Abstract
Breast cancers (BCs) comprise heterogeneous subtypes of various prognoses. An active anti-tumor immune profile usually correlated with a better survival. Two current major challenges of BC research are to understand the inter-relations between BC and anti-tumor immunity, and to identify candidates whose targeting would contribute to enhance anti-tumor efficiency.
Keywords: NK cells, anti-tumor immunity, breast, immuno-editing, immunosurveillance
Innate immunity maintains poised policemen such as natural killer (NK) cells that recognize and destroy cells that turn into troublemakers, as is the case of tumor cells. NK-cells, but also other members of anti-tumor immunity, have this natural ability to distinguish normal cells from “modified” cancer cells through the expression of inhibitory and activating receptors triggered during target-cell recognition. The majority of inhibitory receptors are specific for the different HLA-class I molecules expressed by most normal cells. The main activating receptors recognize specific ligands upregulated upon cellular stress, a consequence of the genomic instability that initiate and drive cancer, and/or signals coming from the altered microenvironment. Accordingly, NK-cells can kill target cells that have lost or express low amounts of HLA-class I molecules and that express activating ligands, both reported features of tumor cells.
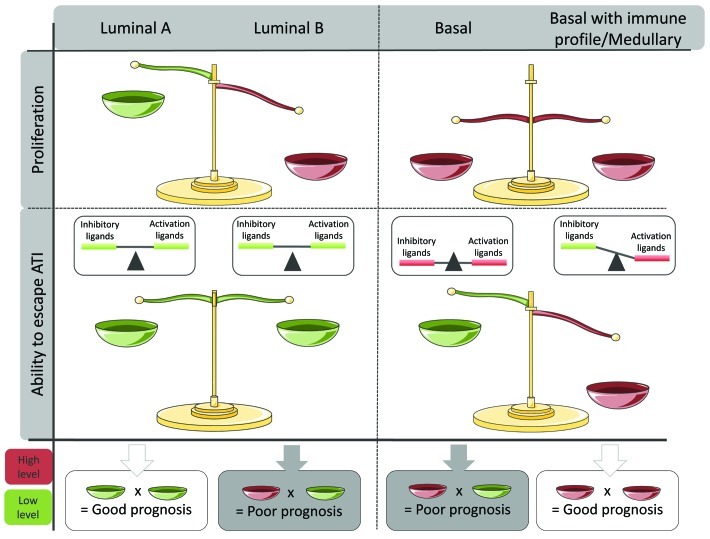
When looking at the expression of NK-cells ligands in breast cancer (BC) patients to understand why immunity fails to control BC occurrence in otherwise healthy individuals, we observed several patterns of ligand expression.1,2 Interestingly, these patterns correspond to different molecular subtypes, themselves characterized by distinct genomic alterations and originating from different precursors.3 The two major BC subtypes are luminal and basal. We observed that luminal BC express lower levels of inhibitory and activating molecules compared with healthy breast tissues, suggesting a poor triggering of NK-cell immunity, and certainly of the other components of anti-tumor immunity as well. In contrast, basal tumors express both high levels of inhibitory ligands and activating ligands. These differences suggested that the phenotype of BC cells at diagnosis was already the result of a more-or-less successful immuno-editing process. Interestingly, a major difference between these two subtypes is disease evolution and clinical outcome. Luminal, but not basal BCs, express hormone receptors and can be subdivided in luminal-A and luminal-B BCs. Luminal-B BCs resist hormone therapy and have a poor prognosis. Luminal-B but not luminal-A BCs are highly proliferative. Thus, within luminal BCs, the main predictor of evolution is proliferation, a feature resulting from intrinsic genomic abnormalities and/or the pro-inflammatory environment. Basal BCs have an overall poor prognosis as compared with luminal BCs. Basal BCs are all highly proliferative and proliferation is therefore neither a determinant nor a predictor of their evolution. Nevertheless, it is possible to identify subgroups of basal BCs with a relatively better prognosis.4 The latter are explicitly characterized by the expression of genes involved in anti-tumor immunity.4-7 Thus, in basal BCs, the main predictor of outcome is the anti-tumor immune response. Why immune response is not as an important predictor of survival in luminal BCs may be because, as mentioned earlier, the involvement of anti-tumor immunity is not the same in the two subtypes and the factor “proliferation” (present in luminal-B but not in luminal-A) eventually outperforms by far the factor “immune response” in survival analyses (Fig. 1).
Figure 1. Involvement of proliferative factors and anti-tumor immunity in the Luminal and basal breast cancer subtypes, at diagnosis, and associated prognosis. Luminal A are poorly proliferative and express low level of both activating and inhibitory receptors of anti-tumor immunity, resulting in a low activation of anti-tumor immunity. Luminal B, which are of poor prognosis, are also poorly immunogenic, but are characterized by a strong proliferative capacity. All basal BCs are highly proliferative and have an overall poor prognosis as compared with luminal BCs. Within basal BCs the cases with the worst prognosis are poorly immunogenic despite the presence of activating ligands of anti-tumor immunity, certainly because of the strong expression of inhibitory ligands and other inhibitory factors such as an increase in Treg recruitment. In this case, the tumor features allowing its proliferation are not constrained, leading to its rapid evolution. A particular subgroup of basal BC can be identified by the presence of an active anti-tumor immune response that can apparently outperform the factor “proliferation” and confer a surprisingly better prognosis to these patients.
Nevertheless, at diagnosis, breast tumors have evolved to become “invisible” to anti-tumor immunity, and those with a “higher” visibility seem to be of better prognosis.4 Most importantly, BC cells seem to have acquired multiple tricks to achieve invisibility and avoid anti-tumor recognition. Indeed, the phenotypic and functional analyzes of NK cells isolated from progressive stages of BC patients revealed that NK-cells are poorly functional in most patients with invasive BC only. These alterations were erased in patients undergoing long-term remission It appeared that: (1) tumor can induce its own tolerance from NK-cells anti-tumor immunity; (2) invasive characteristics and metastasis occurrence are dependent on the inhibition of NK-cell-mediated anti-tumor functions. Our data and those of others converge to show the importance of immune response in limiting both tumor growth and dissemination of metastatic cells.8-10
In addition, we have identified some of the mechanisms possibly used by breast tumor cells to shun NK cells. However, the list remains open and includes so far: inhibition through cell-cell contacts, secretion of inhibitory factors, re-organization of the tumor microenvironment, notably with the increase of Treg recruitment and facilitation of mesenchymal stem cell growth. Such new surrounding environment contributes to support tumor growth in situ or at the site of metastasis, kept protected from immune recognition. Most of these modifications are possible through the massive secretion of TGFβ1, PGE2, as well as several metalloproteases responsible for the downregulation of activating receptors, the shedding of homing molecules or surface beacons that are used as NK-cell ligands, such as sMICA. This is not to mention the direct involvement of these molecules on disease progression, metastatic potential and therapeutic resistance.
Altogether, our works show that BCs leave little chance to anti-tumor immunity, and especially NK cells, to efficiently exert their primary functions. We have demonstrated that when tumor cells cannot be recognized or when the destruction power is altered, innate immunity remain useless. “Stealth” BC cells can thus safely leave the primary tumor site to metastasize. However, in some cases, a residual anti-tumor immunity might be sufficient to tip the scale toward a more favorable prognosis, even in usually very aggressive tumors. The good news is that, if tumor cells develop strategies to overcome destruction by innate immunity, we can elaborate counter-strategies to restore NK-cells functions. Quite surprisingly, this might be more efficient through the targeting of strategies used to escape NK-cells recognition rather than the direct enhancement of anti-tumor immune functions alone. However, considering the startling genomic variability and associated tumorigenic features observed in BCs, the best strategy might certainly rely on combinatorial approaches to successfully target such complex disease.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/18528
References
- 1.Mamessier E, Sylvain A, Thibult ML, Houvenaeghel G, Jacquemier J, Castellano R, et al. Human breast cancer cells enhance self tolerance by promoting evasion from NK cell antitumor immunity. J Clin Invest. 2011;121:3609–22. doi: 10.1172/JCI45816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mamessier E, Sylvain A, Bertucci F, Castellano R, Charaffe-Jaufret E, Finetti P, et al. Human breast tumor cells induced self-tolerance mechanisms to avoid NKG2D- and DNAM1-mediated NK-cells recognition. Cancer Res. 2011 doi: 10.1158/0008-5472.CAN-11-0792. In press. [DOI] [PubMed] [Google Scholar]
- 3.Sorlie T, Tibshirani R, Parker J, Hastie T, Marron JS, Nobel A, et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci USA. 2003;100:8418–23. doi: 10.1073/pnas.0932692100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Teschendorff AE, Miremadi A, Pinder SE, Ellis IO, Caldas C. An immune response gene expression module identifies a good prognosis subtype in estrogen receptor negative breast cancer. Genome Biol. 2007;8:R157. doi: 10.1186/gb-2007-8-8-r157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sabatier R, Finetti P, Mamessier E, Raynaud S, Cervera N, Lambaudie E, et al. Kinome expression profiling and prognosis of basal breast cancers. Mol Cancer. 2011;10:86. doi: 10.1186/1476-4598-10-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sabatier R, Finetti P, Cervera N, Lambaudie E, Esterni B, Mamessier E, et al. A gene expression signature identifies two prognostic subgroups of basal breast cancer. Breast Cancer Res Treat. 2011;126:407–20. doi: 10.1007/s10549-010-0897-9. [DOI] [PubMed] [Google Scholar]
- 7.Bertucci F, Finetti P, Cervera N, Charafe-Jauffret E, Mamessier E, Adelaide J, et al. Gene expression profiling shows medullary breast cancer is a subgroup of basal breast cancers. Cancer Res. 2006;66:4636–44. doi: 10.1158/0008-5472.CAN-06-0031. [DOI] [PubMed] [Google Scholar]
- 8.Dewan MZ, Terunuma H, Ahmed S, Ohba K, Takada M, Tanaka Y, et al. Natural killer cells in breast cancer cell growth and metastasis in SCID mice. Biomed Pharmacother. 2005;59(Suppl 2):S375–9. doi: 10.1016/S0753-3322(05)80082-4. [DOI] [PubMed] [Google Scholar]
- 9.Alexe G, Dalgin GS, Scanfeld D, Tamayo P, Mesirov JP, DeLisi C, et al. High expression of lymphocyte-associated genes in node-negative HER2+ breast cancers correlates with lower recurrence rates. Cancer Res. 2007;67:10669–76. doi: 10.1158/0008-5472.CAN-07-0539. [DOI] [PubMed] [Google Scholar]
- 10.Mahmoud SM, Paish EC, Powe DG, Macmillan RD, Grainge MJ, Lee AH, et al. Tumor-infiltrating CD8+ lymphocytes predict clinical outcome in breast cancer. J Clin Oncol. 2011;29:1949–55. doi: 10.1200/JCO.2010.30.5037. [DOI] [PubMed] [Google Scholar]