Abstract
The use of immunotherapy to treat cancer is rapidly gaining momentum. Using pre-clinical mouse models, we have recently demonstrated potent and long lasting tumor regression can be elicited by immune-stimulating monoclonal antibodies (mAbs) when combined with histone deacetylase inhibitors (HDACi) and believe this therapy will have broad application in humans.
Keywords: HDACi, anticancer immunity, anticancer therapy, apoptosis, epigenetic regulatory agent, immune-stimulating antibodies, immunogenic cell death, vorinostat
There is currently a great expansion of interest in treating cancer with immunotherapy, in particular manipulating the anticancer host immune response with mAbs. The recent FDA approval of the anti-CTLA-4 mAb (ipilumimab) highlights the potential of this type of therapy and provides opportunities for further development in this area. An important function of these immunomodulatory mAbs is their ability to synergise with conventional first line therapy to enhance anti-cancer efficacy.1 In 2006 our laboratory described the combination of an apoptosis-inducing agonistic anti-TRAIL receptor mAb with the immunostimulatory mAbs anti-CD40 and anti-CD137.2 Termed trimAb, this therapy led to potent eradication of subcutaneous solid tumors in a variety of preclinical mouse models. Although highly efficacious, induction of tumor cell apoptosis via an active TRAIL pathway was found to be essential, thus limiting the scope of this combination to TRAIL-sensitive tumors. Considering the ability of the novel anti-cancer agents, histone deacetylase inhibitors (HDACi), to induce potent and specific tumor cell apoptosis independent of TRAIL sensitivity,3,4 we posited that addition of HDACi may broaden the application of this combination therapy.
HDACi are an exciting class of anti-cancer agents demonstrating striking single agent efficacy against hematological malignancies, but less potent activity against solid tumors. HDACi exert multiple biological effects including induction of tumor cell death, blockade of cell cycle progression, induction of cellular senescence and differentiation.5 Furthermore, HDACi are able to enhance tumor cell immunogenicity via the upregulation of MHC, co-stimulatory and adhesion molecules, leading to the generation of IFNγ secreting T cells6 and enhanced killing of tumor cells by CTLs.7 This host component of the HDACi-mediated response is relatively poorly understood and provides impetus to investigate not only the role of the immune system in mediating anti-tumor responses to HDACi, but also to test HDACi in combination with immunotherapy.
In March 2011,8 we published a report demonstrating that the combination of HDACi with immunostimulatory mAbs is highly efficacious for the treatment of solid tumors. The HDACi vorinostat and the agonistic mAb therapy targeting CD40 and CD137 (termed here as bimAb), were individually able to minimally delay the growth of established solid tumors of diverse tissue origins including mammary (4T1.2), colon (MC38) and kidney (Renca) carcinoma. Strikingly however, the combination of vorinostat with bimAb (V/bimAb) induced significant delay in tumor outgrowth and resulted in regression of tumors below palpable detection in in up to 56% of mice. Importantly, V/bimAb was also effective against TRAIL-insensitive tumors. Similar results were achieved with the HDACi panobinostat (P/bimAb) and the anti-tumor effect of both combinations was both well tolerated and long lasting, with mice remaining tumor free for > 100 days. Furthermore, the combination therapy was able to generate a potent and specific memory response as mice previously cured with V/bimAb rejected the same tumor upon rechallenge, however failed to reject tumors of differing tissue origins. We therefore found the combination of HDACi with bimAb to be safe and highly efficacious against established solid tumors of diverse tissue origin, regardless of TRAIL sensitivity.
HDACi have been proposed to possess immunogenic properties and can dictate immunogenicity via upregulation of immune-related molecules on the tumor cell surface. However, we did not detect changes in expression of MHC, co-stimulatory or regulatory molecules after HDACi exposure in the tumors we assessed. Nonetheless, we demonstrated that MC38 tumor cells undergoing apoptosis in response to vorinostat were phagocytosed by bone marrow-derived CD11c+APCs. Cells overexpressing Bcl-2 were resistant to vorinostat-induced apoptosis and were not phagocytosed by APCs. We concluded HDACi-treated tumor cells were an attractive target for APCs and thus sought to determine whether HDACi were engaging the immune system via this mechanism. Immunogenic cell death is apoptosis-dependent. Two hallmarks of immunogenic cell death are the translocation of calreticulin from the endoplasmic reticulum to the external plasma membrane and the release of the nuclear danger signal HMGB1.9 We found that calreticulin was translocated to the surface of vorinostat-treated MC38 cells and HMGB1 was released in to the supernatent in an apoptosis-dependent manner, abrogated by overexpression of Bcl-2 (unpublished data). Similar data has been generated following vorinostat treatment of other solid tumor cells.10 Together data suggests HDACi are indeed multifaceted anticancer agents able to manipulate tumor cell immunogenicity in multiple ways including the induction of immunogenic cell death.
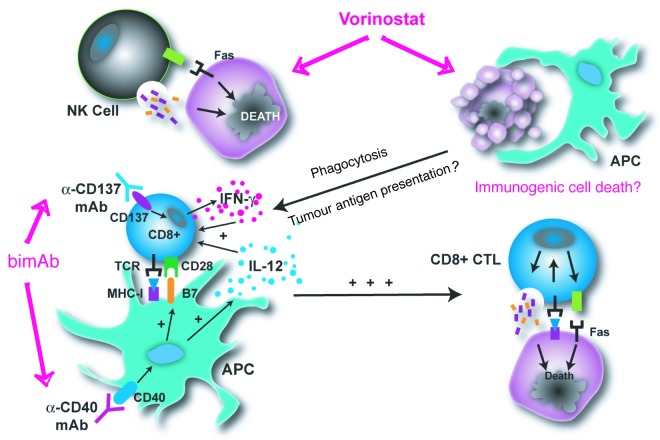
We sought to determine the role of the immune system in the eradication of established tumors by V/bimAb given the potentially immunogenic nature of the therapy. Therapeutic efficacy of V/bimAb was abrogated in mice lacking functional CD8+ T cells while mice lacking functional NK cells only partially responded. We also determined an important role for perforin and IFNγ as mice lacking these effector molecules were completely refractory to therapy. These data highlight the capacity of HDACi/bimAb to generate a potent antitumor immune response mediated by cytotoxic lymphocytes. These data highlight the capacity of HDACi/bimAb to generate a potent antitumour immune response conducted by cytotoxic lymphocytes. As depicted in Figure 1, we propose this response is initiated by tumor cell apoptosis and potential immunogenic cell death mediated by HDACi, and further enhanced by bimAb during antigen processing and presentation and T cell priming.
Figure 1. Mechanism of action of V/bimAb. We propose V/bimAb potently eradicates solid tumors by acting in three major ways. First, vorinostat enhances tumor cell (purple cells) sensitivity to NK cells by altering surface expression of immune-related molecules. Second, vorinostat induces tumor cell-specific apoptosis and immunogenic cell death leading to phagocytosis by APCs. Finally the presence of bimAb enhances antigen processing and presentation of tumor antigens and heightened priming of CD8+ T cells. Together, V/bimAb leads eradication of the tumor in a cytotoxic lymphocyte-dependent manner, utilizing the effector molecules perforin and interferon-γ.
We believe the results generated by this study greatly broaden the application of HDACi in the clinic. The use of HDACi in combination with immunostimulatory mAb therapy was able to induce regression of established solid tumors of various tissue origins in a well-tolerated manner. Furthermore, eradication of the solid tumors was found to be immune-mediated and capable of generating tumor cell-specific memory, with new data suggesting both bimAb and HDACi may initiate this anti-tumor immunity. Two HDACi, including vorinostat, are FDA-approved and many others are in late phase trials. We envisage the data discussed here will lead to the development of more rationale-based combination therapies using these potent pan-HDACi such as the highly efficacious V/bimAb.
Glossary
Abbreviations:
- FDA
Food and Drug Association
- CD
Cluster of differentiation
- TRAIL
TNF-related apoptosis-inducing ligand
- mAb
Monoclonal antibody
- HDACi
Histone deacetylase inhibitor
- IFN
Interferon
- CTL
Cytotoxic lymphocyte
- MHC
Major histocompatibility complex
- HMGB1
High mobility group protein B1
- Bcl-2
B-cell lymphoma 2
Disclosure of Potential Conflicts of Interest
The R.W.J. laboratory has collaborative research grants from Merck & Co and Novartis for studies involving vorinostat and panobinostat, respectively.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/18804
References
- 1.Frew AJ, Johnstone RW, Bolden JE. Enhancing the apoptotic and therapeutic effects of HDAC inhibitors. Cancer Lett 2009; 280:125-33 [DOI] [PubMed] [Google Scholar]
- 2.Uno T, Takeda K, Kojima Y, Yoshizawa H, Akiba H, Mittler RS, Gejyo F, Okumura K, Yagita H, Smyth MJ. Eradication of established tumors in mice by a combination antibody-based therapy. Nat Med 2006; 12:693-8 [DOI] [PubMed] [Google Scholar]
- 3.Ellis L, Bots M, Lindemann RK, Bolden JE, Newbold A, Cluse LA, Scott CL, Strasser A, Atadja P, Lowe SW, Johnstone RW. The histone deacetylase inhibitors LAQ824 and LBH589 do not require death receptor signaling or a functional apoptosome to mediate tumor cell death or therapeutic efficacy. Blood 2009; 114:380-93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lindemann RK, Newbold A, Whitecross KF, Cluse LA, Frew AJ, Ellis L, et al. Analysis of the apoptotic and therapeutic activities of histone deacetylase inhibitors by using a mouse model of B cell lymphoma. Proc Natl Acad Sci USA 2007; 104:8071-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bolden JE, Peart MJ, Johnstone RW. Anticancer activities of histone deacetylase inhibitors. Nat Rev Drug Discov 2006; 5:769-84 [DOI] [PubMed] [Google Scholar]
- 6.Khan AN, Gregorie CJ, Tomasi TB. Histone deacetylase inhibitors induce TAP, LMP, Tapasin genes and MHC class I antigen presentation by melanoma cells. Cancer Immunol Immunother 2008; 57:647-54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Manning J, Indrova M, Lubyova B, Pribylova H, Bieblova J, Hejnar J, et al. Induction of MHC class I molecule cell surface expression and epigenetic activation of antigen-processing machinery components in a murine model for human papilloma virus 16-associated tumours. Immunology 2008; 123:218-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Christiansen AJ, West A, Banks KM, Haynes NM, Teng MW, Smyth MJ, et al. Eradication of solid tumors using histone deacetylase inhibitors combined with immune-stimulating antibodies. Proc Natl Acad Sci USA 2011; 108:4141-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kepp O, Tesniere A, Schlemmer F, Michaud M, Senovilla L, Zitvogel L, et al. Immunogenic cell death modalities and their impact on cancer treatment. Apoptosis. 2009;14:364–75. doi: 10.1007/s10495-008-0303-9. [DOI] [PubMed] [Google Scholar]
- 10.Sonnemann J, Gressmann S, Becker S, Wittig S, Schmudde M, Beck JF. The histone deacetylase inhibitor vorinostat induces calreticulin exposure in childhood brain tumour cells in vitro. Cancer Chemother Pharmacol. 2010;66:611–6. doi: 10.1007/s00280-010-1302-4. [DOI] [PubMed] [Google Scholar]