Abstract
The immune interactions occurring within the tumor microenvironment have a critical role in determining the outcome of colorectal cancer patients. We carried-out an immunohistochemical analysis of tumor infiltrating T-lymphocytes expressing chemokine receptor 7 (CCR7) in a series of colorectal cancer patients enrolled in a prospective clinical trial. We demonstrated that a high tumor infiltration score of this lymphocyte subset is predictive of longer progression free survival and overall survival.
Keywords: CCR7, colorectal cancer, prognostic factor, tumor infiltrating lymphocytes
Adaptive immunity is considered to play a crucial role for a long survival of cancer patients.1 Recent findings support the idea that the immune microenvironment of colorectal cancer has important peculiarities. In fact, primary tumor infiltration by regulatory T cells expressing the FOXP3 transcription factor [CD4+ CD25+FoxP3+ T cells (Treg)], commonly considered as immune-suppressive cells, represents an unpredicted favorable prognostic value both in local2 and in advanced disease.3 Although a clear explanation of this finding is not currently available, several hypotheses have been formulated. We suggested that the presence of Treg is an indirect sign of a preexisting and efficient anti-tumor immune-response.3 In the attempt to clarify this issue we decided to investigate the prognostic value of different tumor infiltrating T lymphocyte subsets in a recent work.4 At this aim we performed an immunohistochemical analysis to evaluate the prognostic value of tumor infiltrating CD8+ T cells expressing the chemokine-receptor-7 (Tccr7) and the possible correlation between tumor infiltration by Tccr7 and Treg. The study involved 76 patients enrolled in a Phase III trial on first line treatment for metastatic colorectal cancer (mCRC), named GOLFIG-2 trial, comparing GOLFIG [Gemcitabine (1,000 mg/m2 on days 1 and 15), oxaliplatin (85 mg/m2 on days 2 and 16), LF (100 mg/m2 on days 1, 2, 15 and 16) and 5-FU (400 mg/m2 as a bolus and 800 mg/m2 as a 24 h infusion on days 1, 2, 15 and 16), followed by subcutaneous rGM-CSF (100 μg, on days 3–7) and ultra-low dose subcutaneous rIL-2 (0.5 × 106 IUs twice a day on days 8–14 and 17–29)] chemo immunotherapy5 vs FOLFOX-4 regimen.
High Tccr7 tumor infiltration was predictive of prolonged Overall Survival (OS) [high vs. low Tccr7 score HR = 0.48 (95% CI; 0.24–0.96); p = 0.03] and prolonged Progression Free Survival (PFS) [high vs. low Tccr7 score: HR = 0.54 (95% CI; 0.28–1.01); p = 0.01] after front-line chemotherapy. Patients with high infiltration by both Tccr7 and Treg presented a favorable outcome, while the outcome was very poor for concomitant low Tccr7 and Treg [HR = 0.32 (95% CI; 0.12–0.87); p = 0.02 for OS HR = 0.43 (95% CI; 0.17–1.06) for PFS; p = 0.01]. These findings led us to conclude that a high Tccr7 tumor infiltration score has a favorable prognostic value for mCRC.4
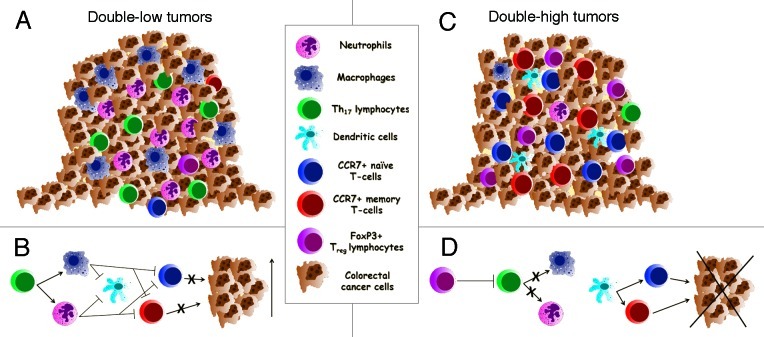
How to interpret these findings in the general scenario of colorectal cancer microenvironment? CCR7 is a chemokine receptor involved in chemotaxis, expressed on naïve-T lymphocytes and on central memory T lymphocytes. CCR7 ability to bind chemokine ligand (CCL)-19 and CCL-21 allows it to drive these different lymphocyte subpopulations at the site of the “immune-attack”: naïve-T lymphocytes may subsequently differentiate in cytotoxic effectors or central memory; whereas central memory T lymphocytes may proliferate and differentiate in activated effector lymphocytes. Both these events may lead to a tumor-specific immune-response with potential antitumor activity. On these basis, patients with a high CCR7 tumor infiltration probably have a still competent immune system with effector arms in full action. Moreover, the presence of Treg may represent an attempt to attenuate a potentially dangerous over-reaction and auto-immunity. In our series, we found that Tccr7 and Treg co-infiltration in the tumor tissue confers a survival advantage but the coordinated expression of the two lymphocyte population doesn’t occur in most tumors. This suggests that other points need to be considered in order to explain the prognostic value of Treg. Ladoire et al. have focused attention on the unique colon tissue microbiological environment, which in specific condition may activate a Th-17-dependent inflammatory response, that in turn, exerts a powerful immune-suppressive and tumor promoting effect. In this view Treg act as specific repressors of IL-17 induced inflammation.6 Furthermore, myeloid derived suppressor cells may be attracted in tumor tissues by different inflammatory cytokines, became tumor-associated macrophages and tumor-associated neutrophils and, in turn, promote tumor progression by means of Th1-immunosuppressive activity.7,8 Colorectal cancer microenvironment appears therefore to modulate tumor progression by counterbalancing effects, inflammatory cytokines driven tumor vs. Tccr7 and Treg anti-inflammatory immune promoting activity (Fig. 1). At this aim, our findings add novel information demonstrating that tumor infiltrating Tccr7 positively affect long-term disease outcome and represent therefore important actors in the scenario of colorectal cancer immune microenvironment.
Figure 1. The figure is representative of the opposite function of different tumor infiltrating immune-cells. (A and B) explain the pro-tumor activity of several infiltrating myeloid derived cells (such as neutrophils and macrophages) driven by a Th-17- mediated inflammation. This condition leads to the suppression of the adaptive antitumor immune response and, consequently, to a low tumor infiltration by either CCR7+ and FoxP3+ lymphocytes (double-low tumors). Conversely, (C and D) show a tumor highly infiltrated by both CCR7+ and FoxP3+ lymphocytes (double-high tumors). In this view, FoxP3+ may act by regulating tumor-dependent inflammation thus enhancing a tumor-specific response, leading to an efficient antitumor activity mediated by CCR7+ lymphocytes.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/19404
References
- 1.Zitvogel L, Kepp O, Kroemer G. Immune parameters affecting the efficacy of chemotherapeutic regimens. Nat Rev Clin Oncol. 2011;8:151–60. doi: 10.1038/nrclinonc.2010.223. [DOI] [PubMed] [Google Scholar]
- 2.Salama P, Phillips M, Grieu F, Morris M, Zeps N, Joseph D, et al. Tumor-infiltrating FOXP3+ T regulatory cells show strong prognostic significance in colorectal cancer. J Clin Oncol. 2009;27:186–92. doi: 10.1200/JCO.2008.18.7229. [DOI] [PubMed] [Google Scholar]
- 3.Correale P, Rotundo MS, Del Vecchio MT, Remondo C, Migali C, Ginanneschi C, et al. Regulatory (FoxP3+) T-cell tumor infiltration is a favorable prognostic factor in advanced colon cancer patients undergoing chemo or chemoimmunotherapy. J Immunother. 2010;33:435–41. doi: 10.1097/CJI.0b013e3181d32f01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Correale P, Rotundo MS, Botta C, Del Vecchio MT, Ginanneschi C, Licchetta A, et al. Tumour infiltration by T-lymphocytes expressing chemokine receptor 7 (CCR7) is predictive of favourable outcome in patients with advanced colorectal carcinoma. Clin Cancer Res. 2012;18:850–7. doi: 10.1158/1078-0432.CCR-10-3186. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 5.Correale P, Tagliaferri P, Fioravanti A, Del Vecchio MT, Remondo C, Montagnani F, et al. Immunity feedback and clinical outcome in colon cancer patients undergoing chemoimmunotherapy with gemcitabine + FOLFOX followed by subcutaneous granulocyte macrophage colony-stimulating factor and aldesleukin (GOLFIG-1 Trial) Clin Cancer Res. 2008;14:4192–9. doi: 10.1158/1078-0432.CCR-07-5278. [DOI] [PubMed] [Google Scholar]
- 6.Ladoire S, Martin F, Ghiringhelli F. Prognostic role of FOXP3+ regulatory T cells infiltrating human carcinomas: the paradox of colorectal cancer. Cancer Immunol Immunother. 2011;60:909–18. doi: 10.1007/s00262-011-1046-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Erreni M, Mantovani A, Allavena P. Tumor-associated Macrophages (TAM) and Inflammation in Colorectal Cancer. Cancer Microenviron. 2011;4:141–54. doi: 10.1007/s12307-010-0052-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, et al. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell. 2009;16:183–94. doi: 10.1016/j.ccr.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]