Abstract
Lymphocytic infiltration is often seen in breast cancer and has been suggested as a marker of host anti-tumor response but its importance in prognosis remains controversial. Our recent study demonstrated an association between tumor-infiltrating CD8+ T lymphocytes in invasive breast cancer and better prognosis.
Keywords: CD8, Immunohistochemistry, breast cancer, immunosurveillance, tissue microarrays
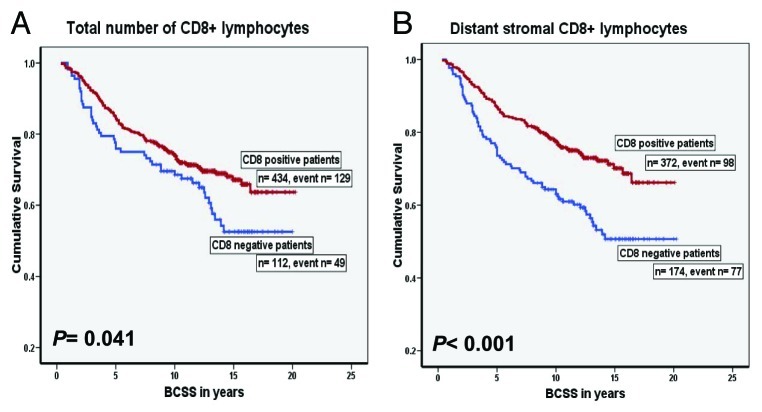
Understanding the role of immune system in cancer development and progression has been one of the most challenging questions in immunology. The formulation of the cancer immunoediting hypothesis not only indicates a role for the immune system in the active elimination of immunogenic tumor cells (immunosurveillance), but also emphasizes the importance of immunity in promoting the outgrowth of less immunogenic tumor cell variants.1 Breast cancer is a major health problem for women worldwide with more than one million cases are diagnosed every year. The efficacy of current treatment is limited by inherent therapeutic resistance and by collateral damage to normal tissues which highlights the need for new highly targeted therapeutic approaches. Infiltration of immune cells is a well-documented observation in breast carcinoma. However, the role of the immune response in patients with breast cancer is still a source of controversy with regards to its relationship with patient outcome. We analyzed a cohort of 1334 unselected breast tumors from patients with long-term follow-up for the density and micro-anatomical localization of CD8+ cytotoxic T lymphocytes using tissue microarrays and immunohistochemistry.2 High numbers of CD8+ cells infiltrating carcinomas were significantly correlated with high histological grade, younger patient age and negative estrogen receptor status. High CD8+ cell counts were associated with improved patient outcome (Fig. 1), independent of the standard prognostic and predictive factors.2
Figure 1. Kaplan-Meier curves of breast cancer specific survival (BCSS) for CD8+ lymphocytic infiltration in the training set. (A) Survival curve for the total number of CD8+ lymphocytes, (B) Survival curve for the distant stromal CD8+ lymphocytes.2
These data indicate that a stratification of the patients based on the assessment of CD8+ cell density could be of interest in clinical practise. Importantly, this methodology uses a routine immunohistochemistry and commercially available antibody to detect a marker that may potentially increase prediction accuracy of assessment of patient prognosis for planning of appropriate adjuvant therapy. Previous studies examining other types of cancer have reached a similar conclusion.3,4 Indeed, assessment of inflammation is recommended for melanoma, Markel cell carcinoma and colorectal carcinoma.5 The results of these studies provide evidence for the equilibrium phase of cancer immunoediting in humans. In the equilibrium phase of cancer immunoediting process, the adaptive immune system prevents tumor growth and also sculpts the immunogenicity of the tumor cells.1 However, breast cancer is a heterogeneous disease with different types having remarkably different biological characteristics and clinical behavior. Not all the results on the role of inflammatory cells in breast cancer are consistent. Another study with similar design to ours found CD8+ cells were of independent value only in estrogen receptor negative carcinomas.6 Similar results have been found using microarray gene expression data.7 Our data showed that the CD8+ cell density was not of prognostic value in patients with HER2 positive carcinomas,2 but others have found an association between immune genes and better prognosis in this tumor type.8 It is likely that different immune mechanisms are important in different types of breast cancer and this deserves further investigation. Also there is evidence that other components of immune response, such as B cells, are important in breast cancer.9
The role of tumor infiltrating leukocytes in cancer formation and progression has been considered one of hallmarks of cancer.10 Hence, harnessing tumor-immune/inflammation interaction has become an important focus of anti-cancer research with the development of tumor vaccines and immune modulating therapies. Given the evidence discussed in this article, the hypothesis that adaptive immunity is implicated in breast cancer prognosis deserves consideration and further investigation. Although early results of immunotherapy in breast cancer have been disappointing, the finding of an association between CD8+ count and better prognosis in our study suggest that further investigation of this mode of treatment should be encouraged.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/18614
References
- 1.Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3:991–8. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 2.Mahmoud SMA, Paish EC, Powe DG, Macmillan RD, Grainge MJ, Lee AHS, et al. Tumor-infiltrating CD8+ lymphocytes predicts clinical outcome in breast cancer. J Clin Oncol. 2011;29:1949–55. doi: 10.1200/JCO.2010.30.5037. [DOI] [PubMed] [Google Scholar]
- 3.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–4. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 4.Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci USA. 2005;102:18538–43. doi: 10.1073/pnas.0509182102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC cancer staging handbook (Springer, New York, 2010). [Google Scholar]
- 6.Baker K, Lachapelle J, Zlobec I, Bismar TA, Terracciano L, Foulkes WD. Prognostic significance of CD8+ T lymphocytes in breast cancer depends upon both oestrogen receptor status and histological grade. Histopathology. 2011;58:1107–16. doi: 10.1111/j.1365-2559.2011.03846.x. [DOI] [PubMed] [Google Scholar]
- 7.Teschendorff AE, Miremadi A, Pinder SE, Ellis IO, Caldas C. An immune response gene expression module identifies a good prognosis subtype in estrogen receptor negative breast cancer. Genome Biol. 2007;8:R157. doi: 10.1186/gb-2007-8-8-r157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rody A, Holtrich U, Pusztai L, Liedtke C, Gaetje R, Ruckhaeberle E, et al. T-cell metagene predicts a favorable prognosis in estrogen receptor-negative and HER2-positive breast cancers. Breast Cancer Res. 2009;11:R15. doi: 10.1186/bcr2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mahmoud SM, Lee AH, Paish EC, Macmillan RD, Ellis IO, Green AR. The prognostic significance of B lymphocytes in invasive carcinoma of the breast. Breast Cancer Res Treat. 2011 doi: 10.1007/s10549-011-1620-1. In press. [DOI] [PubMed] [Google Scholar]
- 10.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]