Abstract
Tumor-intrinsic immuno-resistance is a prerequisite for emergence of follicular lymphomas. Here we show that in vitro, such cells are more resistant to immune cytolysis when grown as follicle-mimicking tridimensional aggregates than when grown as cell suspensions. So in patients, this innate adaptation to tumor immunity might precede its selective pressure.
Keywords: aggregates, immune escape models, lymphoma, transcriptome
The cure of non-Hodgkin lymphomas (NHL) largely depends upon cytolytic lymphocytes recruited during therapy, but most NHL cells appear relatively resistant to their action. It is thus important to understand how they do so. A hallmark of follicular lymphoma is their growth as dense aggregates in lymph nodes, a feature however lacking from all in vitro studies which currently involve cell suspensions only.
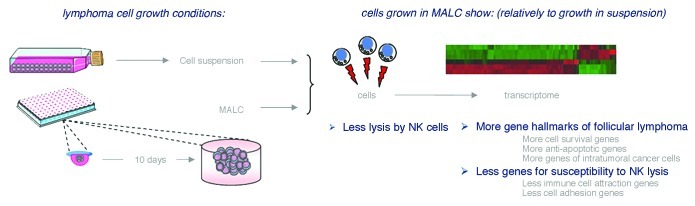
Here we grew lymphoma cells in vitro as tridimensional multicellular aggregates (MALC) to test whether such a mode of cell growth promotes some degree of immuno-resistance. In this aim, MALC of the t(14,18)+ follicular lymphoma cell line RL were produced in vitro by a modified “hanging drop” method.1,2 This type of cell growth formed compact ovoïd aggregates reaching a diameter of ~1 mm by three weeks of culture, which were never produced in the usual cell suspensions in culture flasks. We then asked whether these two kinds of cell growth induced different gene expression profiles. So, the transcriptomes from RL cells grown for 10 d either as MALC or cell suspensions were produced on Affymetrix microarrays (HG-133 plus 2.0) and analyzed by Autocompare.3 451 genes were significantly upregulated in cells grown as MALC comparatively to cell suspensions (> 4×, p < 0.05). These were enriched for genes upregulated in the central area of tumors relative to periphery (p = 10−20)4 and for genes regulated by the histone-lysine N-methyltransferase gene EZH2 (p = 10−12).5 The upregulated MALC genes also reflected the highly relevant hallmarks of follicular lymphoma cells: induction of anti-apoptotic functions (p = 0.0002), pro-survival NFκB cascade (p = 0.0006) and response to hypoxia (p = 0.002). Further, the 241 most downregulated MALC genes (< 0.5×, p < 0.05) indicated shut down of cytokine/cytokine receptor interactions (p = 10−7) and chemokine signaling (p = 10−5). So, lymphoma cells grown in vitro as MALC present a gene expression pattern which is not only highly relevant of follicular lymphoma cells in vivo, but also suggests a pre-adaptation to selective pressure from immunity. This view was strenghened when the lymphoma cells grown either in culture flasks or MALC were dissociated and compared as targets in classical 4 h 51Cr-release lysis assays by allogeneic NK cells from different healthy donors. The lymphoma cells were more resistant to NK lysis when grown as MALC than when grown as cell suspensions (Fig. 1).
Figure 1. Follicular lymphoma cell growth in vitro as MALC increases immune resistance.
Various lymphoma cell-intrinsic immuno-escape pathways such as altered HLA-I, CD58,6 IDO7 and deficient immune synapses8 have been identified. We now propose that growth as cell aggregates represent another tumor cell-intrinsic pathway of immuno-resistance for follicular lymphomas. The transcriptome of follicular lymphoma cells grown as MALC was characteristic of deeply intra-tumoral cancer cells4 and reflected regulation by known drivers of lymphomagenesis.9 Together with these features, cells from MALC showed more anti-apoptotic and “immunosilent” gene expression profiles and they showed higher resistance to NK lysis than cells in suspension. So when grown aggregated in vitro, the lymphoma cells show a remarkable tumor-intrinsic pre-adaptation to further immune pressure. These observations suggest that natural lymphomagenesis in lymphoid organs from patients might also involve such an “innate” predisposition to immune escape.
Glossary
Abbreviations:
- MALC
multicellular aggregates of lymphoma cells
- IDO
Indoleamine 2,3-dioxygenase
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/19365
References
- 1.Timmins NE, Nielsen LK. Generation of multicellular tumor spheroids by the hanging-drop method. Methods Mol Med. 2007;140:141–51. doi: 10.1007/978-1-59745-443-8_8. [DOI] [PubMed] [Google Scholar]
- 2.Dalle S, Dupire S, Brunet-Manquat S, Reslan L, Plesa A, Dumontet C. In vivo model of follicular lymphoma resistant to rituximab. Clin Cancer Res. 2009;15:851–7. doi: 10.1158/1078-0432.CCR-08-1685. [DOI] [PubMed] [Google Scholar]
- 3.Pont F, Familiades J, De´jean S, Fruchon S, Cendron D, Poupot M, et al. The gene expression profile of phosphoantigen-specific human γδ T lymphocytes is a blend of αβ T-cell and NK-cell signatures. Eur J Immunol. 2012;42:228–40. doi: 10.1002/eji.201141870. [DOI] [PubMed] [Google Scholar]
- 4.Nakamura T, Kuwai T, Kitadai Y, Sasaki T, Fan D, Coombes KR, et al. Zonal heterogeneity for gene expression in human pancreatic carcinoma. Cancer Res. 2007;67:7597–604. doi: 10.1158/0008-5472.CAN-07-0874. [DOI] [PubMed] [Google Scholar]
- 5.Nuytten M, Beke L, Van Eynde A, Ceulemans H, Beullens M, Van Hummelen P, et al. The transcriptional repressor NIPP1 is an essential player in EZH2-mediated gene silencing. Oncogene. 2008;27:1449–60. doi: 10.1038/sj.onc.1210774. [DOI] [PubMed] [Google Scholar]
- 6.Challa-Malladi M, Lieu YK, Califano O, Holmes AB, Bhagat G, Murty VV, et al. Combined genetic inactivation of β2-microglobulin and CD58 reveals frequent escape from immune recognition in diffuse large B cell lymphoma. Cancer Cell. 2011;20:728–40. doi: 10.1016/j.ccr.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ninomiya S, Hara T, Tsurumi H, Hoshi M, Kanemura N, Goto N, et al. Indoleamine 2,3-dioxygenase in tumor tissue indicates prognosis in patients with diffuse large B-cell lymphoma treated with R-CHOP. Ann Hematol. 2011;90:409–16. doi: 10.1007/s00277-010-1093-z. [DOI] [PubMed] [Google Scholar]
- 8.Ramsay AG, Clear AJ, Kelly G, Fatah R, Matthews J, Macdougall F, et al. Follicular lymphoma cells induce T-cell immunologic synapse dysfunction that can be repaired with lenalidomide: implications for the tumor microenvironment and immunotherapy. Blood. 2009;114:4713–20. doi: 10.1182/blood-2009-04-217687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lenz G, Staudt LM. Aggressive lymphomas. N Engl J Med. 2010;362:1417–29. doi: 10.1056/NEJMra0807082. [DOI] [PMC free article] [PubMed] [Google Scholar]