Abstract
The efficacy of antineoplastic chemotherapies with anthracyclines or oxaliplatin relies on the induction of immunogenic cell death, thus provoking an anticancer immune response. Recently, we observed that overexpression of CD39, an ATP-degrading enzyme expressed on the cell surface, abolishes the immunogenicity of cell death, thus rendering cancers resistant against chemotherapy.
Keywords: ATP release, anthracyclines, cancer, immunogenic cell death, macroautophagy, oxaliplatin
CD39 (also known as ectonucleoside triphosphate diphosphohydrolase 1, ENTPD1, or nucleotide triphosphate diphosphohydrolase, NTPDase) is an enzyme that expresses on the surface of selected cellular populations such as endothelial cells and regulatory T cells and that degrades extracellular ATP to generate ADP and AMP. The latter product may be converted by yet another ecto-enzyme, CD73, to adenosine.1 Extracellular ATP, ADP, AMP and adenosine act on different types of purinergic and adinosinergic receptors to influence a plethora of biological processes including thrombocyte aggregation, nociception, inflammation and immune responses. Soluble CD39 has been shown to lower extracellular ATP concentrations when applied to animals, thereby reducing the sequels of myocardial or cerebral ischemia.2
CD39 is selectively expressed on the surface of CD4+CD25+Foxp3+ regulatory T cells. In patients with head and neck squamous cell carcinoma, particularly those with active disease, the level of expression of CD39 on regulatory T cells is increased.3 Similarly, in patients with chronic lymphocytic leukemia (CLL), circulating CD4+ (regulatory?) T cells overexpress CD39, and this parameter may have a negative prognostic impact.4 In mice, CD39 expression by regulatory T cells has been shown to facilitate the growth of intraportally injected cancer cells in the liver, presumably through the CD39-dependent inhibition of NK cells.5 It has also been suggested that tumor cell-derived exosomes may mediate immunosuppression by expressing CD39 and CD73,6 although formal evidence in favor of this hypothesis is still elusive. Thus, the available evidence suggests that CD39 is immunosuppressive.
Anticancer chemotherapies are particularly efficient when they elicit immunogenic cell death, which is a type of apoptosis in which dying and dead cells act as a therapeutic vaccine.7 One of the hallmarks of immunogenic cell death is the secretion of ATP,8 which occurs in an active fashion as the cells expose phosphatidylserine on their surface, before they permeabilize their plasma membranes.9 The ATP release induced by anthracyclines and oxaliplatin requires the activation of caspases as well as the pre-mortem activation of autophagy. Inhibition of caspases or suppression of autophagy abolishes ATP release; however caspase activation alone or stimulation of autophagy without cell death is insufficient to stimulate ATP release. Apparently both processes must occur in a sequential fashion (first autophagy, then caspase activation) to allow for ATP to be released into the microenvironment of dying tumor cells.9,10 Either the inhibition of autophagy (by knockdown of essential autophagy-relevant genes including Atg5 and Atg7) or the inhibition of caspases (by transgenic expression of the baculovirus-encoded caspase inhibitor p35) are sufficient to abolish the immunogenicity of cancer cell death induced by anthracyclines or oxaliplatin in vivo.7,10
To assess the importance of the release of ATP into the microenvironment of dying tumor cells in a more direct fashion, we expressed mouse CD39 on the surface of MCA205 fibrosarcomas at the same time as we generated a cell line transduced by an empty vector (control cells). Cytofluorometric analyses revealed that the vast majority (> 90%) of the CD39-transduced cells expressed CD39 at the surface, while control cells lacked detectable CD39 expression.10 We then assessed the effect of this manipulation on the immunogenicity of cell death, using a combined in vitro/in vivo approach. Control cells or CD39+ MCA205 cells were treated with the anthracycline mitoxanthrone in vitro to induce approximately 70% of cell death. Subsequently, the dying cells were inoculated subcutaneously into immunocompetent C57Bl/6 mice. To assess the capacity of these dying cell preparations to vaccinate against tumor antigens, the mice were inoculated one week later with live parental MCA205 cells and tumor growth was monitored. The vast majority of the animals vaccinated with mitoxantrone-treated control cells were protected against the outgrowth of live MCA205 tumors. In contrast, animals vaccinated with dying CD39+ MCA205 cells were not protected, indicating that the expression of CD39 abolishes the immunogenicity of cell death (Fig. 1A).
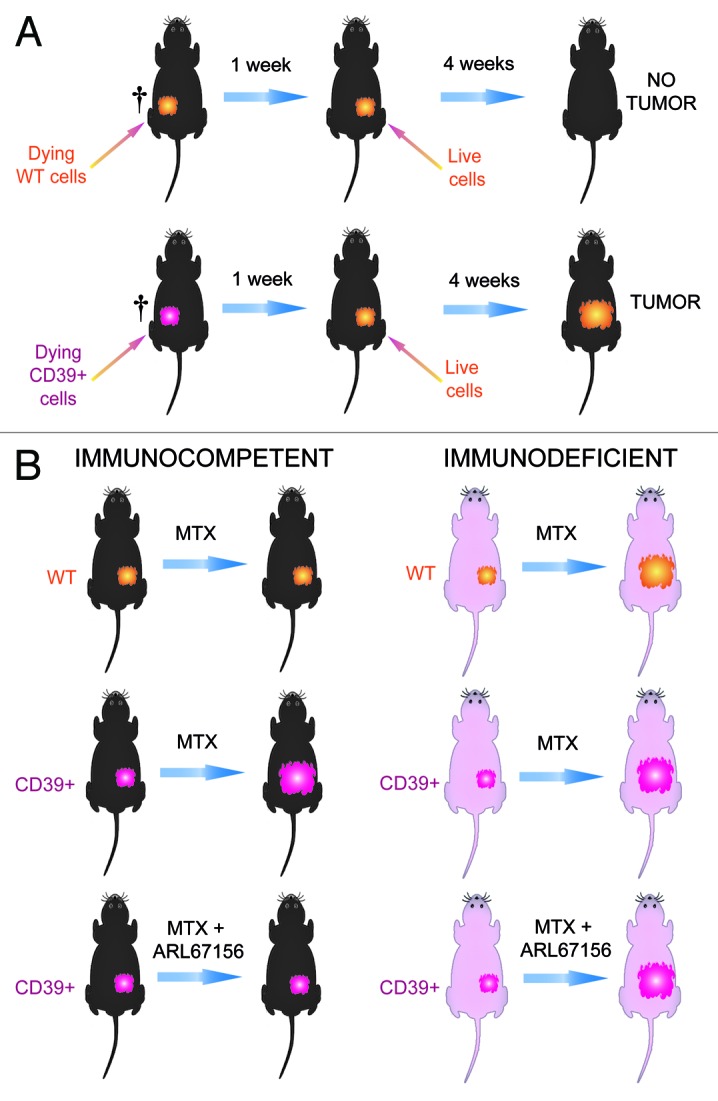
Figure 1. Effects of CD39 overexpression on the immunogenicity of cancer cell death. (A) Vaccination experiments to evaluate immunogenic cell death. Control MCA205 fibrosarcoma cells expressing vector only or MCA205 stably transduced with CD39 were killed by mitoxantrone treatment in vitro. When 70 ± 10% of the cells stained with an Annexin V-FITC conjugate and/or incorporated the vital dye propidium iodine, they were washed and injected subcutaneously into the left flank of C57Bl/6 mice. One week later, the mice were challenged with live MCA205 cells injected into the opposite flank. The absence of tumor growth after four weeks was then interpreted as a sign of a protective anticancer immune response. (B) Immune-dependent chemotherapeutic effects. Control or CD39-expressing cells were injected into C57Bl/6 wild type (WT) or athymic nu/nu mice. The mice then received intraperitoneal injections of mitoxantrone and/or intratumoral injections of the CD39 inhibitor ARL67156 (or PBS as vehicle controls) and tumor growth was monitored for four weeks.
Next, we assessed the impact of CD39 on chemotherapeutic responses. For this we inoculated mice with control or CD39+ MCA205. Once the animals had developed palpable tumors, we treated them with systemic mitoxantrone-based chemotherapy. Importantly, only control tumors growing on immunocompetent mice reduced their growth in response to mitoxantrone. CD39+ MCA205 cancers growing on immunocompetent C57Bl/6 mice or any kind of tumor growing on immunodeficient nu/nu mice failed to reduce their growth rate after mitoxantrone treatment.10 These results indicate that CD39 expression is sufficient to abolish the immune-dependent anticancer effects of anthracyclines in vivo. This immunosuppressive effect of CD39 can be attributed to its ATP-degrading enzyme because intratumoral injection of the pharmacological ecto-ATPase inhibitor, ARL67156, reestablished the efficacy of anthracycline therapy, provided that this treatment was applied to immunocompetent hosts (Fig. 1B).
The aforementioned results underscore the essential contribution of extracellular ATP to the immune response against dying cells, presumably due to its capacity to attract immune effectors including dendritic cells (DC) into the proximity of dying and dead cells10 and to stimulate inflammasome activation in DC.8 In addition, they reveal the intriguing possibility that CD39 expressed on regulatory T cells that infiltrate the tumor bed might exert similar immunosuppressive effects through the depletion of ATP.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/19070
References
- 1.Stagg J, Smyth MJ. Extracellular adenosine triphosphate and adenosine in cancer. Oncogene. 2010;29:5346–58. doi: 10.1038/onc.2010.292. [DOI] [PubMed] [Google Scholar]
- 2.Marcus AJ, Broekman MJ, Drosopoulos JH, Islam N, Pinsky DJ, Sesti C, et al. Metabolic control of excessive extracellular nucleotide accumulation by CD39/ecto-nucleotidase-1: implications for ischemic vascular diseases. J Pharmacol Exp Ther. 2003;305:9–16. doi: 10.1124/jpet.102.043729. [DOI] [PubMed] [Google Scholar]
- 3.Mandapathil M, Szczepanski MJ, Szajnik M, Ren J, Lenzner DE, Jackson EK, et al. Increased ectonucleotidase expression and activity in regulatory T cells of patients with head and neck cancer. Clin Cancer Res. 2009;15:6348–57. doi: 10.1158/1078-0432.CCR-09-1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pulte D, Furman RR, Broekman MJ, Drosopoulos JH, Ballard HS, Olson KE, et al. CD39 expression on T lymphocytes correlates with severity of disease in patients with chronic lymphocytic leukemia. Clin Lymphoma Myeloma Leuk. 2011;11:367–72. doi: 10.1016/j.clml.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun X, Wu Y, Gao W, Enjyoji K, Csizmadia E, Muller CE, et al. CD39/ENTPD1 expression by CD4+Foxp3+ regulatory T cells promotes hepatic metastatic tumor growth in mice. Gastroenterology. 2010;139:1030–40. doi: 10.1053/j.gastro.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clayton A, Al-Taei S, Webber J, Mason MD, Tabi Z. Cancer exosomes express CD39 and CD73, which suppress T cells through adenosine production. J Immunol. 2011;187:676–83. doi: 10.4049/jimmunol.1003884. [DOI] [PubMed] [Google Scholar]
- 7.Zitvogel L, Kepp O, Kroemer G. Immune parameters affecting the efficacy of chemotherapeutic regimens. Nat Rev Clin Oncol. 2011;8:151–60. doi: 10.1038/nrclinonc.2010.223. [DOI] [PubMed] [Google Scholar]
- 8.Ghiringhelli F, Apetoh L, Tesniere A, Aymeric L, Ma Y, Ortiz C, et al. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1beta-dependent adaptive immunity against tumors. Nat Med. 2009;15:1170–8. doi: 10.1038/nm.2028. [DOI] [PubMed] [Google Scholar]
- 9.Martins I, Tesniere A, Kepp O, Michaud M, Schlemmer F, Senovilla L, et al. Chemotherapy induces ATP release from tumor cells. Cell Cycle. 2009;8:3723–8. doi: 10.4161/cc.8.22.10026. [DOI] [PubMed] [Google Scholar]
- 10.Michaud M, Martins I, Sukkurwala AQ, Adjemian S, Ma Y, Pellegatti P, et al. Autophagy-dependent anticancer immune responses induced by chemotherapeutic agents in mice. Science. 2011;334:1573–7. doi: 10.1126/science.1208347. [DOI] [PubMed] [Google Scholar]


