Abstract
Dexamethasone has been a mainstay of anti-myeloma therapy for 20 years. However, it is intensely immunosuppressive and may limit the efficacy of the immune system to control myeloma, and limit the exciting opportunities to use immune stimulating drug therapies such as Lenalidomide to maximize the fight against this disease.
Keywords: NK cells, activating receptors, dexamethasone, lenalidomide, myeloma
There is little doubt that the use of high dose corticosteroid therapy in the form of either dexamethasone or prednisolone when used in combination with either conventional chemotherapy, such as cyclophosphamide, melphalan or adriamycin, or, more latterly, novel agents such as bortezomib or lenalidomide have a major therapeutic impact in the initial therapy of multiple myeloma (MM).1
Malignant plasma cells, in common with other lymphoid cell subtypes, show a high level of sensitivity to corticosteroid-induced apoptosis and therefore these agents are logical choices to control the devastating effects of MM.
Over the past ten years, therapeutic regimens against MM have increasingly adopted novel non-chemotherapeutic drugs. Included in this therapeutic shift have been the immunomodulatory drugs (IMiDs) thalidomide and its pharmacological derivatives lenalidomide and pomalidomide.1,2 Immunologically, IMiDs are a fascinating group of agents which, although the intracellular mechanisms are still to be defined, show potent immunostimulatory activity including enhancement of NK cell function and T-cell co-simulation.3,4
However, as part of the clinical trial development of IMiD therapies, they have often been given in combination with high-dose dexamethasone and compared with control cohorts treated with either dexamethasone alone or conventional dexamethasone-containing salvage chemotherapy.
Given the well-known immunosuppressive effects of high-dose corticosteroids, our previously published analysis examined whether the immunostimulatory capacity of lenalidomide could be maintained in a clinical setting when co-administered with dexamethasone.5 Our correlative study the cellular immunology of patients with relapsed or refractory MM treated on a prospective clinical trial of lenalidomide 15 mg daily for 21 days per 28 day cycle and dexamethasone 20 mg four days per week for three weeks of each 28 day cycle (total per cycle dose = 240 mg). We identified that the absolute numbers of NK cells in the peripheral blood through serial cycles of therapy were well maintained or increased. Conversely, we identified that NK cell cytoxicity as measured by their ability to lyse K562 targets progressively declined over the course of therapy. Similarly, we identified that both absolute number and percent of CD4+ T-cells in the peripheral blood of patients were substantially suppressed at enrolment and did not improve despite patients achieving demonstrable clinical benefit from lenalidomide and dexamethasone therapy.
Our initial clinical investigations lead to an in vitro dissection of the effects of lenalidomide alone or in combination with dexamethasone. We identified that, as shown by others, Lenalidomide did enhance NK cell function in vitro when used alone. However, the presence of even very small doses of dexamethasone resulted in complete suppression of NK cell cytotoxic function.
In order to understand this phenomenon further, we examined the role of other lymphocyte subsets in this culture system and identified that the major immunostimulatory capacity of lenalidomide on NK cell function was via the lenalidomide-driven production of IL-2 from CD4+ T cells. Again when cultured in the presence of dexamethasone, IL-2 production from CD4+ T cells was completely prevented despite the addition of lenalidomide.
We therefore concluded that in a patient with multiple myeloma who already has a substantial numerical defect in CD4+ T cells, the application of both lenalidomide and dexamethasone in combination while very effective therapy against MM, resulted in complete abrogation of the potential immunostimulatory capacity of lenalidomide. Our in vitro analyses also identified that the effects of dexamethasone on CD4+ T-cell IL-2 production was ongoing even once the dexamethasone was removed from the culture system.
The implications of these observations are clear. The presence of dexamethasone in vivo will result in substantial suppression of IMiD-driven NK cell-mediated immune response against MM. Furthermore, the profound suppression of an already defective IL-2 response is likely to blunt any potentially adaptive T-cell response against MM. This phenomenon may underline the reasons why, when used with lenalidomide, high-dose dexamethasone resulted in poorer patient survival compared with low-dose dexamethasone despite the higher rates of response seen in the high-dose dexamethasone arm.6
Further to our initial findings we have also recently identified that dexamethasone results in suppressive alterations of NK cell receptor expression, preventing the lenalidomide-induced expression of the critical NK cell activating receptors NKG2D and NKp46 (unpublished data), resulting in further abrogation of NK cell function. Our observations have recently been confirmed by others, in clinical observations when dexamethasone was used at > 160 mg per cycle.7 We have recently extended these observations via our ongoing analysis of the immunology in patients with newly diagnosed MM enrolled on a range of lenalidomide and dexamethasone dose combinations. We have been able to compare the NK cell function in patients who have yet to be exposed to any therapy and following exposure to either Lenalidomide alone or Lenalidomide in combination with very low dose dexamethasone of 60 mg per cycle. In these comparative analyses we have found, somewhat reassuringly, that if the dexamethasone is used at these very low doses (< 60 mg / cycle) it did not adversely alter the expression NK cell activating receptors (except for DNAM-1) in patients with MM (Fig. 1)8 indicating that NK cell function may well be preserved in vivo.
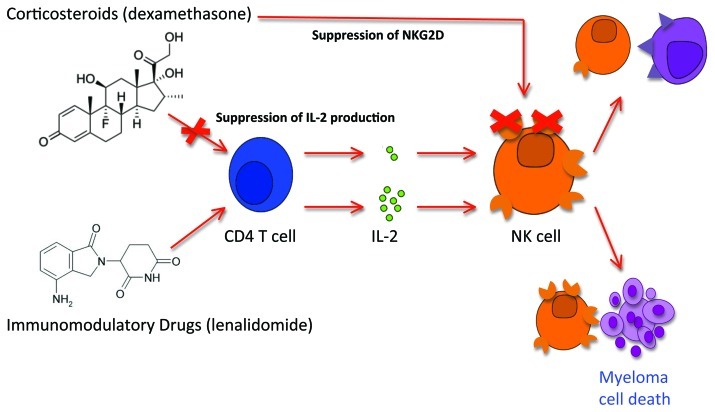
Figure 1. The immune-stimulatory effects of immunomodulatory drugs are inhibited by concurrent corticosteroid administration. Enhancement of NK cell cytotoxicity by immunomodulatory drugs such as lenalidomide require CD4 T cells as intermediates for the production of IL-2. In the presence of corticosteroids, which is routinely used in the treatment of multiple myeloma patients, the NK stimulatory effect is completely abrogated via two mechanisms: (1) suppression of IL-2 production by CD4 T cell; and (2) suppression of the major cytotoxic receptor NKG2D on NK cells. The net result of lenalidomide and dexamethasone combination therapy is the irreversible suppression of NK cell cytotoxicity and hence reduced immune mediated responses against myeloma.
Overall, our findings indicate that the immunostimulatory capacity of IMiDs in clinical practice are lost when used in combination with dexamethasone, potentially denying patients the full benefits of immunological control of MM.9
We would conclude that the future design of clinical trials to examine immunologically mediated mechanisms of action must limit the exposure of patients to immunosuppressive agents such as corticosteroids and similarly must incorporate prospectively immunological biomarkers to ensure that the intended immuno-stimulatory effects are actually delivered.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/18963
References
- 1.Bird JM, Owen RG, D’Sa S, Snowden JA, Pratt G, Ashcroft J, et al. E Low9, J Behrens on behalf of the Haemato-oncology Task Force of the British Committee for Standards in Haematology (BCSH) and UK Myeloma Forum. Guidelines for the diagnosis and management of multiple myeloma 2011. Br J Haematol. 2011;154:32–75. doi: 10.1111/j.1365-2141.2011.08573.x. [DOI] [PubMed] [Google Scholar]
- 2.Lacy MQ, Allred JB, Gertz MA, Hayman SR, Short KD, Buadi F, et al. Pomalidomide plus low-dose dexamethasone in myeloma refractory to both bortezomib and lenalidomide: comparison of 2 dosing strategies in dual-refractory disease. Blood. 2011;118:2970–5. doi: 10.1182/blood-2011-04-348896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davies FE, Raje N, Hideshima T, Lentzsch S, Young G, Tai YT, et al. Thalidomide and immunomodulatory derivatives augment natural killer cell cytotoxicity in multiple myeloma. Blood. 2001;98:210–6. doi: 10.1182/blood.V98.1.210. [DOI] [PubMed] [Google Scholar]
- 4.LeBlanc R, Hideshima T, Catley LP. Immunodulatory drug costimulates T cells via the B7-CD28 pathway. Blood. 2004;103:1787–90. doi: 10.1182/blood-2003-02-0361. [DOI] [PubMed] [Google Scholar]
- 5.Hsu AK, Quach H, Tai T, Prince HM, Harrison SJ, Trapani JA, et al. The immunostimulatory effect of lenalidomide on NK-cell function is profoundly inhibited by concurrent dexamethasone therapy. Blood. 2011;117:1605–13. doi: 10.1182/blood-2010-04-278432. [DOI] [PubMed] [Google Scholar]
- 6.Rajkumar SV, Jacobus S, Callander NS, Fonseca R, Vesole DH, Williams ME, et al. Eastern Cooperative Oncology Group Lenalidomide plus high-dose dexamethasone versus lenalidomide plus low-dose dexamethasone as initial therapy for newly diagnosed multiple myeloma: an open-label randomised controlled trial. Lancet Oncol. 2010;11:29–37. doi: 10.1016/S1470-2045(09)70284-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carter CL, Fleyler S, Smalle N. Effect of combined dexamethasone/lenalidomide therapy on NK cell receptor levels in myeloma patients. Blood. 2011;118:6465–6. doi: 10.1182/blood-2011-08-372680. [DOI] [PubMed] [Google Scholar]
- 8.Hsu AK, Quach H, Tai T, Prince HM, Harrison SJ, Trapani JA, et al. Dexamethasone dose alters expression of NK activationg receptors in vivo. Blood. 2011;117:1605–15. doi: 10.1182/blood-2010-04-278432. [DOI] [PubMed] [Google Scholar]
- 9.Mitsiades CS. How “immunomodulatory” are IMIDs? Blood. 2011;117:1440–1. doi: 10.1182/blood-2010-11-317156. [DOI] [PubMed] [Google Scholar]