in the lysogens. It was possible that the OL3-4 mutation was somehow acting through an effect on PL or its regulation, but we found using PL.lacZ reporters that the basal activity and CI repressibility of PL were not substantially affected by the OL3-4 mutation (data not shown).
Thus, our results provide an explanation for the retention of the third operator at OL, indicating that the primary role of OL3 is to provide for CI negative autoregulation in order to limit the CI concentration in the lysogenic state and allow efficient switching to lytic development.
Localizing CI CTDs at OL3 is sufficient to facilitate repression of PRM
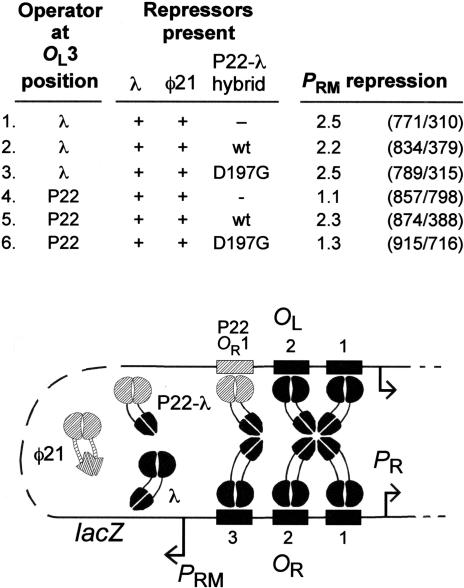
According to our model, the role of OL3 in PRM repression is to localize the CTDs of a dimer for contact with the CTDs of a CI dimer bound at OR3 (Fig. 1D). The P22-λ hybrid repressor of Whipple et al. (1994) carries the DNA-binding NTD of P22 repressor (residues 1-94) fused within the linker region to the CTD of wild-type (wt) λ repressor (residues 112-236). This hybrid protein is functional for binding to P22 operators and for cooperative interactions with dimers of the same type and with dimers of λ repressor (Whipple et al. 1994). Accordingly, we predicted that this hybrid repressor would be able to substitute functionally for wt λ CI if bound to the DNA at the position of OL3.
To test this prediction, we replaced OL3 on the chromosomal PRM reporter constructs with the P22 OR1 operator (Poteete et al. 1980). λ operators are 17-bp-long, and the center-to-center spacing of OL2 and OL3 is 20 bp; in the replacement, the spacing between OL2 and the 18-bp P22 operator was 20.5 bp. The P22-λ hybrid repressor was supplied to these reporters from a plasmid that directs the synthesis of an amount corresponding to roughly 10 lysogen's worth of λ repressor. The lysogenic level of wt λ repressor was supplied in all cases from a λ prophage (λatt80) integrated at the φ80 attachment site. In addition, because the reporter phages carry the immunity region of phage 21 (imm21), the phage 21 repressor was also present. The phages P22 and 21 are homo-immune; the P22 repressor can bind to phage 21 operators and 21 repressor can bind to P22 operators (Ballivet et al. 1977, 1978), and the OR1 operators of these phages are identical (Poteete et al. 1980).
Repression of PRM was measured as PRM-r1 LacZ activity (= unrepressed) divided by the activity of wt PRM. In the absence of the hybrid repressor and with wt OL3, PRM was repressed 2.5-fold (Fig. 3, line 1). As expected, replacement of λOL3 with the P22 operator prevented this repression of PRM by λ repressor (Fig. 3, line 4). (Note that in the absence of repression a PRM `repression' value of 1.1 is obtained because the r1 mutation improves PRM activity by about 10%; Dodd et al. 2001). Because we expect the resident reporter-derived 21 repressor to occupy the OL3 position in this construct, this result indicates that repression of PRM is not enhanced by the binding of just any protein at the OL3 position. The presence of the hybrid repressor restored PRM repression in the case of the reporter construct with the P22 operator at the position of OL3 (2.3-fold repression; Fig. 3, line 5), consistent with the idea that a hybrid repressor dimer is able to bind to the P22 operator using its P22 NTDs and simultaneously contact λ CI dimer bound at OR3 using its λ CTDs (diagram of Fig. 3). Repression was almost as strong as in the native λ situation (Fig. 3, line 1), indicating that the lysogenic concentration of the 21 repressor does not compete significantly with the ∼10 lysogen's worth of hybrid repressor for binding to the P22 operator at OL3.
Figure 3.
CI CTDs at OL3 assist in repression of PRM. The reporter constructs were as in Fig. 2, except that in some cases the λ OL3 operator was substituted by a P22 OR1 operator (gray box). The cartoon shows the interactions expected for line 5. λ repressor was supplied from the λatt80 prophage, and the φ21 repressor was from the λimm21 reporter prophage itself. The P22-λ hybrid repressor (about 10 WLU) was supplied (or not) from plasmid pFW7-280Δ (or parent plasmid pLR1ΔcI) with 5μM IPTG. The P22-λ hybrid repressor bearing the D197G substitution was supplied from pFW7-280Δ D197G, a derivative of pFW7-280Δ. Plasmid pAD325 was the source of the lac repressor. PRM repression values are calculated by dividing the PRM lacZ units for reporters carrying the r1 mutation in OR3 (unrepressible) by the units for reporters where OR3 is wt. Shown are the average results of duplicate assays performed in a single representative experiment. The cells containing either pFW7-280Δ (encoding the hybrid repressor) or pLR1ΔcI (encoding no repressor) were assayed on four separate occasions with similar results, and the cells containing pFW7-280Δ D197G were assayed on two separate occasions with similar results.
This experiment shows that repression of PRM does not require a particular DNA sequence at OL3 or a particular DNA binding domain bound at OL3. Instead, effective repression can be achieved simply by tethering a pair of λ CI CTDs at OL3 for interaction with the CTDs of a CI dimer bound at OR3.
Figure 2 showed that repression of PRM was lost when either OR3 or OL3 was mutated to prevent CI binding. The data in Figure 3 show that a similar loss of repression occurs when both OR3 and OL3 are mutated, indicating that the two mutations are not acting independently, but rather to disrupt the same repressive mechanism. The PRM activity of the OL3-P22 OR3-r1 double mutant (857 units, Fig. 3, line 4) was similar to the activities seen with either single mutant (Fig. 3, line 1, OL3+OR3-r1 = 771 units; line 4, OL3-P22 OR3+ = 798 units).
We wished to test the inference that interaction between CI dimers at bound at OR3 and OL3 involves the same region of the CI CTD that mediates cooperative binding of pairs of dimers to operators separated by two or a few helical turns. To do this, we introduced the amino acid substitution D197G into the λ CI CTD on the P22-λ hybrid repressor. This substitution abolishes cooperativity between CI dimers but does not affect DNA binding or repressor dimerization (Whipple et al. 1994, 1998). In addition, residue D197 lies on the interface between repressor dimers in the crystal structure of the CI C-terminal domain tetramer (Bell et al. 2000). The P22-λ D197G repressor, unlike the wild-type hybrid, was unable to assist repression of PRM (Fig. 3, line 6), supporting the idea that the long-range interaction between OL3 and OR3 is mediated by the same interface used for short-range dimer-dimer interactions.
The OL3–OR3 interaction alone is insufficient for CI autoregulation
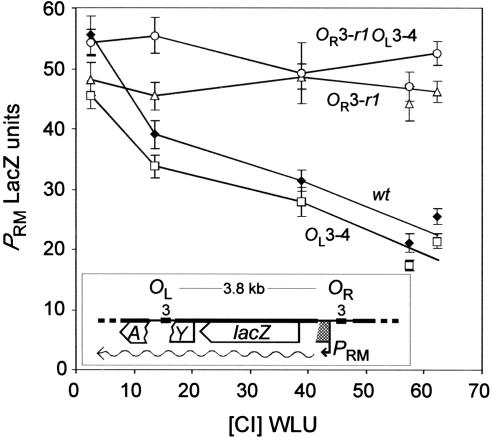
We hypothesized that the CI-mediated interaction between OL3 and OR3 occurs efficiently only because the formation of a CI octamer linking OR1.OR2 and OL1.OL2 juxtaposes OL3 and OR3 suitably for the binding of a CI tetramer. We tested this hypothesis by examining whether OL3 alone was able to assist CI binding to OR3 alone to repress PRM. The PRM reporter construct shown in Figure 4 contains OL3 and OR3 spaced at 3.8 kb (precisely the same distance as in the Fig. 2 constructs) but does not carry OL1, OL2, OR1, or OR2. There was no activation of PRM in these reporters because OR2 is absent. We found that OR3+ versions of this reporter were only slightly repressed at 4 CI WLU (using pZC320cI, data not shown), so we introduced a higher range of CI concentrations (up to 60 WLU) by using a high-copy CI expression plasmid, pZE15cI (Dodd et al. 2001). We found that repression of PRM did occur at these high CI levels as long as OR3 was intact (Fig. 4). Because OL3 is a considerably stronger binding site for CI than OR3 (see Table 1) and because it is clear that OR3 is occupied at these concentrations, we are confident that OL3 was also occupied. However, there was no enhancement of PRM repression by the presence of an intact OL3 at 3.8 kb (Fig. 4). Thus, cooperativity at this distance between CI bound at OL3 and OR3 requires the presence of the other λ operators.
Figure 4.
OR3 and OL3 do not interact in the absence of the other operators. Response to CI of PRM::lacZ operon fusions bearing single OL3 and OR3 operators at OL and OR. The structure of the reporter fusions is shown in the inset. The OR3-r1 and OL3-4 mutations were used to block CI binding to either or both operators. CI was supplied using plasmids pZE15cI and pUHA1 (0-500 μM IPTG). Error bars indicate 95% confidence limits (n = 8).
Table 1.
Parameters used in physicochemical modeling
| Operatora | ΔG (kcal/mole) | Operator | ΔG (kcal/mole) |
|---|---|---|---|
| Or1 | −13.2 | Ol1 | −13.8 |
| Or2 | −10.7 | Ol2 | −12.1 |
| Or3 | −10.2 | Ol3 | −12.4 |
| Or1-Or2 coop | −3.0 | Ol1-Ol2 coop | −2.5 |
| Or2-Or3 coop | −3.0 | Ol2-Ol3 coop | −2.5 |
| Or1-Or3 coop | 0 | Ol1-Ol3 coop | 0 |
| Or1-Or2- Or3 coop | −3.0 | Ol1-Ol2- Ol3 coop | −2.5 |
| Or3 (c12)b | −11.0 | Ol3-4c | −6.2 |
| Or3 (r1)b | −7.3 |
| Parameter | Value | Promoter | LacZ unitsh |
|---|---|---|---|
| Kdimd (M−1) | 6.7 × 107 (ΔGdim = −11.1) | Pr basal | 1056 |
| Knse (M−1) | 2.5 × 104 (ΔGns = −6.2) | Pr repressed | 2 |
| [NS]f | 6.76 × 10−3 M | Prm basal | 45 |
| [CI]lysogenicg | 3.7 × 10−7 M | Prm activated | 360 (265) |
| Prm repressed | 0.5 |
Set as equal to nonspecific binding.
CI dimerization (Koblan and Ackers 1991).
Affinity for nonspecific DNA; see text for details. Kns is a tool to align in vitro and in vivo repressor concentration scales, and we ascribe no particular significance to its value.
Concentration of nonspecific sites from E. coli genome size (4.6 × 106 bp/cell) and 1 molecule/cell = 1.47 nM (Donachie and Robinson 1987).
Calculated from the data of Reichardt and Kaiser (1971) and 1 molecule/cell = 1.47 nM.
Wild-type values; value in parentheses for looped configuration. Pr values for mutants (basal/repressed): Ol3-4 998/2, no Ol 1164/2. Prm values for mutants (basal/activated/repressed): c12 37/240(180)/0.5, r1 47/380(280)/0.5.
Physicochemical modeling of the CI-OL-OR system
Statistical thermodynamic analyses of CI DNase I footprint titrations have allowed resolution of the free energies for interaction of CI with each of the six operators and also for the cooperative interactions between CI bound within the same set of operators (Table 1). However, previous analyses have not taken into account long-range interactions between OL and OR. In order to validate our model of CI regulation and also possibly to derive estimates of the free energies of the long-range interactions, we modified the statistical thermodynamic approach of Shea and Ackers (1985) to incorporate the OL-OR interaction. We then tested whether this new statistical thermodynamic model, with suitable values for the long-range interaction parameters, could simulate our PRM and PR reporter data.
The ΔG for each configuration of the 64 possible species for CI occupancy of OR and OL (six operators, each unoccupied or occupied by CI) can be calculated from the free energies listed in Table 1. We modeled the OL-OR interaction by also considering looped-unlooped equilibria, introducing nine looped configurations and two new cooperative free energy terms, ΔGoct and ΔGtet. DNA looping was permitted for any species in which CI was bound to an adjacent pair of operators at OL and to an adjacent pair of operators at OR (four different four-dimer species, four different five-dimer species, and the single six-dimer species), and the free energy for each such looped configuration was obtained by adding the ΔGoct cooperativity term to the free energy calculated for the unlooped configuration. This cooperativity term reflects a net free energy change due to octamerization of CI bound across OR-OL, together with the cost of formation of a DNA loop (Fig. 1B,C). The ΔGtet cooperativity term was added only in the case where all six operators were occupied (applied in addition to ΔGoct). This cooperativity term reflects an overall favorable free energy change due to tetramerization between CI bound to the remaining operator at OL and to the remaining operator at OR when a CI octamer is already present. Based on the experiment of Figure 4, which showed that a single operator at OL and a single operator at OR do not cooperate, ΔGtet was not applied to species in which octamer formation could not occur.
To generate curves that would simulate our LacZ reporter data (LacZ activity as a function of CI concentration), we assigned LacZ activities for PR and PRM for each operator configuration, based on the LacZ activities measured with our reporter constructs (derepressed or fully repressed for PR; basal, activated, or repressed for PRM; Fig. 2; Dodd et al. 2001). The LacZ values used are listed in Table 1; further details are given in Materials and Methods. CI concentrations were converted to lysogenic units by using a lysogenic CI concentration of 370 nM (Table 1). Initially, we found that the simulations gave CI activities that were ∼10-fold more effective than expected from the reporter data. Binding of CI to nonspecific DNA in vivo should lower the concentration of CI available to interact with specific sites, and we were able to align theoretical repression curves with in vivo repression data by considering this nonspecific binding (Materials and Methods). The parameter KNS (the affinity of CI for a single nonspecific site) was adjusted to give a reasonable fit to reporter data for PRM and for PR in the absence of CI binding to OL.
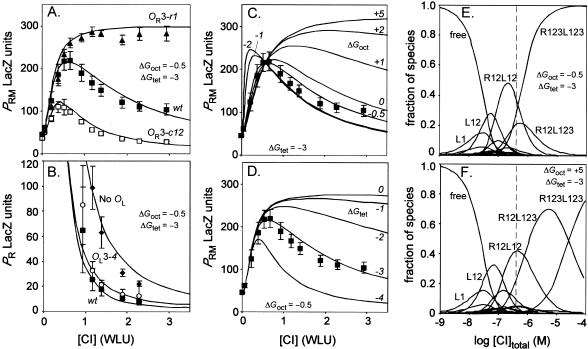
Reasonable fits to all lacZ reporter data for wild-type and mutant PRM and PR promoters in the presence of OL were obtained with free energy values of -0.5 kcal/mole for ΔGoct and -3 kcal/mole for ΔGtet. Figure 5A shows the simulations for PRM when OR is wild-type or carries mutations in OR3 that abolish (r1) or improve (c12) CI binding. Figure 5B shows the simulation of the PR data from Figure 2B. There is a reasonable fit between data and theory at higher CI concentrations, reproducing the weakening of PR repression by the loss of OL and also the very slight effect of the OL3-4 mutation. However, for reasons we do not understand, the theoretical curves predict stronger repression of PR than we observed at CI concentrations below 1 WLU.
Figure 5.
Physicochemical modeling of CI regulation. (A-D) Simulation of PRM and PR lacZ reporter data using a physicochemical model incorporating the long-range CI interactions (see text). Simulations (solid lines) using ΔGoct = -0.5 kcal/mole and ΔGtet = -3.0 kcal/mole for (A) wt PRM or PRM carrying the r1 or the c12 mutation in OR3 (OL present; data from Dodd et al. 2001); (B) the PR reporter data of Fig. 2B. (C,D) The effect of varying ΔGoct from -2 to +5 kcal/mole with ΔGtet fixed at -3.0 kcal/mole (C) or varying ΔGtet from -4 to 0 kcal/mole with ΔGoct fixed at -0.5 kcal/mole (D) on the simulation of the wt PRM data. (E,F) The fraction of each of the 64 possible CI-operator species (looped and unlooped fractions of each species combined) predicted by the model over a range of CI concentrations, in the presence (E; ΔGoct = -0.5, ΔGtet = -3.0 kcal/mol) or effective absence (F; ΔGoct = +5, ΔGtet = -3.0 kcal/mol) of the long-range interactions. Minor species are not labeled; the dashed line shows the lysogenic CI concentration.
The effects of alterations in ΔGoct and ΔGtet on the fit of the simulations with the data are shown for wild-type PRM in Figure 5C,D. With ΔGtet held at -3 kcal/mole, lowering ΔGoct below -0.5 kcal/mole produced a more sensitive CI activation of PRM, whereas increasing ΔGoct values above -1 kcal/mole weakened CI repression of PRM (Fig. 5C). Setting ΔGoct to +5 kcal/mole, effectively eliminating DNA looping, showed the lack of PRM repression that would be expected for a CI mutant specifically defective in octamerization. Lowering ΔGtet while holding ΔGoct at -0.5 kcal/mole also increased CI repression of PRM (Fig. 5D).
Figure 5E shows the population distribution of the 64 different operator occupation species (looped and unlooped configurations are combined) as a function of total repressor concentration, calculated using ΔGoct = -0.5 and ΔGtet = -3 kcal/mole. With these cooperativities, the four-dimer and five-dimer species that are able to form loops are in the looped configuration 69% of the time, whereas the fully liganded R123L123 species is 99.7% looped. Although the model allows the ΔGoct cooperativity term for all potential octameric species, those species able to form an R12L12 octamer account for at least 90% of the possible octamer-containing species at any CI concentration.
Strikingly, despite the large number of possibilities, only five CI-bound species achieve more than a 10% share of the total fraction at any CI concentration (Fig. 5E). At the lysogenic CI concentration, three species make up 90% of the total: the R12L12 (∼40%), the R12L123 complex (∼20%), and the fully occupied R123L123 species (∼30%); with ∼72% of the total being the looped forms of these species. Remarkably, the lysogenic concentration occurs at a region of the graph at which the R12L12 and R123L123 curves have steep and opposite slopes, such that small fluctuations in CI concentration would produce large changes in the ratio of activated to repressed PRM. Thus the lysogenic state seems to be poised for maximal responsiveness in CI negative autoregulation. Figure 5F gives the distribution of species calculated for the case where looping between OR and OL is effectively absent (ΔGoct = +5). The major effect is the change in the fractions of the three lysogenic species, with the R123L123 species populated only at repressor levels well above the lysogenic concentrations, and only the two PRM-activated species prevalent at physiological CI concentrations.
Discussion
The OL-OR model
Our results support the model depicted in Figure 1 describing the CI interactions that regulate transcription from the OL-OR region.
First, OL3 was shown to be necessary for CI repression of PRM at physiological CI concentrations. Blocking binding of λ CI to OL3, either by the OL3-4 mutation (Fig. 2A) or by substitution with a P22 operator (Fig. 3), eliminated repression of PRM by lysogenic CI concentrations in reporter constructs in which OL was located 3.8 kb away from OR. The requirement for OL3 in CI negative autoregulation and efficient prophage induction was confirmed in the native phage context where OL3 and OR3 are separated by 2.4 kb. Because OR3 is necessary for repression of PRM at physiological CI concentrations (Fig. 2A) and is sufficient for repression at high CI concentrations (Fig. 4) and because CI binding at OL3 is unable to repress PRM directly (Fig. 4), we conclude that OL3 works by fostering CI binding at OR3.
Second, the proposal that OL3 facilitates CI binding at OR3 through a direct interaction between the dimers bound at OL3 and OR3 was supported by the finding that OL3 did not contibute significantly to the enhancement of CI repression of PR by OL, indicating that CI at OL3 does not interact significantly with CI bound to OR1 or OR2 (Fig. 2B). The conclusion that CI bound at OL3 interacts primarily with CI bound at OR3 was strengthened by the physicochemical modeling, which indicated (assuming that ΔGoct is the same for all octamers) that L12R12 is the favored octameric species at lower CI concentrations, with the OL3 and OR3 sites becoming filled at higher CI concentrations.
Third, the idea that the sole role of the OL3-bound CI dimer in facilitating PRM repression is to make a favorable protein-protein contact with the dimer bound at OR3 was supported by the demonstration that the CI dimer bound at OL3 could be functionally replaced by a hybrid repressor bearing the λ CI CTD but a heterologous DNA-binding domain. Specifically, OL3 was replaced with a P22 operator, and the hybrid repressor bore the P22 repressor DNA-binding domain (Fig. 3). Moreover, the protein-protein contact of the λ CI CTDs was shown to be similar to that providing short-range cooperativity between CI dimers, because PRM repression was lost when the λ CI CTD of the hybrid repressor carried an amino acid substitution that prevents short-range cooperativity.
Fourth, OL3 and OR3 did not cooperate in the absence of the other operators, even at CI concentrations where OR3 and, presumably, OL3 were occupied (Fig. 4). Thus, the energetic benefit of the OR3-OL3 interaction must not outweigh the energetic cost of DNA looping. This supports the idea that the OR1.OR2-OL1.OL2 interaction, which mediates repression of PR and PL and activation of PRM at low CI concentrations, occurs first and `pays' the cost of the DNA looping. Thus, the looped structure that is formed by the interaction of CI dimers bound at OR1.OR2 and OL1.OL2 presumably juxtaposes OL3 and OR3 so that CI dimers bound at those sites can interact with little additional DNA conformational change.
Fifth, the regulatory model (Fig. 1) was supported by a thermodynamic analysis that incorporated the long-range interactions and was able to simulate PRM and PR reporter data reasonably well. We obtained an estimate of ΔGoct = -0.5 kcal/mole for CI octamerization with a 3.8-kb DNA loop and an estimate of ΔGtet = -3.0 kcal/mole for the OR3-OL3 CI tetramerization reaction, a value similar to short-range cooperativities between CI dimers (Koblan and Ackers 1992). Although these cooperativities are small, they are able to significantly improve CI repression of the PR lytic promoter and give repression of PRM at the lysogenic CI concentration. These long-range cooperativities appear to position the lysogenic state ideally for CI negative autoregulation, such that small changes in CI concentration should produce relatively large compensatory changes in PRM activity.
It is important to note that the values for the long-range cooperativity terms (ΔGoct and ΔGtet) used in our physicochemical model cannot provide structural information. Formally, ΔGoct represents a stabilization of the four-dimer CI complex and ΔGtet represents an additional stabilization of the six-dimer CI complex. Although plausible, it is not certain that CI is able to form an octamer while bound simultaneously to OL and OR. In theory, the four-dimer complex could be a pair of `trans' tetramers, for example, a CI tetramer linking OL1 and OR1 with another tetramer linking OL2 and OR2. Further structural and mutational studies will be necessary to resolve these uncertainties, and also uncertainties about the path that the DNA takes through the CI complex.
The role of DNA in long-range interactions
It is clear that the DNA plays a critical role in the interaction between CI dimers at OL3 and OR3, because the bringing together of these two DNA sites by the CI octamerization reaction is a prerequisite for the cooperative interaction to occur. Such assisted cooperativity is presumably a general feature of large nucleoprotein complexes.
What role does the DNA play in the initial long-range interaction of CI tetramers bound at OL1.OL2 and at OR1.OR2? The ΔGoct cooperativity term represents the net free energy change when the four-dimer complex goes from the unlooped to the looped configuration. ΔGoct is equal to -RT lnKc, where Kc is the equilibrium constant for the intramolecular cyclization reaction (Shore et al. 1981), and can be seen as the sum of two free energy changes, ΔGP and ΔGD. ΔGP is a favorable change due largely to protein-protein association and is equal to -RT lnKa, where Ka is the equilibrium constant for the bimolecular association reaction for protein complexes on separate DNA molecules (Shore et al. 1981). In our case, this is the octamerization reaction for CI tetramers bound to OL and OR when the two sites are unlinked. ΔGD is an unfavorable change due to bringing the two DNA sites together. For a DNA loop of this size, ΔGD is expected primarily to reflect the entropic cost of the loss of freedom for the DNA due to the protein-induced restraint (Rippe 2001).
Neither ΔGP nor ΔGD are known for the long-range CI octamerization reaction. However, provisional estimates of their values indicate that the reaction should be unfavorable, suggesting that there are extra factors favoring the reaction. A provisional estimate for ΔGP (-9.1 kcal/mol) can be obtained from the free energy of association of two CI tetramers in the absence of DNA, as determined by sedimentation equilibrium experiments (Senear et al. 1993). Octamerization of CI bound to short single-operator DNA fragments is only slightly less favorable (Rusinova et al. 1997). Unfortunately, data for CI association in the presence of double operator fragments are not available. Provisional estimates of ΔGD can be obtained from modeling of in vitro DNA cyclization. Because ΔGD = ΔGoct-ΔGP, ΔGoct = -RT lnKc and ΔGP = -RT lnKa (see above), then ΔGD = -RT ln(Kc/Ka). The ratio Kc/Ka is the parameter j (Shore et al. 1981), which is the local concentration of one site on the DNA relative to another site on the DNA. Theoretical models have been developed to permit calculation of j over a large range of DNA separations, and these values fit reasonably well with observed effects of DNA length on the rate of cyclization of linear, naked DNA in vitro (Rippe 2001). We used the equation of Rippe to calculate j for a 3.8-kb separation as 1.1 × 10-8 M (Eq. 3: Kuhn length l = 100 nm, Lm = 0.34 nm per bp, d = 0; Rippe 2001), giving an estimate of ΔGD = 11.3 kcal/mole. Note that, by this calculation, the DNA tether makes the concentration of OL relative to OR ∼sevenfold higher than the concentration of an unlinked OL site in the cell (∼1.5 × 10-9 M; Table 1). Combining these provisional estimates for ΔGP and ΔGD gives a ΔGoct value of +2.2 kcal/mole, considerably higher than our ΔGoct value of -0.5 kcal/mole and a value that would make DNA looping and repression of PRM inefficient (see Fig. 5C). How then can the observed efficiency of the OL-OR interaction in vivo be explained?
There are two reasons why ΔGP might be more favorable than our provisional estimate of -9.1 kcal/mole. First, the in vitro conditions used for measuring CI octamerization may not adequately reflect in vivo conditions; CI may octamerize more readily in solution in vivo. (Note that, if CI octamers were to form too readily, then the OL1.OL2 and OR1.OR2 operators could each become occupied by octamers rather than tetramers, which would prevent DNA looping and cause a loss of regulation of PRM.) Second, CI octamerization may be more favorable when CI dimers are bound to OL and OR DNA than when they are unbound. It is not unreasonable that CI DNA binding could favorably affect the octamerization reaction, though no such effect is seen with single operators (Rusinova et al. 1997).
The observed efficiency of the long-range CI looping reaction could be explained without any change to ΔGP if the long-range DNA interaction in vivo is some 80-fold more efficient than for naked, linear DNA in vitro. A number of in vivo factors seem capable of giving this degree of improvement (Rippe 2001). Long-range interactions may be aided by compaction of the DNA and by changes to DNA flexibility caused by the binding of nucleoid or other proteins. These parameters are represented in the equation for j, but reliable in vivo values are not known (Rippe 2001). Specific DNA bends, either intrinsic or due to bound proteins, if properly located between the interacting sites, can also improve DNA interactions. However, we do not expect this factor to be important in our case because the effect of specific bends is likely to be weak at large distances (Rippe 2001), and we also know that the OL-OR interaction occurs efficiently with two completely different intervening sequences (on the phage chromosome and on the lacZ reporter). A further in vivo factor thought to affect ΔGD is DNA supercoiling. Supercoiling is expected to assist interactions between DNA sites because it should cause the DNA to wind into compact plectonemic structures in which separate DNA segments are more frequently in contact with each other. Physical simulations suggest that DNA supercoiling can improve j by two orders of magnitude (Vologodskii et al. 1992). This activity of supercoiling is also supported by observations of long-range DNA-based protein-protein interactions in vitro, in both eukaryotic (Barton et al. 1997) and prokaryotic systems (Liu et al. 2001), and in vivo in bacteria (see Scheirer and Higgins 2001 and references therein).
Dröge and Müller-Hill (2001) suggested that the location of OL and OR on the same DNA molecule is not important in the CI octamerization reaction and that OL acts solely as a `scaffold' to foster the assembly of a CI tetramer that would not otherwise form in the cell. They speculated that eukaryotic enhancers may act largely as scaffolds for the assembly of unique and potent protein complexes. Although OL is in part acting in this way, we believe that the `tethering' of OL to OR by the intervening DNA is critical. As discussed above, the linkage of OL to OR by 3.8 kb of DNA may improve their interaction ∼560-fold compared to the interaction of unlinked sites within the cell (the product of a sevenfold increase in effective concentration over unlinked sites and an 80-fold improvement if ΔGD is improved by 2.7 kcal/mole in vivo, see above). The idea that the tethering of protein binding sites can significantly increase their relative concentration in vivo, even at such large DNA distances, helps to explain the action of enhancers in more complex cells. The λ CI-OL-OR interaction provides a well defined and tractable experimental system for examining basic questions about the requirements for such long-range interactions on DNA in vivo.
Materials and methods
Strains and media
NK7049 (ΔlacIZYA)χ74 galOP308 StrR Su- from Bob Simons (Simons et al. 1987) was the host for all LacZ assays and phage work. DH5α and MC1061 were hosts for recombinant DNA work. Cells were grown at 37°C in LB (Miller 1972) with addition of ampicillin (100 μg/mL except 30 μg/mL for pZC320) and kanamycin (50 μg/mL for pUHA1). λatt80 was λimm λh φ80att φ80 from Gary Gussin (University of Iowa, Iowa City).
Construction of lacZ reporter fusions
In all constructions, the sequence of all inserted, mutagenized, or PCR-amplified regions of DNA incorporated into constructs was confirmed. Further details of the cloning procedures are available on request.
The construction of the λimm21 phage lacZ reporter vectors λRS45ΔYA (deletion in lacY and lacA; no OL) and λRS45ΔYAOL (carrying PL-.OL1.OL2.OL3; depicted in Fig. 2A) is described in Dodd et al. (2001). λRS45ΔYAOL.OL3-4, λRS45ΔYAOL3, λRS45ΔYAOL3-4, and λRS45ΔYAOL.OL3P22 were made using the same approach. λRS45ΔYAOL.OL3-4 is the same as λRS45ΔYAOL except that the sequence of OL3 bears four changes (underlined) to inactivate CI binding: TATCACTAGAGTTGGTT. λRS45ΔYAOL.OL3P22 is the same as λRS45ΔYAOL except that the sequence of OL3 (17 bp) and one bp to its right were substituted by the 18-bp P22 OR1 operator: ATTAAGTGTTCTTTAAT. In λRS45ΔYAOL3 and λRS45ΔYAOL3-4, OL1 and OL2 have been removed, leaving the sequence GGAGATAATTTATCACCGCAGATGGTTAT (or its OL3-4 derivative) between the BsrGI and SgrAI sites (OL3 and the remainder of OL2 are underlined).
Plasmid-based lacZ fusions were constructed in pTL61T (Linn and St. Pierre 1990), transferred to the phage lacZ reporter vectors for insertion into the E. coli chromosome, and single-copy confirmed, as described in Dodd et al. (2001).
Construction of the PRMwt::lacZ and PRMr1::lacZ operon fusions in pTL61T is described in Dodd et al. (2001); the r1 version carries the OR3-r1 mutation that inactivates CI binding. Construction of PRMΔ(12)3-wt::lacZ and PRMΔ(12)3-r1::lacZ operon fusions, in which OR1 and OR2 have been removed, was the same except that the cloned PCR product contained sequence from the -37 to +62 region of PRM (4 bp of OR2 remain). The pRwt::lacZ operon fusion was made the same way, except that the restriction sites added to the end of the PCR fragment were swapped, so that the fragment was inserted in the reverse orientation.
Expression and quantitation of CI in vivo
The CI expression plasmids pZC320cI (mini-F origin, ApR, Plac+::cI) or pZE15cI (colE1 origin, ApR, Plac+::cI), in combination with pUHA1 (supplies lac repressor), were used to supply IPTG-controlled levels of wt CI to lacZ reporters; cellular CI levels from pZC320cI were previously quantitated using a gel shift assay, by calibration against CI binding activites of extracts from λ lysogens (Dodd et al. 2001). Similar quantitation of CI levels produced by pZE15cI (with pUHA1) gave values of 2.5, 13.5, 38.9, 62.5, and 57.5 WLU (means of two extracts) for IPTG concentrations of 0, 50, 100, 200, and 500 μM, respectively.
The expression plasmid pFW7-280Δ (Whipple et al. 1998) was used to supply IPTG-controlled levels of the P22-λ hybrid repressor, and plasmid pLR1ΔcI (Whipple et al. 1994), which does not encode a repressor, served as the control plasmid. These plasmids are derived from pBR322 and provide resistance to ampicillin. Plasmid pAD325 (Derman et al. 1993) is a derivative of plasmid pACYC184 that contains the lacIq gene and provides resistance to chloramphenicol. Cells containing plasmids pFW7-280Δ (or pLR1ΔcI) and pAD325 were grown in the presence of 100 μg/mL carbenicillin and 25 μg/mL chloramphenicol. LacZ assays for Figures 2 and 4 were carried out using 96-well microtiter plates as described in Dodd et al. (2001), with the modification that for assaying repression of PR, we found that subculturing and a second overnight incubation with IPTG before preparation of logphase cultures for assay was needed to remove residual LacZ. The method used for the LacZ assays of Figure 3 is essentially that of Miller (1972) modified as described in Whipple et al. (1998).
λOL3-4 construction and characterization
An OL-containing PCR fragment containing λ sequences from 35219 to 35858 was inserted into pBluescript KS+ (Stratagene) to give pBS-OL+. The OL3-4 mutation (see above) was introduced into pBS-OL+ using the Quikchange method (Stratagene). The mutant HpaI:35260-PvuI:35790 fragment was used to replace the equivalent wt fragment in pAP831, and the mutation was recombined in vivo from pAP831-OL3-4 onto λimm434 (Dodd et al. 2001), to give λOL3-4.
Physicochemical modeling
The relative probability of each operator configuration, s, is given as
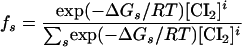 |
where ΔGs is the sum of the contributions of all the free energies (see Table 1) for a given configuration (s), [CI2] is the concentration of free CI dimers, and i is the stoichiometry of CI dimers bound in each s configuration (Shea and Ackers 1985). It was assumed that monomers and dimers of CI are in equilibrium in solution and that only dimers can bind DNA, either specifically or nonspecifically. Results are plotted in terms of total monomer concentration, taking into account nonspecific binding, according to
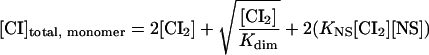 |
where Kdim is the dimerization constant for repressor, KNS is the binding constant describing nonspecific binding of repressor to DNA, and [NS] is the molar concentration of nonspecific sites in an E. coli cell (Table 1). KNS was obtained by simulation (see text).
For each configuration, we assigned basal PR activity when OR1 and OR2 were unoccupied, repressed PR activity if either OR1 or OR2 were occupied, basal PRM activity when OR2 and OR3 were unoccupied, activated PRM activity when OR2 but not OR3 were occupied, and repressed PRM activity when OR3 was occupied. Basal promoter values were taken from reporter activities in the absence of CI; for PRM (wt, r1, and c12), values were from Dodd et al. (2001), and for PR (wt, OL3-4, and no OL), values were from Figure 2B (no IPTG). The activated PRM values for the unlooped species were the maximal activities seen for PRM (wt, r1, and c12) in the absence of OL (Dodd et al. 2001). The activated PRM values for the looped species were set 26% lower, in line with the effect of OL on the maximal activity of PRM-r1 (Dodd et al. 2001). The values used are given in Table 1.
Acknowledgments
We thank Stanley Brown, John Little, Mark Ptashne, Kim Sneppen, and members of the Egan and Hochschild labs for discussions; and Rachel Schubert for comments on the manuscript. This research was supported by the Australian Research Council (J.B.E.) and the U.S. NIH (A.H. and J.B.E.).
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Article and publication are at http://www.genesdev.org/cgi/doi/10.1101/gad.1167904.
References
- Arkin A., Ross, J., and McAdams, H.H. 1998. Stochastic kinetic analysis of developmental pathway bifurcation in phage λ-infected Escherichia coli cells. Genetics 149: 1633-1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aurell E., Brown, S., Johanson, J., and Sneppen, K. 2002. Stability puzzles in phage λ. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 65: 051914. [DOI] [PubMed] [Google Scholar]
- Ballivet M., Reichardt, L.F., and Eisen, H. 1977. Purification and properties of coliphage 21 repressor. Eur. J. Biochem. 73: 601-606. [DOI] [PubMed] [Google Scholar]
- Ballivet M., Eisen, H., and Reichardt, L.F. 1978. Purification and properties of phage P22 c2 repressor. Eur. J. Biochem. 82: 175-180. [DOI] [PubMed] [Google Scholar]
- Barton M.C., Madani, N., and Emerson, B.M. 1997. Distal enhancer regulation by promoter derepression in topologically constrained DNA in vitro. Proc. Natl. Acad. Sci. 94: 7257-7262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell C.E. and Lewis, M. 2001. Crystal structure of the λ repressor C-terminal domain octamer. J. Mol. Biol. 314: 1127-1136. [DOI] [PubMed] [Google Scholar]
- Bell C.E., Frescura, P., Hochschild, A., and Lewis, M. 2000. Crystal structure of the λ repressor C-terminal domain provides a model for cooperative operator binding. Cell 101: 801-811. [DOI] [PubMed] [Google Scholar]
- Burz D.S. and Ackers, G.K. 1996. Cooperativity mutants of bacteriophage λ cI repressor: Temperature dependence of self-assembly. Biochemistry 35: 3341-3350. [DOI] [PubMed] [Google Scholar]
- Carter D., Chakalova, L., Osborne, C.S., Dai, Y.F., and Fraser, P. 2002. Long-range chromatin regulatory interactions in vivo. Nat. Genet. 32: 623-626. [DOI] [PubMed] [Google Scholar]
- Dandanell G., Valentin-Hansen, P., Larsen, J.E., and Hammer, K. 1987. Long-range cooperativity between gene regulatory sequences in a prokaryote. Nature 325: 823-826. [DOI] [PubMed] [Google Scholar]
- Derman A.I., Puziss, J.W., Bassford Jr., P.J., and Beckwith, J. 1993. A signal sequence is not required for protein export in prlA mutants of Escherichia coli. EMBO J. 12: 879-888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd I.B., Perkins, A.J., Tsemitsidis, D., and Egan, J.B. 2001. Octamerization of λ CI repressor is needed for effective repression of P(RM) and efficient switching from lysogeny. Genes & Dev. 15: 3013-3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donachie W.D. and Robinson, A.C. 1987. Cell division: Parameter values and the process. In Escherichia coli and Salmonella typhimurium (ed. F.C. Neidhardt), pp. 1578-1593. American Society for Microbiology, Washington, DC.
- Dröge P. and Müller-Hill, B. 2001. High local protein concentrations at promoters: Strategies in prokaryotic and eukaryotic cells. Bioessays 23: 179-183. [DOI] [PubMed] [Google Scholar]
- Gralla J.D. and Collado-Vides, J. 1996. Organization and function of transcriptional regulatory elements. In Escherichia coli and Salmonella (ed. F.C. Neidhardt), pp. 1232-1245. ASM Press, Washington, DC.
- Hasty J., McMillen, D., and Collins, J.J. 2002. Engineered gene circuits. Nature 420: 224-230. [DOI] [PubMed] [Google Scholar]
- Hochschild A. 2002. The λ switch: cI closes the gap in autoregulation. Curr. Biol. 12: R87-R89. [DOI] [PubMed] [Google Scholar]
- Hochschild A. and Ptashne, M. 1986. Cooperative binding of λ repressors to sites separated by integral turns of the DNA helix. Cell 44: 681-687. [DOI] [PubMed] [Google Scholar]
- Johnson A.D., Poteete, A.R., Lauer, G., Sauer, R.T., Ackers, G.K., and Ptashne, M. 1981. Lambda repressor and cro-components of an efficient molecular switch. Nature 294: 217-223. [DOI] [PubMed] [Google Scholar]
- Koblan K.S. and Ackers, G.K. 1991. Energetics of subunit dimerization in bacteriophage λ cI repressor: Linkage to protons, temperature, and KCl. Biochemistry 30: 7817-7821. [DOI] [PubMed] [Google Scholar]
- ____. 1992. Site-specific enthalpic regulation of DNA transcription at bacteriophage λ OR. Biochemistry 31: 57-65. [DOI] [PubMed] [Google Scholar]
- Linn T. and St. Pierre, R. 1990. Improved vector system for constructing transcriptional fusions that ensures independent translation of lacZ. J. Bacteriol. 172: 1077-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Bondarenko, V., Ninfa, A., and Studitsky, V.M. 2001. DNA supercoiling allows enhancer action over a large distance. Proc. Natl. Acad. Sci. 98: 14883-14888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer R., Meyer, B., and Ptashne, M. 1980. Gene regulation at the right operator (OR) bacteriophage λ. I. OR3 and autogenous negative control by repressor. J. Mol. Biol. 139: 147-161. [DOI] [PubMed] [Google Scholar]
- Miller J.H. 1972. Experiments in molecular genetics, pp. 352-355. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
- Nickels B.E., Dove, S.L., Murakami, K.S., Darst, S.A., and Hochschild, A. 2002. Protein-protein and protein-DNA interactions of sigma70 region 4 involved in transcription activation by λ cI. J. Mol. Biol. 324: 17-34. [DOI] [PubMed] [Google Scholar]
- Pabo C.O., Sauer, R.T., Sturtevant, J.M., and Ptashne, M. 1979. The λ repressor contains two domains. Proc. Natl. Acad. Sci. 76: 1608-1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poteete A.R., Ptashne, M., Ballivet, M., and Eisen, H. 1980. Operator sequences of bacteriophages P22 and 21. J. Mol. Biol. 137: 81-91. [DOI] [PubMed] [Google Scholar]
- Ptashne M. 1998. Agenetic switch: Phage λ and higher organisms. Cell Press & Blackwell Science, Cambridge, MA.
- Reichardt L. and Kaiser, A.D. 1971. Control of λ repressor synthesis. Proc. Natl. Acad. Sci. 68: 2185-2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitzer L.J. and Magasanik, B. 1986. Transcription of glnA in E. coli is stimulated by activator bound to sites far from the promoter. Cell 45: 785-792. [DOI] [PubMed] [Google Scholar]
- Révet B., von Wilcken-Bergmann, B., Bessert, H., Barker, A., and Müller-Hill, B. 1999. Four dimers of λ repressor bound to two suitably spaced pairs of λ operators form octamers and DNA loops over large distances. Curr. Biol. 9: 151-154. [DOI] [PubMed] [Google Scholar]
- Rippe K. 2001. Making contacts on a nucleic acid polymer. Trends Biochem. Sci. 26: 733-740. [DOI] [PubMed] [Google Scholar]
- Rusinova E., Ross, J.B., Laue, T.M., Sowers, L.C., and Senear, D.F. 1997. Linkage between operator binding and dimer to octamer self-assembly of bacteriophage λ cI repressor. Biochemistry 36: 12994-13003. [DOI] [PubMed] [Google Scholar]
- Sarai A. and Takeda, Y. 1989. Lambda repressor recognizes the approximately twofold symmetric half-operator sequences asymmetrically. Proc. Natl. Acad. Sci. 86: 6513-6517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheirer K.E. and Higgins, N.P. 2001. Transcription induces a supercoil domain barrier in bacteriophage Mu. Biochimie 83: 155-159. [DOI] [PubMed] [Google Scholar]
- Senear D.F., Brenowitz, M., Shea, M.A., and Ackers, G.K. 1986. Energetics of cooperative protein-DNA interactions: Comparison between quantitative deoxyribonuclease footprint titration and filter binding. Biochemistry 25: 7344-7354. [DOI] [PubMed] [Google Scholar]
- Senear D.F., Laue, T.M., Ross, J.B., Waxman, E., Eaton, S., and Rusinova, E. 1993. The primary self-assembly reaction of bacteriophage λ cI repressor dimers is to octamer. Biochemistry 32: 6179-6189. [DOI] [PubMed] [Google Scholar]
- Shea M.A. and Ackers, G.K. 1985. The OR control system of bacteriophage λ: A physical-chemical model for gene regulation. J. Mol. Biol. 181: 211-230. [DOI] [PubMed] [Google Scholar]
- Shore D., Langowski, J., Baldwin, R.L., Burz, D.S., and Ackers, G.K. 1981. DNA flexibility studied by covalent closure of short fragments into circles. Proc. Natl. Acad. Sci. 78: 4833-4837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons R.W., Houman, F., and Kleckner, N. 1987. Improved single and multicopy lac-based cloning vectors for protein and operon fusions. Gene 53: 85-96. [DOI] [PubMed] [Google Scholar]
- Vologodskii A.V., Levene, S.D., Klenin, K.V., Frank-Kamenetskii, M., and Cozzarelli, N.R. 1992. Conformational and thermodynamic properties of supercoiled DNA. J. Mol. Biol. 227: 1224-1243. [DOI] [PubMed] [Google Scholar]
- Whipple F.W., Kuldell, N.H., Cheatham, L.A., and Hochschild, A. 1994. Specificity determinants for the interaction of λ repressor and P22 repressor dimers. Genes & Dev. 8: 1212-1223. [DOI] [PubMed] [Google Scholar]
- Whipple F.W., Hou, E.F., and Hochschild, A. 1998. Amino acid-amino acid contacts at the cooperativity interface of the bacteriophage λ and P22 repressors. Genes & Dev. 12: 2791-2802. [DOI] [PMC free article] [PubMed] [Google Scholar]