Abstract
Myeloid-derived suppressor cells (MDSC) induced during neoplasia display potent pro-tumorigenic activities. Tumor-derived factors influence MDSC development, yielding monocytic and granulocytic subsets. In contrast to monocytic MDSC, little is known about how granulocytic MDSC develop. We demonstrated that tumor-derived G-CSF drives granulocytic MDSC formation, thus providing new insights into myeloid-tumor biology.
Keywords: Immunosuppression, immunosurveillance, inflammation and cancer, myeloid-derived suppressor Cells, tumor progression
Despite significant progress in cancer diagnosis and treatment, many patients still succumb to invasive or metastatic disease. One potentially important reason for limited therapeutic efficacy reflects the overall therapeutic strategy. Current paradigms for understanding the biology of disease are heavily focused on the “cell-intrinsic” (genetic and epigenetic) events that govern neoplastic development.1 However, it is now well-regarded that tumor cell interactions with the host are critical to achieve full malignant capability,2,3 suggesting that this host-dependent arm of the neoplastic process has important implications for prognosis and therapy.
Compelling studies reveal that myeloid-derived suppressor cells (MDSC), a relatively newly discovered leukocyte population induced in both cancer patients and animal models, promote neoplastic progression through multiple mechanisms, including immune suppression and angiogenesis.2,4,5 MDSC are found systemically in the blood and secondary lymphoid tissues, as well as locally at sites of disease activity. They constitute heterogeneous populations of monocytic and granulocytic cells reflecting a continuum of differentiation stages. In mouse models, MDSC subsets can be distinguished from other regulatory myelo-monocytic populations on the basis of unique phenotypic profiles. Monocytic MDSC are CD11b+Ly6ChiLy6G- (or CD11b+Gr-1lo), whereas granulocytic MDSC are CD11b+Ly6CloLy6G+ (or CD11b+Gr-1hi).2,6 Although much attention has been dedicated to unraveling mechanisms by which the MDSC subsets mediate immune suppression and tumor progression, a larger gap remains in our understanding of the mechanisms that initiate their development. Enhancing our understanding of the molecular basis for MDSC subset development could facilitate the identification of new biomarkers or therapeutic targets to improve responses to immunotherapy.
It is thought that the inappropriate secretion of hematopoietic growth factors by tumors can alter normal myelopoiesis and lead to the accumulation of dysfunctional myeloid populations, like MDSC. As noted earlier, MDSC fall into monocytic and granulocytic subsets, although both have been shown to be equally immunosuppressive.6 It turns out that granulocytic cell types comprise a major component of the MDSC response;2,6 yet, the underlying reasons for this remain unclear. Although a number of tumor-derived factors (TDF) have been linked to diverse elements of MDSC biology, namely VEGF, GM-CSF, IL-1β, IL-6, IL-10 or PGE2,2,4 none have been rigorously tested to explain their connection with the granulocytic MDSC response. Since the overall MDSC response is a manifestation of deregulated myelopoiesis, we hypothesized that the inappropriate production of certain hematopoietic growth factors, like granulocyte-colony stimulating factor (G-CSF), is an underexplored key initiator of granulocytic MDSC development.
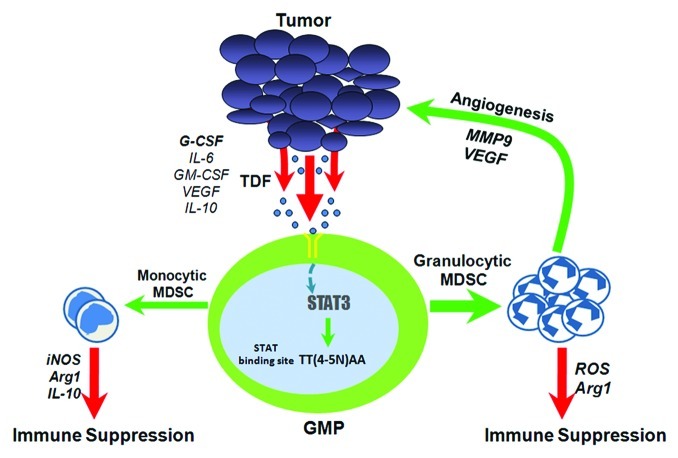
Ordinarily, endogenous G-CSF regulates granulopoiesis and has a vital role in neutrophil mobilization in response to diverse insults.7 Exogenous G-CSF is also important to overcome neutropenia caused by various anti-neoplastic treatments. However, G-CSF paradoxically can elicit adverse effects and inhibit innate and adaptive immunity.8 The notion that G-CSF may not always be beneficial to the host is also supported by the findings that G-CSF is aberrantly expressed in human neoplasia.9 But how would G-CSF fit into the pathway of MDSC generation? The connection between G-CSF and MDSC would likely converge at the level of STAT3 based on the knowledge that G-CSF signaling is strongly STAT3-dependent7 and that elevated STAT3 activity is important for the accumulation of MDSC2 (Fig. 1). Thus, we took a mechanistic approach to dissect a potentially new role of G-CSF in myeloid-tumor biology.10
Figure 1. Granulocytic MDSC development via G-CSF-dependent mechanisms. Aberrant myelopoiesis from bone marrow progenitors (i.e., granulocyte-macrophage/GMP) is initiated by TDF, many of which function through STAT3. We propose that aphysiologic levels of tumor-derived G-CSF constitute a relevant myelopoietic growth factor, which triggers STAT3 activation in GMP ensuing G-CSF receptor engagement. Activated STAT3 then translocates to the nucleus where it binds to specific elements of myelopoietic target genes that in turn alter normal myeloid cell differentiation, perhaps skewing development in the direction of granulocytic MDSC. Several STAT3 target genes have been previously described.2 A few examples of relevant TDF, as well as mechanisms of granulocytic and monocytic MDSC-mediated tumor progression are shown. Such TDF may influence MDSC in multiple ways, including how MDSC function or mobilize to sites of pathologic challenges.
First, we observed abundant amounts of G-CSF in several, but not all mouse tumor models tested. Interestingly, G-CSF production directly correlated with granulocytic MDSC generation. To test the hypothesis that G-CSF production and the granulocytic MDSC response are causally linked, three systematic in vivo approaches were then taken: (1) RNA interference to silence G-CSF production in a G-CSF-expressing tumor; (2) ectopic overexpression of G-CSF in a non-G-CSF-expressing tumor; and (3) direct injection of recombinant G-CSF protein. Overall, we found that G-CSF loss-of-function decreased granulocytic MDSC generation, while G-CSF gain-of-function (via transfection or direct injection of protein) had the opposing impact.10 In addition to its effects on granulocytic MDSC burden, we observed that tumor-derived G-CSF significantly increased tumor growth rate. By cell depletion studies, we determined that the G-CSF-tumor effect was largely due to the induced granulocytic MDSC response.
These data are consistent with a new cyclical paradigm that the neoplastic process influences granulocytic MDSC development through the secretion of G-CSF, and that the accumulation of granulocytic MDSC in turn further nurtures tumor growth/progression (Fig. 1). Therefore, the identification of myeloid-reactive targets, such as G-CSF, provides the rationale to target G-CSF for prognostic or therapeutic purposes in appropriate subsets of patients where such cytokine levels are demonstrable. Monitoring changes in sera/plasma G-CSF levels, along with other clinicopathologic parameters, may define a “biomarker signature” of disease status. Therapeutic approaches that target granulocytic MDSC-initiating TDF may offer additional ways to abrogate MDSC-mediated mechanisms of tumor progression to enhance the efficacy of immune-based interventions. Such approaches would be akin to those used for TNF blockade (e.g., Remicade®) in patients with certain autoimmune disorders or VEGF blockade (e.g., Avastin®) in patients with certain solid cancers to reduce disease-associated cytokine levels below a ‘pathologic threshold’.
It is also noteworthy that the administration of G-CSF protein led to the induction of granulocytic-like MDSC, which strongly recapitulated the phenotypic, functional and molecular characteristics observed with tumor-induced granulocytic MDSC.10 These data argue that high concentrations of circulating G-CSF can impair myeloid cell development/differentiation, leading to granulocytic MDSC formation. Thus, the development of granulocytic MDSC in response to G-CSF exposure may also have important implications in non-neoplastic settings and explain, in part, the basis of immune suppression or tolerance under such clinical situations.8
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/19334
References
- 1.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–74. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Egeblad M, Nakasone ES, Werb Z. Tumors as organs: complex tissues that interface with the entire organism. Dev Cell. 2010;18:884–901. doi: 10.1016/j.devcel.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ostrand-Rosenberg S, Sinha P. Myeloid-derived suppressor cells: linking inflammation and cancer. J Immunol. 2009;182:4499–506. doi: 10.4049/jimmunol.0802740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Filipazzi P, Huber V, Rivoltini L. Phenotype, function and clinical implications of myeloid-derived suppressor cells in cancer patients. Cancer Immunol Immunother. 2011;61:255–63. doi: 10.1007/s00262-011-1161-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Youn JI, Nagaraj S, Collazo M, Gabrilovich DI. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J Immunol. 2008;181:5791–802. doi: 10.4049/jimmunol.181.8.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Panopoulos AD, Watowich SS. Granulocyte colony-stimulating factor: molecular mechanisms of action during steady state and ‘emergency’ hematopoiesis. Cytokine. 2008;42:277–88. doi: 10.1016/j.cyto.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rutella S, Zavala F, Danese S, Kared H, Leone G. Granulocyte colony-stimulating factor: a novel mediator of T cell tolerance. J Immunol. 2005;175:7085–91. doi: 10.4049/jimmunol.175.11.7085. [DOI] [PubMed] [Google Scholar]
- 9.Bharadwaj U, Li M, Zhang R, Chen C, Yao Q. Elevated interleukin-6 and G-CSF in human pancreatic cancer cell conditioned medium suppress dendritic cell differentiation and activation. Cancer Res. 2007;67:5479–88. doi: 10.1158/0008-5472.CAN-06-3963. [DOI] [PubMed] [Google Scholar]
- 10.Waight JD, Hu Q, Miller A, Liu S, Abrams SI. Tumor-derived G-CSF facilitates neoplastic growth through a granulocytic myeloid-derived suppressor cell-dependent mechanism. PLoS One. 2011;6:e27690. doi: 10.1371/journal.pone.0027690. [DOI] [PMC free article] [PubMed] [Google Scholar]