Abstract
The emerging evidence that DNA vaccines elicit a protective immune response in rodents, dogs and cancer patients, coupled with the US Food and Drug Administration (FDA) approval of an initial DNA vaccine to treat canine tumors is beginning to close the gap between the optimistic experimental data and their difficult application in a clinical setting. Here we review a series of conceptual and biotechnological advances that are working together to make DNA vaccines targeting molecules that play important roles during cancer progression (oncoantigens) a promise with near-term clinical impact.
Keywords: DNA vaccination, ErbB-2, In vivo electroporation, Mammary cancer, Oncoantigens, immunotherapy
Once we made a promise we swore we’d always remember
No retreat, baby, no surrender
Blood brothers in a stormy night
With a vow to defend
No retreat, baby, no surrender
No Surrender, Bruce Springsteen
Introduction
The promise of antitumor vaccines rests on their ability to trigger a long-lasting protection against a tumor with virtually no side effects. This promise is underpinned by data from countless vaccination-tumor challenge experiments.1-3 It must be admitted that these vaccines have generated both waves of enthusiasm and total disillusionment. Even so, the fascinating possibility of controlling tumor spread through a vaccine-elicited adaptive immune response is such as to continue to spur the search for better tumor antigens and more efficient vaccination modalities.4
DNA vaccines are a precision tool for activating effective immunity against cancer.5 Their potential impact is illustrated by the first one approved by FDA in the January 2010 for the treatment of advanced canine melanoma, namely OnceptTM (Merial, Duluth, GA), a plasmid coding for human tyrosinase.6 The emerging information should also speed up the translation of new immunotherapies to patients since initiation and progression of canine and human cancers are influenced by similar factors.
However, DNA vaccines are still a promise that may appear far from being an established cancer therapy. Here we are teasing apart a few of the technological and conceptual advances working together to make DNA vaccines targeting oncoantigens, i.e., molecules that play important roles during cancer progression and that can be attached by immune reactivity, a promise with near-term clinical impact.
DNA Vaccines: What are They?
In DNA vaccines the antigen is replaced by its blueprint. When administered, DNA causes the expression of the protein that induce the immune response. Recombinant DNA technologies have facilitated the preparation of plasmids as compared with conventional vaccines. Adjuvant sequences can be embedded in the plasmid. These reasons make DNA vaccination popular in preclinical experiments involving rodents where it elicits cell and antibody-mediated responses to a variety of antigens. The amount of protein expressed, its persistence and the kind of antigen presentation are key variables that determine the efficacy of the protection thus elicited. See references 5 and 7 for excellent reviews on DNA vaccination in cancer.
Basically, a DNA vaccine consists in a circular double strand bacterial DNA (the plasmid) in which a few eukaryotic and synthetic sequences has been inserted. To minimize the possibility of plasmid integration into host chromosomes, these are limited to the antigen sequence, an ubiquitary enhancer-promoter driving its expression and a transcription termination site. To permit insertion of the sequence coding for the antigen, the plasmid should also contain a synthetic 100 base pairs DNA sequence (the multiple cloning site). The presence of a “relaxed origin of replication” and an antibiotic-resistance gene allows efficient plasmid replication in bacterial cells and their selection, two key aspects for high-scale plasmid production. When employment of a plasmid in clinical trials is envisaged, the antibiotic-resistance should not involve antibiotics commonly used in human infections and should be less likely to elicit allergic reactions. The pVAX1 plasmid (Invitrogen) containing the Kanamycin resistance gene meets the FDA’s requirements for vectors to be used in patients.
In Vivo Electroporation: The DNA Vaccinologist’s Dirty Little Secret
There are several ways of delivering a naked plasmid. Its intramuscular injection is enough to elicit an immune response in experimental rodents and humans.8,9 The response is enhanced by the manipulation of the muscle through substances toxic for muscle cells10 or its surgical exposure.11 The plasmid can be enclosed in cationic liposomes to protect it against nucleases12 or shot into the dermis after coating it on gold particles by exploiting a “gene gun”13 or jet-injectors.14 Lastly, a rapid immune response follows tattooing of a plasmid into the skin.15
Just as adjuvants are the “immunologist’s dirty little secret”16 to increase the efficacy of a vaccine, electroporation is the dirty little secret that enhances the efficacy of DNA vaccines:
(A) by facilitating plasmid entry into target cells;
(B) by acting as an adjuvant that induces local tissue damage eventually attracting an inflammatory infiltrate.
Delivery of short electrical pulses to the injection site causes temporary permeabilization of the cell membrane. Entry of the plasmid into the cell is facilitated and inter-individual variability of the response is minimized.17,18 Sophisticated electroporators have rendered plasmid electroporation a common way of securing efficient DNA immunization in animals19 and patients.20 Electroporators deliver pulses at a voltage that may be fixed or regulated to take account of tissue resistance. The number and kind of electric pulses, their voltage and their delivery through plaques applied on the skin or by needles determine the kind and intensity of the response elicited. In part this is due to the efficacy of the penetration of the plasmid into the cell. On the other hand, the kind of injury provoked by both needle insertion and electric pulses play a role in deciding the form and extent of cell death and the release of necrotic debris or apoptotic bodies. The type and amount of cell death influences the recruitment of distinct reactive cells releasing idiosyncratic repertoires of cytokines. These inflammatory cells are differently able to both take up and present the antigen secreted by transfected cells and cross present antigen-expressing cell debris released by the dying cells.
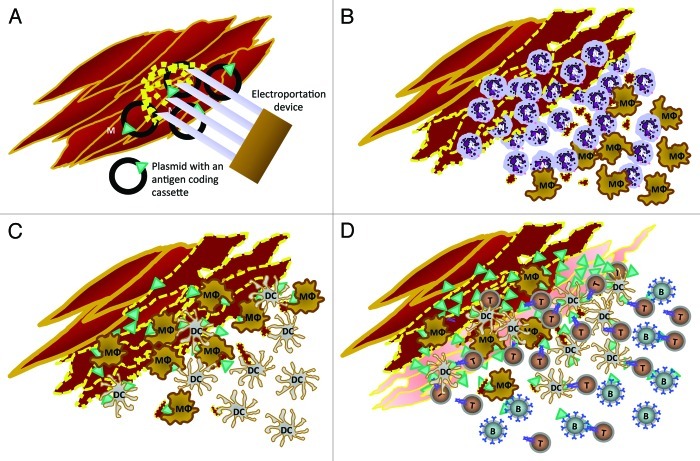
The Igea Cliniporator is a fixed voltage, clinical device adopted to improve the penetration of bleomicin into cancer lesions.21 It can also be set to improve DNA vaccination. A morphological analysis of the events following intramuscular injection and electroporation of a plasmid22 shows that damaged myofibers first become infiltrated by granulocytes, then by macrophages, dendritic cells (DC) and later by B and T cells (Fig. 1). The numerous contacts between macrophages, DC, T cells and antigen expressing myofibers suggests that both a direct antigen presentation and macrophage and DC-mediated antigen cross-presentation may occur in the electroporated muscle. By contrast, the reaction of the draining lymph nodes is weak.
Figure 1. Cellular events following intramuscular electroporation of 50 µg of plasmid in 20 µl of saline through Cliniporator (Igea, Carpi, Italy). Insertion of the electroporator needles into the quadriceps muscle of the mouse and delivery of two low voltage pulses of 150V of 25 ms with a 300 µs interval (A). One-6 h later the damaged myofibers and cell debris (dotted yellow) are surrounded by polymorphonuclear (N) and mononuclear leukocytes (MΦ) (B). One or two days later mature and differentiated tissue macrophages (MΦ) and dendritic cells (DC) progressively become prominent among inflammatory cells infiltrating the numerous necrotic myofibers (C). On the third-fourth day from the electroporation, intact and regenerating muscular fibers (pale red) are overexpressing the protein encoded by the plasmid (green triangles), while the area is being infiltrated by B (B), T (T) and dendritic cells (DC). Interestingly, CD11b+ macrophages and CD11c+ DC and, later, CD4+ T cells are often in direct contact with antigen expressing muscle cells or antigen expressing fragments and each other (D).
Exogenous Adjuvants
As the environment plays a major role in modulating the response that ensues after delivery of a plasmid, we have set out to determine whether adjuvants enhance the immune response elicited in mice by the intramuscular injection or electroporation of a plasmid (RRTErbB-2) coding for the extracellular and transmembrane domains of rat ErbB-2 oncogene.11,23,24
LAG-3 (lymphocyte activation gene-3/CD223) is a type I transmembrane protein associated with the T cell receptor (TCR)–CD3 complex. Administration of soluble LAG-3, generated by fusing the extracellular domain of murine LAG-3 to murine IgG2a Fc portion, in the area in which RRTErbB-2 has been injected potentiates the vaccination by eliciting a stronger CD8+ T cell activity, a sustained secretion of both interferon-gamma (IFNγ) and IL-4, and the expansion of CD8+/CD11b+/CD28+ effector and CD8+/CD11b+/CD28- effector-memory T cells. Combination of plasmid injection with soluble LAG-3 also leads to higher titers of anti-rat ErbB-2 antibodies with a faster shift toward IgG2a. In mammary cancer prone rat ErbB-2-transgenic BALB/c mice (BALB-neuT mice) this enhanced response goes along with a sustained protection against the onset of mammary lesions.25
Remarkably, even the higher immune response that follows RRTErbB-2 electroporation in BALB-neuT mice is further enhanced by intravenous administration of BAT monoclonal antibodies (mAb) (CureTech). The stronger anti-rat ErbB-2 T cell and antibody-mediated immune response thus elicited results in even better protection.26 These BAT mAb bind the programmed death-1 (PD-1) receptor of the B7 family expressed on T cells after activation.27 Following the interaction with its ligands (PDL-1 and PDL-2) PD-1 downregulates TCR signals and promotes T cell anergy and apoptosis. By blocking this suppressive loop, BAT mAb enhance T-cell response and leads to potent anti-tumor immunity both in mouse models and in patients with advanced hematologic cancer.27
Lactoferrin, a glycoprotein of the transferrin superfamily, plays several distinct activities in immunity. We have shown that repeated oral administrations of a recombinant human lactoferrin (Talactoferrin, TLF, Agennix) hamper the onset of mammary carcinomas in BALB-neuT mice.28 Association of oral TLF with RRTErbB-2 electroporation extends tumor-free interval, decreases the number of palpable tumors/mouse, and augments vaccine-induced response as compared with sole RRTErbB-2 electroporation.
Build-In Adjuvants
Hypomethylated CpG dinucleotide-containing motifs present or inserted in the bacterial plasmid trigger innate immune activities and help to shape the adaptive response elicited by the vaccine. Similarly, pattern recognition receptors that sense intracellular DNA can be used to harness the intrinsic immune-stimulating properties of DNA vaccines. A plasmid encoding the cytosolic DNA-dependent activator of interferon regulatory factors co-injected with antigen-encoding plasmids enhances antigen-specific proliferation and induction of effector and memory T cells.29
In principle, cytokines are excellent adjuvants for DNA vaccines, either as recombinant proteins or as embodied genes. They may increase the response and thus allow selective activation of particular reaction mechanisms. We have shown that the nonapeptide VQGEESNDK corresponding to the 163–171 amino acids of the human IL1β retains several of the immunostimulatory properties while it is free from the potent proinflammatory properties of the entire molecule and increases the immunogenicity of poorly immunogenic tumors.30 When BALB-neuT mice were immunized with RRTErbB-2 plasmids in which the sequence of this nonapeptide was inserted, a higher anti-rat ErbB2 antibody response accompanied by a massive lymphocyte infiltration of the mammary lesions went along with a stronger protection against mammary tumors.31
One of the most effective non-DNA vaccines against ErbB-2, the “Triplex ” vaccine, is based on mammary carcinoma cells expressing both the rat ErbB-2 protein and allogeneic class I major histocompatibility complex (MHC) glycoproteins administered in combination with recombinant IL-12.32 By replacing the recombinant cytokine with cytokine gene-engineered cells, it was possible to compare the efficacy of IFNγ, IL-2, IL-12, and IL-15. In cancer prone ErbB-2 transgenic mice IL-12 was vastly superior to other cytokines.33 The translation of the Triplex design from cell-based to a DNA vaccine, using a mixture of three plasmids: RRTErbb2, a plasmid encoding full length H-2Dq MHC gene and another plasmid encoding mouse IL-12, showed that cell-based and the DNA Triplex vaccines were equally effective.34 However, in most instances vaccination with the RRTErbb2 plasmid alone elicited a protection similar to that obtained with the Triplex plasmids. This finding was surprising since our previous experience with cell-based vaccines had shown that the Triplex design yielded vastly superior protection from tumor onset in comparison to any subset of its components.32 Our results show that the strong immunity elicited by the RRTErbB-2 plasmid, already helped by embedded CpG sequences and by electroporation, is less dependent than analogous cell vaccines on the adjuvant activity of cytokines.
Lastly, construction of more sophisticated plasmids results in the induction of better immune responses by simultaneously relieving from suppression. Plasmids can thus be endowed with two expression cassettes, one coding for the antigen, the other expressing a shRNA able to silence those molecules that negatively control the immune response.35 This shRNA-mediated interference with regulatory mechanisms only concerns plasmid-transfected antigen-presenting cells. When the ability of these transfected cells to induce an efficient immune response is disturbed by a tumor, neutralization of tumor-borne regulatory factors may result in an efficient presentation of oncoantigen peptides.36
The Sequence Coding for the Antigen
Fine modifications of the sequence coding for the antigen protein lead to major differences in protein processing and immunogenicity.5 Addition of a signal peptide or an ubiquitine signal to the N-terminus of the antigen sends the encoded protein toward the extracellular microenvironment through the endoplasmic reticulum or toward the proteasome for processing and presentation by MHC class I glycoproteins,37 whereas it goes to the plasma membrane when idrophobic sequences are added to the C-terminus. Addition of a lysosomal targeting signal drives its presentation by MHC class II glycoproteins.38
The adaptive response elicited by the antigen can be “helped” by inserting additional sequences and obtaining an encoded protein fusing the antigen with these sequences.5 The fusion proteins containing tumor antigens and epitopes of the tetanus toxin engage T helper cells induced by a previous anti-tetanus immunization in the response against the tumor antigen.
By exploiting receptors of different kind, the fusion antigen can be selectively targeted toward antigen presenting cells to empower them to in vivo priming of CD8+ T cells. When mice were electroporated with a plasmid encoding amino acid 364–391 of human ErbB-2 fused with the Fc domain of a human IgG1 (Fc-ErbB-2364–391), the better CD8 T cell-mediated response toward human ErbB-2 epitopes was paralleled by a stronger protection against a challenge of human ErbB-2+ mammary cancer cells.39
Another way we explored to get a more selective delivery of tumor antigens is to generate a plasmid coding for the antigen fused with the extracellular domain of CTLA-4, a receptor binding to the co-stimulatory B7 (CD86) molecules on DC. Following the injection in mice of a plasmid coding for the NH-terminal 222-amino acids of human ErbB-2 fused with the extracellular domain of human CTLA-4 we observed an enhanced anti-human ErbB-2 humoral and T cell mediated response and protection against a challenge with mouse tumor cells expressing human ErbB-2. A similar plasmid encoding a fusion CTLA-4-ErbB-2 mouse protein markedly delayed the onset of mammary carcinomas in BALB-neuT mice.40
We also searched for the smallest rat ErbB-2 epitope still able to elicit a protective response. Our starting point was the observation that RRTErbB-2 plasmid elicits a similar or even better response than the one encoding the full length rat ErbB-2.11 Then, we produced seven plasmids encoding the transmembrane domain associated with decreasing fragments of the extracellular domain.41 When these plasmids were electroporated in wild-type BALB/c mice, the intensity of the response and protection against a challenge of rat ErbB-2+ murine cells decreased with the shortening of the ErbB-2 fragment encoded. Surprisingly, immunization with the RRTErbB-2311–689 plasmid encoding a fragment lacking the first 311 amino acids of the ErbB-2 extracellular domain still afforded full protection. In BALB-neuT mice electroporation of RRTErbB-2311–689 induces a protection comparable to that elicited by RRTErbB-2. Its location in the transfected cell and the conformation acquired by the short fragment encoded probably allows a favorable exposition of critical ErbB-2 epitopes.41
One of the problems linked to the use of oncoantigens as a target is that they are self-tolerated molecules. Therefore, a vaccine should be able to induce an autoimmune response overcoming central and peripheral tolerance to self.42,43 Both the induction and the persistence of an immune response are markedly hindered by natural Treg cells that expand in response to the overexpression of self oncoantigens by the tumor. The “regulatory memory” constantly tuning down the immune response to self antigens44 makes the induction of an effective response to oncoantigens difficult, and make it necessary to continuously boost the vaccination to maintain its efficacy.45
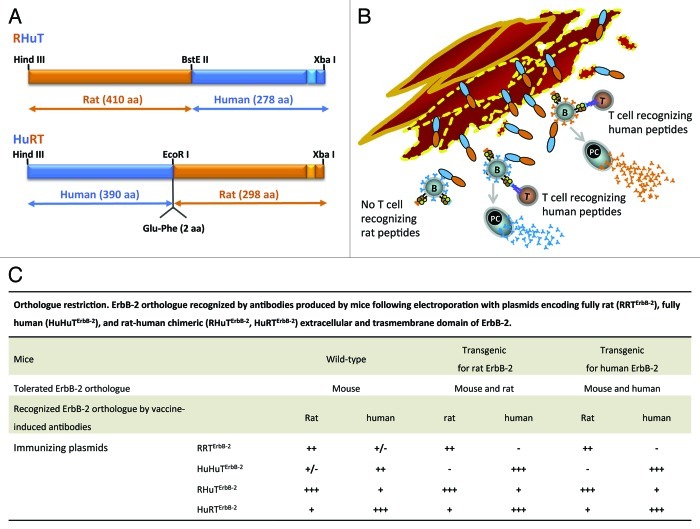
Vaccination with xenogeneic ortholog antigens is an effective way to break self tolerance. Heterologous antigens activate both bystander anergic B and T cells and prime T cells. However, the cross-reactive response to self antigens may be of low intensity and affinity.46 When wild-type mice and mice transgenic and tolerant for the rat or the human ErbB-2 are immunized with plasmids encoding the extracellular and transmembrane domains of the fully human (HuHuTErbB-2) or fully rat ErbB-2 (RRTErbB-2), the response shows a consummate specificity to the rat or human ErbB-2 ortholog used as antigen but a poor cross-reactivity toward the ortholog expressed by the mouse and its tumors (Fig. 2). This “orthologue restriction” corresponds to a similar restriction in the ability to inhibit the growth of tumors expressing the human or rat ErbB-2 ortholog.22 To overcome it we immunized mice with plasmids coding for ErbB-2 proteins composed in part by rat and by human sequences. The homologous moiety guarantees the specificity of the response while the hetereologous moiety ensures better overcoming of tolerance (Fig. 2). Vaccination of wild-type mice and mice transgenic for the rat or the human ErbB-2 with these chimeric plasmids elicits a stronger and more cross-reactive response and a better protection than fully human or fully rat plasmids against carcinomas overexpressing either rat or human ErbB-2.22,45,47
Figure 2. Immunogenicity of chimeric proteins coded by rat-human (RHuTErbB-2) and human-rat (HuRTErbB-2) plasmids. RHuTErbB-2 encodes for a protein in which the 410 NH2-terminal residues are from the rat ErbB-2 extracellular domain and the remaining residues from human ErbB-2. HuRTErbB-2 encodes for a protein in which the 390 NH2-terminal residues are from human ErbB-2 and the remainder from rat ErbB-2 (A). The ability of B cells (B) to present not-tolerated peptides contributes to production of an antibody response to both the tolerated and not-tolerated moieties of the antigen. Following a DNA electroporation of a rat ErbB-2 tolerant mouse muscle with a plasmid encoding for a rat (orange) and human (blue) chimeric ErbB-2 protein (RHuTErbB-2 or HuRTErbB-2), T cells (T) recognizing the xenogeneic human peptides expands. The expanded T cells interact and provide helper signals to B cells by recognizing not-tolerated human peptides (blue triangles) presented by MHC II molecules on the cell membrane of B cells. The interaction between T and B cells recognizing human moiety of the encoded vaccine leads to the production by plasmacells (PC) of antibodies (blue Y) to the xenogeneic human moiety. By contrast, the interaction of expanded T cells with B cells specific for a tolerated rat moiety help to break immune tolerance to self protein leading to the production by plasmacells (PC) of antibodies (orange Y) to the tolerated rat moiety (B). ErbB-2 ortholog recognized by antibodies produced by mice following electroporation with plasmids encoding fully rat (RRTErbB-2), fully human (HuHuTErbB-2), and rat-human chimeric (RHuTErbB-2, HuRTErbB-2) extracellular and trasmembrane domain of ErbB-2 (C).
Oncoantigens, Why?
Tumor’s genetic instability often thwarts the immune attack because it results in the selection of clones that do no longer express the target antigen or express it in a way that it cannot be perceived by T cells.48,49 A conceptual quantum jump in planning new generation vaccines is to target the vaccine against oncoantigens since these are molecules not (easily) disposable during tumor progression nor are they easy to hide because of their indispensable role. Oncoantigen-loss variants can occur, but they will get a crippled tumorigenic potential and negative selection.50 Otherwise, the function of the targeted oncoantigen can be taken by its replacement by one that will offer a further target.51
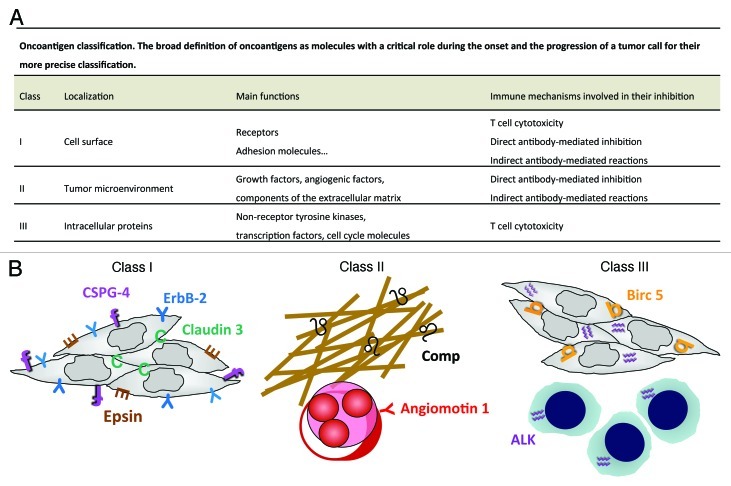
Besides its selection of antigen-loss variants, a tumor escapes T cell recognition through impairment of antigen processing machinery and downmodulation of MHC glycoproteins. However, because of their function, oncoantigens are often accessible to vaccine-induced antibodies.52 Their diverse functions and localizations (Fig. 3) thus allows their targeting by different immune mechanisms, among which those mediated by antibody may acquire a prominent role. Antibodies may directly block their function, activate complement-mediated lythic and inflammatory mechanisms, mediate antibody and complement dependent cytotoxicity, and give rise to immune complexes triggering additional immune responses and epitope spreading.55,58
Figure 3. Precise oncoantigen classification (A). Validated class I, II and III oncoantigens present in various tumor compartments currently targeted by our therapeutic vaccines (B). Comp,53 (black ♌) and Angiomotin 1,54 (red Y) are validated class I oncoantigens expressed on tumor extracellular matrix and on tumor vasculature respectively (left panel). ErbB-2,55 (blue Y), Epsin, (brown E), Claudin,53 (green C) and CSPG4,56 (purple f) are class II oncoantigens expressed on tumor cell membrane (middle panel). The protein product of the NPM-ALK translocation,57 (ALK, purple ♒) and BIRC5,53 (orange b) are class III oncoantigens present in the cytoplasm and nucleus of carcinomas and lymphomas (right panel). Several additional molecule endowed with the properties of oncoantigens have been validated in immunization assay by other laboratories.58
The evidence of an important role of vaccine-elicited antibodies in controlling the onset and expansion of a tumor constitutes a second conceptual quantum leap afforded by vaccines against oncoantigens. Despite the persistent T cell chauvinisms, DNA vaccines against oncoantigens should be planned to elicit both humoral and cellular immunity and not solely a T cell-mediated immunity.32,51,59
On the Search for Fresh Oncoantigens
The “rite of passage” that marks a tumor antigen to become a verified oncoantigen has two phases:
(A) The disclosure of a functional role of the molecule in fostering the transformed phenotype of a given (human) neoplasm, i.e. the “onco” portion of the oncoantigen definition;
(B) The evidence that the immune system recognizes the molecule and mounts an effective response affecting tumor progression, i.e. the “antigen” part.
The identification of oncoantigens moves from the consideration that the progression of carcinogenesis requires the activation of specific genes and may be altered when these are inhibited. The characterization of genes differentially activated during cancer progression should lead to the identification of prominent targets against which to trigger the immune response. Microarray transcription analysis highlights several gene signatures that differentiate successive stages of tumor progression in transgenic mice. Of the transcripts upregulated going from pre-neoplasia to overt cancer, only those that have an ortholog in humans, low expression in normal human tissues, and a high and homogeneous expression in human cancers are selected as “putative” oncoantigens.53 The most promising are those that may be targeted by both antibodies and T cells. They are then validated by immunizing cancer-prone transgenic mice with DNA vaccines targeting them.53,55 For a provisional inventory of putative and verified oncoantigens see reference 52.
In many cases vaccines targeting oncoantigens afford cancer-prone genetically engineered mice a life-long protection. By contrast, in the management of minimal residual disease or in the presence of recurrences and metastases, their potential is limited.52,58,60 Greater efficacy could be achieved by targeting oncoantigens expressed by cancer initiating cells (CIC) responsible for the progression, metastasis, resistance to therapy, and recurrence of many kinds of tumors. To identify oncoantigens expressed by CIC of mammary cancer we are performing a comparative transcriptomic analysis of the bulk of tumor cells from transgenic mice with that of CIC-enriched serial sphere passages of the same tumor cells.61 Subsequent integration of these mouse data with those from human breast cancer will allow the selection of oncoantigens involved in the maintenance of the CIC-like features of mouse and human cancer. Fresh DNA vaccines targeting these newly identified CIC oncoantigens may extend vaccine efficacy.36
Models for Oncoantigen Validation
During the long-lasting relationship between a tumor and its environment, antibodies against oncoantigens play diverse roles. These roles are prominent precisely because an oncoantigen is both a tumor target and a molecule with a functional role in tumor progression.55 The importance of vaccine-elicited antibodies against oncoantigens is one of the lessons emerging from the studies on genetically engineered mice developing autochthonous cancers. Immunization experiments carried on these mice, on the other hand, illustrate the limit of an anti-oncoantigen vaccine. It can keep an incipient tumor at bay for a very long time.23 However, the efficacy of vaccine-induced protection fades when the vaccine is first administered to mice already bearing clinically evident tumors.23,57 In a therapeutic setting the protection afforded by an effective oncoantigen vaccine is often similar to that observed in “successful” clinical trials with anti-tumor vaccines: a statistically significant delay in tumor progression, but of little weight in the life of a patient.
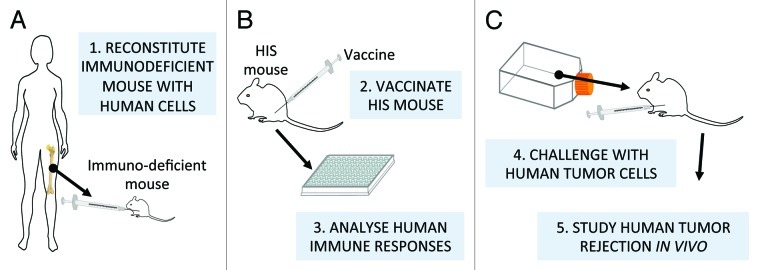
Since experimental models are so critical in assessment of the weight of the immune mechanisms involved in tumor protection, those that ever more closely reproduce the human situation should be sought. Genetically engineered mice with profound and stable immunodeficiencies have been transplanted with a human immune system and used to investigate and manipulate, in a murine context, human immunity against human tumors.62 Even in their present, imperfect state of development, these mice can be used not only to study the development of human immune components, but also to better define a few critical features of vaccines against oncoantigens (Fig. 4).
Figure 4. Human immune system-engrafted (HIS) mice for validation of oncoantigen vaccines. Human hematopoietic stem cells implanted in severely immunodeficient mice give rise to a (more or less) functional human immune system (A) that can be subsequently vaccinated with human tumor antigens to investigate and manipulate in an experimental context human anti-tumor immunity, (B and C). Note that most current protocols use human progenitors from cord blood for the engraftment, hence the final step shown in the figure, i.e. challenge with human (adult) tumor cells, could entail MHC allogeneic interactions. Human immune system-engrafted mice are undergoing continuous improvements, because the interactions of xenogeneic human and murine molecules is not always functional. Novel models include additional genes encoding for human MHC, to humanize antigen presentation, or human species-specific cytokines to enhance human cell long-term survival and functional differentiation.63
Lastly, it should be borne in mind that many aspects of the immune response can be captured by mathematical models ranging from system-level models that describe the immune system as a whole to extremely fine-grained ones that analyze a single cellular or molecular response.64 The latest generation of models is beginning to tackle practical problems. Both the general field of vaccines and tumor immunology especially can derive great practical benefits.65
Climbing Over the Limit
Experiments with genetically engineered mice have furnished bitter evidence of the limit of DNA vaccines against oncoantigens. When an oncoantigen vaccine is administered to a healthy mouse genetically predestined to die because of a specific cancer, repeated boosts keep it tumor free for a period of time that may equate its natural life span. However, when the same vaccine is administered to a mouse with more advanced stages of microscopic lesions, the protection gradually fades away.66 As mentioned above, vaccine combination with adjuvants of different kind variously extends the efficacy of vaccine-elicited protection.25,26 A further extension is obtained by manipulating the sequence coding for the antigen protein.45 However, as the time of the first vaccine administration approaches the stages of a clinical tumor diagnosis, vaccine efficacy disappears. A pattern of this kind is shared by different combinations of vaccine in genetically engineered mice confining the exploitation of oncoantigen vaccines to the awkward field of tumor prevention.58 It also spurs the search for a way to make oncoantigen vaccine of significance in tumor therapy. Probably multiple factors contribute to the shortfall of vaccine efficacy. The progression of tumor renders the microscopic lesions and incipient tumors less permeable to immune attack. Moreover, a series of factors released by the tumor together with the increasing overexpression of the targeted oncoantigen results in an expansion of regulatory cells that suppress the induction, effector and memory phases of anti-tumor immune response. The importance of these tumor-driven negative regulations is evident in mice bearing slow growing autochthonous cancer as well as in patients with a clinically evident tumor.
Almost paradigmatically BALB-neuT mice show that during the slow progression of their mammary lesions immature myeloid cells,67 suppressive NKT cells68 and natural Treg cells42 expand. As the availability of anti-CD25 mAb allows a simple even if gross interference with Treg cells, we assessed how a temporary interference with Treg cells extend vaccine efficacy. In BALB-neuT mice central tolerance activated by ErbB-2 expression in the thymus and its overexpression in the mammary lesions lead to the deletion of CD8+ T cell clones reacting at high affinity with dominant ErbB-2 epitopes. Because of this deletion, anti-ErbB-2 DNA vaccination elicits solely CD4+ cell expansion and anti-ErbB-2 antibodies.23,69,70 However, the combination of DNA vaccination with the depletion of Treg cells triggers a higher antibody response combined with the onset of a CD8+ cytotoxic response against the ErbB-2 immunodominant epitope. This unexpected cytotoxic response is due to the freeing of a latent pool of low-avidity CD8+ T cells bearing cryptic TCR from Treg constraints. The stronger and composite response elicited inhibits cancer progression at stages in which the vaccine alone is ineffective. While dramatic, this extension of vaccine efficacy is no longer able to secure mice survival when diffuse and invasive microscopic cancers become palpable.43
These results suggest that a more sophisticated interference with tumor-borne regulatory and suppressor mechanisms may serve to endow oncoantigen vaccines with therapeutic efficacy. The exploitation of the plasmids both coding for the antigen and expressing shRNA able to silence molecules that negatively control the immune response may offer new ways to extend oncoantigen vaccine efficacy.36
Conclusions: First Fulfillments of the Promise
The antibody and cell mediated response elicited by the electroporation of HuRTErbB-2 plasmid in healthy dogs and in pet dogs after the surgical removal of mammary cancer proves the efficacy of DNA vaccines electroporation in higher animals (Cavallo F, unpublished results). Moving from this evidence, we started a clinical trial in pet dogs with advanced mucosal malignant melanoma to assess the potential of the electroporation of a plasmid coding for the human chondroitin sulfate proteoglycan-4 (CSPG4), a molecule highly expressed by human and dog melanomas whereas it has a restricted distribution in normal tissues.71 Dogs with biopsy-confirmed CSPG4+ malignant melanoma and absence of metastases beyond the first regional lymph nodes were subjected to surgery and then electroporated at monthly intervals with a plasmid coding for human CSPG4. In all dogs the vaccine elicited antibodies to human CSPG4. Their cross-reactivity with the canine ortholog shows that the plasmid coding for this heterologous molecule effectively breaks immune tolerance to dog CSPG4. So far, the intensity of the elicited immune response appears to go along with the extension of the time of tumor recurrence and dog survival (Cavallo F et al., in preparation).
Preliminary data from ongoing trials with cancer patients are showing that DNA vaccines induce immune responses that goes along with an inhibition of tumor progression.52,72 When plasmids are electroporated, the response elicited in patients with recurrent prostate cancer is much higher than that triggered by the sole injection of the plasmid.20 Italian authorities have recently approved a phase I/II clinical trial based on repeated electroporations of the chimeric RHuTErbB-2 plasmid in patients in whom a primary advanced ErbB-2+ squamous carcinomas of the oral cavity, oropharynx, hypopharynx has been surgically removed (EudraCT, 2011-001104-34). Both the implemented dog trial with CSPG4 and the incipient trial with RHuTErbB-2 plasmids are aimed to exploit the immune response not to cure an existing cancer, but to extend the tumor free survival and delay tumor relapse.
Admittedly, all the forthcoming and ongoing clinical trials are still far from fulfilling the therapeutic promise of DNA vaccine against oncoantigens, but hopefully they are putting a solid case for it. No retreat and no surrenders!
Acknowledgments
The experimental data discussed here have been obtained thanks to grants from the Italian Association for Cancer Research (AIRC; IG 5377 and 11675 to F.C.; IG 10353 to P.L.L.), the Italian Ministry for the Universities and Research, The University of Torino, the Regione Piemonte (Piattaforma Innovativa, ImmOnc), and the EU Consortium of Anticancer Antibody Development (EUCAAD) 200755.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/19127
References
- 1.Klein G, Sjogren HO, Klein E, Hellstrom KE. Demonstration of resistance against methylcholanthrene-induced sarcomas in the primary autochthonous host. Cancer Res. 1960;20:1561–72. [PubMed] [Google Scholar]
- 2.Musiani P, Modesti A, Giovarelli M, Cavallo F, Colombo MP, Lollini PL, et al. Cytokines, tumour-cell death and immunogenicity: a question of choice. Immunol Today. 1997;18:32–6. doi: 10.1016/S0167-5699(97)80012-6. [DOI] [PubMed] [Google Scholar]
- 3.Van Pel A, Boon T. Protection against a nonimmunogenic mouse leukemia by an immunogenic variant obtained by mutagenesis. Proc Natl Acad Sci U S A. 1982;79:4718–22. doi: 10.1073/pnas.79.15.4718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gilboa E. The promise of cancer vaccines. Nat Rev Cancer. 2004;4:401–11. doi: 10.1038/nrc1359. [DOI] [PubMed] [Google Scholar]
- 5.Rice J, Ottensmeier CH, Stevenson FK. DNA vaccines: precision tools for activating effective immunity against cancer. Nat Rev Cancer. 2008;8:108–20. doi: 10.1038/nrc2326. [DOI] [PubMed] [Google Scholar]
- 6.Bergman PJ. Cancer immunotherapy. Top Companion Anim Med. 2009;24:130–6. doi: 10.1053/j.tcam.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 7.Wei WZ, Jacob J, Radkevich-Brown O, Whittington P, Kong YC. The “A, B and C” of Her-2 DNA vaccine development. Cancer Immunol Immunother. 2008;57:1711–7. doi: 10.1007/s00262-008-0464-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ulmer JB, Donnelly JJ, Parker SE, Rhodes GH, Felgner PL, Dwarki VJ, et al. Heterologous protection against influenza by injection of DNA encoding a viral protein. Science. 1993;259:1745–9. doi: 10.1126/science.8456302. [DOI] [PubMed] [Google Scholar]
- 9.Wang R, Doolan DL, Le TP, Hedstrom RC, Coonan KM, Charoenvit Y, et al. Induction of antigen-specific cytotoxic T lymphocytes in humans by a malaria DNA vaccine. Science. 1998;282:476–80. doi: 10.1126/science.282.5388.476. [DOI] [PubMed] [Google Scholar]
- 10.Danko I, Fritz JD, Jiao S, Hogan K, Latendresse JS, Wolff JA. Pharmacological enhancement of in vivo foreign gene expression in muscle. Gene Ther. 1994;1:114–21. [PubMed] [Google Scholar]
- 11.Rovero S, Amici A, Di Carlo E, Bei R, Nanni P, Quaglino E, et al. DNA vaccination against rat her-2/Neu p185 more effectively inhibits carcinogenesis than transplantable carcinomas in transgenic BALB/c mice. J Immunol. 2000;165:5133–42. doi: 10.4049/jimmunol.165.9.5133. [DOI] [PubMed] [Google Scholar]
- 12.Perrie Y, Frederik PM, Gregoriadis G. Liposome-mediated DNA vaccination: the effect of vesicle composition. Vaccine. 2001;19:3301–10. doi: 10.1016/S0264-410X(00)00432-1. [DOI] [PubMed] [Google Scholar]
- 13.Fynan EF, Webster RG, Fuller DH, Haynes JR, Santoro JC, Robinson HL. DNA vaccines: protective immunizations by parenteral, mucosal, and gene-gun inoculations. Proc Natl Acad Sci U S A. 1993;90:11478–82. doi: 10.1073/pnas.90.24.11478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haensler J, Verdelet C, Sanchez V, Girerd-Chambaz Y, Bonnin A, Trannoy E, et al. Intradermal DNA immunization by using jet-injectors in mice and monkeys. Vaccine. 1999;17:628–38. doi: 10.1016/S0264-410X(98)00242-4. [DOI] [PubMed] [Google Scholar]
- 15.Bins AD, Jorritsma A, Wolkers MC, Hung CF, Wu TC, Schumacher TN, et al. A rapid and potent DNA vaccination strategy defined by in vivo monitoring of antigen expression. Nat Med. 2005;11:899–904. doi: 10.1038/nm1264. [DOI] [PubMed] [Google Scholar]
- 16.Janeway CA., Jr Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb Symp Quant Biol. 1989;54:1–13. doi: 10.1101/sqb.1989.054.01.003. [DOI] [PubMed] [Google Scholar]
- 17.Mir LM, Bureau MF, Gehl J, Rangara R, Rouy D, Caillaud JM, et al. High-efficiency gene transfer into skeletal muscle mediated by electric pulses. Proc Natl Acad Sci U S A. 1999;96:4262–7. doi: 10.1073/pnas.96.8.4262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murakami T, Sunada Y. Plasmid DNA Gene Therapy by Electroporation: Principles and Recent Advances. Curr Gene Ther. 2011;11:447–56. doi: 10.2174/156652311798192860. [DOI] [PubMed] [Google Scholar]
- 19.Curcio C, Khan AS, Amici A, Spadaro M, Quaglino E, Cavallo F, et al. DNA immunization using constant-current electroporation affords long-term protection from autochthonous mammary carcinomas in cancer-prone transgenic mice. Cancer Gene Ther. 2008;15:108–14. doi: 10.1038/sj.cgt.7701106. [DOI] [PubMed] [Google Scholar]
- 20.Low L, Mander A, McCann K, Dearnaley D, Tjelle T, Mathiesen I, et al. DNA vaccination with electroporation induces increased antibody responses in patients with prostate cancer. Hum Gene Ther. 2009;20:1269–78. doi: 10.1089/hum.2009.067. [DOI] [PubMed] [Google Scholar]
- 21.Quaglino P, Mortera C, Osella-Abate S, Barberis M, Illengo M, Rissone M, et al. Electrochemotherapy with intravenous bleomycin in the local treatment of skin melanoma metastases. Ann Surg Oncol. 2008;15:2215–22. doi: 10.1245/s10434-008-9976-0. [DOI] [PubMed] [Google Scholar]
- 22.Quaglino E, Riccardo F, Macagno M, Bandini S, Cojoca R, Ercole E, et al. Chimeric DNA vaccines against ErbB2+ carcinomas: from mice to humans. Cancers. 2011;3:3225–41. doi: 10.3390/cancers3033225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Quaglino E, Iezzi M, Mastini C, Amici A, Pericle F, Di Carlo E, et al. Electroporated DNA vaccine clears away multifocal mammary carcinomas in her-2/neu transgenic mice. Cancer Res. 2004;64:2858–64. doi: 10.1158/0008-5472.CAN-03-2962. [DOI] [PubMed] [Google Scholar]
- 24.Amici A, Smorlesi A, Noce G, Santoni G, Cappelletti P, Capparuccia L, et al. DNA vaccination with full-length or truncated neu induces protective immunity against the development of spontaneous mammary tumors in HER-2/neu transgenic mice. Gene Ther. 2000;7:703–6. doi: 10.1038/sj.gt.3301151. [DOI] [PubMed] [Google Scholar]
- 25.Cappello P, Triebel F, Iezzi M, Caorsi C, Quaglino E, Lollini PL, et al. LAG-3 enables DNA vaccination to persistently prevent mammary carcinogenesis in HER-2/neu transgenic BALB/c mice. Cancer Res. 2003;63:2518–25. [PubMed] [Google Scholar]
- 26.Quaglino E, Mastini C, Iezzi M, Forni G, Musiani P, Klapper LN, et al. The adjuvant activity of BAT antibody enables DNA vaccination to inhibit the progression of established autochthonous Her-2/neu carcinomas in BALB/c mice. Vaccine. 2005;23:3280–7. doi: 10.1016/j.vaccine.2005.01.086. [DOI] [PubMed] [Google Scholar]
- 27.Berger R, Rotem-Yehudar R, Slama G, Landes S, Kneller A, Leiba M, et al. Phase I safety and pharmacokinetic study of CT-011, a humanized antibody interacting with PD-1, in patients with advanced hematologic malignancies. Clin Cancer Res. 2008;14:3044–51. doi: 10.1158/1078-0432.CCR-07-4079. [DOI] [PubMed] [Google Scholar]
- 28.Spadaro M, Curcio C, Varadhachary A, Cavallo F, Engelmayer J, Blezinger P, et al. Requirement for IFN-gamma, CD8+ T lymphocytes, and NKT cells in talactoferrin-induced inhibition of neu+ tumors. Cancer Res. 2007;67:6425–32. doi: 10.1158/0008-5472.CAN-06-4080. [DOI] [PubMed] [Google Scholar]
- 29.Lladser A, Mougiakakos D, Tufvesson H, Ligtenberg MA, Quest AF, Kiessling R, et al. DAI (DLM-1/ZBP1) as a genetic adjuvant for DNA vaccines that promotes effective antitumor CTL immunity. Mol Ther. 2011;19:594–601. doi: 10.1038/mt.2010.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Forni G, Musso T, Jemma C, Boraschi D, Tagliabue A, Giovarelli M. Lymphokine-activated tumor inhibition in mice. Ability of a nonapeptide of the human IL-1 beta to recruit anti-tumor reactivity in recipient mice. J Immunol. 1989;142:712–8. [PubMed] [Google Scholar]
- 31.Rovero S, Boggio K, Di Carlo E, Amici A, Quaglino E, Porcedda P, et al. Insertion of the DNA for the 163-171 peptide of IL1beta enables a DNA vaccine encoding p185(neu) to inhibit mammary carcinogenesis in Her-2/neu transgenic BALB/c mice. Gene Ther. 2001;8:447–52. doi: 10.1038/sj.gt.3301416. [DOI] [PubMed] [Google Scholar]
- 32.Nanni P, Nicoletti G, De Giovanni C, Landuzzi L, Di Carlo E, Cavallo F, et al. Combined allogeneic tumor cell vaccination and systemic interleukin 12 prevents mammary carcinogenesis in HER-2/neu transgenic mice. J Exp Med. 2001;194:1195–205. doi: 10.1084/jem.194.9.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De Giovanni C, Nicoletti G, Landuzzi L, Astolfi A, Croci S, Comes A, et al. Immunoprevention of HER-2/neu transgenic mammary carcinoma through an interleukin 12-engineered allogeneic cell vaccine. Cancer Res. 2004;64:4001–9. doi: 10.1158/0008-5472.CAN-03-2984. [DOI] [PubMed] [Google Scholar]
- 34.De Giovanni C, Nicoletti G, Palladini A, Croci S, Landuzzi L, Antognoli A, et al. A multi-DNA preventive vaccine for p53/Neu-driven cancer syndrome. Hum Gene Ther. 2009;20:453–64. doi: 10.1089/hum.2008.172. [DOI] [PubMed] [Google Scholar]
- 35.Yen MC, Lin CC, Chen YL, Huang SS, Yang HJ, Chang CP, et al. A novel cancer therapy by skin delivery of indoleamine 2,3-dioxygenase siRNA. Clin Cancer Res. 2009;15:641–9. doi: 10.1158/1078-0432.CCR-08-1988. [DOI] [PubMed] [Google Scholar]
- 36.Bolli E, Quaglino E, Arigoni M, Lollini PL, Calogero R, Forni G, et al. Oncoantigens for an immune prevention of cancer. Am J Cancer Res. 2011;1:255–64. [PMC free article] [PubMed] [Google Scholar]
- 37.Grant EP, Michalek MT, Goldberg AL, Rock KL. Rate of antigen degradation by the ubiquitin-proteasome pathway influences MHC class I presentation. J Immunol. 1995;155:3750–8. [PubMed] [Google Scholar]
- 38.Rodriguez F, Harkins S, Redwine JM, de Pereda JM, Whitton JL. CD4(+) T cells induced by a DNA vaccine: immunological consequences of epitope-specific lysosomal targeting. J Virol. 2001;75:10421–30. doi: 10.1128/JVI.75.21.10421-10430.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zizzari IG, Veglia F, Taurino F, Rahimi H, Quaglino E, Belleudi F, et al. HER2-based recombinant immunogen to target DCs through FcγRs for cancer immunotherapy. J Mol Med (Berl) 2011;89:1231–40. doi: 10.1007/s00109-011-0794-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sloots A, Mastini C, Rohrbach F, Weth R, Curcio C, Burkhardt U, et al. DNA vaccines targeting tumor antigens to B7 molecules on antigen-presenting cells induce protective antitumor immunity and delay onset of HER-2/Neu-driven mammary carcinoma. Clin Cancer Res. 2008;14:6933–43. doi: 10.1158/1078-0432.CCR-08-1257. [DOI] [PubMed] [Google Scholar]
- 41.Rolla S, Marchini C, Malinarich S, Quaglino E, Lanzardo S, Montani M, et al. Protective immunity against neu-positive carcinomas elicited by electroporation of plasmids encoding decreasing fragments of rat neu extracellular domain. Hum Gene Ther. 2008;19:229–40. doi: 10.1089/hum.2006.196. [DOI] [PubMed] [Google Scholar]
- 42.Ambrosino E, Spadaro M, Iezzi M, Curcio C, Forni G, Musiani P, et al. Immunosurveillance of Erbb2 carcinogenesis in transgenic mice is concealed by a dominant regulatory T-cell self-tolerance. Cancer Res. 2006;66:7734–40. doi: 10.1158/0008-5472.CAN-06-1432. [DOI] [PubMed] [Google Scholar]
- 43.Rolla S, Ria F, Occhipinti S, Di Sante G, Iezzi M, Spadaro M, et al. Erbb2 DNA vaccine combined with regulatory T cell deletion enhances antibody response and reveals latent low-avidity T cells: potential and limits of its therapeutic efficacy. J Immunol. 2010;184:6124–32. doi: 10.4049/jimmunol.0901215. [DOI] [PubMed] [Google Scholar]
- 44.Rosenblum MD, Gratz IK, Paw JS, Lee K, Marshak-Rothstein A, Abbas AK. Response to self antigen imprints regulatory memory in tissues. Nature. 2011 doi: 10.1038/nature10664. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Quaglino E, Mastini C, Amici A, Marchini C, Iezzi M, Lanzardo S, et al. A better immune reaction to Erbb-2 tumors is elicited in mice by DNA vaccines encoding rat/human chimeric proteins. Cancer Res. 2010;70:2604–12. doi: 10.1158/0008-5472.CAN-09-2548. [DOI] [PubMed] [Google Scholar]
- 46.Jacob J, Radkevich O, Forni G, Zielinski J, Shim D, Jones RF, et al. Activity of DNA vaccines encoding self or heterologous Her-2/neu in Her-2 or neu transgenic mice. Cell Immunol. 2006;240:96–106. doi: 10.1016/j.cellimm.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 47.Jacob JB, Quaglino E, Radkevich-Brown O, Jones RF, Piechocki MP, Reyes JD, et al. Combining human and rat sequences in her-2 DNA vaccines blunts immune tolerance and drives antitumor immunity. Cancer Res. 2010;70:119–28. doi: 10.1158/0008-5472.CAN-09-2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3:991–8. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 49.Norell H, Carlsten M, Ohlum T, Malmberg KJ, Masucci G, Schedvins K, et al. Frequent loss of HLA-A2 expression in metastasizing ovarian carcinomas associated with genomic haplotype loss and HLA-A2-restricted HER-2/neu-specific immunity. Cancer Res. 2006;66:6387–94. doi: 10.1158/0008-5472.CAN-06-0029. [DOI] [PubMed] [Google Scholar]
- 50.Nanni P, Pupa SM, Nicoletti G, De Giovanni C, Landuzzi L, Rossi I, et al. p185(neu) protein is required for tumor and anchorage-independent growth, not for cell proliferation of transgenic mammary carcinoma. Int J Cancer. 2000;87:186–94. doi: 10.1002/1097-0215(20000715)87:2<186::AID-IJC5>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 51.Cavallo F, De Giovanni C, Nanni P, Forni G, Lollini PL. 2011: the immune hallmarks of cancer. Cancer Immunol Immunother. 2011;60:319–26. doi: 10.1007/s00262-010-0968-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lollini PL, Nicoletti G, Landuzzi L, Cavallo F, Forni G, De Giovanni C, et al. Vaccines and Other Immunological Approaches for Cancer Immunoprevention. Curr Drug Targets. 2011;12:1957–73. doi: 10.2174/138945011798184146. [DOI] [PubMed] [Google Scholar]
- 53.Calogero RA, Quaglino E, Saviozzi S, Forni G, Cavallo F. Oncoantigens as anti-tumor vaccination targets: the chance of a lucky strike? Cancer Immunol Immunother. 2008;57:1685–94. doi: 10.1007/s00262-008-0481-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Holmgren L, Ambrosino E, Birot O, Tullus C, Veitonmäki N, Levchenko T, et al. A DNA vaccine targeting angiomotin inhibits angiogenesis and suppresses tumor growth. Proc Natl Acad Sci U S A. 2006;103:9208–13. doi: 10.1073/pnas.0603110103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cavallo F, Calogero RA, Forni G. Are oncoantigens suitable targets for anti-tumour therapy? Nat Rev Cancer. 2007;7:707–13. doi: 10.1038/nrc2208. [DOI] [PubMed] [Google Scholar]
- 56.Wang X, Wang Y, Yu L, Sakakura K, Visus C, Schwab JH, et al. CSPG4 in cancer: multiple roles. Curr Mol Med. 2010;10:419–29. doi: 10.2174/156652410791316977. [DOI] [PubMed] [Google Scholar]
- 57.Chiarle R, Martinengo C, Mastini C, Ambrogio C, D’Escamard V, Forni G, et al. The anaplastic lymphoma kinase is an effective oncoantigen for lymphoma vaccination. Nat Med. 2008;14:676–80. doi: 10.1038/nm1769. [DOI] [PubMed] [Google Scholar]
- 58.Lollini PL, Cavallo F, Nanni P, Forni G. Vaccines for tumour prevention. Nat Rev Cancer. 2006;6:204–16. doi: 10.1038/nrc1815. [DOI] [PubMed] [Google Scholar]
- 59.Curcio C, Di Carlo E, Clynes R, Smyth MJ, Boggio K, Quaglino E, et al. Nonredundant roles of antibody, cytokines, and perforin in the eradication of established Her-2/neu carcinomas. J Clin Invest. 2003;111:1161–70. doi: 10.1172/JCI17426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cavallo F, De Giovanni C, Nanni P, Forni G, Lollini PL. 2011: the immune hallmarks of cancer. Cancer Immunol Immunother. 2011;60:319–26. doi: 10.1007/s00262-010-0968-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Grange C, Lanzardo S, Cavallo F, Camussi G, Bussolati B. Sca-1 identifies the tumor-initiating cells in mammary tumors of BALB-neuT transgenic mice. Neoplasia. 2008;10:1433–43. doi: 10.1593/neo.08902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shultz LD, Ishikawa F, Greiner DL. Humanized mice in translational biomedical research. Nat Rev Immunol. 2007;7:118–30. doi: 10.1038/nri2017. [DOI] [PubMed] [Google Scholar]
- 63.Manz MG, Di Santo JP. Renaissance for mouse models of human hematopoiesis and immunobiology. Nat Immunol. 2009;10:1039–42. doi: 10.1038/ni1009-1039. [DOI] [PubMed] [Google Scholar]
- 64.Adam J, Bellomo N. A survey of models for tumor-immune system dynamics. Boston: Birkhauser, 1997. [Google Scholar]
- 65.Lollini P-L, Motta S, Pappalardo F. Modeling tumor immunology. Math Mod Meth Appl Sci. 2006;16:1091–1124. doi: 10.1142/S0218202506001479. [DOI] [Google Scholar]
- 66.Cavallo F, Offringa R, van der Burg SH, Forni G, Melief CJ. Vaccination for treatment and prevention of cancer in animal models. Adv Immunol. 2006;90:175–213. doi: 10.1016/S0065-2776(06)90005-4. [DOI] [PubMed] [Google Scholar]
- 67.Melani C, Chiodoni C, Forni G, Colombo MP. Myeloid cell expansion elicited by the progression of spontaneous mammary carcinomas in c-erbB-2 transgenic BALB/c mice suppresses immune reactivity. Blood. 2003;102:2138–45. doi: 10.1182/blood-2003-01-0190. [DOI] [PubMed] [Google Scholar]
- 68.Park JM, Terabe M, Donaldson DD, Forni G, Berzofsky JA. Natural immunosurveillance against spontaneous, autochthonous breast cancers revealed and enhanced by blockade of IL-13-mediated negative regulation. Cancer Immunol Immunother. 2008;57:907–12. doi: 10.1007/s00262-007-0414-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rolla S, Nicoló C, Malinarich S, Orsini M, Forni G, Cavallo F, et al. Distinct and non-overlapping T cell receptor repertoires expanded by DNA vaccination in wild-type and HER-2 transgenic BALB/c mice. J Immunol. 2006;177:7626–33. doi: 10.4049/jimmunol.177.11.7626. [DOI] [PubMed] [Google Scholar]
- 70.Spadaro M, Ambrosino E, Iezzi M, Di Carlo E, Sacchetti P, Curcio C, et al. Cure of mammary carcinomas in Her-2 transgenic mice through sequential stimulation of innate (neoadjuvant interleukin-12) and adaptive (DNA vaccine electroporation) immunity. Clin Cancer Res. 2005;11:1941–52. doi: 10.1158/1078-0432.CCR-04-1873. [DOI] [PubMed] [Google Scholar]
- 71.Mayayo SL, Prestigio S, Maniscalco L, Rosa GL, Aricò A, Maria RD, et al. Chondroitin sulfate proteoglycan-4: A biomarker and a potential immunotherapeutic target for canine malignant melanoma. Vet J. 2011;190:e26–30. doi: 10.1016/j.tvjl.2011.02.020. [DOI] [PubMed] [Google Scholar]
- 72.Stevenson FK, Mander A, Chudley L, Ottensmeier CH. DNA fusion vaccines enter the clinic. Cancer Immunol Immunother. 2011;60:1147–51. doi: 10.1007/s00262-011-1042-2. [DOI] [PMC free article] [PubMed] [Google Scholar]