Abstract
This note challenges the current idea that a key role of T cells in tumor regression is to directly kill tumor cells. It favors the view that TIL are keys but act indirectly by helping other immune cells to damage the tumor and its stroma.
Keywords: T lymphocytes, immunotherapy, innate cells, kinetics, stroma, tumor regression
The Current Model and its Limits
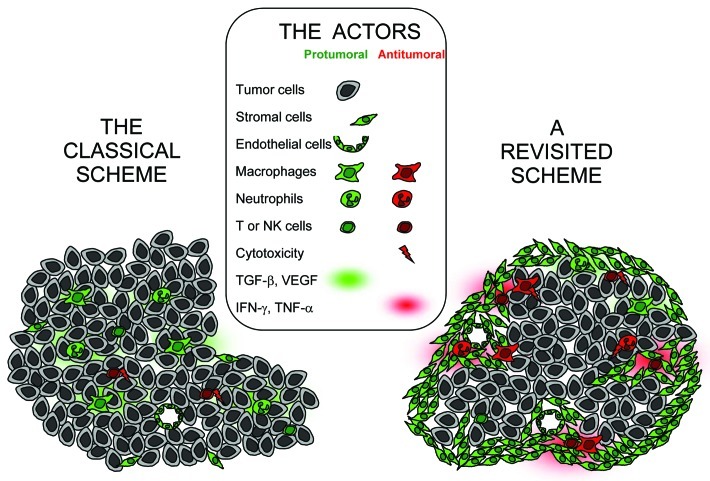
It is now clear that solid tumors can be infiltrated by immune cells able to play both pro- and anti-tumoral roles. Based on the fact that in most (though not all) human cancers, a good prognosis is associated with the presence of tumor-infiltrating lymphocytes (TIL), the current model places T cells as central actors of the antitumor immune action, i.e., they are expected to directly kill tumor cells, and to be the main final effectors of tumor cell death (Fig. 1, left). A frequently overlooked paradox is that TIL present in primary tumors cannot be the ones that will attack tumors in patients after resection of their primary tumor; at most, they are indicative of an ongoing T cell response, hopefully with clones persisting in patients after resection, with a role in immune surveillance (an issue distinct from tumor regression) and a better outcome.
Figure 1. Cellular and molecular actors in the tumor ecosystem after induction of an anti-tumor immune response. In the classical scenario (left), there is no specific structuration of the tumor. All the cellular actors are viewed as protumoral, and once an anti-tumor response is induced, cytotoxic T and NK cells are considered as the sole final effectors, acting through direct tumor cell killing. In a revisited scheme (right), tumor and stromal regions are clearly distinct, with most immune cells in the stroma, and a large spectrum of pro- and anti-tumoral effectors exist. The action of T cells is to a large extent indirect, and involves cellular interactions in the stroma. This scheme does not take into account the time sequence following which various immune cells are recruited at the tumor site to participate to tumor destruction.
In many murine tumor models, tumor growth is accelerated when animals are depleted of circulating T cells (almost always CD8). The importance of perforin in this issue remains controversial.1,2 The fact that tumors grow despite the presence of TIL is generally explained by the fact that TIL become unresponsive in an immunosuppressive microenvironment and thus, cannot do their job to attack tumor cells. Another frequent argument in favor of a direct cytotoxic effect of anti-tumoral T cells is the therapeutic success of adoptive transfer of anti-tumor T lymphocytes in some human cancers.3 In these trials, the existence of interactions between transferred T cells and tumor cells has been illustrated but such interactions have never been quantified.
However, in a murine model of adoptive transfer of T cells, it has been shown that when tumors melt, the vast majority of transferred T cells accumulated at the periphery of the tumor, the tumor nest being mainly invaded by Gr1+ innate cells (neutrophils and/or inflammatory monocytes).4 In addition, the slow rate at which cytotoxic T cells can kill tumor cells in situ5 is hardly compatible with the idea that such an effect may be efficient against rapidly dividing tumor cells. Conversely, innate cells like NK, γδT cells and macrophages act rapidly to kill and/or phagocyte. In addition, efficacy of monoclonal antibodies in cancer therapy is strongly associated with Fc receptor-dependent mechanisms and cell-mediated cytotoxicity which are properties shared by these potent innate effectors.
Thus, all these observations do not fit well with the current model of TIL killing directly tumor cells and of cytotoxic T cells being the main final effectors against tumor cells. Despite this, the current model is largely prevailing, with major consequences. Indeed, even if other concepts are taken into account, such as the importance of limiting immunosuppression,6 or making the appropriate combinations of chemo- and immunotherapy,7 most clinical trials of active immunotherapy still aim at generating highly reactive T cells as the main way to optimize a tumor attack. We consider that this point of view needs to be revisited.
A Closer Look at the T Cell Environment in the Tumor
Data obtained in human and murine tumors are consistent with the idea that the anti-tumoral action of T cells is indirect. The localization of immune cells in human tumors is only known for tumors in progression, when they are resected. In these tumors, when present, the highest density of TIL is found in the peritumoral stroma, not in the cancer nest itself (ref. 8 and Salmon et al., in revision). A good prognosis is in general not associated with abundant CD8 in the cancer nest (even if there are a few exceptions, such as seen in ref. 9), but with abundant T cells in the stroma, either CD410 or coexistence of CD4 and CD8.11,12 Even NK cells, when present, are more abundant in the stroma.13 It is difficult to draw firm conclusions concerning the ability of such cells to contribute to tumor regression, since, as mentioned above, these data only characterize the properties of cells that will participate in cancer immunosurveillance after tumor resection. However, if an efficient anti-tumoral immune response able to lead to tumor regression could be triggered, it would have to start with such an intratumoral distribution. Data obtained with murine tumors, and further developed below, are also in favor of the idea that the anti-tumoral action of T cells is mostly indirect.
The peritumoral stroma is a complex ecosystem and a key battlefield
Tumor cells are embedded in a stroma providing nutrients and growth factors. The vasculature, although built out of control, feeds the tumor (blood) and allows metastasic spread (lymph). Tumor-associated fibroblasts secrete growth factors and extracellular matrix elements, mandatory for tumor growth and emigration. Tissue remodeling and fibrosis are supported by myeloid cells (macrophages and neutrophils) making metalloproteases, angiogenic factors and TGFβ. This last molecule contributes to suppress an aggressive action of the immune system. Most studies consider only this first set of tumor stroma features (for a review, see e.g., ref. 14).
However, and despite its absence of an obvious spatial organization, the same stroma, or at least part of it, may also be viewed as a tertiary lymphoid organ, starting point of an efficient anti-tumor response. Thus, Eberl and colleagues have shown that lymphoid stromal cells, which contribute to the normal development of secondary lymphoid organs, are present in the peritumoral stroma.15 These lymphoid stromal cells could interact with myeloid cells to attract many different cell types and start the auto-organization of a lymphoid organ. In line with this, the presence of lymphoid-like structure in human non-small cell lung carcinoma has been associated with a better prognosis.16
It seems reasonable to conclude that multiple cellular interactions taking place in this microenvironment could cooperate for the anti-tumor effector phase (Fig. 1, right). In particular, rather than acting as direct killers of tumor cells, CD8 T cells may act through the release of various cytokines, and could activate/recruit other immune cells at the tumor site. IFNγ can activate macrophages, and tissue-infiltrating neutrophils are very potently triggered to release H2O2 by TNFα produced by lymphocytes or macrophages.17 NK cells are also able to produce IFNγ and to activate other effectors, or to help the maturation of dendritic cells.18 A consequence of this T cell contribution would be the triggering of an inflammatory response arising within the stroma.
As a result of the cellular interactions taking place in the tumor stroma, multiple activated effectors may target not only tumor cells but also the tumor vasculature. Destruction of the microvasculature involving T cells, granulocytes or macrophages has been described in mouse models of transplanted tumors and may involve IFNγ-dependent anti-angiogenesis.19,20 This is also true for human tumors. For instance, in a phase I clinical trial, the group of Dranoff tested the clinical effects of an anti-CTLA-4 Ab in patients that had been previously vaccinated with irradiated autologous tumor cells engineered to secrete GM-CSF. They observed tumor regressions with necrosis and vasculopathy, associated with inflammatory cells, particularly at the periphery of the lesion.6,21 Although it remains unclear if neutrophil recruitment precedes or follows vasculature damage, it is clear that vasculature damage is a key for tumor necrosis. Other stromal cells, like fibroblasts activation protein-α+ (FAP) cells may be appropriate targets for immune cells. Indeed, their destruction facilitates anti-tumor immune response and tumor growth control.22
An anti-tumoral action of T cells within the stroma cannot be performed by any type of activated T cell. It requires an antigen-specificity. Indeed, tumor-specific T cells are active against the tumor whereas concanavalin-A-activated T cells are not.23 The reason is probably that cytokine secretion by memory cells is almost instantaneous (already quite large four to six hours after stimulation), but it is expected to cease rapidly when T cells are no longer stimulated or when they are stimulated in an immunosuppressive environment, see e.g. ref. 24. Thus, T cells need to be reactivated locally, e.g., by tumor-associated antigens that could be presented by dendritic cells/macrophages in the tumor stroma and even by neutrophils,17 endothelial cells or fibroblasts. The molecular and cellular mechanisms for antigen capture and presentation to CD4 and CD8 T cells in this context would deserve a careful study. Highly infiltrated tumors may be of favorable prognosis in this setting, in that local concentration of T cells may allow sustained T cell activation and epitope spreading in the tumor area as suggested by T. Boon and colleagues.25
Together, these data underline that the role played by anti-tumor specific T cells in tumor regression could to a large extent be through a destabilization of the stroma ecosystem. Like Janus, the tumor stroma is two-faced. On one hand it may play a pro-tumoral role, and on the other it may contribute to its own destruction, and thus have an anti-tumoral role.
Kinetic dependence of anti-tumoral actions
If there is a consensus for considering that macrophages and neutrophils have an important role for fighting infections, the same cells are usually considered as detrimental for the efficiency of anti-tumor responses. It seems more appropriate to us to consider that these innate cells may have both pro- and anti-tumoral roles. To understand this apparent paradox, one has to take into account the importance of kinetics in immune responses.
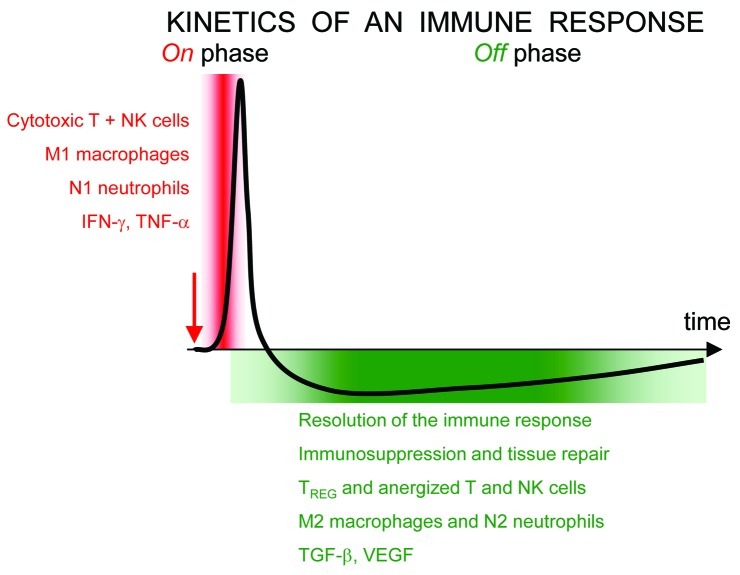
The same cellular or molecular actor may play very different roles in different contexts, and depending whether their abundance varies sharply or is sustained. Efficient immune responses are most often transient (compare, e.g., acute vs. chronic infections) with “on” and “off” phases and positive and negative feedbacks (Fig. 2). Appropriate termination of an immune response is absolutely essential to prevent a cytokine storm, organ specific autoimmune diseases with tissue destruction and to let wound healing take place.
Figure 2. After an initial stimulus (red arrow), immune responses are always transient. The initial and brief “on” phase is followed by a prolonged “off” phase. For each group of stimulated cells, cytotoxic activities and release of inflammatory cytokines occur only during the on phase, whereas immunosuppression, fibrosis, vascularisation are favored during the off phase. Within a tissue or a tumor, non-synchronized responses for different groups of cells may coexist. All types of immune cells may participate to both phases.
The same categories of cells and even molecules may be involved in these “on” and “off” phases. For instance, in the case of tissue injury, macrophages first phagocyte and kill intruders before repairing collateral damages. The phenotype of these macrophages changes with time.26 Similarly, if the chronic presence of neutrophils within tumors seems to be pro-tumoral, neutrophils may also play a major role in anti-tumoral responses. Indeed, the sudden immune response triggered by Bacillus Calmette-Guerin for treating bladder cancer involves a neutrophil attack.27,28
Opposite effects may also be exerted by one molecule on tumor-related phenomenon, as illustrated with IFNγ. In general, a sudden increase of IFNγ is anti-angiogenic, with important consequences for anti-tumoral actions. But the sustained secretion of IFNγ may also be pro-angiogenic, e.g., for IFNγ secreted by special NK cells in the uterus, that help the remodelling of the uterine vasculature necessary for the implantation of the fetus in the placenta.29 It is likely that both the kinetics of IFNγ secretion (rapid change vs. chronicity associated with the development of adaptive responses, or desensitization) and the cellular context, are key to the dominant effect exerted by IFNγ.
In conclusion, the view that myeloid cells and granulocytes may only benefit to tumors is largely skewed by the time window that has been most studied: growing tumors, associated with an exhausted anti-tumor response, in its “off,” or repair phase. A simple snapshot indicating that T cells, macrophages or neutrophils are abundant in a tumor provides no information about the phase of the immune response in which they are involved, neither on their ability to participate to an anti-tumor attack, if appropriately stimulated.
Which Scenarios for T Cells and Other Partners in this Context?
When a primary tumor has developed, the immune system could be stimulated to play two roles. One is to contribute to tumor regression (together with surgery, chemo- and radiotherapy). The second is to prolong the dormancy of micrometastases, or even eliminate them. The mechanisms necessary for these two actions are not necessarily the same ones. Concerning a contribution of the immune system to tumor regression, discussed in this paper, one can conclude the following from the above analysis. In order to exploit the properties of the immune system, one should trigger a sudden attack that should rapidly reach some success, as efficient immune responses are always transient. It is likely that the intensity of the attack can be larger if it is local. This would justify the use of peritumoral vaccination, whenever possible. Such a vaccination should aim at activating a set of cellular actors, not only T cells. The targeted structure should not necessarily be tumor cells, but also components of the stroma (FAP+ fibroblasts or endothelial cells) (see30 for an excellent review).
Such a result may be reached by mimicking in the peritumoral region a microbial attack, aiming at activating locally both the innate system and T cells in an antigen-specific way, if possible. Such a view has already been pioneered by William Coley, a century ago.31 However, its translation in a clinical protocol requires a proper design in order to obtain an efficient, necessarily transient response always followed by an “off” phase. Improperly designed, one may trigger an excessive, counterproductive “off” phase. It may be helpful to limit the importance of this “off” phase, e.g., with an anti-TGFβ.32
It is obviously important to test this set of notions in animal models in which immune stimulations do result in tumor regression and not only in slowing its growth, as reported in most papers in tumor-murine models.
Acknowledgments
We would like to thank Emmanuel Donnadieu for critical reading of this manuscript.
Glossary
Abbreviations:
- TIL
tumor-infiltrating lymphocytes
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/18800
References
- 1.MacGregor JN, Li Q, Chang AE, Braun TM, Hughes DP, McDonagh KT. Ex vivo culture with interleukin (IL)-12 improves CD8(+) T-cell adoptive immunotherapy for murine leukemia independent of IL-18 or IFN-gamma but requires perforin. Cancer Res. 2006;66:4913–21. doi: 10.1158/0008-5472.CAN-05-3507. [DOI] [PubMed] [Google Scholar]
- 2.Dobrzanski MJ, Reome JB, Dutton RW. Role of effector cell-derived IL-4, IL-5, and perforin in early and late stages of type 2 CD8 effector cell-mediated tumor rejection. J Immunol. 2001;167:424–34. doi: 10.4049/jimmunol.167.1.424. [DOI] [PubMed] [Google Scholar]
- 3.Dudley ME, Yang JC, Sherry R, Hughes MS, Royal R, Kammula U, et al. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol. 2008;26:5233–9. doi: 10.1200/JCO.2008.16.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blohm U, Potthoff D, van der Kogel AJ, Pircher H. Solid tumors “melt” from the inside after successful CD8 T cell attack. Eur J Immunol. 2006;36:468–77. doi: 10.1002/eji.200526175. [DOI] [PubMed] [Google Scholar]
- 5.Breart B, Lemaitre F, Celli S, Bousso P. Two-photon imaging of intratumoral CD8+ T cell cytotoxic activity during adoptive T cell therapy in mice. J Clin Invest. 2008;118:1390–7. doi: 10.1172/JCI34388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hodi FS, Mihm MC, Soiffer RJ, Haluska FG, Butler M, Seiden MV, et al. Biologic activity of cytotoxic T lymphocyte-associated antigen 4 antibody blockade in previously vaccinated metastatic melanoma and ovarian carcinoma patients. Proc Natl Acad Sci USA. 2003;100:4712–7. doi: 10.1073/pnas.0830997100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zitvogel L, Kepp O, Kroemer G. Immune parameters affecting the efficacy of chemotherapeutic regimens. Nat Rev Clin Oncol 20118:151-60 [DOI] [PubMed] [Google Scholar]
- 8.Ohtani H. Focus on TILs: prognostic significance of tumor infiltrating lymphocytes in human colorectal cancer. Cancer Immun. 2007;7:4. [PMC free article] [PubMed] [Google Scholar]
- 9.Naito Y, Saito K, Shiiba K, Ohuchi A, Saigenji K, Nagura H, et al. CD8+ T cells infiltrated within cancer cell nests as a prognostic factor in human colorectal cancer. Cancer Res. 1998;58:3491–4. [PubMed] [Google Scholar]
- 10.Wakabayashi O, Yamazaki K, Oizumi S, Hommura F, Kinoshita I, Ogura S, et al. CD4+ T cells in cancer stroma, not CD8+ T cells in cancer cell nests, are associated with favorable prognosis in human non-small cell lung cancers. Cancer Sci. 2003;94:1003–9. doi: 10.1111/j.1349-7006.2003.tb01392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hiraoka K., Miyamoto M., Cho Y., Suzuoki M., Oshikiri T., Nakakubo Y., et al. Concurrent infiltration by CD8+ T cells and CD4+ T cells is a favourable prognostic factor in non-small-cell lung carcinoma. Br J Cancer 200694:275-80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Al-Shibli KI, Donnem T, Al-Saad S, Persson M, Bremnes RM, Busund LT. Prognostic effect of epithelial and stromal lymphocyte infiltration in non-small cell lung cancer. Clin Cancer Res. 2008;14:5220–7. doi: 10.1158/1078-0432.CCR-08-0133. [DOI] [PubMed] [Google Scholar]
- 13.Carrega P, Morandi B, Costa R, Frumento G, Forte G, Altavilla G, et al. Natural killer cells infiltrating human nonsmall-cell lung cancer are enriched in CD56 bright CD16(-) cells and display an impaired capability to kill tumor cells. Cancer. 2008;112:863–75. doi: 10.1002/cncr.23239. [DOI] [PubMed] [Google Scholar]
- 14.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature 2008454:436-44 [DOI] [PubMed] [Google Scholar]
- 15.Peduto L, Dulauroy S, Lochner M, Spath GF, Morales MA, Cumano A, et al. Inflammation recapitulates the ontogeny of lymphoid stromal cells. J Immunol. 2009;182:5789–99. doi: 10.4049/jimmunol.0803974. [DOI] [PubMed] [Google Scholar]
- 16.Dieu-Nosjean MC, Antoine M, Danel C, Heudes D, Wislez M, Poulot V, et al. Long-term survival for patients with non-small-cell lung cancer with intratumoral lymphoid structures. J Clin Oncol 200826:4410-7 [DOI] [PubMed] [Google Scholar]
- 17.Nathan C. Neutrophils and immunity: challenges and opportunities. Nat Rev Immunol. 2006;6:173–82. doi: 10.1038/nri1785. [DOI] [PubMed] [Google Scholar]
- 18.Zitvogel L, Terme M, Borg C, Trinchieri G. Dendritic cell-NK cell cross-talk: regulation and physiopathology. Curr Top Microbiol Immunol. 2006;298:157–74. doi: 10.1007/3-540-27743-9_8. [DOI] [PubMed] [Google Scholar]
- 19.Qin Z, Schwartzkopff J, Pradera F, Kammertoens T, Seliger B, Pircher H, et al. A critical requirement of interferon gamma-mediated angiostasis for tumor rejection by CD8+ T cells. Cancer Res. 2003;63:4095–100. [PubMed] [Google Scholar]
- 20.Qin Z, Blankenstein T. CD4+ T cell–mediated tumor rejection involves inhibition of angiogenesis that is dependent on IFN gamma receptor expression by nonhematopoietic cells. Immunity. 2000;12:677–86. doi: 10.1016/S1074-7613(00)80218-6. [DOI] [PubMed] [Google Scholar]
- 21.Hodi FS, Butler M, Oble DA, Seiden MV, Haluska FG, Kruse A, et al. Immunologic and clinical effects of antibody blockade of cytotoxic T lymphocyte-associated antigen 4 in previously vaccinated cancer patients. Proc Natl Acad Sci USA. 2008;105:3005–10. doi: 10.1073/pnas.0712237105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kraman M, Bambrough PJ., Arnold JN, Roberts EW, Magiera L, Jones JO, et al. Suppression of antitumor immunity by stromal cells expressing fibroblast activation protein-alpha. Science 2010330:827-30 [DOI] [PubMed] [Google Scholar]
- 23.Hamzah J, Nelson D, Moldenhauer G, Arnold B, Hammerling GJ, Ganss R. Vascular targeting of anti-CD40 antibodies and IL-2 into autochthonous tumors enhances immunotherapy in mice. J Clin Invest. 2008;118:1691–9. doi: 10.1172/JCI33201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Isogawa M, Furuichi Y, Chisari FV. Oscillating CD8(+) T cell effector functions after antigen recognition in the liver. Immunity. 2005;23:53–63. doi: 10.1016/j.immuni.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 25.Boon T, Coulie PG, Van den Eynde BJ, van der Bruggen P. Human T cell responses against melanoma. Annu Rev Immunol. 2006;24:175–208. doi: 10.1146/annurev.immunol.24.021605.090733. [DOI] [PubMed] [Google Scholar]
- 26.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549–55. doi: 10.1016/S1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 27.Simons MP, O'Donnell MA, Griffith TS. Role of neutrophils in BCG immunotherapy for bladder cancer. Urol Oncol. 2008;26:341–5. doi: 10.1016/j.urolonc.2007.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ludwig AT, Moore JM, Luo Y, Chen X, Saltsgaver NA, O'Donnell MA, et al. Tumor necrosis factor-related apoptosis-inducing ligand: a novel mechanism for Bacillus Calmette-Guerin-induced antitumor activity. Cancer Res. 2004;64:3386–90. doi: 10.1158/0008-5472.CAN-04-0374. [DOI] [PubMed] [Google Scholar]
- 29.Ashkar AA, Di Santo JP, Croy BA. Interferon gamma contributes to initiation of uterine vascular modification, decidual integrity, and uterine natural killer cell maturation during normal murine pregnancy. J Exp Med. 2000;192:259–70. doi: 10.1084/jem.192.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bissell MJ, Hines WC.Why don't we get more cancer? A proposed role of the microenvironment in restraining cancer progression. Nat Med 201117:320-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoption Cann SA, van Netten JP, van Netten C. Dr William Coley and tumour regression: a place in history or in the future. Postgrad Med J. 2003;79:672–80. [PMC free article] [PubMed] [Google Scholar]
- 32.Takaku S, Terabe M, Ambrosino E, Peng J, Lonning S, McPherson JM, et al. Blockade of TGF-beta enhances tumor vaccine efficacy mediated by CD8(+) T cells. Int J Cancer. 2010;126:1666–74. doi: 10.1002/ijc.24961. [DOI] [PMC free article] [PubMed] [Google Scholar]