Abstract
Interleukin (IL)-21, a cytokine produced by activated conventional CD4+ T lymphocytes and Natural Killer T cells, drives anti-tumor immunity in the skin and kidney. However IL-21 is also pro-inflammatory in many tissues and promotes colitis-associated colon cancer. Understanding the biology of IL-21 in these different situations is needed to ensure maximal therapeutic benefit.
Keywords: IL-17A, IL-6, STAT3, Tregs, colitis-associated colon cancer
Introduction
The functional relationship between inflammation and cancer was first proposed by Rudolf Virchow nearly 150 y ago, when he noticed leucocytes in tumors and the surrounding stroma. Many epidemiologic and molecular studies since then showed that tumors can develop at sites of infection and/or chronic inflammation (Table 1).1 It is however also clear that inflammation-driven cell proliferation alone is not per se sufficient to cause cancer. For example in patients with psoriasis, the chronic inflammatory process sustains hyperproliferation of keratinocytes but does not increase the risk of skin cancer.2 By contrast, cells that proliferate in an environment rich in growth factors, activated stroma, DNA-damaging agents and mutagenic insults have a much higher probability of becoming neoplastic. Finally, inflammatory cells in the tumor microenvironment may exert protective antitumor activity.3 The specific nature of the inflammatory response and the tissue context may thus determine the beneficial vs. the detrimental effects of inflammation in carcinogenesis.
Table 1. Cancer types associated with infection or chronic inflammation.
| Inflammatory condition | Related cancer type | |
|---|---|---|
| Barrett’s esophagus |
Esophageal adenocarcinoma |
|
| Lung inflammation (e.g., caused by smoking, asbestos, infection) |
Lung cancer |
|
| HBV- and HCV-induced chronic hepatitis |
Hepatocellular carcinoma |
|
| Primary sclerosing cholangitis |
Cholangiocarcinoma |
|
| Chronic cholecystitis |
Gallbladder cancer |
|
|
H. pylori-induced gastritis |
Gastric cancer |
|
| Chronic pancreatitis |
Pancreatic carcinoma |
|
| Inflammatory bowel disease (i.e., ulcerative colitis and Crohn’s disease) |
Colorectal cancer |
|
| Endometriosis |
Endometrial carcinoma |
|
| Chronic pelvic inflammation |
Ovarian cancer |
|
| Schistosomiasis |
Bladder cancer |
|
| Chronic prostatitis |
Prostate cancer |
|
| Virus-induced lymphohistiocytosis | T cell lymphoma |
The tumor microenvironment contains both innate immune cells (e.g., macrophages, neutrophils, mast cells, myeloid-derived suppressor cells, dendritic cells, natural killer cells) and adaptive immune cells (T and B lymphocytes). Tumor-associated macrophages (TAMs) and T cells are the most frequent immune cells within the tumor microenvironment. TAMs generally have a phenotype described as “M2” or “alternatively activated macrophages,” oriented toward suppressing adaptive immunity and promoting tissue repair and angiogenesis.4 High numbers of M2 TAMs correlate with poor prognosis in many cancers.4 Tumor progression can also be sustained by T helper (Th) type 2 cells and regulatory T cells (Tregs).3 In contrast, dendritic cells and M1 macrophages producing IL-12, cytotoxic CD8+ T cells and interferon (IFN)-γ-producing Th1 cells can mediate anti-tumor immunity, and their presence is associated with better survival in some cancers (i.e., colon cancer, melanoma, multiple myeloma, and pancreatic cancer).3 However, the anti-tumor activity of these specific cell subsets cannot be considered as a given since there is evidence that CD8+ T cells and Th1 cells can also promote rather than inhibit tumorigenesis in some circumstances.3 The factors that render specific T cell subsets anti-tumorigenic in some cancers and pro-tumorigenic in others remain unknown, even though the profile of cytokines produced by these cells, rather than the type of cell infiltrate, seems to play a decisive role in influencing the growth and activity of the tumor cells.
Role of IL-21 in Immunotherapy
IL-21 is a member of a large family of cytokines (IL-2, IL-4, IL-7, IL-9 and IL-15) whose receptors share a common receptor γ chain (γc).5 IL-21 is made by a range of activated CD4+ Th cells, including Th1 and Th17 cells, activated NKT cells, and T follicular helper cells.5 IL-21 drives B cell differentiation into plasma cells, regulates immunoglobulin production, controls the proliferation and/or effector function of both CD4+ and CD8+ T cells, limits the differentiation of Tregs and can stimulate epithelial cells and fibroblasts to produce inflammatory mediators.5 Like other cytokines in this family, IL-21 has potent anti-tumor effects due to its ability to expand the pool of cytotoxic CD8+ T cells, NK cells and NKT cells.5 Beneficial IL-21-mediated anti-tumor responses have been observed in several independent experimental models, where mice inoculated with transplantable syngeneic tumor lines (e.g., colon carcinoma, fibrosarcoma, pancreatic carcinoma, renal cell carcinoma, melanoma) were successfully treated with IL-21 via cytokine-gene transfection, plasmid delivery or injection of the recombinant protein.6 In view of its anti-tumor activity documented in pre-clinical studies, IL-21-based therapy has been proposed in the management of malignant neoplasias. In Phase I and Phase IIa clinical trials, IL-21 was well tolerated and showed anti-tumor activity in patients with renal cell carcinoma and metastatic melanoma.6
However, before generally considering IL-21 as an anti-tumor cytokine, it should be taken into consideration that the majority of preclinical studies investigating the role of IL-21 in tumor development have been conducted on implanted tumor models. It remains unclear whether the anti-tumor activity of IL-21 can be generalized to spontaneously arising tumors, including those boosted by chronic inflammatory processes.
Involvement of IL-21 in Immunopathology and Colitis-Associated Colon Cancer
IL-21 can trigger inflammatory pathways and promote tissue damage in many organs. A pathogenic role of IL-21 has been described in psoriasis, rheumatoid arthritis, Type I diabetes, systemic lupus erythematosus, graft-vs. host disease, and inflammatory bowel diseases (IBD),5 and IL-21 blockers are now ready to move to the clinic. IBD is the general term for Crohn’s disease and ulcerative colitis (UC), the two major chronic inflammatory disorders of the intestine. IBD, and particularly UC, represents an excellent model for understanding the contribution of IL-21 in tumorigenesis, since it is very well known that patients with UC have enhanced risk of colon cancer.1 We have recently shown that IL-21 is produced in excess not only in the mucosa of patients with UC, but also in the neoplastic areas of patients with UC-associated colon cancer and sporadic colorectal cancer.7 Using the mouse model of colitis-associated colon cancer induced by administration of azoxymethane (AOM) followed by repeated oral administration of dextran sulfate sodium (DSS), we showed that IL-21 is over-produced in the colonic neoplastic areas and that mice deficient in IL-21 had less colonic inflammation and were largely protected against the development of colitis-associated colon cancer.7 Wild-type mice given a neutralizing IL-21 antibody developed fewer smaller tumors than mice treated with a control antibody.7 In line with these data, another study by Jauch and colleagues, documented the tumor-promoting effect of IL-21 in a very similar model of colitis-associated colon cancer.8
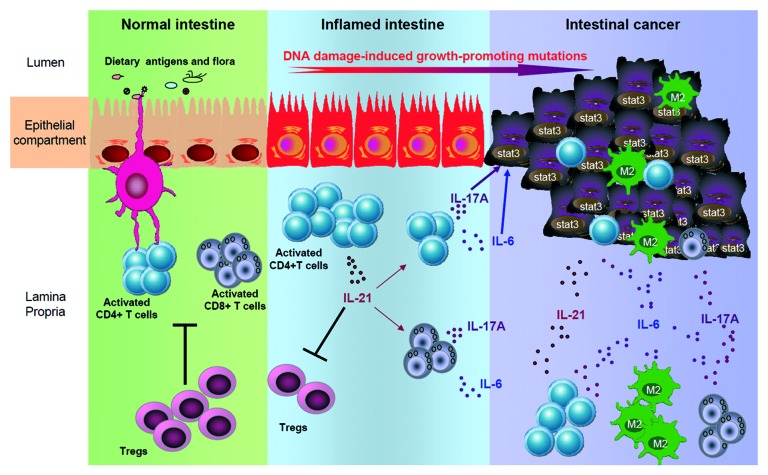
Analysis of mechanisms by which IL-21 promotes malignant cell growth in the colon revealed that IL-21 sustains CD4+ T cell infiltration in the tumor and peri-tumor areas and enhances the production of IL-6 and IL-17A (Fig. 1).7 This is particularly interesting since these two cytokines are known to be involved in tumor-associated inflammation and tumorigenesis in the colon.9 Mice deficient in IL-21 show a marked downregulation of the transcription factor STAT3, both in the lamina propria and gut epithelium, and reduced expression of Bcl-XL, a STAT3-induced anti-apoptotic protein.7 This finding is consistent with the demonstration that human colon cancers overexpress active STAT3, and that STAT3 activation in tumor cells and tumor-associated inflammatory cells sustains tumor progression by augmenting tumor survival, angiogenesis and suppressing anti-tumor immunity . It remains to be determined whether, following AOM+DSS treatment, STAT3 is directly activated by IL-21 or is dependent on enhanced production of IL-6 and IL-17A, given that these two cytokines activate directly STAT3 in tumor cells.9 This later hypothesis seems to be more plausible because mouse colonic epithelial cells do not express the IL-21 receptor and correspondingly fail to activate STAT3 when stimulated with IL-21.7
Figure 1. Putative functions of IL-21 in promoting colitis-associated colon cancer. In normal intestine APC-driven T cell activation is counter-regulated by Tregs. During inflammation, activated T cells produce increased level of IL-21 that damps Treg differentiation and, in parallel, promotes T cell expansion and subsequent secretion of pro-tumorigenic cytokines (e.g., IL-6 and IL-17A). These cytokines target DNA mutation-sensitized epithelial cells promoting their proliferation through STAT3 activation and fostering tumor development. Finally, tumor-infiltrating cells sustain and amplify this pro-tumoral milieu by secreting IL-21, IL-6 and IL-17A.
IL-21 suppresses the peripheral differentiation of Foxp3+ regulatory T cells,5 so it was expected that the milder colitis and reduced CD4+ T cell infiltrate seen in IL-21-deficient mice might be associated with higher percentage of Foxp3-expressing T cells. These findings may however appear surprising, because as pointed out above Tregs inhibit anti-tumor immunity and hence promote tumorigenesis. In this context, it is however noteworthy that the presence of Tregs has been associated with anti-tumorigenic responses in gastrointestinal cancers.10
Conclusion
It is now evident that cytokines have powerful effects on tumor development, as they generate an attractive environment for the growth and spread of malignant cells. Among these cytokines, particular attention has recently been paid to IL-21, since it seems to play a decisive role in controlling tumorigenesis. Data emerging from pre-clinical and clinical studies indicates that forced overexpression of IL-21 in tumor cells suppresses their growth, through enhanced antitumor immunity. Nonetheless, studies in models of colitis-associated colon cancer strongly support the tumor-promoting role of IL-21, raising the possibility that IL-21 could have opposing functions on the growth of tumors, depending on the tissue context and the local immune activation. Future work is needed to confirm these observations as well as to determine whether IL-21 is also involved in the development of other cancers complicating the natural history of patients with chronic inflammatory processes (e.g., Helicobacter pylori-induced gastritis). If this is the case, the use of IL-21 blockers could represent an attractive and novel approach for preventing and/or treating inflammation-associated malignancies as well as the underlying inflammation.
Acknowledgments
This work received support from the “Fondazione Umberto di Mario,” Rome, the Broad Medical Research Program Foundation, A.I.R.C. (grant n° 9148), and Giuliani SpA, Milan, Italy.
Disclosure of Potential Conflicts of Interest
GM has filed a patent entitled “A treatment for inflammatory diseases” (patent Nr. 08154101.3), while the remaining authors have no conflict of interests to disclose.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/19122
References
- 1.Kundu JK, Surh YJ. Inflammation: gearing the journey to cancer. Mutat Res. 2008;659:15–30. doi: 10.1016/j.mrrev.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 2.Nickoloff BJ, Ben-Neriah Y, Pikarsky E. Inflammation and cancer: is the link as simple as we think? J Invest Dermatol. 2005;124:x–xiv. doi: 10.1111/j.0022-202X.2005.23724.x. [DOI] [PubMed] [Google Scholar]
- 3.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–99. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mantovani A, Schioppa T, Porta C, Allavena P, Sica A. Role of tumor-associated macrophages in tumor progression and invasion. Cancer Metastasis Rev. 2006;25:315–22. doi: 10.1007/s10555-006-9001-7. [DOI] [PubMed] [Google Scholar]
- 5.Spolski R, Leonard WJ. Interleukin-21: basic biology and implications for cancer and autoimmunity. Annu Rev Immunol. 2008;26:57–79. doi: 10.1146/annurev.immunol.26.021607.090316. [DOI] [PubMed] [Google Scholar]
- 6.Søndergaard H, Skak K. IL-21: roles in immunopathology and cancer therapy. Tissue Antigens. 2009;74:467–79. doi: 10.1111/j.1399-0039.2009.01382.x. [DOI] [PubMed] [Google Scholar]
- 7.Stolfi C, Rizzo A, Franzè E, Rotondi A, Fantini MC, Sarra M, et al. Involvement of interleukin-21 in the regulation of colitis-associated colon cancer. J Exp Med. 2011;208:2279–90. doi: 10.1084/jem.20111106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jauch D, Martin M, Schiechl G, Kesselring R, Schlitt HJ, Geissler EK, et al. Interleukin 21 controls tumour growth and tumour immunosurveillance in colitis-associated tumorigenesis in mice. Gut. 2011;60:1678–86. doi: 10.1136/gutjnl-2011-300612. [DOI] [PubMed] [Google Scholar]
- 9.Terzicá J, Grivennikov S, Karin E, Karin M. Inflammation and colon cancer. Gastroenterology. 2010;138:2101–14.e5. doi: 10.1053/j.gastro.2010.01.058. [DOI] [PubMed] [Google Scholar]
- 10.Erdman SE, Sohn JJ, Rao VP, Nambiar PR, Ge Z, Fox JG, et al. CD4+CD25+ regulatory lymphocytes induce regression of intestinal tumors in ApcMin/+ mice. Cancer Res. 2005;65:3998–4004. doi: 10.1158/0008-5472.CAN-04-3104. [DOI] [PubMed] [Google Scholar]