Abstract
ALDHbright cells in human tumor cells lines, xenografts and lesions have been shown to have characteristics of cancer stem cells (CSC). We have shown that these cells are recognized by ALDH1A1-specific CD8+ T cells in vitro and in vivo. The results support the potential of ALDH1A1-based immunotherapy to target CSC.
Keywords: ALDH1A1, CTL, adoptive therapy, cancer stem cells, xenograft
Cancer Stem Cells and ALDH1A1
Not without controversy, cancer stem cells (CSC) are considered capable of symmetrical or asymmetric self-renewal, resistance to standard chemo- or radio- therapies, less differentiated, and tumorgenic, the latter evident by ability in low numbers to establish tumors in immunodeficient mice.1,2 As the failure of current therapies to control cancer can be attributed to their inability to eliminate CSC, there is a critical need to develop strategies that eliminate these “stem cell-like” tumor cells.
Employing the methods of flow cytometry-based studies of hematopoietic and leukemic stem cells, highly enriched populations of tumor cells with “stem cell-like” properties have been isolated from many types of human carcinomas. Most recently, it is based on elevated expression of aldehyde dehydrogenase (ALDH) activity. In combination with DEAB, an inhibitor of the ALDH1A1, -A2, -A3 and ALDH3A1 isoforms, the flow reagent ALDEFLUOR identifies ALDH+ cells.3 Expression of ALDH1A1, which metabolizes genotoxic aldehydes, is critical to the chemoradio-resistance of normal stem cells and tumor cells. However, while it is not absolute that all CSC are ALDH+ cells, isolated populations of ALDH+ tumor cells certainly have characteristics distinct from the remaining tumor cell population.
Identification of ALDH1A1 as a Shared Tumor Antigen (TA)
In a collaborative study with Dr. Theresa Whiteside, my laboratory identified ALDH1A188–96 peptide as a shared CTL-defined human tumor antigen (TA) in squamous cell carcinoma of the head and neck (SCCHN).4 Given that only one or two exchanges distinguish the peptides encoded by codons 88–96 of the ALDH1/3 isoforms, particular attention was paid to ensure that the HLA-A2-restricted CTL were specific for the ALDH1A188–96 peptide. Furthermore, ALDH1A1 expression at the protein level correlated with the severity of oral mucosal dysplasia and less differentiated and more aggressive SCCHN lesions. These findings suggested that it might be an early marker for development of SCCHN and an attractive target for immunoprevention as well as therapy of this disease.
Somewhat unappreciated at first, as one became more aware of the roles of ALDH1A1 in retinoic acid metabolism and detoxification of genotoxic aldehydes, its identification as a TA gained our attention. In line with the work of Mackenzie and colleagues dealing with SCCHN CSC,5 ALDH1A1+ cells isolated from several of our SCCHN cell lines used in our studies were CD44+, tended to have a primitive morphology and clonogenic, whereas ALDH1A1neg cells were not. These findings combined with the seminal identification of ALDH+ breast cancer cells as CSC6 prompted focusing our attention on targeting CSC in human cancers with ALDH1A1-specific CTL.7
Targeting ALDHbright Cells with ALDH1A1-Specific CTL
In ensure the purity of the ALDH+ cells being analyzed in our studies, ALDHbright cells, which have a mean fluorescence intensity (MFI) twice that of the bulk ALDH+ cell population in a sample, were isolated. Re-analysis showed that sorted ALDHbright cells were ~95% ALDH+, whereas sorted “bulk” ALDH+ cells consisted of only 65% ALDH+ cells. Critically, the ALDHbright cells sorted from HLA-A2+ breast MDA-MB-231, SCCHN PCI-13 and pancreas MIA PaCa-2 cell lines were tumorigenic. Furthermore, qRT/PCR analysis of sorted ALDHbright cells from these cell lines indicated the dominant expression of ALDH1A1 mRNA relative to other ALDH1/3 isoform mRNA.
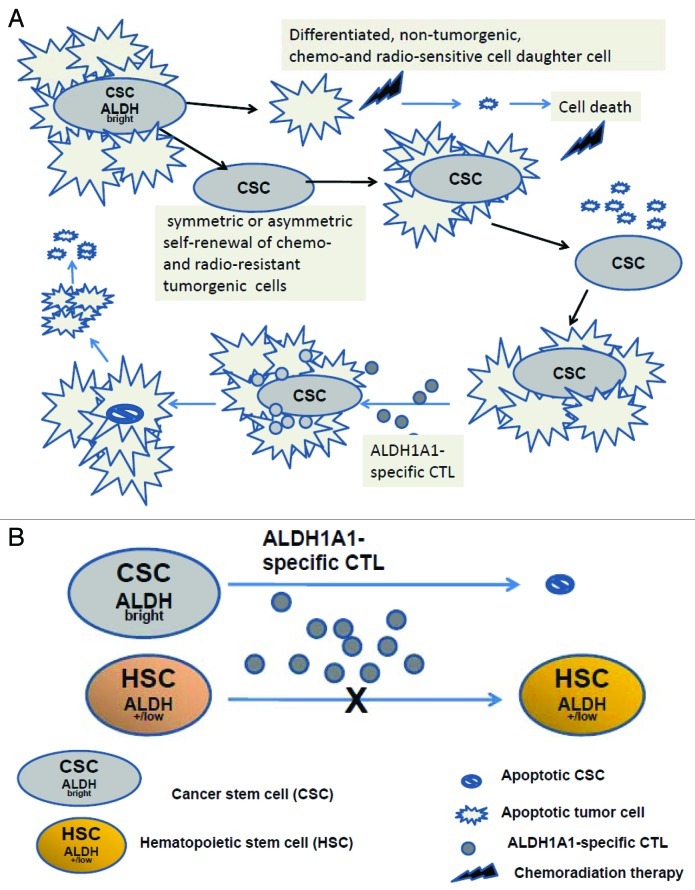
Most importantly, whereas recognition of SCCHN cell lines by ALDH1A1-specific CTL in ELISPOT IFNγ assays requires pretreatment with IFNγ (to upregulate antigen processing and presentation), recognition of sorted ALDHbright cells does not. One presumes this is due to elevated ALDH1A1 expression in these cells and sufficient HLA class I/ALDH1A1 peptide complexes for CTL recognition and detection in this assay. The research subsequently was facilitated by (1) using artificial antigen presenting cells to induce ALDH1A1-specific CTL from normal donor and patient-derived lymphocytes8; T-cell effector yields were 10 to 30× that obtained previously, and (2) direct detection of the elimination by ALDH1A1-specific CTL of ALDH+/ALDHbright cells in cell lines and disaggregated xenografts and surgically removed patient lesions samples in a flow-based ALDEFLUOR assay (Fig. 1).
Figure 1. ALDHbright CSC but not normal ALDH+/low cells are recognized by HLA-A2 restricted, ALDH1A1-specific CTL. (A) Unlike bulk population of tumor cells, ALDHbright CSC are resistant to chemoradiation therapy but sensitive to ALDH1A1-specific CTL resulting in control of tumor growth. (B) Relative to levels of ALDH1A1 expressed by ALDHbright CSC, normal CD34+ HSC are ALDH+/low and not recognized by ALDH1A1-specific CTL.
Of the in vivo-based assays performed to show the efficacy of ALDH1A1-specific CTL-based therapy performed in collaboration with Dr. Hui Wang’ laboratory, particularly important was adoptive transfer of CTL to mice following surgical removal of primary MDA-MB-231-derived orthotopic xenografts. These mice generally succumb to metastases within two months. Following their surgery, however, treatment of mice with ALDH1A1-specific CTL until all untreated control mice died increased their survival past seven months.
Given that CD34+ hematopoietic stem cells (HSC) express ALDH1A1,3 starting from our initial study, it has been critical to show that HSC are not recognized by ALDH1A1-specific CTL. To date, there is no indication that HSC, even pretreated with IFNγ, are recognized in vitro by ALDH1A1-specific CTL; most likely, because the ALDH1A1 level is lower than in tumor cells. In ancillary mouse experiments, immunization of mice with dendritic cells transfected with aldh1a1 cDNA (mouse ALDH1A1 homolog) induced aldh1a1-reactive T cells and had no overall effect on hematopoiesis using absolute numbers and CD4+/CD8+ T cell ratios as surrogate markers.
Future Directions
Future research efforts require the use of primary tumors arising in genetically modified mouse models of human cancer to demonstrate efficacy of ALDH1A1-based- active and passive immunotherapy in eliminating CSC and controlling tumor growth and metastases. However, any type of therapy promotes tumor escape. To minimize this, we are investigating the efficacy of combinatorial strategies to target CSC by combining ALDH1A1-specific CTL with stem cell-signaling pathway inhibitors9 and antibody-based therapies.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/18826
References
- 1.Dick JE. Stem cell concepts renew cancer research. Blood. 2008;112:4793–807. doi: 10.1182/blood-2008-08-077941. [DOI] [PubMed] [Google Scholar]
- 2.Visvader JE. Cells of origin in cancer. Nature. 2011;469:314–22. doi: 10.1038/nature09781. [DOI] [PubMed] [Google Scholar]
- 3.Storms RW, Trujillo AP, Springer JB, Shah L, Colvin OM, Ludeman SM, et al. Isolation of primitive human hematopoietic progenitors on the basis of aldehyde dehydrogenase activity. Proc Natl Acad Sci USA. 1999;96:9118–23. doi: 10.1073/pnas.96.16.9118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Visus C, Ito D, Amoscato A, Maciejewska-Franczak M, Abdelsalem A, Dhir R, et al. Identification of human aldehyde dehydrogenase 1 family member A1 as a novel CD8+ T-cell-defined tumor antigen in squamous cell carcinoma of the head and neck. Cancer Res. 2007;67:10538–45. doi: 10.1158/0008-5472.CAN-07-1346. [DOI] [PubMed] [Google Scholar]
- 5.Locke M, Heywood M, Fawell S, Mackenzie IC. Retention of intrinsic stem cell hierarchies in carcinoma-derived cell lines. Cancer Res. 2005;65:8944–50. doi: 10.1158/0008-5472.CAN-05-0931. [DOI] [PubMed] [Google Scholar]
- 6.Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1:555–67. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Visus C, Wang YY, Lozano-Leon A, Ferris RL, Silver S, Szczepanski MJ, et al. Targeting ALDHbright human carcinoma initiating cells with ALDH1A1-specific CD8+ T cells. Clin Cancer Res. 2011;17:6174–84. doi: 10.1158/1078-0432.CCR-11-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sluijter BJ, van den Hout MF, Stam AG, Lougheed SM, Suhoski MM, van den Eertwegh AJ, et al. 4-1BB-mediated expansion affords superior detection of in vivo primed effector memory CD8(+) T cells from melanoma sentinel lymph nodes. Clin Immunol. 2010;137:221–33. doi: 10.1016/j.clim.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 9.Liu S, Wicha MS. Targeting breast cancer stem cells. J Clin Oncol. 2010;28:4006–12. doi: 10.1200/JCO.2009.27.5388. [DOI] [PMC free article] [PubMed] [Google Scholar]